Abstract
The rapid pace of innovations and the frequency of replacement of electrical and electronic equipment has made waste printed circuit boards (WPCB) one of the fastest growing waste streams. The frequency of replacement of equipment can be caused by a limited time of proper functioning and increasing malfunctions. Resource utilization of WPCBs have become some of the most profitable companies in the recycling industry. To facilitate WPCB recycling, several advanced technologies such as pyrometallurgy, hydrometallurgy and biometallurgy have been developed. Bioleaching uses naturally occurring microorganisms and their metabolic products to recover valuable metals, which is a promising technology due to its cost-effectiveness, environmental friendliness, and sustainability. However, there is sparse comprehensive research on WPCB bioleaching. Therefore, in this work, a short review was conducted from the perspective of potential microorganisms, bioleaching mechanisms and parameter optimization. Perspectives on future research directions are also discussed.
1. Introduction
Rapid technological innovation has greatly shortened the life cycle of electronic products [1,2]. The massive generation of waste electrical and electronic equipment (WEEE) has displayed exponential growth with a rate of more than 3–5% annually. In 2016, the global amount of electronic waste, so called e-waste, was approximately 44.7 million metric tons (Mt) and has reached 57 Mt in 2021 [3,4]. Printed circuit boards (PCBs) are a major and critical constituent of electrical and electronic equipment [5] and the resultant waste PCBs (WPCBs) account for approximately 3–6 wt.% of total e-waste [6,7]. These WPCBs contain various precious metals, such as gold (Au), silver (Ag) and palladium (Pd) with concentrations tens or hundreds of times larger than than the natural ores [8,9] and have been regarded as an “urban mine” (Figure 1). According to the content of Au, the WPCBs could be divided into low (100 g/t), medium (100–400 g/t), and high (400 g/t) grades [10]. Their net present value varies from 6.8 million € for medium-grade ones to 63.0 million € for high-grade ones [11].
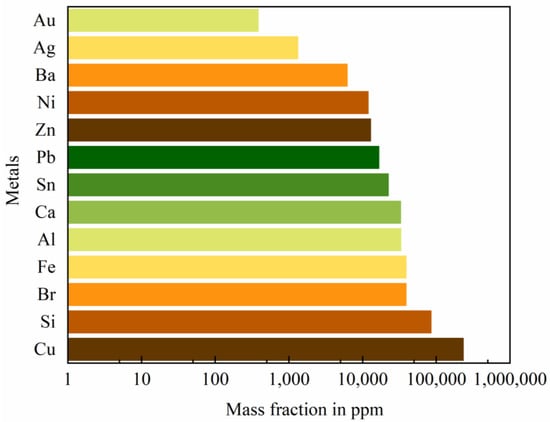
Figure 1.
Metal component in WPCBs [6].
In the past few decades, landfilling, combustion and informal processing activities [12], such as acid stripping and open burning have led to significant environmental pollution. Oloruntoba et al. [13] assessed the status of polybrominated diphenyl ethers (PBDEs) contamination at e-waste dumpsites in Lagos, Nigeria. PBDE levels across the soil profile (0–45 cm depth) showed an increase in PBDE accumulation in the topsoil and migration into the sub-soil. Sediments and rainwater ponds around the dumpsites were found to be contaminated as well. Abubakar et al. [14] studied the heavy metal concentrations, in relation to threshold values, and assessments of risk for noncarcinogenic and cancer risk threat. Pb had the highest mean concentration of 0.0693 ppm, Cu 0.0525 parts per million (ppm), and Cd 0.0042 ppm. The informal e-waste burning had resulted in the substantially high levels of air pollution identified at the treatment points and in turn posed a threat to the environment and public health. Recently, many advanced technologies have been developed to facilitate metal recovery from WPCBs, including pyrometallurgy, hydrometallurgy, physico-mechanical separation, electrolysis, supercritical fluid, and bioleaching (Table 1). Among them, the pyrometallurgical processes are generally operated at 300–900 °C [15,16] and have the disadvantage of high energy consumption and expensive capital investment [17]. Hydrometallurgical processes use cyanide, halide, thiourea and thiosulfate to recover metals [18], consuming large amounts of chemical reagents as well as producing a large volume of effluents. Mechanical beneficiation operations such as gravity air classifiers, eddy current separation, and magnetic separation have been widely used in e-waste recycling plants worldwide. However, the recovered metals are mixed, and need be refined [19].

Table 1.
The comparison of different technologies of WPCB treatment.
Bioleaching uses a variety of microorganisms, including chemolithotrophic prokaryotes, heterotrophic bacteria and fungi to mobilize metals from WPCBs based on the three following mechanisms (Figure 2). (1) Transformation of organic or inorganic acids (protons); (2) Oxidation and reduction reactions; and (3) Excretion of complexing agents [38]. It overcomes the problems of high energy consumption, serious environmental pollution and complex operation and thus has been regarded as a promising method for metals recovery [39]. Recently, different leaching techniques have been developed. In laboratory-scale investigations, percolator leaching, submerged leaching and column leaching are employed. For industrial-scale purposes, dump leaching, heap leaching, underground leaching and tank leaching are applied [40]. Kumar et al. [41] used Pseudomonas balearica SAE1 to achieve the dissolution of Au and Ag, resulting in a recovery of 68.5% Au, and 33.8% Ag, at pH of 9.0, pulp density of 10 g/L, temperature of 30 °C, and glycine concentration of 5 g/L. Zhou et al. [42] proposed biological detoxification and comprehensive utilization of non-metal residue from waste copper clad laminate. The leaching rate of metal was close to 100% and was 8.7% higher than that by dilute sulfuric acid leaching. It further demonstrated the potential of microorganisms and the feasibility of the bioleaching approach. The bioleaching of WPCBs has been attempted however, there are sparse systematic studies on this topic. The bioleaching mechanism and optimization of leaching conditions are not clear, and need be further probed. Therefore, a short review was conducted from the perspective of potential microorganisms, bioleaching mechanism and parameters optimization. The perspectives on future research directions were also discussed. Promotion of experimental scale studies to a larger scale in practice is expected to speed up e-waste recycling and result in zero waste buildup in China.
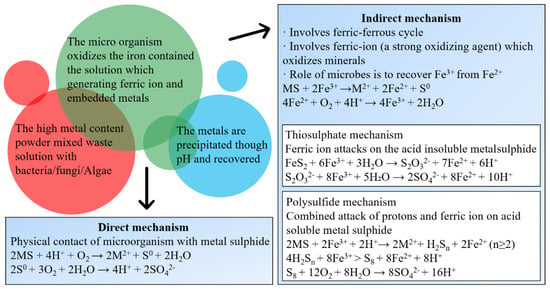
Figure 2.
Bioleaching mechanisms of metals from WPCBs [25].
2. Microorganisms
Microbes were found to have the ability to extract metals a long time ago, therefore microbial technology has been more developed. It has long been noticed that initially microorganisms were only used to extract metals from mine resources. The use of microbial interactions on mineral substances is gaining practical significance. Leaching metals from ores makes it possible to obtain solutions, for example copper or uranium, from which these metals can be recovered using hydrometallurgical processes. Microbes also offer the possibility to recover metals from e-waste. In the bioleaching process, the growth of microorganisms usually involves two stages. The reaction of organic sugar and acid with WPCBs takes place in the first stage, while the second—the development of microorganisms [43]. Most often, bioleaching is carried out in an acidic environment, using processes such as the oxidation of sulphur or its reduced compounds to sulfuric acid and the production of, for example, organic acids in oxygen respiratory cycles or as a result of fermentation of carbohydrates. The microorganisms involved in the leaching include not only bacteria (Acidithiobacillus, Thiobacillus), but also fungi (Penicillium/Aspergillus/Fusarium/Alternaria Candida). Iron and sulfur-oxidizing bacteria (e.g., Acidithiobacillus ferrooxidans, Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans) are widely used in bio-hydrometallurgical process [44]. Generally, there are two different ways of heterotrophic and autotrophic bioleaching (Figure 3), and three groups of microorganisms could be microbial candidates applied for WPCB recovery, including chemolithotrophic prokaryotes (e.g., Acidithiobacillus thiooxidans, Acidithiobacillus ferrooxidans), heterotrophic bacteria (e.g., Chromobacterium violaceum, Pseudomonas sp., and Bacillus megaterium) and fungi (e.g., Aspergillus niger, Penicillium simplicissimum).
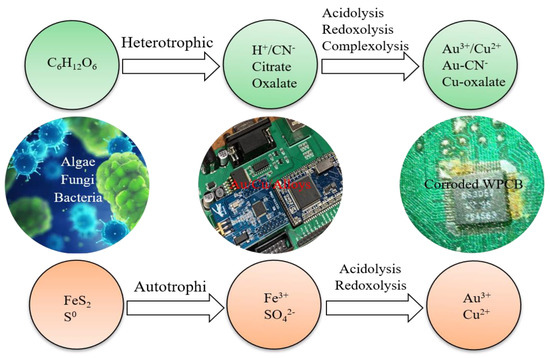
Figure 3.
Heterotrophic and autotrophic bioleaching of WPCBs [26].
2.1. Autotrophic Bioleaching
The chemolithotrophic organisms use atmospheric carbon dioxide as a carbon source and inorganic compounds such as ferrous iron (Fe2+), elemental sulfur (S°) and/or reduced sulfur compounds (S8, S2O32−, H2S and polysulfide) as an energy source [45]. These characteristics facilitate metal dissolution through a series of bio-oxidants and bioleaching reactions [46]. According to their preferred temperatures, the chemolithotrophic organisms could be classified as mesophiles (28–37 °C), moderate thermophiles (40–60 °C) and thermophiles (60–80 °C). Most of them grow at a low pH of 2.0 or below, and have a high tolerance for heavy metal toxicity [47]. Acidithiobacillus ferrooxidans (A. ferrooxidans) was the most well-known and extensively studied microorganism in biometallurgical applications. Bai et al. [48] used A. ferrooxidans to leach heavy metals from WPCB sludge. The leaching rates of Cu, Ni, and Zn reached 76%, 74%, and 72%, respectively, under the optimized conditions of FeSO4·7H2O concentration of 60 g/L, initial pH of 0.5, reaction time of 6 days. In addition to A. ferrooxidans, other species such as Acidiferrobacter thiooxydans (A. thiooxydans), Leptospirillum ferriphilum (L. ferriphilum), Ferrimicrobium acidiphilum (F. acidiphilum), Sulfobacillus thermosulfidooxidans (S. thermosulfidooxidans) were also investigated [49]. Ilyas et al. [50] found the selected moderately thermophilic strains of a mixed adapted consortium of acidophilic chemolothotrophic and acidophilic heterotrophic bacteria could recover 80% Zn, 64% Al, 86% Cu and 74% Ni from WPCB after an acid pre-leaching of 27 days and a bioleaching of 280 days. As compared to using single microbial species, the utilization of different types of chemolithotrophic organisms might yield a better result. It is worth noting that WPCBs are alkaline in nature and more acid was needed to neutralize them, so as to maintain an optimum pH for the microorganisms.
2.2. Heterotrophic Bioleaching
Heterotrophic bacteria and fungi are the main microorganisms of heterotrophic bioleaching, relying on organic compounds as energy sources in their metabolism. In the growth phase, they secrete different organic acids such as lactic, citric, oxalic and gluconic acids as well as enzymes [51], which could be used in the leaching process. In contrast to chemolithotrophic organisms, they can tolerate a wider range of pH as well as complex metals and are employed for treating moderately alkaline wastes [52]. More importantly, in the case of some types of WPCB that lack metal sulfides and cannot provide a sufficient supply of energy sources, heterotrophic bioleaching is regarded as a more promising method.
2.2.1. Heterotrophic Bacteria
Heterotrophic bacteria produce acids during bacterial leaching. The byproducts could be utilized to dissolve metals. Cyanogenic bacteria that produce CN are considered the most typical ones. According to the report [53], the first acidophilic heterotrophic bacterium which is indigenous and active in mineral leaching environments was isolated and characterized some 40 years after the iron/sulfur-oxidizing chemolithotroph T. ferrooxidans and 70 years after the sulfur-oxidizing acidophile T. thiooxidans. After that, more active metal-solubilizing bacteria were isolated and characterized. Various Pseudomonas species were used in the bioleaching of Cu, Au, Ag, Pt, and Zn. Chromobacterium violaceum was used in the bioleaching of Au from high-grade cell phone WPCBs, reaching a recovery tate of 10.8% Au in 8 days [54].
2.2.2. Fungi
Fungi produce a large amount of organic complexing agents, including citric acid, tartaric acid, oxalic acid, and even carboxylic acids, inducing the solubilization of metals from WPCBs by regulating redox potential and acidity during the fungal bioleaching process, and acidolysis, and redoxolysis mechanisms have been described [55]. Typically, this process is carried out at a relatively higher pH (9.0–10.5) and the fungi are able to adapt to high pulp densities of 10% (w/v) [56].
Fungal species like Penicillium chrysogenum, Aspergillus niger and yeast have been thoroughly investigated for bioleaching of metals from solid industrial wastes [57]. According to recent studies, the Penicillium chrysogenum strain KBS3 achieved a maximum solubilization of nickel (55%), copper (67%), magnesium (69%), cobalt (60%), and zinc (65%) from mine tailings [58]. A study was carried out on Aspergillus niger in the bioleaching of metals from fly ash [59]. A recovery rate of 56.1% for Cu, 15.7% for Al, 20.5 % for Pb, 49.5% for Zn and 8.1% for Sn were achieved.
It is worth mentioning that the metals in WPCBs could be extracted by bioleaching, however, a significant amount of nonmetallic fraction is left out and remains of great environmental concern. Numerous attempts have revealed that the microorganisms not only have the ability for metal extraction but also are promising for the degradation of plastics. An increasing number of isolates of bacterial (e.g., Bacillus, Rhodococcus), fungal (e.g., Aspergillus, Penicillium), and bacterial consortia (e.g., Souda and Agios, Bacillus cereus and Bacillus sphaericus) with degradation properties and effects on organic compounds have been identified [60]. Senophiyah-Mary et al. [61] revealed that the microbes used for bioleaching had potential for deteriorating plastics, particularly with the assistance of sunlight, UV, etc.
3. Bioleaching Mechanisms
It should be noted that the mechanisms of microbial interaction are not very clear. The stages of contact and non-contact mechanisms (which are currently known) are presented in Figure 4. For A. ferrooxidans, the main mechanism is indirect, where metals are dissolved through the prior oxidation of ferrous ions. In the case of A. thiooxidans, conversion of metals into a soluble state is mostly done by sulfuric acid production [62]. According to literature sources, in some cases contact bioleaching dominates over the non-contact one, influencing a larger metal recovery process. Mishra et al. [41] explained that it was mainly because the direct mechanism involved direct physical contact of microorganisms with target material surfaces.
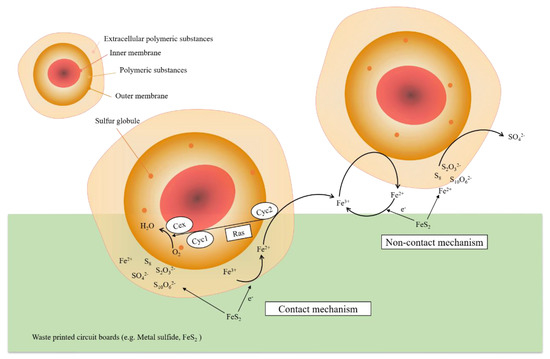
Figure 4.
Critical steps in contact and non-contact mechanisms [28].
3.1. Contact Bioleaching
In contact bioleaching, there is physical contact of the microorganisms with metal sulfide (e.g., Pyrite, FeS2). Metal solubilization takes place when bacteria such as A. ferrooxidans oxidize the metal sulfides and directly obtain electrons from the reduced materials. When the Fe3+ from the extracellular polymeric substances (EPS) layer accepts an electron, it will be reduced to Fe2+ and diffuse towards the outer membrane, where the ion may be re-oxidized to Fe3+ again. The hydrolytic reaction of Fe3+ may also be inhibited in acid conditions.
S0 + O2 + H2O → H2SO3
2H2SO3 + O2 → 2H2SO4
Me + Fe3+ + H+ + O2 → Me2+ + Fe2+ + H2O
4FeS2 + 14O2 + 4H2O → 4FeSO4 + 4H2SO4
4FeSO4 + O2 + 2H2SO4 → 2Fe2(SO4)3 + 2H2O
Silva et al. [63] studied the influence of contact mechanisms during Cu bioleaching from WPCBs using a partition system. A reduction of 25% in the extraction was observed, when the contact mechanism was disabled. The bacterial attachment to the WPCB surface was proven to be 4.3 × 107 cells/g, implying that the disabling of bacterial attachment was the only reason for the decrease in the extraction efficiency. To better understand the mineralogical effect of EPS, the micromorphologies of residues after bioleaching were analyzed [64]. Intensive adsorption sites such as rills and micropores were observed on the mineral surface, implying a stronger contact mechanism. Sulfur-oxidizers such as A. thiooxidans generally showed a greater dependence on these for their adsorption behavior. Interestingly, the size of the micropores was found to be consistent with the cell size of A. caldus.
3.2. Non-Contact Bioleaching
In non-contact bioleaching, a ferric-ferrous cycle exists, which involves planktonic (free-living) bacteria oxidizing Fe2+ to Fe3+, as well as converting sulfur species to sulfuric acid; and the Fe3+ ion accepting electrons from metal sulfides and reducing them to Fe2+ in turn. In the process, metal solubilization could be described according to the following reaction:
4Fe2+ + O2 + 4H+ → 4Fe3+ + 2H2O
2Fe3+ + Me0 → 2Fe2+ + Me2+
MeS + Fe2(SO4)3 → MeSO4 + 2FeSO4 + S0
Gu et al. [65] studied the effect of modified electrode by nitrogen-doped carbon nanotubes in bioleaching copper from WPCB by A. ferrooxidans. The results indicated that the Fe2+ in the culture medium was used as a nutrient substance by A. ferrooxidans, and Fe2+ was oxidized to Fe3+. The resultant Fe3+ would oxidize the copper into a copper ion. Meanwhile, the Fe3+ became Fe2+.
Cu0 + 2Fe3+ → Cu2+ + 2Fe2+
2Fe2+ + O2 + 4H+ → 2Fe3+ + 2H2O
2Fe3+ + Me0 → 2Fe2+ + Me2+
Dong et al. [66] recovered vanadium from low grade stone coal using Bacillus mucilaginosus. Bioleaching behavior was elucidated through the bacteria-mineral contact leaching and non-contact leaching test. In non-contact bioleaching, there is no need for contact between solids and microorganisms. Only the molecular organic acids in the metabolites interacted with the minerals. The macromolecular compounds in the metabolites cannot form complexes with the metallic elements in the minerals, reducing the opportunities for bacteria to utilize the nutrients in the minerals, thus affecting the growth and metabolism of the bacteria.
4. Biochemical Process
Naturally, microorganisms have developed many other processes that influence the biogeochemical cycles of elements (weathering/biooxidation/biotransformation/bioaccumulation/biosorption/bioprecipitation) [67]. Of course, biosorption/bioprecipitation are typical processes that can contribute to the recovery of metals from WPCBs using biological materials. Until now, modern biotechnology has enhanced the ability of metal extraction and optimized performance by understanding the fundamental mechanisms, aimed at economic gain and sustainable development [68].
4.1. Biosorption
Biosorption is a passive physico-chemical and metabolically-independent process, based on the ability of living as well as dead microorganisms to utilize a variety of mechanisms, including complexation, chelation, microprecipitation, and microbial reduction, or combinations of them [69]. It does not require large financial outlays, is characterized by high efficiency and minimization of wastes of chemical or biological origin. Moreover, unlike conventional methods (filtration or adsorption on activated carbon), it enables biomass regeneration and metal recovery. In general, the process is mediated through [37]: (1) Extracellular enrichment and precipitation; (2) Exchange and adsorption of cell surfaces; (3) Intracellular transformation. On a cellular scale, metal ions are initiated on the cell surface or active site of biosorbents by the microorganism’s generated ligands through a chelation process [70].
A vast array of biosorbents with potential for metal recovery have been exploited, most of them prepared from natural or waste biomass. A strain of Penicillium expansum was used to recover metals, and the resultant product obtained is a highly concentrated solution of lanthanum (up to 390 ppm) and terbium (up to 1520 ppm) [71]. Chlorella vulgaris was investigated to recover neodymium from an aqueous solution derived from hard drive disk magnets. The maximum experimental neodymium uptakes at 21, 35 and 50 °C and an initial concentration of 250 mg/L were 126.13, 157.40 and 77.10 mg/g, respectively, at the optimal pH of 5 [71]. Recently, nanomaterials (i.e., nanocellulose structures) and hybrid bio-nanomaterials (assemblies of biological molecules and inorganic nanostructures) with large specific surface areas that could enhance biodegradability and better separation of metal ions, have received attention and are considered the new generation of biosorbents [72].
4.2. Bioprecipitation
Bioprecipitation refers to the process of formation of mineral phases (bioprecipitates or biominerals) by the activity of microorganisms. In the process, microorganisms facilitate precipitation by catalyzing oxidative and reductive processes resulting in the precipitation of soluble metals and non-metals [73]. On a cellular scale, bioreduction of metals takes place either by direct contact with the cell surface or through extracellular electron shuttles. Some metal nanoparticles, such as gold elements, were observed depositing on the outer surface of cells. Desulfovibrio desulfuricans was one of the microorganisms that was successfully used to recover Au3+ as Au0 from test solutions and from waste electronic scrap leachate. When processing the aqua regia leachate of Central Processing Units, the Saccharomyces cerevisiae were able to rapidly and selectively collect aqueous Au(III) ions from the aqua regia leachate at pH 1.2 within 10 min [74]. By using the sulfate reducing inversed fluidized bed bioreactors, the removal efficiencies of Cu and Zn reached 90% at an initial concentration of 25 mg/L [75].
4.3. Parameter Optimization
The bioleaching efficiency is usually not high enough. In most cases, the process is rather time-consuming. In order to improve the efficiency, the bioleaching kinetics were investigated, and the results demonstrated that the interruption in bacterial growth, the formation of precipitates, the toxicity resulting from the increasing level of leached metals, and the increase in the pH of the solutions are all related to the process. In this case, the optimal parameters such as initial pH, temperature, pulp density are considered the critical factors that affect the bioleaching process (Table 2). Understanding their effects well should be beneficial for optimizing their performance.
4.3.1. pH
System pH is an important factor for bioleaching with acidophiles. SOB present in the leaching medium contribute to the system’s acidity which helps in maintaining the system’s pH. This is achieved by the oxidation of sulfur by sulfur-oxidizing bacteria. The optimal pH is 2.0–3.5 for the normal growth of A. ferrooxidans. Crust et al. [76] investigated the effect of pH on the metal dissolution. The leaching of Cu and Ni was seen to be best at an initial pH 1.8, whereas that of Al and Zn had comparable dissolution efficiencies at pH 1.8 and 2.0. At increased pH values (>2 or 2.25), the formation and precipitation of jarosite and/or secondary passivation products was detected, which were unfavorable to the leaching. Gu et al. [65] studied the variation of pH during bioleaching. The pH rose quickly within 5 days and slowly after 7 days, finally reached a steady state. The metabolism of A. ferrooxidans was a process of H+ consumption, and the procedure of zero-valent metal turning into metal ions was also a process which could consume H+.
4.3.2. Inoculum Volume
Crust et al. [76] investigated the effect of inoculum variation (5–20 (v/v)) on the metal dissolution. At higher initial inoculum, the costs involved for preparation and consumption of chemicals will be higher but the metal recoveries will be the same as for the 10% (v/v) initial inoculum. The oxido-reductive potential (ORP) values were nearly similar at higher inoculums indicating a strong oxidizing environment due to the rapid oxidation of Fe2+. Fu et al. [77] studied the effect of inoculation volume on metals leaching by A. ferrooxidans at 20–35 °C. In the early stage (<50 days), the higher the inoculation number of bacteria was, the higher the leaching rate. On the hand, the number of bacteria on WPCB particles per unit area increased, the contact between bacteria and the active site on the particle surface increased, and metal erosion was accelerated. In the middle-late period (50–80 d), with the growth and reproduction of bacteria and the dissolution of metals, the bacterial number and the ferric-ion concentration in the system with less initial inoculation increased, while the metal-leaching efficiency in the system with more inoculation decreased due to the precipitation of jarosite and the lack of nutrients. Considering the industrial application cost, the optimal inoculation was determined as 5%.
4.3.3. Fe2+ Concentration
Iron oxidizing bacteria derive energy by the oxidation of Fe2+ ions resulting in the production of biogenic Fe3+, which is a powerful oxidizing agent for metals leaching. Therefore, the Fe2+ concentration in the medium can affect the metabolism of microbes and the metals leaching rate. Crust et al. [76] investigated the effect of initial Fe2+ concentration (1–9 g/L) on the dissolution of Cu, Al, Ni and Zn. With the initial concentration increasing from 7 to 9 g/L, increases of 20% for Cu and 10% for other metals were observed. The maximum ORP values were obtained operating at 9 g/L initial Fe2+. Gu et al. [65] investigated the variation of Fe2+ concentration during the bioleaching process by A. ferrooxidans. The initial Fe2+ concentration was 8.09 g/L and it declined obviously in the later bioleaching period. Much research indicates that the lower the Fe2+ concentration the final medium has, the better the bioleaching rate the group gets.
4.3.4. Pulp Density
Pulp density also affects the recovery process due to the higher concentration of toxic materials and the change in pH. Garg et al. [78] used mixed microbial consortia of iron and sulfur-oxidizing microorganisms to dispose of waste mobile phone WPCBs for batch bioleaching at varying pulp densities of 7%, 10% and 15% (w/v). The Ni recovery was 20.39% and 47.9% at a pulp density of 7% and 15%. Crust et al. [76] studied the effect of pulp density in the range 2.5% to 15% (w/v). The dissolution of Cu was higher at 2.5% (w/v), whereas the dissolution of Al, Zn and Ni were higher at 10% (w/v). The dissolution of Cu at 10% PD was 91%. Considering dissolution of all targeted metals, 10% (w/v) was the best in order to achieve maximum metal recoveries. The increasing solid content had a significant effect on the pH changes of the bioreactor. At ≤10% (w/v), the diffusion of O2 and/or CO2 is efficient for oxidation of Fe2+, contributing towards higher metal dissolution.
4.3.5. Particle Size
A mechanical activation was discovered to trigger physicochemical changes in solid materials, such as structural defects, phase transformations, and amorphization, to improve their leaching activity [79]. A two-step crushing process has been developed, and heat pretreatment technology before the crushing process has been proposed, as well as for reducing particle size and improving the breakage and liberation effects of WPCBs [80]. During the crushing stage, it should be noted that the micro-cracks produced were beneficial for the growth of bacteria, thereby enhancing the bioleaching efficiency of copper [81].
4.3.6. Catalyst
Studies have shown that graphite can be used as a catalyst to increase the bioleaching rate. In the case of sphalerite, graphite has been found to influence microbial populations [82]. Tong et al. [83] investigated the effect of graphite on leaching behavior of WPCBs. In the absence of graphite, the copper leaching rate was 90% after 5 days. Leaching performance increased with the addition of graphite and it reached 100% with 2.5 g/L of graphite. Gu et al. [35] studied the effect of graphene on bioleaching by Acidithiobacillus ferrooxidans. The Cu leaching rate increased from 74% to 84% by adding graphene in the culture solution. The transformation between Fe2+ and Fe3+ was actuated by A. ferrooxidans and graphene. The graphene could be recycled and reused by treating with HNO3. They also explored the bioleaching efficiency of Cu driven by nitrogen-doped carbon nanotube modified electrodes [65]. It reached 99% and 20% higher than that of the control. The electrical conductivity and specific surface area which the modified electrode had, provided a good platform for the metabolism of A. ferrooxidans and the transfer of electrons.
4.3.7. Bioreactor
Crust et al. [76] investigated the optimal values for key parameters at the shake-flask level and tested the feasibility of these optimal conditions in bench-scale bioreactors. Maximum recovery values of 98.1% Cu, 55.9% Al, 79.5% Ni and 66.9% Zn were achieved under the optimum parameters within 8 days in the laboratory-scale experiments. Under the same optimized conditions, 97.3% Cu, 55.8% Al, 79.3% Ni and 66.8% Zn were bioleached in bench-scale reactors.
4.3.8. Hybrid Bioleaching
Even if the above parameters are optimized, the individual work of those microorganisms is still slow, especially compared to pyrometallurgy and hydrometallurgy processes. Therefore, several studies have been carried out on hybrid bioleaching for better bioleaching effects. For example, Sheel and Pant [84] achieved 90% recovery of Au from e-waste by using the combination of ammonium thiosulfate and Lactobacillus acidophilus. Sinha et al. [85] developed a novel biorecovery process followed by electrochemical treatment to recover copper, achieving 92.7% Cu recovery. Dolker et al. [86] applied the chemical-biological hybrid systems to the bioleaching process, increasing Li leaching by 25% and cobalt biosorption by 98%. Gomes et al. [87] linked electrodialytic remediation with microbial metabolism, higher mobilization of metals was observed when associating both methods, with higher metal concentrations in both anode and cathode compartments, in particular for Cu and Cr. All of them exhibited greater bioleaching potential than individual cultures.

Table 2.
Recent research on the bioleaching of WPCB.
Table 2.
Recent research on the bioleaching of WPCB.
| Microorganisms | WPCB | R | Leaching Efficiencies | References |
|---|---|---|---|---|
| A. ferrooxidans | Mobile phone PCB with size of 37–150 µm | Stirring rate of 170 rpm, temperature of 30 °C, initial pH of 1, pulp density of 9.25 g/L, Fe3+ concentration of 4.17 g/L | Up to 99% Cu and Ni after 55 days | [88] |
| Small pieces with size of <15 mm) | Ambient temperature (20–35 °C), WPCB concentration of 5.0% (w/w), inoculation volume of 5% (v/v). | 95.92% of Cu, 93.53% of Al, 92.58% of Zn, 65.27% of Ni, and 95.33% of Sn | [77] | |
| A. niger | Less than 300 mm | Pulp densities of 0.5–20 g/L, stirring rate of 120 rpm and ambient temperature | 100% of Zn, 80.39% of Ni and 85.88% of Cu in 30 days. | [89] |
| A. Ferrooxidans and A. acidophilum | Particle size of 0.075–1 mm | Pulp density of 7.5 g/L, pH of 2.5, stirring rate of 170 rpm, temperature of 30 °C | 96% Cu, 94.5% Zn, 75% Ni, and 74.5% Pb in 18 days | [90] |
| A. Ferrooxidans and A. Thiooxidans | Particle size of less than 100 µm | Stirring rate of 180 rpm, temperature of 30 °C, pulp density of 15 g/L | Cu of 86%, Zn of 100% and Ni of 100% after leaching in 25 days | [62] |
| A. Ferrooxidans, F. acidiphilum, and L. ferriphilum | Desktop-computer motherboards | Stirring rate of 170 rpm, temperature of 45 °C, pH of 1.6, pulp density of 5% (w/v), concentration of Fe3+ of 9 g/L | 100% after adding 2.5 g/L graphite in 5 days | [83] |
| A. ferrooxidans, L. ferrooxidans and A. thiooxidans | Mobile phone PCB with size of 2 × 2 cm2 | Initial Fe2+ concentration of 9 g/L, pulp density of 10% (w/v), inoculum of 10% (v/v) and initial pH of1.8 | 97.3% Cu, 55.8% Al, 79.3% Ni and 66.8% Zn in bench-scale reactor. | [76] |
5. Conclusions and Perspective
Summarizing the peer-reviewed review of the program for bioleaching of used PCBs, the bioleaching process offers benefits in terms of low-cost and environmental friendliness and also shows promise in the recovery of metals from WPCBs. Chemolithotrophs, bacteria and fungi can be candidates for the microorganisms involved in the bioleaching process. The chemolithotrophs are ferrous and/or reduced sulfur oxidizers, thriving at a low pH, while heterotrophic bacteria and fungi are considered as organotrophs and tolerate a wider range of pH. Their contact and non-contact mechanisms were analyzed, as well as the biochemical processes of biosorption and bioprecipitation, and it was found that contact leaching played as greater role in this process. As shown by the scientific research, microorganisms adapt to various pH conditions, raise the temperature and density of the pulp in the appropriate range, mechanical activation can strengthen and improve their leaching efficiency. However, there is still much room for improvement in bioleaching and further research is required in industrial applications.
Firstly, most microorganisms have a shortage of time-consuming and environmental limitations. Genetic modification could provide the engineered microorganisms with higher efficiency and rapid adaption to environmental changes.
Secondly, hybrid bioleaching should be developed, and the fundamental mechanisms need to be further probed since some remain uncertain. Moreover, the e-waste usually contains lots of complex materials that might have negative effects on microorganisms’ growth and hinder their metabolism. The collection and classification of different types of waste are key processes for providing pure raw materials, and the pretreatment of WPCBs, such as dismantling and shredding, is an important process for removing toxic components and improving bioleaching efficiency.
Lastly, the bioleaching conditions should be optimized as they affect the biological activities of microorganisms. Novel bioreactors should be built, such as light induced systems and energy harvesting systems [91,92]. The addition of various catalysts also needs to be tested, which might help improve bioleaching efficiency.
Author Contributions
Z.Y., X.J., conceptualization; X.J., S.Y., methodology; X.J., M.Y., data curation; X.J., S.Y., writing—original draft; M.Y., A.W., writing—review and editing; X.J., Z.Y., funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant no. LTY21B070002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qiu, R.; Lin, M.; Ruan, J.; Fu, Y.; Hu, J.; Deng, M.; Tang, Y.; Qiu, R. Recovering full metallic resources from waste printed circuit boards: A refined review. J. Clean. Prod. 2020, 244, 118690. [Google Scholar] [CrossRef]
- Yao, Z.; Ling, T.; Sarker, P.K.; Su, W.; Liu, J.; Wu, W.; Tang, J. Recycling difficult-to-treat e-waste cathode-ray-tube glass as construction and building materials: A critical review. Renew. Sustain. Energy Rev. 2018, 81, 595–604. [Google Scholar] [CrossRef]
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor—2017: Quantities, Flows and Resources; United Nations University (UNU): Bonn, Germany; International Telecommunication Union (ITU): Geneva, Switzerland; International Solid Waste Association (ISWA): Vienna, Austria, 2017. [Google Scholar]
- Wang, Q.; Zhang, B.; Yu, S.; Xiong, J.; Yao, Z.; Hu, B.; Yan, J. Waste-printed circuit board recycling: Focusing on preparing polymer composites and geopolymers. ACS Omega 2020, 5, 17850–17856. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Lin, M.; Yao, Z.; Zhu, J.; Ruan, J.; Tang, Y.; Qiu, R. A novel approach of accurately rationing adsorbent for capturing pollutants via chemistry calculation: Rationing the mass of CaCO3 to capture Br-containing substances in the pyrolysis of nonmetallic particles of waste printed circuit boards. J. Hazard. Mater. 2020, 393, 122410. [Google Scholar] [CrossRef]
- Korf, N.; Løvik, A.N.; Figi, R.; Schreiner, C.; Kuntz, C.; Mählitz, P.M.; Rösslein, M.; Wäger, P.; Rotter, V.S. Multi-element chemical analysis of printed circuit boards–challenges and pitfalls. Waste Manag. 2019, 92, 124–136. [Google Scholar] [CrossRef]
- Kaya, M. Waste printed circuit board (WPCB) recycling: Conventional and emerging technology approach. Encycl. Renew. Sustain. Mater. 2020, 4, 677–694. [Google Scholar]
- Li, Y.; Lin, M.; Ni, Z.; Yuan, Z.; Liu, W.; Ruan, J.; Tang, Y.; Qiu, R. Ecological influences of the migration of micro resin particles from crushed waste printed circuit boards on the dumping soil. J. Hazard. Mater. 2020, 386, 121020. [Google Scholar] [CrossRef]
- Han, J.; Duan, C.; Lu, Q.; Jiang, H.; Fan, X.; Wen, P.; Ju, Y. Improvement of the crushing effect of waste printed circuit boards by co-heating swelling with organic solvent. J. Clean. Prod. 2019, 214, 70–78. [Google Scholar] [CrossRef]
- Oguchi, M.; Murakami, S.; Sakanakura, H.; Kida, A.; Kameya, T. A preliminary categorization of end-of-life electrical and electronic equipment as secondary metal resources. Waste Manag. 2011, 31, 2150–2160. [Google Scholar] [CrossRef]
- Adamo, I.D.; Ferella, F.; Gastaldi, M.; Maggiore, F.; Rosa, P.; Terzi, S. Towards sustainable recycling processes: Wasted printed circuit boards as a source of economic opportunities. Resour. Conserv. Recycl. 2019, 149, 455–467. [Google Scholar]
- Işıldar, A.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N. Electronic waste as a secondary source of critical metals: Management and recovery technologies. Resour. Conserv. Recycl. 2018, 135, 296–312. [Google Scholar] [CrossRef]
- Oloruntoba, K.; Sindiku, O.; Osibanjo, O.; Weber, R. Polybrominated diphenyl ethers (PBDEs) concentrations in soil, sediment and water samples around electronic wastes dumpsites in Lagos, Nigeria. Emerg. Contam. 2022, 8, 206–215. [Google Scholar] [CrossRef]
- Abubakar, A.; Zangina, A.S.; Maigari, A.I.; Badamasi, M.M.; Ishak, M.Y.; Abdullahi, A.S.; Haruna, J.A. Pollution of heavy metal threat posed by e-waste burning and its assessment of human health risk. Environ. Sci. Pollut. Res. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Becci, A.; Amato, A.; Fonti, V.; Karaj, D.; Beolchini, F. An innovative biotechnology for metal recovery from printed circuit boards. Resour. Conserv. Recycl. 2020, 153, 104549. [Google Scholar] [CrossRef]
- Yu, S.; Su, W.; Wu, D.; Yao, Z.; Liu, J.; Tang, J.; Wu, W. Thermal treatment of flame retardant plastics: A case study on a waste TV plastic shell sample. Sci. Total Environ. 2019, 675, 651–657. [Google Scholar] [CrossRef]
- Xia, M.; Bao, P.; Liu, A.; Wang, M.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X. Bioleaching of low-grade waste printed circuit boards by mixed fungal culture and its community structure analysis. Resour. Conserv. Recycl. 2018, 136, 267–275. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Guo, Y.; An, N.; Liang, C.; Ge, Z. Bioleaching of gold from waste printed circuit boards by alkali-tolerant Pseudomonas fluorescens. Hydrometallurgy 2020, 194, 105260. [Google Scholar] [CrossRef]
- Ogunniyi, I.O.; Vermaak, M.K.G.; Groot, D.R. Chemical composition and liberation characterization of printed circuit board comminution fines for beneficiation investigations. Waste Manag. 2009, 29, 2140–2146. [Google Scholar] [CrossRef] [Green Version]
- Dinç, N.İ.; Tosun, A.U.; Baştürkcü, E.; Özer, M.; Burat, F. Recovery of valuable metals from WPCB fines by centrifugal gravity separation and froth flotation. J. Mater. Cycles Waste Manag. 2022, 24, 224–236. [Google Scholar] [CrossRef]
- Bilesan, M.R.; Makarova, I.; Wickman, B.; Repo, E. Efficient separation of precious metals from computer waste printed circuit boards by hydrocyclone and dilution-gravity methods. J. Clean. Prod. 2021, 286, 125505. [Google Scholar] [CrossRef]
- Li, J.; Gao, B.; Xu, Z. New technology for separating resin powder and fiberglass powder from fiberglass–resin powder of waste printed circuit boards. Environ. Sci. Technol. 2014, 48, 5171–5178. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Xiong, J.; Yu, S.; Su, W.; Wu, W.; Tang, J.; Wu, D. Kinetic study on the slow pyrolysis of nonmetal fraction of waste printed circuit boards (NMF-WPCBs). Waste Manag. Res. 2020, 38, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Yu, S.; Wu, D.; Lü, X.; Tang, J.; Wu, W.; Yao, Z. Pyrolysis treatment of nonmetal fraction of waste printed circuit boards: Focusing on the fate of bromine. Waste Manag. Res. 2020, 38, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Gurgul, A.; Szczepaniak, W.; Zabłocka-Malicka, M. Incineration and pyrolysis vs. steam gasification of electronic waste. Sci. Total Environ. 2018, 624, 1119–1124. [Google Scholar] [CrossRef]
- Liu, K.; Huang, S.; Jin, Y.; Ma, L.; Wang, W.; Lam, J.C. A green slurry electrolysis to recover valuable metals from waste printed circuit board (WPCB) in recyclable pH-neutral ethylene glycol. J. Hazard. Mater. 2022, 433, 128702. [Google Scholar] [CrossRef]
- Qi, Y.; Yi, X.; Zhang, Y.; Meng, F.; Shu, J.; Xiu, F.; Sun, Z.; Sun, S.; Chen, M. Effect of ionic liquid [MIm] HSO4 on WPCB metal-enriched scraps refined by slurry electrolysis. Environ. Sci. Pollut. Res. 2019, 26, 33260–33268. [Google Scholar] [CrossRef]
- Chu, Y.; Chen, M.; Chen, S.; Wang, B.; Fu, K.; Chen, H. Micro-copper powders recovered from waste printed circuit boards by electrolysis. Hydrometallurgy 2015, 156, 152–157. [Google Scholar] [CrossRef]
- Zhihui, W.; Shuqi, Y.; Jinghao, L.; Yufeng, W.U.; Jiamei, Y.U. Research progress of hydrometallurgy technology for leaching precious metals in waste printed circuit board. Environ. Chem. 2021, 40, 886–895. [Google Scholar]
- Birloaga, I.; Vegliò, F. Hydrometallurgical Processing of Waste Printed Circuit Boards; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–113. [Google Scholar]
- Zhu, P.; Tang, J.; Tao, Q.; Wang, Y.; Wang, J.; Li, Z.; Cao, Z.; Qian, G.; Theiss, F.; Frost, R.L. The kinetics study of dissolving SnPb solder by hydrometallurgy. Environ. Eng. Sci. 2019, 36, 1236–1243. [Google Scholar] [CrossRef]
- Li, K.; Xu, Z. A review of current progress of supercritical fluid technologies for e-waste treatment. J. Clean. Prod. 2019, 227, 794–809. [Google Scholar] [CrossRef]
- Chen, J.; Meng, T.; Leng, E.; Jiaqiang, E. Review on metal dissolution characteristics and harmful metals recovery from electronic wastes by supercritical water. J. Hazard. Mater. 2022, 424, 127693. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Zhang, F. Degradation of brominated epoxy resin and metal recovery from waste printed circuit boards through batch sub/supercritical water treatments. Chem. Eng. J. 2013, 219, 131–136. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Dong, B.; Zhuang, X.; Zhao, J.; Zhang, C.; Wang, J.; Shih, K. Catalytic effect of graphene in bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 2017, 171, 172–178. [Google Scholar] [CrossRef]
- Trivedi, A.; Hait, S. Bioleaching of Selected Metals from E-Waste Using Pure and Mixed Cultures of Aspergillus Species; Springer: Berlin/Heidelberg, Germany, 2020; pp. 271–280. [Google Scholar]
- Huang, H.; Jia, Q.; Jing, W.; Dahms, H.; Wang, L. Screening strains for microbial biosorption technology of cadmium. Chemosphere 2020, 251, 126428. [Google Scholar] [CrossRef] [PubMed]
- Asghari, I.; Mousavi, S.M.; Amiri, F.; Tavassoli, S. Bioleaching of spent refinery catalysts: A review. J. Ind. Eng. Chem. 2013, 19, 1069–1081. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Hasan, M.; Mishra, Y.K.; Pandey, A.K.; Tiwary, B.N.; Kuhad, R.C.; Gupta, V.K.; Thakur, V.K. Environmentally sound system for E-waste: Biotechnological perspectives. Curr. Res. Biotechnol. 2019, 1, 58–64. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, H.S.; Kumar, S. Bioleaching of gold and silver from waste printed circuit boards by Pseudomonas balearica SAE1 isolated from an e-waste recycling facility. Curr. Microbiol. 2018, 75, 194–201. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Y.; Cheng, H.; Liu, X.; Tian, Z.; Zhang, L.; Kang, X.; Ge, Y.; Peng, J.; Sun, J. A novel process for the biological detoxification of non-metal residue from waste copper clad laminate treatment: From lab to pilot scale. J. Clean. Prod. 2020, 255, 120116. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, T.; Awual, M.R.; Rahman, A.; Sultana, M.; Abd Aziz, A.; Monir, M.U.; Teo, S.H.; Hasan, M. Advances in sustainable approaches to recover metals from e-waste-A review. J. Clean. Prod. 2020, 244, 118815. [Google Scholar] [CrossRef]
- Becci, A.; Amato, A.; Rodríguez-Maroto, J.M.; Beolchini, F. Bioleaching of End-of-Life Printed Circuit Boards: Mathematical Modeling and Kinetic Analysis. Ind. Eng. Chem. Res. 2021, 60, 4261–4268. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Potysz, A.; Pędziwiatr, A.; Hedwig, S.; Lenz, M. Bioleaching and toxicity of metallurgical wastes. J. Environ. Chem. Eng. 2020, 8, 104450. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
- Bai, J.; Weihua, G.; Changzhong, L.; Wenyi, Y.; Chenglong, Z.; Jingwei, W.; Bin, D.; Kaimin, S. Bioleaching for Extracting Heavy Metals from Electronic Waste Sludge; Elsevier: Amsterdam, The Netherlands, 2019; pp. 525–551. [Google Scholar]
- Johnson, D.B. Biomining—biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef]
- Ilyas, S.; Ruan, C.; Bhatti, H.N.; Ghauri, M.A.; Anwar, M.A. Column bioleaching of metals from electronic scrap. Hydrometallurgy 2010, 101, 135–140. [Google Scholar] [CrossRef]
- Gu, T.; Rastegar, S.O.; Mousavi, S.M.; Li, M.; Zhou, M. Advances in bioleaching for recovery of metals and bioremediation of fuel ash and sewage sludge. Bioresour. Technol. 2018, 261, 428–440. [Google Scholar] [CrossRef]
- Natarajan, G.; Ramanathan, T.; Bharadwaj, A.; Ting, Y. Bioleaching of metals from major hazardous solid wastes. Microbiol. Miner. Met. Mater. Environ. 2015, 2025, 229–262. [Google Scholar]
- Johnson, D.B.; Roberto, F.F. Heterotrophic Acidophiles and Their Roles in the Bioleaching of Sulfide Minerals; Springer: Berlin/Heidelberg, Germany, 1997; pp. 259–279. [Google Scholar]
- Chi, T.D.; Lee, J.; Pandey, B.D.; Yoo, K.; Jeong, J. Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Miner. Eng. 2011, 24, 1219–1222. [Google Scholar] [CrossRef]
- Işıldar, A.; Vossenberg, J.V.D.; Rene, E.R.; Hullebusch, E.D.V.; Lens, P.N. Biorecovery of Metals from Electronic Waste; Springer: Berlin/Heidelberg, Germany, 2017; pp. 241–278. [Google Scholar]
- Brandl, H.; Bosshard, R.; Wegmann, M. Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Mishra, D.; Rhee, Y.H. Microbial leaching of metals from solid industrial wastes. J. Microbiol. 2014, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Chi, R.; Lee, J. Fungal bioleaching of metals from mine tailing. Min. Proc. Ext. Met. Rev. 2013, 34, 185–194. [Google Scholar] [CrossRef]
- Wu, H.; Ting, Y. Metal extraction from municipal solid waste (MSW) incinerator fly ash—Chemical leaching and fungal bioleaching. Enzym. Microb. Technol. 2006, 38, 839–847. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Senophiyah-Mary, J.; Loganath, R.; Shameer, P.M. Deterioration of cross linked polymers of thermoset plastics of e-waste as a side part of bioleaching process. J. Environ. Chem. Eng. 2018, 6, 3185–3191. [Google Scholar] [CrossRef]
- Mostafavi, M.; Mirazimi, S.; Rashchi, F.; Faraji, F.; Mostoufi, N. Bioleaching and kinetic investigation of WPCBs by A. ferrooxidans, A. thiooxidans and their mixtures. J. Chem. Pet. Eng. 2018, 52, 81–91. [Google Scholar]
- Silva, R.A.; Park, J.; Lee, E.; Park, J.; Choi, S.Q.; Kim, H. Influence of bacterial adhesion on copper extraction from printed circuit boards. Sep. Purif. Technol. 2015, 143, 169–176. [Google Scholar] [CrossRef]
- Feng, S.; Li, K.; Huang, Z.; Tong, Y.; Yang, H. Specific mechanism of Acidithiobacillus caldus extracellular polymeric substances in the bioleaching of copper-bearing sulfide ore. PLoS ONE 2019, 14, e213945. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Dong, B.; Zhuang, X.; Zhao, J.; Zhang, C.; Wang, J.; Shih, K. Enhanced bioleaching efficiency of copper from waste printed circuit board driven by nitrogen-doped carbon nanotubes modified electrode. Chem. Eng. J. 2017, 324, 122–129. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Liu, Y.; Zhao, Y. Blank roasting and bioleaching of stone coal for vanadium recycling. J. Clean. Prod. 2020, 243, 118625. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Rathna, R.; Nakkeeran, E. Biological Treatment for the Recovery of Minerals from Low-Grade Ores; Elsevier: Amsterdam, The Netherlands, 2020; pp. 437–458. [Google Scholar]
- Marra, A.; Cesaro, A.; Rene, E.R.; Belgiorno, V.; Lens, P.N. Bioleaching of metals from WEEE shredding dust. J. Environ. Manag. 2018, 210, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Di Piazza, S.; Cecchi, G.; Cardinale, A.M.; Carbone, C.; Mariotti, M.G.; Giovine, M.; Zotti, M. Penicillium expansum Link strain for a biometallurgical method to recover REEs from WEEE. Waste Manag. 2017, 60, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Calderón, O.A.; Abdeldayem, O.M.; Pugazhendhi, A.; Rene, E.R. Current updates and perspectives of biosorption technology: An alternative for the removal of heavy metals from wastewater. Curr. Pollut. Rep. 2020, 6, 8–27. [Google Scholar] [CrossRef]
- Rene, E.R.; Sahinkaya, E.; Lewis, A.; Lens, P.N. Sustainable Heavy Metal Remediation: Volume 1: Principles and Processes; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Saitoh, N.; Fujimori, R.; Nakatani, M.; Yoshihara, D.; Nomura, T.; Konishi, Y. Microbial recovery of gold from neutral and acidic solutions by the baker’s yeast Saccharomyces cerevisiae. Hydrometallurgy 2018, 181, 29–34. [Google Scholar] [CrossRef]
- Janyasuthiwong, S.; Rene, E.R.; Esposito, G.; Lens, P.N. Effect of pH on Cu, Ni and Zn removal by biogenic sulfide precipitation in an inversed fluidized bed bioreactor. Hydrometallurgy 2015, 158, 94–100. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Panda, S. Intensified acidophilic bioleaching of multi-metals from waste printed circuit boards (WPCBs) of spent mobile phones. J. Chem. Technol. Biotechnol. 2020, 95, 2272–2285. [Google Scholar] [CrossRef]
- Fu, K.; Tian, L.; Hou, P.; Long, M.; Chen, S.; Lin, H. Stirred-tank leaching of coarse-grained waste, printed circuit boards with Acidithiobacillus ferrooxidans. Physicochem. Probl. Miner. Process. 2021, 57, 153–163. [Google Scholar] [CrossRef]
- Garg, H.; Nagar, N.; Ellamparuthy, G.; Angadi, S.I.; Gahan, C.S. Bench scale microbial catalysed leaching of mobile phone PCBs with an increasing pulp density. Heliyon 2019, 5, e2883. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Lu, L.; Zhuang, X.; Zhao, J.; Yuan, W.; Zhang, C.; Wang, J. Improved bioleaching efficiency of metals from waste printed circuit boards by mechanical activation. Waste Manag. 2019, 98, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Guo, J.; Zhu, G.; Zhang, Z.; Zhao, P.; Xiangnan, Z.; Zhang, B. Liberation enhancement and copper enrichment improvement for waste printed circuit boards by heating pretreatment. Waste Manag. 2020, 106, 145–154. [Google Scholar] [CrossRef]
- Chen, J.; Tang, D.; Zhong, S.; Zhong, W.; Li, B. The influence of micro-cracks on copper extraction by bioleaching. Hydrometallurgy 2020, 191, 105243. [Google Scholar] [CrossRef]
- Mehrabani, J.V.; Shafaei, S.Z.; Noaparast, M.; Mousavi, S.M. Bioleaching of different pyrites and sphalerite in the presence of graphite. Geomicrobiol. J. 2017, 34, 97–108. [Google Scholar] [CrossRef]
- Tong, L.; Zhao, Q.; Kamali, A.R.; Sand, W.; Yang, H. Effect of graphite on copper bioleaching from waste printed circuit boards. Minerals 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Sheel, A.; Pant, D. Recovery of gold from electronic waste using chemical assisted microbial biosorption (hybrid) technique. Bioresour. Technol. 2018, 247, 1189–1192. [Google Scholar] [CrossRef]
- Sinha, R.; Chauhan, G.; Singh, A.; Kumar, A.; Acharya, S. A novel eco-friendly hybrid approach for recovery and reuse of copper from electronic waste. J. Environ. Chem. Eng. 2018, 6, 1053–1061. [Google Scholar] [CrossRef]
- Dolker, T.; Pant, D. Chemical-biological hybrid systems for the metal recovery from waste lithium ion battery. J. Environ. Manag. 2019, 248, 109270. [Google Scholar] [CrossRef]
- Gomes, H.I.; Funari, V.; Dinelli, E.; Soavi, F. Enhanced electrodialytic bioleaching of fly ashes of municipal solid waste incineration for metal recovery. Electrochim. Acta 2020, 345, 136188. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Hait, S. Biometallurgical recovery of metals from waste printed circuit boards using pure and mixed strains of Acidithiobacillus ferrooxidans and Acidiphilium acidophilum. Process Saf. Environ. Prot. 2020, 143, 262–272. [Google Scholar] [CrossRef]
- Sahu, M.; Hajra, S.; Kim, H.; Rubahn, H.; Kumar Mishra, Y.; Kim, H.J. Additive manufacturing-based recycling of laboratory waste into energy harvesting device for self-powered applications. Nano Energy 2021, 88, 106255. [Google Scholar] [CrossRef]
- Sahu, M.; Hajra, S.; Panda, S.; Rajaitha, M.; Panigrahi, B.K.; Rubahn, H.; Mishra, Y.K.; Kim, H.J. Waste textiles as the versatile triboelectric energy-harvesting platform for self-powered applications in sports and athletics. Nano Energy 2022, 97, 107208. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).