Abstract
Advanced oxidation processes (AOPs) based on peroxydisulfate (PDS) or peroxymonosulfate (PMS) activation have attracted much research attention in the last decade for the degradation of recalcitrant organic contaminants. Sulfate (SO4•−) and hydroxyl (•OH) radicals are most frequently generated from catalytic PDS/PMS decomposition by thermal, base, irradiation, transition metals and carbon materials. In addition, increasingly more recent studies have reported the involvement of singlet oxygen (1O2) during PDS/PMS-based AOPs. Typically, 1O2 can be produced either along with SO4•− and •OH or discovered as the dominant reactive oxygen species (ROSs) for pollutants degradation. This paper reviews recent advances in 1O2 generation during PDS/PMS activation. First, it introduces the basic chemistry of 1O2, its oxidation properties and detection methodologies. Furthermore, it elaborates different activation strategies/techniques, including homogeneous and heterogeneous systems, and discusses the possible reaction mechanisms to give an overview of the principle of 1O2 production by activating PDS/PMS. Moreover, although 1O2 has shown promising features such as high degradation selectivity and anti-interference capability, its production pathways and mechanisms remain controversial in the present literatures. Therefore, this study identifies the research gaps and proposes future perspectives in the aspects of novel catalysts and related mechanisms.
1. Introduction
Nowadays the wide occurrence of emerging organic and refractory pollutants in the soil and aquatic environment has raised much concern about water and food security. Advanced oxidation processes (AOPs) are feasible options to remove these undesirable pollutants by producing reactive oxidizing species (ROSs), such as hydroxyl radicals (•OH, E0 = 2.8 V) and sulfate radicals (SO4•−, E0 = 2.5 − 3.1 V) [1,2]. In the past two decades, AOPs using peroxydisulfate (PDS, S2O82−) and peroxymonosulfate (PMS, HSO5−) as oxidants have attracted much attention for the non-selective degradation of a wide range of pollutants. Because PDS and PMS are solid powders, they can be easily delivered. Compared to H2O2, the anions of PDS/PMS can remain stable in water much longer until being properly activated. In addition, PMS and PDS-based AOPs can be smoothly carried out over a broad solution pH range from acid to alkaline while H2O2-based Fenton processes require strict acidic conditions. Typically, PDS and PMS should be properly activated by breaking the O-O bond with the aid of ultraviolet, heat, alkali or metallic catalysts and so on. SO4•− and •OH are commonly produced as the main ROSs [3]. Despite how promising both SO4•− and •OH are for degrading organic contaminants, their widespread application to wastewater has been limited by the presence of radical quenchers, such as some inorganic anions and naturally-occurring organic compounds [4,5]. However, many recent articles also indicate that singlet oxygen (1O2, a non-radical reactive oxidizing species) can be produced for pollutants degradation via a non-radical process instead of radical attacking pathways [6]. Compared to SO4•− and •OH, 1O2 shows higher selectivity to electron-rich organics because of its electrophilic nature [7,8,9]. This advantage is conducive to the degradation of micropollutants, such as pharmaceuticals and endocrine-disrupting compounds (EDCs), in the coexistence of salinity and other organic matter.
The other well-known method for 1O2 generation is photosensitized excitation of molecular oxygen. Photosensitizers including dyes, porphyrins, transition metal complexes and semiconductors act as the medium by transferring the light energy to ground dioxygen [10]. Other approaches to form 1O2 involve the use of chemicals like potassium perchromate [11], ozonide [12], H2O2-hypochlorous [13], periodate [14], and bismuth oxides [15]. But these processes have to use toxic sensitizers or consume too much bismuth precursors. As an alternative, activating PDS/PMS is a feasible and green chemical process for 1O2 production.
Current studies suggest that catalyst is influential to regulate the oxidizing species formed during PDS/PMS-based AOPs. For example, 1O2 has been frequently found as the primary reactive species by using carbonaceous catalysts in PDS and PMS activation [16], but SO4•− and •OH are usually found to be the dominant species when PDS/PMS are activated by homogeneous heat, UV, and transition metals [3,17]. The difference between PDS and PMS leads to different activation pathways. In particular, a study by Wang and others indicated that SO4•− was mainly formed during MnO2-induced activation of PMS [18], but 1O2 was evolved when PMS was replaced by PDS [19]. Given the increasing interest in PDS/PMS, some recent works have reviewed the application of PDS/PMS-based AOPs for water treatment [2,3,4,17,20,21]. Most of these reviews focus on the generation of SO4•−, however, a comprehensive assessment of 1O2 formation during PDS/PMS activation is rarely reported.
The present study reviews recent progress of 1O2 formation in PDS/PMS activating systems. A brief introduction of the chemistry of 1O2, performance of various types of catalysts, and insights into the possible mechanisms are critically discussed here. Finally, the research barriers and future perspectives are summarized in the last part. This review will provide more guidance for future research of PDS/PMS activation for water purification.
2. Chemical Feature of 1O2 and Its Oxidation
Singlet oxygen (1O2) is the excited molecular oxygen, and broadly refers to two low-lying states oxygen species O2 (1Δg) and O2 (1Σg+). Due to its short lifetime (10−12 s), O2 (1Σg+) is easily converted to lower excited O2 (1Δg) (lifetime 10−3–10−6 s) [22]. Herzberg first defined this excited oxygen with higher energy as 1O2, but its importance was not recognized until 1964 when scientists established its role in chemical oxidation [23]. 1O2 is a non-radical species with energy of 94.2 kJ per mole above ground-state molecular oxygen. As a mild oxidizing species, its standard redox potential (E0 = 1.52 V) is significantly lower than that of •OH (E0 = 2.8 V) and SO4•− (E0 = 2.5 − 3.1 V) [24,25]. The generation of 1O2 has been reported in a relatively broad pH range by various approaches (Figure 1a) including: (1) photosensitized excitation of triplet dioxygen [10,26]; (2) reaction of H2O2 with NaClO [13]; (3) released from phosphite ozonides, bismuth oxides and some organic peroxides [12,15]; (4) proper activation of inorganic oxidants, such as PDS/PMS and periodate [7,14,27].
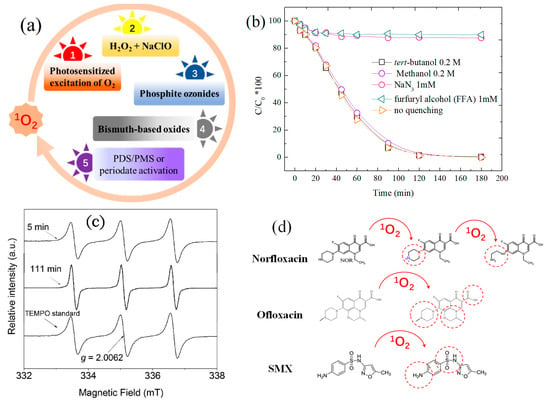
Figure 1.
(a) Reported pathways of 1O2 evolution; (b) the quenching effect of 1O2 on phenol degradation with β-MnO2 (data reorganized from [19]); (c) EPR characteristic spectrum of TEMP-1O2 (figure reprinted from [31]). The EPR spectrometer settings were as follows: modulation frequency, 100 kHz; modulation width, 0.079 mT; scanning field, 335 ± 10 mT; amplitude: 2–500; time constant, 0.1–0.3 s; sweep time, 4 min; microwave power, 4 mW; and microwave frequency, 9.41 GHz.; (d) oxidation pathways of some emerging contaminants by 1O2 [8,34,38], reprinted with permission from Elsevier.
Due to its specific characteristics, 1O2 has been applied in organic synthesis by selective oxidation [28], aqueous pollutant degradation [7], pathogenic bacteria inactivation [29] and medical cancer therapy [30]. The involvement of 1O2 in these fields can be monitored by mainly three approaches including chemical probing tests, electron paramagnetic resonance (EPR) spectrometry and also chemiluminescence detection. Sodium azide (NaN3) and furfuryl alcohol (FFA) are two popular 1O2 quenchers. The reaction rate of 1O2 with NaN3 and FFA have been tested to be 1×109 M−1•s−1 and 1.2×108 M−1•s−1, respectively [19]. However, 1O2 is not sensitive to alcohols, such as methanol, ethanol and tert-butanol, which are normally used for SO4•− and •OH quenching. Therefore, the presence of 1O2 can be identified by using different chemical quenchers as indicated in Figure 1b. Moreover, 1O2 can be captured by a spin trapping agent, 2,2,6,6-tetramethyl-4-piperidinol (TEMP), to form 2,2,6,6-tetramethyl-4-piperidone-N-oxyl (TEMPO) which could be detected by EPR with an intensive 1:1:1 signal (Figure 1c) [31]. In addition, direct or indirect chemiluminescence is also effective for the detection of 1O2. For example, 1O2 could be measured by solid-state near infrared spectroscopy at 1270 nm, which is the radiative photon released from 1O2 because of its intrinsic energy difference compared to ground state oxygen [32]. Fluorescence detection is another helpful technique for 1O2 detection. Yuan et al. reported a conjugated probe consisting of a photosensitizer and a fluorogenic dye that were linked by aminoacrylate (AA) [33]. By reacting with 1O2, the AA linker was cleaved and the probe divided to yield green fluorescence.
As an excited oxygen species, 1O2 shows high affinity for electrophilic oxidation of electron-rich compounds with unsaturated C=C bonds as well as sulfide and amine groups. This natural utility endows 1O2 with the capability to selectively degrade pharmaceutical pollutants. Zhou et al. investigated the degradation of sulfamethoxazole by 1O2, and found that sulfanilic group rather than isoxazole ring was the attack site [34]. Electron transfer and formation of endoperoxide intermediate were possible pathways involved in the 1O2-induced sulfamethoxazole oxidation, specifically resulting in hydroxylation of the aniline ring, amine oxidation and oxidative coupling of the two intermediates (Figure 1d). Gao et al. discovered that the piperazinyl, oxazinyl and carboxylic substituents of ofloxacin were prone to be attacked by 1O2 in the LaBO3/PMS reaction system [8]. Hydroxyl addition, C-N cleavage, demethylation and decarboxylation were possible degradation pathways for 1O2-mediated oxidation of ofloxacin (Figure 1d). Liu et al. found that the olefinic bonds and C-N bonds of carbamazepine were susceptible to electrophilic 1O2 oxidation during PMS activation with N-doped carbon fiber aerogel [9].
An earlier study indicated that the interaction between chlorophenols and PMS would produce SO4•−, •OH as well as 1O2, which then react with phenols or chlorophenols to generate hydroperoxides, and the later intermediate could dehydrate with the formation of p-benzoquinone (Figure 2) [35]. Under alkaline conditions, the reaction rate of 1O2 toward dissociated chlorophenols is in the range of 1.60 × 108 M−1•s−1 to 1.93 × 108 M−1•s−1, which can be slightly influenced by substitutes. In addition, organic sulfides and disulfides were reported to be oxidized to sulfoxides and thiolsulfinates, respectively [10]. Charge transfer and radical propagation were suggested as the two main mechanisms for 1O2 oxidation. However, it should be noted that 1O2 is not as strong as radicals of SO4•− and •OH for the depletion of wastewater COD or TOC [36]. Given its anti-interference and selective feature, 1O2 is frequently studied for tumor cell inactivation via protein oxidation [37].
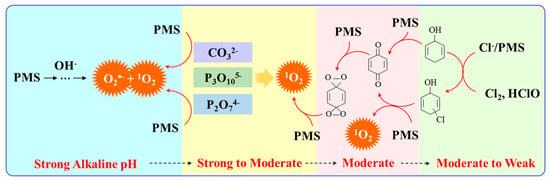
Figure 2.
Evolution of 1O2 in homogeneous activation systems requiring different alkaline conditions.
3. Evolution of 1O2 by Activating PDS/PMS in Homogenous Systems
Although thermo-, UV- and alkali- catalytic activation have proven to be effective for both PDS and PMS, most of these homogeneous systems degrade organic contaminants by forming free radicals, such as SO4•− or •OH. Instead, as indicated in Table 1, the evolution of 1O2 has been recently discovered when PMS was activated by quinones, phenols, alkali, etc.

Table 1.
Performance and mechanism for PDS/PMS oxidation of pollutants in typical homogenous systems.
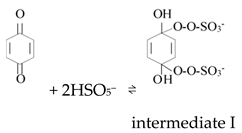
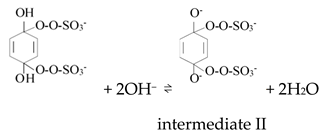
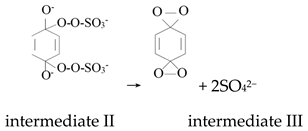
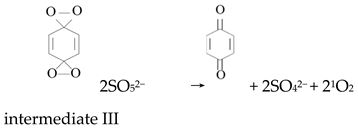
Ketones, quinones, and phenols exist ubiquitously in water and soils. Studies have found that these hydrocarbons can activate PMS, and further suggested that 1O2 might be the main oxidizing species. In 1974, Montgomery discovered that the decomposition rate of PMS was substantially proportional to the amount of ketone at low range of concentrations [39]. Lange and Brauer further verified that 1O2 was formed during ketone-catalyzed PMS activation by using infrared phosphorescence [40]. Similar to ketones with carbonyl groups, Zhou et al. found that benzoquinone (BQ) could effectively activate PMS to degrade sulfamethoxazole, and the degradation rate increased as solution pH increased from 7 to 10 [41]. Radical trapping tests indicated that 1O2 rather than SO4•− and •OH was produced in the BQ/PMS system. The proposed mechanism for BQ-mediated activation of PMS, as illustrated in Figure 2, can be described by Equations (1)–(4). Firstly, PMS anions are added to the carbonyl group of BQ via nucleophilic attack and converted to peroxide adduct labeled as intermediate I as shown in Equation (1). Under alkaline conditions, intermediate I undergoes dehydrogenation and forms intermediate II (Equation (2)), which is further transformed into dioxirane (intermediate III, Equation (3)). Finally, the dioxirane reacts with ionized PMS at a stoichiometric ratio of 1:2, producing 1O2 and BQ again (Equation (4)). It is noteworthy that, as stated by Gallopo et al., dioxirane can be formed as a key intermediate during the reaction between PMS and ketones or BQ through 18O labeling and kinetic studies [42]. Zhang et al. further detected dioxirane and 1O2 by using droplet spray ionization mass spectrometry (DSI-MS) as well as oxygen isotope analysis [43].
Apart from ketones and quinones, phenols are reactive enough to activate PMS because the phenolic group is easily oxidized to quinone byproducts. Zhou et al. found that PMS could be effectively activated to produce 1O2 by phenol at pH 8.5 and 10, in which phenol was oxidized to benzoquinone to promote PMS activation [34]. The chemical structure of phenols is a critical influencing factor for the overall reaction because the substituents and their positions on phenol could remarkably affect the yield of quinone intermediates. At acidic pH, phenols are poorly dissociated so that they can hardly form intramolecular complex with ionized PMS in such molecular state. Therefore, phenols are more likely to react with PMS in alkaline conditions [35]. In addition, it should be noted that the reaction between chloride and PMS at acidic pH would generate reactive chlorine species such as Cl2 and HClO, and further led to organic chlorination. But this undesirable side reaction was highly inhibited because 1O2 was mainly produced at alkali pH so that chloride showed negligible effect on phenol transformation [44].
In fact, alkali is a typical homogeneous PDS/PMS catalyst, and has been employed for in situ field remediation. The activation of PDS by alkali generates SO4•−, •OH and superoxide (O2•−). Unlike PDS, the activation of PMS by alkali initiates non-radical attack for contaminants degradation. Qi et al. found that alkali can activate PMS at ambient temperature to generate O2•− and 1O2 for oxidative degradation of acid orange 7 (AO7) [45]. At lower NaOH concentrations (0.6–1.0 mM), both O2•− and 1O2 were formed, but only O2•− was generated at higher doses of NaOH (8.0 mM). It was proposed that O2•− formed and then recombined to generate one 1O2 and one hydrogen peroxide. But under extremely high pH conditions, the transformation of O2•− to 1O2 was suppressed when the pH was higher than the pKa of O2•− as indicated by Equation (5) [46]. It was worth noting that in the studies from Qi [45] and Lou [47], the addition of p-BQ completely inhibited the oxidation reaction in the PMS/base system. But Zhou’s report pointed out that p-BQ could effectively activate PMS to generate 1O2 under alkaline conditions [41]. Thus the role of p-BQ in PMS activation might be closely related to its dose and pH conditions:
2O2•− + 2H+ → H2O2 + 1O2
Base activation normally requires continuous addition of alkali to maintain desirable pH value. Instead, some studies have developed new effective homogeneous activators, such as chloride, carbonate and BO2− as shown in Figure 2. For example, Lou et al. used polyphosphates to enhance the alkali activation of PMS, in which both O2•− and 1O2 were detected as the main ROSs [47]. Polyphosphates are nucleophiles which promote the breakage of the peroxide O-O bond to speed up 1O2 formation. Like polyphosphate, BO2− is also a nucleophile (Nu) which can bond with PMS to generate a NuOH+ intermediate, and then convert into 1O2 with excellent anti-interference performance [48]. Moreover, Nie et al. found that CO32− could activate PMS for the degradation of pharmaceuticals, phenols and dyes [49]. Species like 1O2 and O2•− were identified, and the system showed good resistance toward the interference of Cl−, NO2−, HCO3− and humic acid.
Chloride is another promoter for PMS activation under both acid and alkaline conditions. Wang et al. used the Cl−/PMS process for the treatment of coking wastewater concentrate, in which 1O2, hypochlorous acid and chlorine radicals were identified [50]. They discovered that the efficiency of the PMS/base/Cl− system was vastly influenced by the dosage of Cl− and NaOH [51]. The degradation of model pollutant was remarkably enhanced by Cl− at low alkalinity, but inhibited when NaOH concentration was up to 2 mM. High alkalinity reduced the formation of organic halides in the PMS/base/Cl− reaction system, which would be significant for the treatment of saline wastewater.
4. Evolution of 1O2 by Activating PDS/PMS with Metal-Free Carbon Catalysts
Metal-free carbon-based materials (MFCMs) are emerging heterogeneous catalysts for PDS/PMS-based oxidation processes in recent years. They have received much attention because of their attractive advantages over metal-based catalysts, such as less cost, no secondary pollution, and their chemical, thermal and mechanical stabilities. MFCMs including reduced graphene oxide (GO), carbon nanotubes (CNTs), nano-diamonds (NDs), carbon spheres (CS) and biochar in different sizes and specifications are investigated as potential PMS/PDS catalysts [16]. Unlike metal-catalyzed processes, organic pollutants can be removed via not only catalytic degradation but also adsorption by MFCMs.
To date, both radical and non-radical oxidations have been reported for MFCMs-mediated PMS/PDS AOPs (Table 2) [16,21]. The non-radical pathway is a potential route to resolve the influence of background organic and inorganic matters on the degradation of targeted pollutants. In a non-radical reaction system, MFCMs can serve as medium for electron transfer between PMS/PDS and organic pollutants. As listed in Figure 3a and Table 2, there are different active sites involved in activating PMS for ROSs generation. They include: (i) delocalized π-electrons (C-π) [52]; (ii) structural defects and vacancies [53]; (iii) heteroatoms bonded to carbon in the form of C=N-C and N-(C)3 [54]; and (iv) C=O and pyran-like oxygen functional groups [55] produced at vacancy defective edges (Equations (6)–(12)):
HSO5− + C-π → SO4•− + OH− + C-π+
HSO5− + C-π+ → SO5•− + H+ + C-π
HSO5− + C=C=O → SO4•− + OH− + C=C-O+
HSO5− + C=C-O+ → SO5•− + H+ + C=C=O
HSO5− + C=N-C+ → SO5•− + H+ + C-N-C
SO5•− + SO5•− → 2SO42− + 1O2
[PDS/PMS] + [sp2/sp3-moieties] → non-radical / free-radical

Table 2.
Performance and mechanism of typical MFCMs catalysts for PDS/PMS oxidation of pollutants.
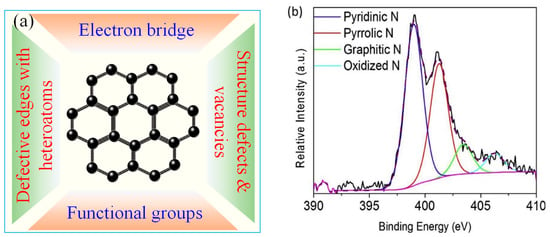
Figure 3.
(a) The proposed active sites of MFCMs for 1O2 evolution via catalytic PDS/PMS activation. (b) The XPS spectra of N 1s for doping N element in different states [67], reprinted with permission from Elsevier.
However, since most MFCMs are constructed with multiple structure and surface characteristics, the identification of the intrinsic active site remains difficult and controversial.
4.1. Carbon Nanotubes (CNTs)
CNTs are one dimensional quantum wires fabricated by rolling 2D graphite sheets. CNTs belong to the family of fullerene, and consist of sp2-hybridized atomic carbon in a hexagonal network. Based on their structural differences, there are single-walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs). With excellent adsorptive ability and electron conductivity, CNTs can act as good electron shuttle between organic pollutants and persulfate oxidants [56] so both CNTs-mediated electron transfer from organics to persulfate and 1O2 are responsible for a nonradical degradative route [57]. Yun et al. explored the role of PDS during its activation with nFe0 and CNTs [58]. Their results indicated that the radical oxidation process was dominant in the nFe0/PDS system but a non-radical mechanism was discovered in the CNTs/PDS system. Further chronoamperometric tests revealed that CNTs served as electron bridge for PDS and organic pollutants.
Noteworthily, the presence of different oxygen functional groups (OFGs) available on the surface of CNTs also play an important role because they directly influence the zeta potential of CNTs [59]. The removal of oxygen contents, especially the blockage of carboxylate group (-COOH) reduction to other carbonyl (C=O) and hydroxyl (-OH) groups through a annealing route is recommended to increase the zeta potential of CNTs. Consequently, this will facilitate the adsorptive uptake of PDS because of weaker electrostatic repulsion, and favor non-radical oxidation route of the targeted pollutants. PDS hydrolysis catalytically occurs when PDS complex with CNTs, and the surface nucleophilic C=O was found to be the crucial sites for the generation of O2•− [60]. The produced O2•− then recombines and is finally converted to 1O2 with the production of hydrogen peroxide as well.
In other cases, surface N-doped CNTs were also investigated compared to pristine material. Sun et al. reported that CNTs doped with 0.88 at.% of nitrogen could achieve a better efficiency (as high as 7-fold) than pristine CNTs for catalytic PMS oxidation of phenol, but showed decreased efficiency on PDS activation. Free sulfate radicals were discovered to be responsible for phenol degradation in the same study [61]. However, some researchers also obtained a contrasting result that non-radical oxidation was dominant in the N-CNTs/PMS system [62]. Because of greater electronegativity, it was postulated that the doped N would enhance the interaction between the carbon atoms and PMS, and thus boost electron transfer.
4.2. GO/rGO
Graphene oxide (GO) is a single-atomic layered carbon material laced with various oxygen-containing groups that fabricated by powerful oxidation of graphite. As a graphene-based material, GO and its reduced derivative rGO have shown great potential in the hydrophilic adsorption of organics and PMS/PDS catalysis, due to various structural defects, vacancies and C=O functional groups [53]. As indicated by density functional theory (DFT) calculations, vacancies and defective edges of rGO would prolong the O-O bond of PMS molecules, enhance adsorption and direct electron transfer, thus facilitate final break-up of O-O bond to initiate nonradical oxidation [63].
The increase of carbonyl groups and graphitization degree would create more vacancies and defects to enhance the catalytic performance of GO/rGO [27]. A simple but effective strategy is to modulate rGO by heteroatom doping [64]. Carbon doped with N is expected to possess more lattice defects for regulating the electronic structure, such as sp2-hybridized carbon skeletons [65,66]. Kang et al. reported that N-doping could significantly improve the activity of reduced graphene oxide (N-rGO) for PMS activation using urea as the nitrogen source [67]. It was found that pyridine N, pyrrole N, graphite N, and oxidized N in N-rGO catalyst accounted for 49.7%, 35.2%, 9.0%, and 5.9%, respectively (Figure 3b). •OH, SO4•− and 1O2 were potential active species for pollutant degradation. As previous reported, pyrrolic N sites were important to adsorb and activate PMS to form 1O2 [68]. Doping nitrogen into carbon matrix is not only beneficial to PMS adsorption due to the increase of surface basicity, but also facilitates electron transfer to the negatively charged PMS, thereby enhancing the catalytic activity.
Besides, the co-doping of other heteroatoms also results in synergistic increase in catalytic performance to generate ROSs. Sun et al. introduced sulfur and nitrogen element into rGO to synthesize a catalyst named i-rGO-NS [69]. The XPS characterizations suggested that the additional sulfur doping increased the content of graphite N (33.74%) and change the distribution of N atom in RGO. Sulfur dopants existed in the form of thiophene S and oxidized S. Compared to rGO, i-rGO-N and metal catalysts, i-rGO-NS showed better reactivity to PMS oxidation of methyl p-hydroxybenzoate (MP). Quenching tests and EPR results indicated that 1O2 was the dominant species but •OH and SO4•− scarcely contributed to MP removal. In general, pyrrole N and pyridine N could activate the π electrons of the sp2 carbon atoms on rGO, and induce the activation of PMS to produce SO4•−, while graphite N could promote the transfer of electrons to PMS to generate 1O2 by neighboring carbon atoms [70]. In addition, thiophene S could mediate the redistribution of charge density to promote the creation of 1O2 [71]. Chen et al. reported that the sole N-atom doping could interrupt the spin and charge dispersion of the uniform sp2-hybridized configuration, leading to graphene chemical inert. However, the co-doping of a second dopant B-atom would activate carbon atoms adjoining to the N-atom. This provided higher electron and spin density, which accelerated the PMS activation through non-radical mechanism [72].
In contrast, high temperature heating of carbon catalysts brings about challenges of poor material dispersion and hydrophilic-to-hydrophobic transformation. As an alternative solution, Zhang et al. anchored amino-functionalized mesoporous silica (NH2-MCM-41) to N-doped GO materials (NG) to improve the hydrophilicity of NG materials, greatly improving the performance on simultaneous adsorption and degradation of contaminants [73]. The introduction of amino groups realized the inversion of negative charge to positive charge, enhanced the electrostatic interaction between the surface of NG and phenolic pollutants, and facilitated the removal of pollutants. Additionally, amino groups would increase the electron transfer capacity of NG to promote catalytic activation [74]. The mesoporous channel of NH2-MCM-41/NG provided effective transport and reaction units for PMS, pollutants and also 1O2.
4.3. Biochar
Biochar is derived from biomass carbonization under an oxygen-free environment. Biochar is cheap and widely available, and functions as a way for carbon sequestration. In recent years, biochar has attracted significant attentions for PDS/PMS activation. Biomass type and pyrolysis temperature are important factors influencing the biochar structural features. For example, biochar obtained through high-temperature pyrolysis (800 °C) have shown structural oxygen defects, which acted as electron conductor moieties for molecular O2 activation via non-radical routes [75]. This finding provided a novel approach to obtain biochar with vacancy defects capable of catalytic pollutants degradation through a non-radical pathway. Moreover, Huang et al. has explored the role of ketone structure of sludge-derived biochar [76]. The formation of 1O2 was detected for the mineralization of BPA. It was deduced that creation of epoxy structure was a possible course to generate 1O2 for ketone-catalyzed PMS decomposition as presented in Equations (13)–(16):
BC-RCOR* + HSO5− → BC-RCOHR*(O-O-SO3−)
BC-RCOHR*(O-O-SO3−) + OH− → BC-RCO−-R*(O-O-SO3−) + H2O
BC-RCO−-R*(O-O-SO3−) → BC-RCOOR* + SO42−
BC-RCOOR* + SO52− → BC-RCOR* + SO42− + 1O2
Additionally, a recent study revealed the role of doped N and S for the catalytic activity of modified biochar [77]. The N-doped biochar gave a positive while S-doping demonstrated a negative effect on biochar-catalyzed PMS activation for metolachlor degradation. It was suggested that N-doping would augment more positive charge of the neighboring C atoms to interact with negatively charged HSO5− species. However, in the case of S-doping, there was insignificant charge transfer due to the disruption of charge redistribution, which referred to breakage of charge balance in covalent carbon electron system. The synergistic effect of the heteroatom N-doping and the prevailing structural defects of graphene both contributed to induce non-radical pathway for the catalytic PMS oxidation of phenol [78]. Yin et al. prepared N-doped sludge-derived biochar (SDBC) with similar Raman spectral characteristics to graphene oxide [79]. They discovered that SDBC could efficiently activate PDS for the removal of sulfamethoxazole through 1O2-mediated degradation, in which 94.6% of sulfamethoxazole (SMX) and 58% of TOC were removed after 180 min of reaction. Furthermore, a high value of Id/Ig of SDBC indicated abundant amount of defect sites inside the carbon layer structure which were possible catalytic sites.
Regarding nitrogen tailing, sewage sludge usually contains N from microbial cells and can be utilized to produce low-N doped sludge biochar. Mian et al. investigated the effectiveness of chemically treated sludge-based biochar for the degradation of organic dyes [80]. It was disclosed that pyridinic-N active sites were the main contributor for the catalytic degradation through non-radical pathway, while pyrrolic-N, activated C(+) as well as surface area acted as active sites for the adsorptive uptake of the pollutants under consideration. Due to the remarkable role of doped N, some studies attempted to increase the N doping amount. Hu et al. doped nitrogen into sludge-derived biochar using urea as a supplementary N source [81]. BET tests and Raman spectroscopy unveiled that the addition of urea improved the specific surface area and the number of active sites for interaction with PMS. Compared to non-doped sludge biochar (C-700), the new N-doped catalyst NC-700 exhibited better activity to remove organic pollutants by synergistic effect of adsorption and catalytic PMS oxidation. The adsorption capacity of methylene blue (MB) on NC-700 reached 35.83 mg/g and the removal rate of MB in NC-700/PMS system was 98.7% after 20 min. The chemical quenching and EPR tests clearly supported that large amount of 1O2 but little •OH and SO4•− were produced in the reaction system, affirming the non-radical pathway induced by biochar.
4.4. Other MFCMs
Many other MFCMs in different dimensions and structure also have shown good reactivity for PDS/PMS activation. Graphited nanodiamond (G-ND) demonstrated superior activation for both PMS and PDS when compared with other metal-free catalysts such as graphene, CNTs, graphite, and fullerene. For example, G-ND showed excellent catalytic performance in persulfate system for the mineralization of phenolic compounds and pharmaceuticals through non-radical pathway [82]. It was deduced from different analysis that G-ND provided surface binding sites for both PDS and phenol molecule to a close proximity. In the formed charge transfer complex, phenol acted as an electron donor and PDS served an electron acceptor, while G-ND functioned as a facile electron transfer mediator channel. Moreover, no inhibition was observed in the existence of oxidant scavengers as well as unwanted natural organic matters. Additionally, the temperature effect on the proportion of graphitic natural carbon in the sp2/sp3 configurations of NDs has been investigated in detail [83]. It was revealed that higher annealing temperature (1100 °C) treatment provided more graphitic shell than the lower annealing temperature (900 °C). The NDs catalyst obtained at 1100 °C (S-ND-1100) contributed to non-radical oxidation route, but the NDs-based catalyst achieved at 900 °C provided radical-dominated oxidation route during PMS activation.
Typically, PDS and PMS were adsorbed and activated on a carbon surface [84]. Jiang et al. successfully developed a metal-free porous carbon aerogel (CA) through the hydrothermal carbonization route by using D-glucose, ammonium persulfate, and aniline [85]. The sp2-hybridized moieties available on CA surface would interact with PDS and dissociate the O-O bonds of PDS. Then the active complex acquired from the first stage initiated the oxidation of rhodamine B (RhB) directly via electron transfer mechanism without the generation of free radicals. In another study, both urea and NaHCO3 were used to functionalize chitosan-derived carbon nanosheets with graphene-like structures [86]. The as-obtained material reflected great potential for the oxidation of recalcitrant pollutants by activating PMS to produce 1O2 as the main ROSs.
5. Evolution of 1O2 by Activating PDS/PMS with Metal Catalysts and Their Composite
Transition metals and metal oxides, such as Co, Mn, Fe, Cu, are effective catalysts for activating PDS/PMS, normally without extra assistance of light and heat. The activation processes with transition metals highly rely on the interaction between PDS/PMS and active redox sites, during which •OH and SO4•− are typically produced as the primary oxidative species. However, thanks to the improvement of analytical techniques, some recent studies found that 1O2 can also be generated from multiple non-radical pathways in metal/PDS or metal/PMS system configurations, including PDS/PMS self-decomposition, recombination of O2•−, and the mutual effect between catalysts and PDS/PMS [87,88]. Interestingly, these processes could take place either simultaneously or coupled with radical oxidations as illustrated in Table 3 and Table 4, which summarize the 1O2 evolution by activating PDS/PMS via heterogeneous transition metals.

Table 3.
Performance and mechanism of typical heterogeneous metallic catalysts for PDS/PMS oxidation of pollutants.

Table 4.
Performance and mechanism of metal-carbon nanocomposite catalysts for PDS/PMS oxidation of pollutants.
5.1. Iron-Based Catalysts
Iron-based materials (e.g., zero-valent iron, Fe3O4) are widely used in AOPs because they are cheap and environmental-friendly. In general, the PMS/PDS activation with iron-based materials is accompanied by transformation from Fe(II) to Fe(III) and the generation of •OH and SO4•− (Equations (17)–(19)) [89]. Therefore, the amount of structural Fe(II) is a critical factor for catalytic PMS/PDS oxidation of organic pollutants:
Fe2+ + HSO5− → Fe3+ + SO4•− + OH−
Fe2+ + S2O82− → Fe3+ + SO4•− + SO42−
SO4•− + H2O → SO42− + •OH + H+
Yet non-radical oxidation is rarely reported for the Fe0/PMS or Fe0/PDS systems. The involvement of non-radical process can be observed under some specific conditions. In the study reported by Li et al., the spike of Cu2+ could enhance efficiency of the Fe0/PMS system by producing 1O2, O2•− and also •OH [90]. Similarly, the corrosion of Fe0 produces Fe2+ to activate PMS to form •OH. However, the process could be interfered by Cu2+ spiking. Cu2+ would transform to Cu0, Cu+ and Cu2O by reacting with Fe0. The newly formed surface composite layer mediates PMS decomposition to a new pathway with production of 1O2 and O2•−. Yang et al. obtained the same results that 1O2 and O2•− were dominant species and coexisted with •OH in the Fe0-montmorillonite/PMS system [91]. Montmorillonite would alter the Fe0 surface oxidation layer which may affect the activation process [92].
Another common iron catalyst is the nanoscale Fe3O4 which exhibits better stability than zero valent iron. The catalysis ability of Fe3O4 relies on its surface structural Fe(II) but not released Fe2+ ions. To overcome magnetic aggregation, Fe3O4 nanoparticles can be typically immobilized on functional supports to yield a controllable structure. The composite materials activate PMS/PDS with diverse active components so as to form multiple ROSs. Pi et al. obtained OBC-Fe3O4 via coating Fe3O4 nanoparticles onto oxidized biochar (OBC) with better adsorption and pollutant degradation performance than Fe3O4 and oxidized biochar [93]. A stable chemical bond was established between spherical Fe3O4 and OBC. The oxygen content of the catalyst increased after reaction, indicating that the oxygen-containing functional group as a bridge for electron transfer played an important role in the process of adsorption and degradation. Fe3O4 activated PDS to produce radicals of •OH and SO4•− [94], while the sp2-hybrid C atom with defective structures and ketone groups mediated electron transfer to generate 1O2. Thus multiple ROSs including SO4•−, •OH and 1O2 would participate in the degradation of tetracycline. Moreover, OBC as carrier of Fe3O4 promoted the adsorption of tetracycline on the catalyst surface, thereby increasing the interaction between ROSs and tetracycline and enhancing its degradation. Liu et al. designed a core-shell iron-carbon nanocomposite catalyst (Fe@CNs as shown in Figure 4a) using sodium alginate as a template to activate PDS and degrade bisphenol A [95]. Under the protection of the carbon shell, the overall iron leaching was less than 3 μg·L−1 as the solution pH was 5~9, far lower than the permissible wastewater discharge standard. The carbon component not only offered larger surface area for the uniform distribution of active sites, but also acted as an excellent electron transfer carrier for PDS catalytic oxidation processes, while the incorporation of Fe enhanced redox activity of the catalyst to favor the PDS activation as evidenced by linear sweep voltammetry (LSV) tests (Figure 4b) [96]. In other similar research works using Fe3C/NC [97] and Fe-N/C [98], it was verified that Fe-C composite could express great synergy for catalytic PMS activation. For example, Fe-N/C could exhibit 34-fold higher reactivity than N/carbon alone towards bisphenol F degradation [98]. Based on the radical scavenging and EPR tests, 1O2 was identified as the main reactive species and coexisted with SO4•− and •OH under the catalysis of Fe-C composite. N-doped C region acted as the active center for electron transfer, and Fe affected the electronic state of the adjacent C region and increased the charge density for PMS activation, which is in consistent with the process of PDS activation [99].
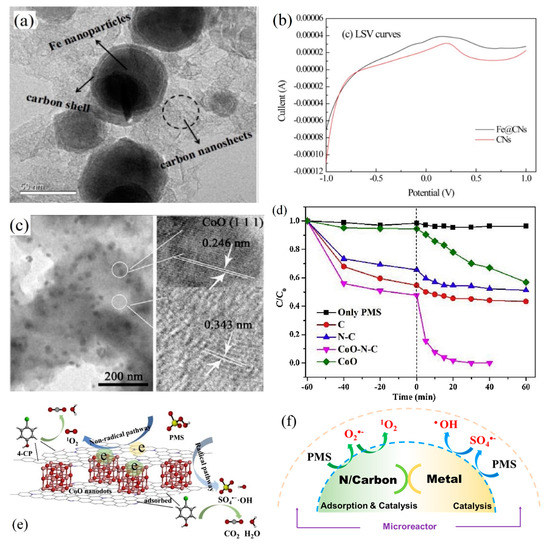
Figure 4.
(a) TEM images of core-shell structure and (b) LSV curves of Fe@CNs [94], reprinted with permission from Elsevier; (c) TEM image of CoO nanodots distribution inside the carbon layers [102], reprinted with permission from Elsevier; (d) performance of CoO-N-C composite in comparison with other catalyst; (e) its catalytic mechanism [102], reprinted with permission; (f) schematic diagram of multi-ROSs generation in PMS/PDS activation systems with carbon/metal composite catalysts.
Overall, the reaction between reductive Fe and PDS/PMS generally underwent radical processes involving SO4•− and •OH oxidations. The modification of Fe-based catalysts with carbon or copper materials would form different reactive sites for collaborative PDS/PMS activation. Carbon and Cu species are able to activate PDS/PMS to form 1O2, while Fe0 or FeII would accelerate regeneration of reactive activators. In addition to Fe/C and Fe/Cu composites, novel Fe-based glasses have attracted increasing research interest for catalytic activation of PDS/PMS. The Fe-based glasses can be easily prepared with unique atomic packing structure and present in the form of ribbons rather than powders. Zero-valent iron inside glasses could provide abundant reactive sites for peroxides activation but with much lower mass loss so as to ensure an excellent reusability [100,101]. Given the superior activity of Fe-based glasses, future efforts to tune their properties to activate PDS/PMS to produce multiple ROSs including 1O2 are highly desirable.
5.2. Cobalt-Based Catalysts
Among transition metal ions (Fe2+, Co2+, Mn2+, Ni2+), Co2+ shows the best catalytic performance for PMS activation [103]. However, excessive Co dispersed in water causes more hazardous impacts on both the environment and public health than other metal ions [64]. As an alternative, heterogeneous catalysts, especially cobalt-containing materials, such as CoOOH [104], and CoFe2O4-x [105], show excellent performance in activating PMS for the generation of 1O2. Zhang et al. focused on Co-OOH nanoparticles owing to the good hydrophilic and electronic transmission rate [104]. They observed that 2,4-DCP could be completely degraded in the CoOOH/PMS system within 120 min, whereas the degradation rates for 2,4-DCP in Co3O4/PMS and CoFe2O4/PMS system were 33% and 73%, respectively. In the Co-catalyzed systems, the redox cycle of Co(III)/Co(II), as evidenced by XPS analysis, was the driving force for PDS/PMS activation as elucidated in Equations (20) and (21). Meanwhile, this redox cycling could be enhanced in the presence of the sufficient surface hydroxyl groups on CoOOH, which expedited the regeneration of CoOH+ intermediate to promote catalytic oxidation rate. In addition to sulfate radicals, 1O2 was produced via self-decomposition of PMS in the CoOOH/PMS system at a rate constant of 0.2 M−1•s−1 as shown in Equation (22) (P4 in Figure 5). The main cause of the 1O2 formation was attributed to the recombination of O2•− (Equation (23)).
≡Co(III) + HSO5− → ≡Co(II) + SO5•− + H+
≡Co(II) + HSO5− → ≡Co(III) + SO4•− + OH−
HSO5− + SO52− → HSO4− + SO42− + 1O2
2O2•− +2H2O → 1O2+ H2O2 + 2OH−
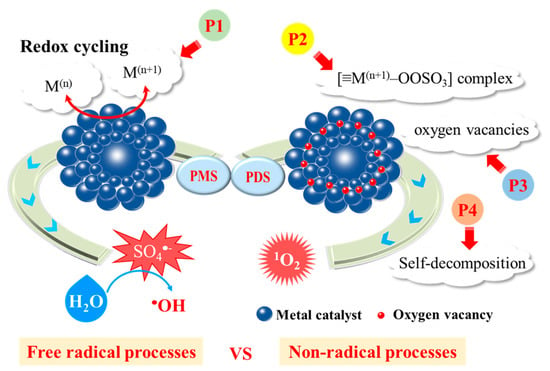
Figure 5.
Schematic illustration of pathways of PMS/PDS activation in metal-catalyzed heterogeneous systems. P1: redox cycling; P2: formation of metal-PMS/PDS complex; P3: generation of oxygen vacancies; P4: PMS/PDS self-decomposition.
For the systems containing both radical and non-radical processes, some water matrices may function as the influential factor that regulates the contribution for 1O2. In a PMS activation system with Co3O4 nanowires as the catalyst, the effect of carbonate ions (CO32−) was investigated for bisphenol A degradation [106]. It was revealed that •OH and SO4•− were the main ROSs in the absence of CO32− (P1 in Figure 5), but in the presence of CO32−, a faster contaminant degradation rate was obtained because of the enhanced formation of 1O2, especially when the solution pH rose up to the pHPZC of Co3O4. Carbonate anions could suppress Co dissolution and facilitate the conversion of catalytic center from Co(II) to Co(III), while the system switched from radical oxidation to 1O2-dominated non-radical process. These findings endorsed that the coupling of Co(III) and CO32−/OH− would have a synergistic effect by functioning as electron and proton acceptors, instead of a simple Co(II)/Co(III) redox cycling. The metal particles tend to aggregate in the water phase, and Co2+ ions would leach once the pH value is not well controlled. To overcome these limitations, nano-carbon materials, including two- or three-dimensional carbon materials, are commonly employed for metal-carbon nanocomposites fabrication. Co immobilized with carbon can offer higher catalytic efficiency for PMS or PDS activation. The co-doping strategy can not only adjust the electronic structure of the carbon catalyst, but also prevent metal leaching and simplify catalyst recovery. The immobilization of Co on carbon materials, as shown in Figure 4c, would also reduce secondary contamination of Co leaching [107]. The Co-C interaction could increase the Fermi level and chemical activity of functionalized C atoms to enhance PMS or PDS activation for pollutants degradation (Figure 4d). Several points were proposed for the reaction mechanism. First, the involvement of adsorptive carbon can facilitate the enrichment of aqueous pollutants and PMS ions to the microenvironment of internal active sites [108]. Second, the reactive Co species effectively activate PMS or PDS with production of SO4•− and •OH to achieve free radical oxidation. Third, the Co and N doping produced more defect sites and carbon graphitization, which could promote electron transfer and activate adjacent C atoms for 1O2 based non-radical degradation (Figure 4e). Moreover, the Co/Fe co-doping into plain N-C was expected to form synergistic effect for more efficient catalysis [109,110]. The existence of binary metals would accelerate redox cycling like a Fenton-like reaction. The simultaneous generation of multi-ROSs in both carbon-mediated and metal-mediated PMS/PDS activation systems facilitated deeper degradation of target pollutants (Figure 4f).
5.3. Manganese-Based Catalysts
Mn-associated catalysts are effective PMS/PDS activators with advantageous features like Mn being an Earth-abundant element and less toxic in nature, as compared to Co [111]. For example, a series of manganese nano catalysts with different oxidative states demonstrated potential catalysis for atrazine elimination through radical and non-radical activation of PMS [112]. α-MnO2 nanowires revealed higher catalytic performance due to their ability to facilitate electron transfer to maintain the redox cycle between Mn(IV) and Mn(III). In another study, both α-MnO2 and β-MnO2 (one-dimensional) displayed relatively effective PDS activation for selective mineralization of organic pollutants in wastewater [19]. Huang et al. found that 1O2 could be formed in the PMS/MnO2 system under acidic conditions [113]. A metastable manganese intermediate (≡MnIV−O−O−SO3) formed when S2O82− attached on the MnO2 surface. ≡MnIV−O−O−SO3 would further react with S2O82− to break the Mn(IV)−O bond along with the formation of O2•−. Afterward, 1O2 was generated as the primary ROSs through direct oxidation of O2•− by Mn(IV), O2•− recombination, and the reaction between O2•− and metastable manganese intermediates at neutral pH (Equations (24)–(26)). Besides, Mn-doped graphite-based carbon nitride (MnCN) also provided good catalysis for PMS oxidation of acetaminophen (ACT) [114]. As indicated in the XPS spectrum, 40% of Mn existed in the Mn(III) state, while N coordinated with Mn as Mn-N. Under optimized conditions, 100% of ACT was removed within 15 min in the MnCN/PMS system. PMS would attach to Mn-N and produce superoxide anions which later transformed to 1O2. Compared to phenols and nitrobenzene, ACT exhibited significant degradation by 1O2 via attacking electron-donating acylamino groups:
2[≡Mn(IV)−OH]III + HS2O8− → 2[≡Mn(IV)−O−O−SO3]II + 3H+
2[≡Mn(IV)−O−O−SO3]II + 4H2O + S2O82− → 2[≡Mn(III)−OH]II + 4SO42− + 2O2•− + 8H+
[≡Mn(IV)−O−O−SO3]II + O2•− + OH− → [≡Mn(III)−OH]II + SO42− + 1O2
Oxygen vacancies could be important active site for Mn oxides (P3 in Figure 5). For instance, Jie et al. manufactured the MnO2-x rattle-type microspheres that a large number of oxygen-defective MnO2 nanoflakes vertically arranged on the surface (OD-MnO2-x-RM), and altered the amount of oxygen vacancies by H2 reduction treatment for various treatment times (20, 40, 60 and 80 min) [115]. PMS was activated to form 1O2 due to the presence of oxygen vacancies and unique nanoarchitecture of the MnO2-x rattle-type microspheres catalyst. The turnover frequency of the optimized catalyst sample OD-MnO2-x-RM (40 min) revealed it as the best-performing catalyst even though OD-MnO2-x-RM (60 min) possessed the richest oxygen vacancies.
5.4. Copper-Based Catalysts
Copper oxide (CuO) is considered as one of the most promising catalysts for the activation of PDS and PMS when evaluated in term of cost and availability. The activity and stability of nanocrystals is strongly dependent on orientation, dimension as well as the crystallographic structure. Du et al. found that sheet-like CuO with preferential exposed crystal facet (001) exhibited much higher reactivity toward catalytic PDS activation than spindle-like CuO [88]. The activation of PDS on CuO mainly followed a non-radical process [116]. To control the morphology and structure of CuO catalyst, Wang et al. applied polyethylene glycol as a structure directing agent [117]. They noticed that CuO-3 with controlled structure reflected better catalytic potential for PMS activation and relatively higher degradation of phenolic compounds and associated organic pollutants found in water. The complex intermediate ≡Cu(II)−(O)OSO3− on catalyst surface was proposed to react with PMS to produce O2•−. It was verified that 1O2 rather than •OH and SO4•− was the main ROSs, and O2•− could be an important precursor of 1O2 in the PMS/CuO-3 system. Interestingly, some reports also found that the CuO/PMS system could perform efficient for saline wastewater treatment due to the good anti-interference nature [118,119].
Another strategy refers to the use of functional support to obtain hybrid structure as well as relocate site electrons. It was reported that the Cu-O-C bond formed by immobilizing CuO on two-dimensional rGO greatly promoted catalytic PDS oxidation for trichlorophenol [88]. The confinement of rGO in hybrid material improved interfacial electron mobility between catalyst and PMS [120]. Also the Cu-O-rGO composite showed better potential to produce more oxygen vacancies for 1O2 generation. Artificial creation of oxygen vacancies effectively modulates the electronic structure of metal oxides, including CuO. This kind of modulation has been proven efficient for boosting catalytic performance [121]. Yu et al. verified that the incorporation of copper into zinc ferrite catalyst could harvest rich oxygen vacancies. The co-participation of Fe and Cu moieties contribute more active sites for catalytic PMS decomposition, and 1O2 and O2•− were detected as the dominant ROSs. According to their results, 96.6% of ciprofloxacin (CIP) was mineralized within 15 min, and the catalyst exhibited good stability and reusability [122]. Furthermore, an easy hydrothermal-calcination route was applied to synthesize CuO-CeO2 composite for the activation of PMS to generate 1O2 [123]. The rate constant noted for the CuO-CeO2/PMS system was 7–11 times higher than that observed in other systems, such as PMS alone, or CeO2/PMS, and CuO/PMS systems. Better electron transfer and more oxygen vacancies reflected the synergy between CuO and CeO2, which contributed to remarkable 1O2 generation during PMS decomposition.
The surface structure of catalysts could be an influential factor. Jawad et al. reported that the incorporation of non-redox MgO into CuO/Fe3O4 catalyst would surprisingly enhance the catalytic performance on PMS activation, and also switch the activation mechanism from a free-radical pathway with generation of SO4•− to 1O2-based non-radical process [124]. The Cu(II)/Cu(III) redox pair no longer acted as the catalytic center, but the incorporation of MgO facilitated the formation of deficient copper [≡Cu(III)–OH]II and the enrichment of extensive ionic PMS. Then [≡Cu(III)–OH]II reacted with PMS to form [≡Cu(III)–OOSO3] complex (Equation (27), P2 in Figure 5). In other cases, divalent copper complex in form of [≡Cu(II)–OOSO3] acted as this vital intermediate [120]. The electron transfer from SO52− to ≡Cu sites ultimately demonstrated the decomposition of [≡Cu–OOSO3] to form O2•− as a precursor of 1O2 (Equation (28)):
[≡Cu(III)−OH]II + HSO5− → [≡Cu(III)−(O)OSO3−]I + H2O
2[≡Cu(III)−(O)OSO3]I + HSO5− +3H2O → 2[≡Cu(II)−OH]I + 2O2•− + 3SO42− + 7H+
5.5. Other Metallic Catalysts
Addtionally, metal oxides of perovskites (ABO3 structure) [8] and spinel (AB2O4 structure) [125] have attracted increasing interest due to their high stability and strong oxidation potential. At octahedral and tetrahedral sites, different types of cations with similar values of crystal field stabilization energies can substitute the metal situating in crystal lattice and form partial oxygen defects for the regulation of the band structure and the recycle of redox pairs [126]. The oxygen vacancies on the surface of metal/metal oxide play an important role in the generation of 1O2 and efficient activation of PMS. The surface and lattice oxygen vacancies are expected to facilitate oxygen adsorption and storage, and accelerate oxygen mobility, which are important for rapid generation of O2•− and their following conversion to 1O2 [105,127]. Gao et al. prepared LaBO3 perovskites with different B site metal (B= Fe, Zn, Mn and Ni) to investigate the effect of B site metals on the PMS activation and 1O2 generation route [8]. It was observed that as high as 21.8% of oxygen defects was monitored for LaNiO3. Ofloxacin (OFX) was completely degraded by the LaNiO3/PMS system, which could be assigned to the effect of oxygen defects on 1O2 generation. The surface oxygen defects of perovskite could lower the energy barrier of spontaneous PMS decomposition on LaBO3 surface, which is an important pathway for the formation of 1O2. Chen et al. also suggested that the cobalt ions in the tetrahedral sites were inclined to be substituted by manganese ions with larger ionic radius [128] accompanied by the generation of vacancies on the O sites [125]. Meanwhile, some active oxygen might react with HSO5− to produce 1O2. More oxygen vacancies would facilitate interfacial electron transfer of PMS activation [129,130].
In addition to the usual transition metals, noble metals also show potential capability to activate PMS with generation of 1O2 for the degradation of selective organic pollutants. Ahn et al. found that noble metals including Pt, Pd, Au, and Ag immobilized on Al2O3 or TiO2 could mediate electron transfer from organics to PMS to achieve non- radical oxidation [131]. The catalytic performance exhibited a dependency on the type of noble metal in an order of Pd > Pt ≈ Au ≫ Ag. To further understand the intrinsic catalytic mechanism, Wang et al. anchored Pd particles in the cavity of g-C3N4 as a heterogeneous catalyst (Pd/g-C3N4) to activate PMS with generation of 1O2 and O2•− for bisphenol A degradation [132]. Noteworthily, less than 10% of bisphenol A could be removed by g-C3N4/PMS alone, while 91% of bisphenol A could be degraded in 60 min by Pd/g-C3N4/PMS. However, it was observed that Pd0 might convert to Pd(II) as indicated by the XPS results that the Pd0/PdII ratio would decrease from 2.02 to 1.19 after the reaction (Equations (29)–(34)). The catalytic ability was significantly influenced by solution pH and reached maximum at pH 9 because 1O2 would attack deprotonated organic compounds at a higher oxidation rate compared to undissociated ones. The mechanism involves the following points: (i) H2O2 and Pd0·OH formed by the reaction between HSO5− and H2O under catalysis of Pd0 (Equations (29)–(30)); (ii) the disassociation of Pd0·OH generates 1O2 according to Equations (31) and (32); (iii) PMS was catalyzed by Pd0 into intermediate •OHPd0SO4•− (Equation (33)), which was then decomposed into PdII, SO42− and H+ (Equation (34)):
HSO5− + H2O → H2O2 + HSO4−
2Pd0 + H2O2 → 2Pd0·OH
2Pd0·OH → H2O + Pd0·O + Pd0
2Pd0·O → 2Pd0 + 1O2
HSO5− + Pd0 → •OHPd0SO4•−
•OHPd0SO4•− + H2O →···→ PdII + SO42− + 2H+
6. Implications for In Situ Applications and Future Perspectives
6.1. Implications for In Situ Applications
The wide occurrence of emerging organic contaminants, such as personal care products and pharmaceuticals (PCPPs), endocrine disrupting chemicals (EDCs), pesticides and surfactants, in natural environment has forced rapid development of PDS/PMS-based AOPs for in situ environmental remediation. Efficiency of conventional PDS/PMS oxidation processes is usually affected by practical matrix conditions, such as temperature, solution pH and salinity. Typically, it is generally recognized that high salinity is a big roadblock for the degradation of organic contaminants in AOPs. Radicals of •OH and SO4•− can easily reacted with Cl−, NO3− to form corresponding byproducts of Cl• and NO3•, and even suppressed in the existence of carbonate and phosphate. This inhibition under high salinity seems to be greatly weakened during non-radical AOPs [118]. An efficient destruction of bisphenol A in high salinity water was observed during 1O2-dominated PMS activation by using nitrogen-doped carbon as the catalyst [133]. Anions including Cl−, NO3−, HCO3−, H2PO4− even in concentrations up to 500 mM exhibited insignificant effects on bisphenol A degradation. This insensitivity to the water matrix is related to the unstable nature of PMS. The unsymmetrical PMS easily undergo self-decomposition under nucleophilic attack by high dose of Cl−, HCO3−, and H2PO4− with production of 1O2. Unlike SO4•− and •OH, 1O2 is a moderate oxidant that unable to oxidize these anions to anion-derived radicals. Besides, soil nature organic matter (NOM) is a complex factor for PMS/PDS activation. SO4•− and •OH radicals are likely to oxidize these background organic constituents so that displaying suppression for target pollutants degradation, but NOM with abundant quinone or semiquinone groups is also a potent PMS activator in alkaline conditions as indicated in Section 3. Moreover, NOM in aquatic systems commonly acts as photosensitizer for 1O2 formation rather than quencher [26], so the negative effect of NOM in 1O2-dominated system might be marginally limited [19,91]. A bench column study by Yang et al. showed that HCO3− and Cl− did not show detrimental effects on TCE degradation and the effect of NOM were negligible at high PMS dosage during in situ chemical oxidation of trichloroethylene (TCE) with bimetallic Fe-Mn oxide as the catalyst [134]. Their EPR and radical scavenging results implied that SO4•−, •OH and 1O2 contributed to TCE degradation. Involvement of various highly ROSs during AOPs resulted in high rate of TCE degradation and dichlorination compared to conventional H2O2-based in situ oxidation. Besides, solution pH is another influential parameter depending on characteristics of activator. In homogeneous activation systems, 1O2 could be directly generated via PDS/PMS activation under neutral (6.5 ± 0.3) and alkaline condition because the surface hydroxyl groups could improve the chemical binding with PDS/PMS. In heterogeneous system, metal could activate PMS with formation of 1O2 in a broader pH range [91]. The nature of catalyst structure, singlet oxygenation and electron transfer are crucial factors behind the formation of 1O2 [90].
In addition, the impact of subsurface minerals on PDS/PMS-based in situ oxidation cannot be ignored. Studies by Zhu et al. indicated that PDS interacting with different crystalline MnO2 forms would transform to 1O2 as the reactive species for phenol abatement. Ahmad et al. and Yu et al. found that synthetic birnessite (manganese oxide) and goethite (iron oxide) were effective mineral for both PDS and PMS activation during in situ chemical oxidation [135,136]. Non-radical pathways accounted for oxidation in birnessite and goethite catalytic PMS systems, and this process could be promoted in presence of soil organic matter. It was concluded that the PDS/PMS decomposition mostly relied on the nature of the mineral surface as well as the rate of metal dissolution. Once persulfate was injected into the contaminated plume, its decomposition occurred due to frequent interaction with aquifer materials including soil organic matter and minerals. Sra et al. verified that the injection of unactivated persulfate into a gasoline source zone could abate a maximum of 46%–86% of gasoline contaminants after two months of remediation [137].
6.2. Future Perspectives
Despite the fact PDS/PMS-based AOPs with production of 1O2 have shown interesting properties in bench scale studies, there are several issues deserve further scientific investigations.
First, except for NaN3, FFA, more suitable quenchers and quantitative methods are needed to further prove key role of singlet oxygen in the rapid degradation of pollutants.
Second, although MFCMs were recognized as desirable potential catalysts, discrepant catalytic activity has been obtained due to different material configuration and surface functional groups. Further studies have to be carried out to figure out the effect of MFCMs characteristics on the reaction efficiency and its key relationship with ROSs production.
Third, the adoption of PDS/PMS-based AOPs for full scale applications largely relies on high pollutant degradation efficacy and cost-effective catalysts. Thus, a novel cheap and stable catalyst which can activate PDS/PMS to exploit multiple oxidation pathways would be truly desirable. In general, 1O2 is recognized to be more selective to mildly oxidize electron-rich substrates and shows much stronger anti-interference capability towards inorganic ions as well as natural organic matters. Therefore, 1O2 can be properly used for disinfection of pathogenic bacteria. By contrast, SO4•− and •OH exhibit more powerful oxidation ability but poor resistance to background water impurities because of radical quenching. Therefore, degradation via multiple oxidation pathways involving different ROSs, such as SO4•−, O2•−, •OH and 1O2 in the same reaction system, is expected to reach a higher oxidation efficiency, especially for wastewaters containing antibiotics and antibiotic resistant bacteria. Finally, the novel techniques available for PDS activation are still limited as compared to PMS-based AOPs. It is well-known that the commercial PDS is cheaper than PMS, and efficient PDS-based AOPs are expected to produce less sulfate ion than PMS-based AOPs after reactions since PMS only constitutes 1/3 of Oxone®. It is thus crucial to develop more PDS-based catalysts with efficient generation of different ROSs for the sake of future commercialization.
7. Conclusions
This paper presents an overview of 1O2 formation via non-radical activation of PDS/PMS in both homogenous and heterogeneous reaction systems. In homogeneous systems, ketones, quinones and alkaline are effective to activate PMS to generate 1O2 while PDS is more likely to be decomposed with generation of radicals. For heterogeneous systems, MFCMs including CNTs, reduced GO and biochar materials have received much attentions. N-doping and structure tailoring endow MFCMs with more lattice vacancies and defect sites for the exploitation of PDS/PMS. In addition, ketone functional groups are able to provide additionally accessible active sites for MFCMs, and the catalytic efficiency could be significantly tuned by controlling the number of ketone groups. Furthermore, the effectiveness of transition metals such as Co, Cu and Mn were discussed in regard of activating PDS or PMS to initiate 1O2 production under some specific conditions. Surface complexation and redox reactions were proposed as the main mechanisms for metal-mediated activation. Additionally, composite catalysts with multiple functions were discussed. Metal would be doped or immobilized into carbon and membrane to show synergistic effect with less metal leaching, enhanced catalytic stability and reusability. Overall, 1O2 can be formed either as the main ROSs to dominate the oxidative degradation or co-exist with radicals including SO4•−, O2•− and •OH. It is largely evidenced that catalysts, oxidant type, reaction parameters are all influential factors for 1O2 production.
Author Contributions
G.X.: Wrote the manuscript. T.X.: Wrote and revised the manuscript. M.F.: Wrote the part of non-metal carbon activation. Y.X.: Wrote the part of homogeneous activation. T.Z.: Wrote the part of manganese and copper oxide activation. H.T.M.: Prepare part of in situ application; J.B.: Manuscript structure guidance. J.D.: Writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The research is supported by Natural Science Foundation of China (41907153), the special fund from the Hubei Provincial Engineering Research Center of Systematic Water Pollution Control (20190729), Natural Science Foundation of Hubei Province (2018CFB262) and Fundamental Research Funds for the Central Universities of the China University of Geosciences (Wuhan) (CUG170646).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Samuel Raphael Ackah for the effort of language editing.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Liang, C.; Su, H.-W. Identification of Sulfate and Hydroxyl Radicals in Thermally Activated Persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; MosbÆK, H.; Siegrist, R.L.; Bjerg, P.L. In Situ Chemical Oxidation of Contaminated Soil and Groundwater Using Persulfate: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Liu, Y.; Kim, S.H.; Dionysiou, D.D. Facile preparation of porous Mn/Fe3O4 cubes as peroxymonosulfate activating catalyst for effective bisphenol A degradation. Chem. Eng. J. 2019, 376, 119193. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Shao, Z.; Wang, S. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B Environ. 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Singlet-Oxygen Generation in Alkaline Periodate Solution. Environ. Sci. Technol. 2015, 49, 14392–14400. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Tian, X.; Nie, Y.; Yang, C.; Zhou, Z.; Wang, Y. Promoted peroxymonosulfate activation into singlet oxygen over perovskite for ofloxacin degradation by controlling the oxygen defect concentration. Chem. Eng. J. 2019, 359, 828–839. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, C.; Wang, Z.; Ding, H.; Deng, H.; Yang, G.; Li, J.; Zheng, H. Urea-assisted one-step fabrication of a novel nitrogen-doped carbon fiber aerogel from cotton as metal-free catalyst in peroxymonosulfate activation for efficient degradation of carbamazepine. Chem. Eng. J. 2020, 386, 124015. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233, 351–371. [Google Scholar]
- Peters, J.W.; Bekowies, P.J.; Winer, A.M.; Pitts, J.N. Potassium perchromate as a source of singlet oxygen. J. Am. Chem. Soc. 1975, 97, 3299–3306. [Google Scholar] [CrossRef]
- Stephenson, L.M.; McClure, D.E. Mechanisms in phosphite ozonide decomposition to phosphate esters and singlet oxygen. J. Am. Chem. Soc. 1973, 95, 3074–3076. [Google Scholar] [CrossRef]
- Foote, C.S.; Wexler, S.; Ando, W.; Higgins, R. Chemistry of singlet oxygen. IV. Oxygenations with hypochlorite-hydrogen peroxide. J. Am. Chem. Soc. 1968, 90, 975–981. [Google Scholar] [CrossRef]
- Du, J.; Xiao, G.; Xi, Y.; Zhu, X.; Su, F.; Kim, S.H. Periodate activation with manganese oxides for sulfanilamide degradation. Water Res. 2020, 169, 115278. [Google Scholar] [CrossRef]
- Yu, K.; Yang, S.; Boyd, S.A.; Chen, H.; Sun, C. Efficient degradation of organic dyes by BiAgxOy. J. Hazard. Mater. 2011, 197, 88–96. [Google Scholar] [CrossRef]
- Yu, J.; Feng, H.; Tang, L.; Pang, Y.; Zeng, G.; Lu, Y.; Dong, H.; Wang, J.; Liu, Y.; Feng, C.; et al. Metal-free carbon materials for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Prog. Mater. Sci. 2020, 111, 100654. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Wang, Y.; Indrawirawan, S.; Duan, X.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. New insights into heterogeneous generation and evolution processes of sulfate radicals for phenol degradation over one-dimensional α-MnO2 nanostructures. Chem. Eng. J. 2015, 266, 12–20. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ. Sci. Technol. 2019, 53, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef] [PubMed]
- Min, D.B.; Boff, J.M. Chemistry and Reaction of Singlet Oxygen in Foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Asghar, A.; Abdul Raman, A.A.; Wan Daud, W.M.A. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Mostafa, S.; Rosario-Ortiz, F.L. Singlet Oxygen Formation from Wastewater Organic Matter. Environ. Sci. Technol. 2013, 47, 8179–8186. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Lim, T.-T. Graphene-and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef]
- García-Fresnadillo, D. Singlet Oxygen Photosensitizing Materials for Point-of-Use Water Disinfection with Solar Reactors. ChemPhotoChem 2018, 2, 512–534. [Google Scholar] [CrossRef]
- Pibiri, I.; Buscemi, S.; Piccionello, A.P.; Pace, A. Photochemically produced singlet oxygen. ChemPhotoChem 2018, 2, 535–547. [Google Scholar] [CrossRef]
- Chen, L.; Yamane, S.; Mizukado, J.; Suzuki, Y.; Kutsuna, S.; Uchimaru, T.; Suda, H. ESR study of singlet oxygen generation and its behavior during the photo-oxidation of P3HT in solution. Chem. Phys. Lett. 2015, 624, 87–92. [Google Scholar] [CrossRef]
- Rkanofsky, J. Assay for Singlet-Oxygen Generation by Peroxidases Using 1270-nm Chemiluminescence, InSinglet Oxygen, UV-A, and Ozone; Academic Press: San Diego, CA, USA, 2000; Volume 319, pp. 59–67. [Google Scholar]
- Yuan, Y.; Zhang, C.-J.; Xu, S.; Liu, B. A self-reporting AIE probe with a built-in singlet oxygen sensor for targeted photodynamic ablation of cancer cells. Chem. Sci. 2016, 7, 1862–1866. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Pang, S.Y.; Yang, Y.; Ma, J.; Gu, J.; Li, J.; Wang, Z.; Wang, L.H.; et al. Activation of peroxymonosulfate by phenols: Important role of quinone intermediates and involvement of singlet oxygen. Water Res. 2017, 125, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Wang, Y.J.; Chen, C.B.; Fu, X.Z.; Cui, S.; Lu, J.Y.; Liu, H.Q.; Li, W.W. Interactions between chlorophenols and peroxymonosulfate: pH dependency and reaction pathways. Sci. Total Environ. 2019, 664, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Stanbury, D.M.; Bounds, P.L. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic. Biol. Med. 2010, 49, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.V.; Sokolovski, S.G.; Goltsov, A.; Gekaluyk, A.S.; Saranceva, E.I.; Bragina, O.A.; Tuchin, V.V.; Rafailov, E.U. Laser-induced generation of singlet oxygen and its role in the cerebrovascular physiology. Prog. Quantum Electron. 2017, 55, 112–128. [Google Scholar] [CrossRef]
- Niu, X.-Z.; Busetti, F.; Langsa, M.; Croué, J.-P. Roles of singlet oxygen and dissolved organic matter in self-sensitized photo-oxidation of antibiotic norfloxacin under sunlight irradiation. Water Res. 2016, 106, 214–222. [Google Scholar] [CrossRef]
- Montgomery, R.E. Catalysis of peroxymonosulfate reactions by ketones. J. Am. Chem. Soc. 1974, 96, 7820–7821. [Google Scholar] [CrossRef]
- Lange, A.; Brauer, H.D. On the formation of dioxiranes and of singlet oxygen by the ketone-catalysed decomposition of Caro’s acid Journal of the chemical society perkin transactions. J. Chem. Soc. Perkin Trans. 1996, 25, 805–811. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.Y.; Li, J.; Lu, X.T.; Yuan, L.P. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015, 49, 12941–12950. [Google Scholar] [CrossRef]
- Gallopo, A.R.; Edwards, J.O. Kinetics and mechanism of the oxidation of pyridine by Caro’s acid catalyzed by ketones. J. Org. Chem. 1981, 46, 1684–1688. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, K.; He, J.; Li, N.; You, H.; Jiang, J. Droplet spray ionization mass spectrometry for real-time monitoring of activation of peroxymonosulfate by 1,4-benzoquinone. Microchem. J. 2018, 139, 437–442. [Google Scholar] [CrossRef]
- Li, C.-X.; Chen, C.-B.; Wang, Y.-J.; Fu, X.-Z.; Cui, S.; Lu, J.-Y.; Li, J.; Liu, H.-Q.; Li, W.-W.; Lau, T.-C. Insights on the pH-dependent roles of peroxymonosulfate and chlorine ions in phenol oxidative transformation. Chem. Eng. J. 2019, 362, 570–575. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef]
- Burns, J.M.; Cooper, W.J.; Ferry, J.L.; King, D.W.; DiMento, B.P.; McNeill, K.; Miller, C.J.; Miller, W.L.; Peake, B.M.; Rusak, S.A.; et al. Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]
- Lou, X.; Fang, C.; Geng, Z.; Jin, Y.; Xiao, D.; Wang, Z.; Liu, J.; Guo, Y. Significantly enhanced base activation of peroxymonosulfate by polyphosphates: Kinetics and mechanism. Chemosphere 2017, 173, 529–534. [Google Scholar] [CrossRef]
- Rao, L.; Yang, Y.; Chen, L.; Liu, X.; Chen, H.; Yao, Y.; Wang, W. Highly efficient removal of organic pollutants via a green catalytic oxidation system based on sodium metaborate and peroxymonosulfate. Chemosphere 2020, 238, 124687. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Zhang, W.; Yan, C.; Xu, W.; Wu, L.; Ye, Y.; Hu, Y.; Dong, W. Enhanced removal of organic contaminants in water by the combination of peroxymonosulfate and carbonate. Sci. Total Environ. 2019, 647, 734–743. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Treatment of membrane filtration concentrate of coking wastewater using PMS_chloridion oxidation process. Chem. Eng. J. 2019, 122361. [Google Scholar] [CrossRef]
- Yang, F.; Huang, Y.; Fang, C.; Xue, Y.; Ai, L.; Liu, J.; Wang, Z. Peroxymonosulfate/base process in saline wastewater treatment: The fight between alkalinity and chloride ions. Chemosphere 2018, 199, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Luo, Z.; Wei, Z.; Luo, S.; Spinney, R.; Yang, W.; Dionysiou, D.D. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies. Curr. Opin. Chem. Eng. 2018, 19, 51–58. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Ao, Z.; Zhou, L.; Wang, G.; Wang, S. Unveiling the active sites of graphene-catalyzed peroxymonosulfate activation. Carbon 2016, 107, 371–378. [Google Scholar] [CrossRef]
- Chen, H.; Carroll, K.C. Metal-free catalysis of persulfate activation and organic-pollutant degradation by nitrogen-doped graphene and aminated graphene. Environ. Pollut. 2016, 215, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Miao, W.; Fang, X.; Tang, Y.; Wu, D.; Mao, S. MOF-derived metal-free N-doped porous carbon mediated peroxydisulfate activation via radical and non-radical pathways: Role of graphitic N and CO. Chem. Eng. J. 2020, 380, 122584. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.; Yuan, X.; Yu, Z.; Zhang, H.; Duan, X.; Wang, S. Activation of Peroxydisulfate on Carbon Nanotubes: Electron-Transfer Mechanism. Environ. Sci. Technol. 2019, 53, 14595–14603. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.-T.; Lee, J.H.; Kim, J.; Park, H.-D.; Lee, J. Identifying the Nonradical Mechanism in the Peroxymonosulfate Activation Process: Singlet Oxygenation Versus Mediated Electron Transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef]
- Yun, E.-T.; Yoo, H.-Y.; Bae, H.; Kim, H.-I.; Lee, J. Exploring the Role of Persulfate in the Activation Process: Radical Precursor Versus Electron Acceptor. Environ. Sci. Technol. 2017, 51, 10090–10099. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.; Nie, G.; Zhang, H.; Duan, X.; Wang, S. Insights into the Electron-Transfer Regime of Peroxydisulfate Activation on Carbon Nanotubes: The Role of Oxygen Functional Groups. Environ. Sci. Technol. 2020, 54, 1267–1275. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kwan, C.; Suvorova, A.; Ang, H.M.; Tadé, M.O.; Wang, S. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals. Appl. Catal. B Environ. 2014, 154, 134–141. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.; Wang, S. The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Ao, Z.; Zhou, L.; Sun, H.; Wang, G.; Wang, S. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl. Catal. Environ. 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Wang, X.; Qin, Y.; Zhu, L.; Tang, H. Nitrogen-Doped Reduced Graphene Oxide as a Bifunctional Material for Removing Bisphenols: Synergistic Effect between Adsorption and Catalysis. Environ. Sci. Technol. 2015, 49, 6855–6864. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, Y.; Lyu, L.; Zeng, Q.; Xing, X.; Hu, C. Electronic Structure Modulation of Graphitic Carbon Nitride by Oxygen Doping for Enhanced Catalytic Degradation of Organic Pollutants through Peroxymonosulfate Activation. Environ. Sci. Technol. 2018, 52, 14371–14380. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Sheng, L.; Zhou, Q.; Wei, T.; Feng, J.; Fan, Z. Edge-Nitrogen-Rich Carbon Dots Pillared Graphene Blocks with Ultrahigh Volumetric/Gravimetric Capacities and Ultralong Life for Sodium-Ion Storage. Adv. Energy Mater. 2018, 8, 1802042. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Wang, S. Catalytic degradation of antibiotics by metal-free catalysis over nitrogen-doped graphene. Catal. Today 2018. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, J. Nitrogen-doped graphene as peroxymonosulfate activator and electron transfer mediator for the enhanced degradation of sulfamethoxazole. Chem. Eng. J. 2019, 375, 122041. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.; Guo, L.; Zhai, Z.; Fang, Y.; Zhang, X.; Sharma, V.K. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B Environ. 2019, 251, 335–345. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Hu, Z.-T.; Sun, Y.-M.; Webster, R.D.; Li, S.-Z.; Lim, T.-T. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes. Appl. Catal. B Environ. 2018, 225, 243–257. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Z.; Zhu, Y.; Huang, Z.; Cui, Y.; Yang, J. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene. Appl. Catal. B Environ. 2018, 220, 635–644. [Google Scholar] [CrossRef]
- Chen, X.; Duan, X.; Oh, W.-D.; Zhang, P.-H.; Guan, C.-T.; Zhu, Y.-A.; Lim, T.-T. Insights into nitrogen and boron-co-doped graphene toward high-performance peroxymonosulfate activation: Maneuverable N-B bonding configurations and oxidation pathways. Appl. Catal. B Environ. 2019, 253, 419–432. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, T.; Sun, D.; Chen, W.; Li, G.; Li, Y. NH2-MCM-41 supported on nitrogen-doped graphene as bifunctional composites for removing phenol compounds: Synergistic effect between catalytic degradation and adsorption. Carbon 2019, 147, 312–322. [Google Scholar] [CrossRef]
- Deng, B.; Wang, D.; Jiang, Z.; Zhang, J.; Shi, S.; Jiang, Z.-J.; Liu, M. Amine group induced high activity of highly torn amine functionalized nitrogen-doped graphene as the metal-free catalyst for hydrogen evolution reaction. Carbon 2018, 138, 169–178. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Z.; Zhang, H.; Di, G.; Qiu, Y.; Yin, D.; Wang, S. Hydrochars from pinewood for adsorption and nonradical catalysis of bisphenols. J. Hazard. Mater. 2020, 385, 121548. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-C.; Jiang, J.; Huang, G.-X.; Yu, H.-Q. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate. J. Mater. Chem. A 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Qian, X.; Chen, L.; Cai, T. Nitrogen-doping positively whilst sulfur-doping negatively affect the catalytic activity of biochar for the degradation of organic contaminant. Appl. Catal. B Environ. 2020, 263, 118348. [Google Scholar] [CrossRef]
- Wu, D.; Song, W.; Chen, L.; Duan, X.; Xia, Q.; Fan, X.; Li, Y.; Zhang, F.; Peng, W.; Wang, S. High-performance porous graphene from synergetic nitrogen doping and physical activation for advancednonradical oxidation. J. Hazard. Mater. 2020, 381, 121010. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.-S.; Ren, N. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2020, 392, 123681. [Google Scholar] [CrossRef]
- Hu, W.; Xie, Y.; Lu, S.; Li, P.; Xie, T.; Zhang, Y.; Wang, Y. One-step synthesis of nitrogen-doped sludge carbon as a bifunctional material for the adsorption and catalytic oxidation of organic pollutants. Sci. Total Environ. 2019, 680, 51–60. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.-i.; Weon, S.; Choi, W.; Hwang, Y.S.; Seo, J.; Lee, C.; Kim, J.-H. Activation of Persulfates by Graphitized Nanodiamonds for Removal of Organic Compounds. Environ. Sci. Technol. 2016, 50, 10134–10142. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Zhang, H.; Saunders, M.; Sun, H.; Shao, Z.; Wang, S. Nanodiamonds in sp2/sp3configuration for radical to nonradical oxidation: Core-shell layer dependence. Appl. Catal. B Environ. 2018, 222, 176–181. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Chen, X.; Huang, Y.; Peng, K.; Huang, Y.; Yan, Y.; Chen, Y. Pig manure-derived nitrogen-doped mesoporous carbon for adsorption and catalytic oxidation of tetracycline. Sci. Total Environ. 2020, 708, 135071. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, Y.; Zhou, M.; Liang, L.; Li, K. Oxidation of Rhodamine B by persulfate activated with porous carbon aerogel through a non-radical mechanism. J. Hazard. Mater. 2018, 358, 53–61. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Zhang, P.-H.; Webster, R.D.; Lim, T.-T. Surface construction of nitrogen-doped chitosan-derived carbon nanosheets with hierarchically porous structure for enhanced sulfacetamide degradation via peroxymonosulfate activation: Maneuverable porosity and active sites. Chem. Eng. J. 2020, 382, 122908. [Google Scholar] [CrossRef]
- Ahn, Y.-Y.; Bae, H.; Kim, H.-I.; Kim, S.-H.; Kim, J.-H.; Lee, S.-G.; Lee, J. Surface-loaded metal nanoparticles for peroxymonosulfate activation: Efficiency and mechanism reconnaissance. Appl. Catal. B Environ. 2019, 241, 561–569. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Si, F.; Yao, C.; Du, M.; Hussain, I.; Kim, H.; Huang, S.; Lin, Z.; Hayat, W. Persulfate non-radical activation by nano-CuO for efficient removal of chlorinated organic compounds: Reduced graphene oxide-assisted and CuO (001) facet-dependent. Chem. Eng. J. 2019, 356, 178–189. [Google Scholar] [CrossRef]
- Xiong, X.; Sun, B.; Zhang, J.; Gao, N.; Shen, J.; Li, J.; Guan, X. Activating persulfate by Fe0 coupling with weak magnetic field: Performance and mechanism. Water Res. 2014, 62, 53–62. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Zhao, P.; Zhou, P.; Liu, Y.; Cheng, X.; Wang, J.; Yang, B.; Guo, H. Enhanced kinetic performance of peroxymonosulfate/ZVI system with the addition of copper ions: Reactivity, mechanism, and degradation pathways. J. Hazard. Mater. 2020, 393, 122399. [Google Scholar] [CrossRef]
- Yang, S.; Wu, P.; Liu, J.; Chen, M.; Ahmed, Z.; Zhu, N. Efficient removal of bisphenol A by superoxide radical and singlet oxygen generated from peroxymonosulfate activated with Fe0-montmorillonite. Chem. Eng. J. 2018, 350, 484–495. [Google Scholar] [CrossRef]
- Kim, C.; Ahn, J.-Y.; Kim, T.Y.; Shin, W.S.; Hwang, I. Activation of Persulfate by Nanosized Zero-Valent Iron (NZVI): Mechanisms and Transformation Products of NZVI. Environ. Sci. Technol. 2018, 52, 3625–3633. [Google Scholar] [CrossRef] [PubMed]
- Pi, Z.; Li, X.; Wang, D.; Xu, Q.; Tao, Z.; Huang, X.; Yao, F.; Wu, Y.; He, L.; Yang, Q. Persulfate activation by oxidation biochar supported magnetite particles for tetracycline removal: Performance and degradation pathway. J. Clean. Prod. 2019, 235, 1103–1115. [Google Scholar] [CrossRef]
- Feng, M.; Qu, R.; Zhang, X.; Sun, P.; Sui, Y.; Wang, L.; Wang, Z. Degradation of flumequine in aqueous solution by persulfate activated with common methods and polyhydroquinone-coated magnetite/multi-walled carbon nanotubes catalysts. Water Res. 2015, 85, 1–10. [Google Scholar] [CrossRef]
- Liu, L.; Xu, X.; Li, Y.; Su, R.; Li, Q.; Zhou, W.; Gao, B.; Yue, Q. One-step synthesis of “nuclear-shell” structure iron-carbon nanocomposite as a persulfate activator for bisphenol A degradation. Chem. Eng. J. 2020, 382, 122780. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, W.; Xu, Y.; Zhu, Z.; Liu, Z.; Cui, F. Iron sludge-derived magnetic Fe0/Fe3C catalyst for oxidation of ciprofloxacin via peroxymonosulfate activation. Chem. Eng. J. 2019, 365, 99–110. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, Y.; Nie, W.; Tang, H. Efficient degradation of drug ibuprofen through catalytic activation of peroxymonosulfate by Fe3C embedded on carbon. J. Environ. Sci. 2019, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, H.; Yang, C.; Li, X.; Lin, Y.; Yin, K.; Sun, J.; Teng, Q.; Du, C.; Zhong, Y. High-performance porous carbon catalysts doped by iron and nitrogen for degradation of bisphenol F via peroxymonosulfate activation. Chem. Eng. J. 2020, 392, 123683. [Google Scholar] [CrossRef]
- Liu, K.; Yu, M.; Wang, H.; Wang, J.; Liu, W.; Hoffmann, M.R. Multiphase Porous Electrochemical Catalysts Derived from Iron-Based Metal–Organic Framework Compounds. Environ. Sci. Technol. 2019, 53, 6474–6482. [Google Scholar] [CrossRef]
- Liang, S.-X.; Wang, X.; Zhang, W.; Liu, Y.-J.; Wang, W.; Zhang, L.-C. Selective laser melting manufactured porous Fe-based metallic glass matrix composite with remarkable catalytic activity and reusability. Appl. Mater. Today 2020, 19, 100543. [Google Scholar] [CrossRef]
- Liang, S.-X.; Jia, Z.; Zhang, W.C.; Li, X.F.; Wang, W.M.; Lin, H.C.; Zhang, L.C. Ultrafast activation efficiency of three peroxides by Fe78Si9B13 metallic glass under photo-enhanced catalytic oxidation: A comparative study. Appl. Catal. B Environ. 2018, 221, 108–118. [Google Scholar] [CrossRef]
- Xie, M.; Tang, J.; Fang, G.; Zhang, M.; Kong, L.; Zhu, F.; Ma, L.; Zhou, D.; Zhan, J. Biomass Schiff base polymer-derived N-doped porous carbon embedded with CoO nanodots foradsorption and catalytic degradation of chlorophenol by peroxymonosulfate. J. Hazard. Mater. 2020, 384, 121345. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, D.; Li, X.; Feng, W.; Lyu, C.; Zhang, Y. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water. J. Hazard. Mater. 2020, 384, 121350. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Hong, J.; Dong, Z.; Wang, J. Degradation of bisphenol A by persulfate activation via oxygen vacancy-rich CoFe2O4-x. Chemosphere 2019, 221, 412–422. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Fan, Z.; Liu, F.; Li, A. Carbonate-enhanced catalytic activity and stability of Co3O4 nanowires for 1O2-driven bisphenol A degradation via peroxymonosulfate activation: Critical roles of electron and proton acceptors. J. Hazard. Mater. 2020, 393, 122395. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, H.; Qian, Z.; Ouyang, T.; Sun, H.; Qin, J.; Tadé, M.O.; Wang, S. Bread-making synthesis of hierarchically Co@C nanoarchitecture in heteroatom doped porous carbons for oxidative degradation of emerging contaminants. Appl. Catal. B Environ. 2018, 225, 76–83. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Xu, X.; Cao, R.; Yang, H.; Xu, X.; Li, J. MOF-derived CoN/N-C@SiO2yolk-shell nanoreactor with dual active sites for highly efficient catalytic advanced oxidation processes. Chem. Eng. J. 2020, 381, 122670. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Chen, L.; Cai, T. Degradation of norfloxacin by CoFe alloy nanoparticles encapsulated in nitrogen doped graphitic carbon (CoFe@N-GC) activated peroxymonosulfate. Chem. Eng. J. 2020, 392, 123725. [Google Scholar] [CrossRef]
- Liu, C.; Liu, S.; Liu, L.; Tian, X.; Liu, L.; Xia, Y.; Liang, X.; Wang, Y.; Song, Z.; Zhang, Y.; et al. Novel carbon based Fe-Co oxides derived from Prussian blue analogues activating peroxymonosulfate: Refractory drugs degradation without metal leaching. Chem. Eng. J. 2020, 379, 122274. [Google Scholar] [CrossRef]
- Taujale, S.; Baratta, L.R.; Huang, J.; Zhang, H. Interactions in Ternary Mixtures of MnO2, Al2O3, and Natural Organic Matter (NOM) and the Impact on MnO2 Oxidative Reactivity. Environ. Sci. Technol. 2016, 50, 2345–2353. [Google Scholar] [CrossRef]
- Zeng, Z.; Khan, A.; Wang, Z.; Zhao, M.; Mo, W.; Chen, Z. Elimination of atrazine through radical/non-radical combined processes by manganese nano-catalysts/PMS and implications to the structure-performance relationship. Chem. Eng. J. 2020, 397, 125425. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Y.; Singewald, K.; Liu, C.-C.; Saxena, S.; Zhang, H. Effects of MnO2of different structures on activation of peroxymonosulfate. Chem. Eng. J. 2019, 370, 906–915. [Google Scholar] [CrossRef]
- Fan, J.; Qin, H.; Jiang, S. Mn-doped g-C3N4 composite to activate peroxymonosulfate for acetaminophen degradation: The role of superoxide anion and singlet oxygen. Chem. Eng. J. 2019, 359, 723–732. [Google Scholar] [CrossRef]
- Yu, J.; Zeng, T.; Wang, H.; Zhang, H.; Sun, Y.; Chen, L.; Song, S.; Li, L.; Shi, H. Oxygen-defective MnO2−x rattle-type microspheres mediated singlet oxygen oxidation of organics by peroxymonosulfate activation. Chem. Eng. J. 2020, 394, 124458. [Google Scholar] [CrossRef]
- Xing, S.; Li, W.; Liu, B.; Wu, Y.; Gao, Y. Removal of ciprofloxacin by persulfate activation with CuO: A pH-dependent mechanism. Chem. Eng. J. 2020, 382, 122837. [Google Scholar] [CrossRef]
- Wang, S.; Gao, S.; Tian, J.; Wang, Q.; Wang, T.; Hao, X.; Cui, F. A stable and easily prepared copper oxide catalyst for degradation of organic pollutants by peroxymonosulfate activation. J. Hazard. Mater. 2020, 387, 121995. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, Y.; Huang, W.; Xue, C.; Zhu, Y.; Wang, Q.; Liu, D. Innovatively employing magnetic CuO nanosheet to activate peroxymonosulfate for the treatment of high-salinity organic wastewater. J. Environ. Sci. 2020, 88, 46–58. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Huang, W.; Wei, X.; Huang, W. Biochar supported CuO composites used as an efficient peroxymonosulfate activator for highly saline organic wastewater treatment. Sci. Total Environ. 2020, 721, 137764. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Ali, J.; Ifthikar, J.; Aregay, G.G.; Zhu, J.; Chen, Z.; Chen, Z. Non-radical PMS activation by the nanohybridmaterial with periodic confinement of reduced graphene oxide (rGO) and Cu hydroxides. J. Hazard. Mater. 2020, 392, 122316. [Google Scholar] [CrossRef]
- Dong, X.; Duan, X.; Sun, Z.; Zhang, X.; Li, C.; Yang, S.; Ren, B.; Zheng, S.; Dionysiou, D.D. Natural illite-based ultrafine cobalt oxide with abundant oxygen-vacancies for highly efficient Fenton-like catalysis. Appl. Catal. B Environ. 2020, 261, 118214. [Google Scholar] [CrossRef]
- Yu, R.; Zhao, J.; Zhao, Z.; Cui, F. Copper substituted zinc ferrite with abundant oxygen vacancies for enhanced ciprofloxacin degradation via peroxymonosulfate activation. J. Hazard. Mater. 2020, 390, 121998. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D.; Zhao, Y.; Li, S.; Wei, X.; Meng, F.; Huang, W.; Lei, Z. Singlet oxygen dominated peroxymonosulfate activation by CuO-CeO2for organic pollutants degradation: Performance and mechanism. Chemosphere 2019, 233, 549–558. [Google Scholar] [CrossRef]
- Jawad, A.; Zhan, K.; Wang, H.; Shahzad, A.; Zeng, Z.; Wang, J.; Zhou, X.; Ullah, H.; Chen, Z.; Chen, Z. Tuning of Persulfate Activation from a Free Radical to a Nonradical Pathway through the Incorporation of Non-Redox Magnesium Oxide. Environ. Sci. Technol. 2020, 54, 2476–2488. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bi, S.; Zhao, G.; Chen, Y.; Hu, Y. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: Mechanisms and intermediates identification. Sci. Total Environ. 2020, 711, 134715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.; Dong, G.; Feng, J.; Wei, T.; Ren, Y.; Ma, J. Oxygen vacancies induced heterogeneous catalysis of peroxymonosulfate by Ni-doped AgFeO2materials: Evolution of reactive oxygen species and mechanism. Chem. Eng. J. 2020, 388, 124371. [Google Scholar] [CrossRef]
- Deng, J.; Cheng, Y.-Q.; Lu, Y.-A.; Crittenden, J.C.; Zhou, S.-Q.; Gao, N.-Y.; Li, J. Mesoporous manganese Cobaltite nanocages as effective and reusable heterogeneous peroxymonosulfate activators for Carbamazepine degradation. Chem. Eng. J. 2017, 330, 505–517. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Peng, W.; Fang, Z.; Liu, J. Peroxymonosulfate activation for efficient sulfamethoxazole degradation by Fe3O4/β-FeOOH nanocomposites: Coexistence of radical and non-radical reactions. Chem. Eng. J. 2019, 356, 904–914. [Google Scholar] [CrossRef]
- Lu, S.; Wang, G.; Chen, S.; Yu, H.; Ye, F.; Quan, X. Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for degradation of organic pollutants. J. Hazard. Mater. 2018, 353, 401–409. [Google Scholar] [CrossRef]
- Ahn, Y.-Y.; Yun, E.-T.; Seo, J.-W.; Lee, C.; Kim, S.H.; Kim, J.-H.; Lee, J. Activation of Peroxymonosulfate by Surface-Loaded Noble Metal Nanoparticles for Oxidative Degradation of Organic Compound. Environ. Sci. Technol. 2016, 50, 10187–10197. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, D.; Liu, M.; Zhao, X. Insights into heterogeneous catalytic activation of peroxymonosulfate by Pd/g-C3N4: The role of superoxide radical and singlet oxygen. Catal. Commun. 2017, 102, 85–88. [Google Scholar] [CrossRef]
- Luo, R.; Li, M.; Wang, C.; Zhang, M.; Nasir Khan, M.A.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Li, J. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol Aunder high salinity condition. Water Res. 2019, 148, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cai, J.; Wang, X.; Li, Y.; Wu, Z.; Wu, W.D.; Chen, X.D.; Sun, J.; Sun, S.P.; Wang, Z. A Bimetallic Fe-Mn Oxide-Activated Oxone for In Situ Chemical Oxidation (ISCO) of Trichloroethylene in Groundwater: Efficiency, Sustained Activity, and Mechanism Investigation. Environ. Sci. Technol. 2020, 54, 3714–3724. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Teel, A.L.; Watts, R.J. Activationof Peroxymonosulfate by Subsurface Minerals. J. Contam. Hydrol. 2016, 191, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Teel, A.L.; Watts, R.J. Persulfate activation by subsurface minerals. J. Contam. 2010, 115, 34–45. [Google Scholar] [CrossRef]
- Sra, K.S.; Thomson, N.R.; Barker, J.F. Persulfate injection into a gasoline source zone. J. Contam. Hydrol. 2013, 150, 35–44. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).