Abstract
Studies on the association of maternal diabetes with motor development in children provide inconsistent findings. We searched MEDLINE/PubMed, EMBASE, Emcare, PsycINFO, and Google Scholar databases for primary observational, case–control, or cohort studies that report on the motor development of children exposed to maternal diabetes during pregnancy. Quality appraisal and data extraction were performed independently and in duplicate. A meta-analysis of summary measures was performed using random-effect models. Eighteen studies were identified for inclusion, however, only 13 were included in the meta-analysis. Exposure to maternal diabetes during pregnancy was associated with a lower pooled motor development in children and a decrease in both gross and fine motor development. Among all other factors, pre-existing diabetes and other gestational comorbidities, such as hypertension and obesity, or low socioeconomic status, also affect child development. Therefore, among children of diabetic mothers, those with other gestational comorbidities or pre-existing diabetes were more likely to be at risk developmentally.
1. Introduction
Children with typical development follow a pattern of developing gross and fine motor skills that allows them to know when they are developing well. The development of gross motor skills includes the assessment of muscle control, coordination, and locomotion, while the development of fine motor skills includes the control and coordination of body segments to achieve more complex movement and perceptual skills [1]. Delayed gross and fine motor development is usually noticed when the child fails to meet the normative development milestones by the normative age [2]. Poor motor development in infants and children may have long-term negative consequences for a child’s later development [3], and it is often indicative of more generalised developmental delays and disabilities in children [4].
It is a widely held view that motor development in children can be influenced by both genetics and environmental factors [5]. There are known predictors of motor development delay, such as low birth weight and premature birth [6], pregnancy complications [7], low maternal intelligence [8], and low education level [9]. There is also evidence that maternal diabetes can have deleterious effects on the developing foetus, as well conditions such as maternal hyperglycaemia, ketonaemia, and recurrent changes in glucose status [7,10]. Iron deficiency is also associated with diabetes [11] and can also adversely affect neurodevelopment in humans [12]. During pregnancy, a hyperglycaemic environment of the intrauterine life negatively impacts foetal neural development [13].
The association of maternal diabetes with motor development in child has been evaluated by several case–control [14,15] or cohort studies [13,16,17], with controversial conclusions. For example, in a recent study by Alamolhoda et al. [18] in Iran, findings showed that gestational diabetes (GDM) can be a powerful risk factor for motor developmental delay after adjusting for crucial variables. On the contrary, two retrospective longitudinal cohort studies based on smaller sample sizes from the United States [19,20] and Sweden [21] revealed that maternal diabetes was not related with the motor development of children.
With the advent of better perinatal care, many women are now closely monitored for blood glucose, and this has greatly reduced adverse outcomes for infants since the 1980s [22]. However, it is necessary to obtain more data to evaluate the association between maternal diabetes and motor developmental delay in children. It is suggested that motor developmental delays may be more subtle and harder to detect because results from studies are mixed and inconsistent [7,23], and the neuroplasticity of the developing brain means that remediation may be possible [24]. To address this knowledge gap, a systematic literature review and meta-analysis are needed to confirm whether exposure to maternal diabetes during intrauterine life is associated with delayed motor development in children.
Systematically synthesised information on the associations between intrauterine exposure to diabetes and motor development is lacking. This study will shed light on this topic and assist in identifying research gaps and provide scientific evidence regarding this association. This will help in identifying children at risk of developmental delay and would provide healthcare professionals with more information to deliver early diagnosis and interventions.
2. Materials and Methods
We performed a systematic review and meta-analysis in accordance with a published protocol (PROSPERO registration number: CRD42020182739). The Joanna Briggs Institute (JBI) methodology for systematic reviews and the Preferred Reporting Items for Systematic Review [25] and Meta-Analysis (PRISMA) guidelines [26] were used to report the findings.
2.1. Review Question and Eligibility
This review aimed to address the following question: Is there an association between exposure to maternal diabetes during pregnancy and motor development in children?
This review considered studies that report on children born to mothers with diabetes. Participants must be children of either gender, aged 12 years or less. The exposure of interest is maternal diabetes. More specifically, maternal diabetes includes diabetes in pregnancy, whether pre-existing or gestational. We included outcomes of motor development, such as fine motor and gross motor milestones. Only motor development outcomes measured by standardised tools were included. This review considered all analytical observational studies that have evaluated the impact of intrauterine foetal exposure to maternal diabetes, with no limitation as to the type of maternal diabetes. We excluded studies with unclear indicators of maternal diabetes during pregnancy, and studies published in languages other than English. There was no limitation on the date of publication.
2.2. Search Strategy and Study Selection
The initial search for this review was performed in duplicate using the search strategy outlined in the electronic Supplementary Material (Supplementary Text S1). The search was performed from June–September 2020. The search was carried out using keywords and medical subject heading terms (MeSH) that were modified for each database. MEDLINE/PubMed, Excerpta Medica dataBASE (EMBASE), Emcare, PsycINFO, and Google Scholar databases were searched using the identified index terms and search strategy. Following the search, identified citations were collated and uploaded into EndNote, and then duplicates were removed. The reference list of all included studies was screened for additional studies. Titles and abstracts were screened by two independent reviewers (V.K., M.J.) for assessment against the inclusion criteria for the review. Potentially relevant studies were retrieved in full, and their citation details were imported into the Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI) [25]. The selected full text citations were assessed in detail against the inclusion criteria by two independent reviewers (V.K., M.J.). Reasons for the exclusion of full text studies that did not meet the inclusion criteria were recorded and reported in the systematic review (Supplementary Table S1), leaving the other citations to be appraised. Any disagreements that arose between the reviewers at each stage of the study selection process were resolved through discussion with and inclusion of a third reviewer (M.A.).
2.3. Data Extraction and Assessment of Methodological Quality
Data were extracted from papers included in the review using the standardised data extraction tools in JBI SUMARI by two independent reviewers (Supplementary Tables S2 and S3). Each reviewer independently assessed the methodological quality of the included studies using standardised critical appraisal instruments from the JBI. All studies, regardless of the results of their methodological quality assessment, underwent data extraction and synthesis (where possible). Any discrepancy in quality assessment between reviewers was resolved through discussion.
2.4. Data Synthesis and Analysis
Data were pooled in a statistical meta-analysis using JBI SUMARI [25] and meta-analysis was performed using random-effect models. Summary measures of the effect size for standardised motor development outcomes were expressed as mean differences. Where means, standard deviations (SD), and effect estimates were not available, they were calculated from data where possible. Where SD data were not available, and standard error data were available, standard error values were converted into SD values. Means, SDs, and sample sizes for the group of children born to mothers with diabetes and those without diabetes were used to calculate effect size. An effect size of 0.2 was considered small, an effect size of 0.5 was considered moderate, and an effect size of 0.8 was considered large [27]. Statistical heterogeneity was quantified using I2 statistics. We were not able to perform stratified analysis by type of diabetes for the gross and fine motor scores given the lack of available data related to the type of diabetes or the limited number of studies.
3. Results
The search strategy identified 1279 records, of which 44 duplicates were removed (Figure 1). There were 1203 records excluded at the title and abstract level, leaving 33 articles to be screened at the full text level. Of these, 15 were excluded with reasons, leaving 18 studies included in the review (Table 1).
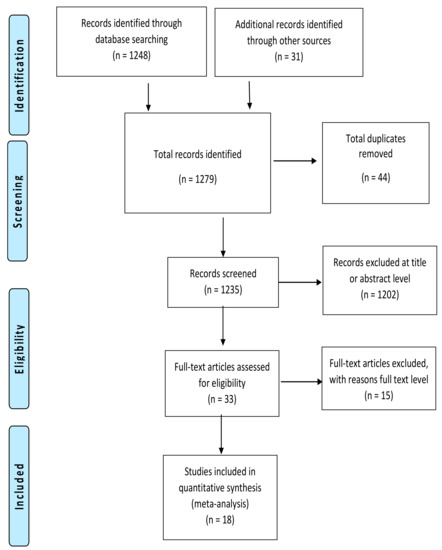
Figure 1.
PRISMA flow diagram: Summary of studies search and selection process

Table 1.
Characteristics of included studies.
3.1. Methodological Quality
Overall, the case–control studies were of a high quality (Supplementary Tables S4 and S5). Other than Biesenbach et al. [14], which scored positively on 8 out of 10 questions, all studies scored positively on all questions. For the cohort studies, excluding all non-applicable results, all other questions resulted in yes answers, except for three studies where there were 2 no answers for one study [28], and 1 no answer for two studies [29,30]
3.2. Characteristics of Included Studies
Of the 18 studies included, 10 were case–control studies, and 8 were cohort studies. Most studies came from high human development index countries, with the exception of Mexico, a medium development index country. In this review, four of the included studies used the same cohort [31,32,33,34] but reported on a different aspect of motor development; therefore, all were included in the review and only one was included in the meta-analysis (Table 2 and Table 3). Three studies used multiple regression analysis in reporting its findings and could not be included in our meta-analysis as the statistical coefficient of motor development cannot be reliably extracted from the regression equations (see Table 3). This resulted in the exclusion of these studies prior to our meta-analysis [16,17,35]. Outcome measures were heterogeneous across studies. We present the main characteristics of included studies in Table 1, Table 2 and Table 3. We subdivided motor development outcomes in this meta-analysis into three domains: general motor development, gross motor development, and fine motor development. The results of all effect sizes for the motor development outcomes are presented in forest plots in Figure 2, Figure 3 and Figure 4.

Table 2.
Summary of results of included studies.

Table 3.
Summary of studies on motor development included in this review.
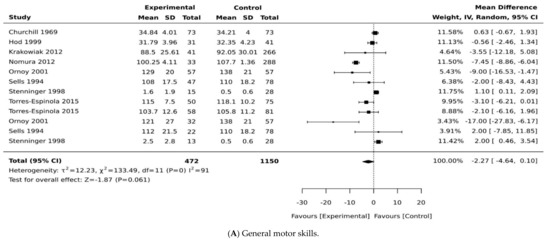
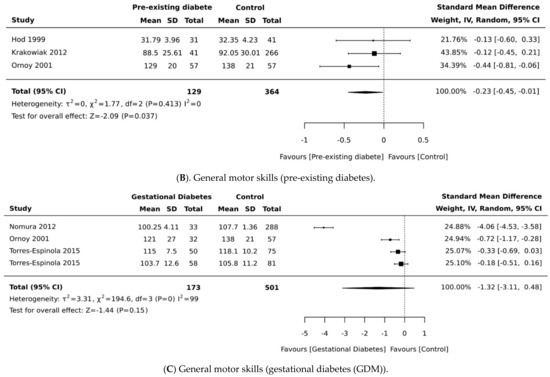
Figure 2.
Effects of maternal pregnancy on general motor development. Forest plots comparing the differences in (A) motor development in all type of diabetes, (B) motor development between children born to mothers with pre-existing diabetes and no diabetes, and (C) forest plots comparing the differences in motor development between children born to mothers with and without gestational diabetes.
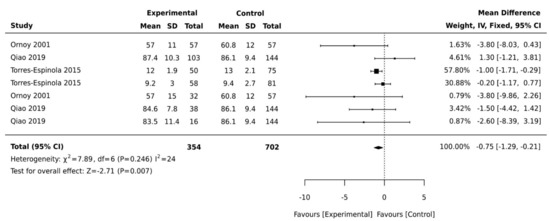
Figure 3.
Forest plots comparing the differences in gross motor development between children born to mothers with and without maternal diabetes.
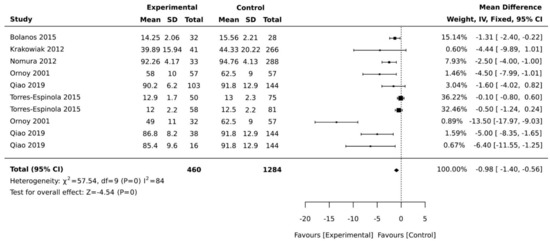
Figure 4.
Forest plots comparing the differences in fine motor development between children born to mothers with and without maternal diabetes.
3.3. Exposure to Maternal Diabetes and Motor Development
We identified eight studies that examined the association of maternal diabetes with measures of motor development (Table 1). Only seven studies were pooled in a meta-analysis for general motor scores. Daraki et al. [17] reported adjustable data related to children born to mothers with and without diabetes, but these data could not be pooled because they were difficult to extract. In this study, the authors reported no association between glucose intolerance and obesity in early pregnancy and motor development in children. However, the study did not provide data for the matched comparison groups or a measure of error to compare motor scores among groups. One study [20] provided data for two cohorts, “early entry” and “late entry”, with no clear definition for the groups. In the meta-analysis, we pooled the standard deviation of the mean (SDM) using both cohorts. In another study [21], data were provided for children according to their experience of “hypoglycaemia” after birth, and both cohorts were included in the meta-analysis. Ornoy et al. [32] provided extractable data for two types of diabetes (pre-existing and gestational diabetes) and both groups were included in the main meta-analysis. In Torres-Espinola et al. [30], both cohorts of children assessed at 6 months and 12 months were included in the meta-analysis.
In the main meta-analysis including children born to mothers with pre-existing diabetes and GDM, the pooled weighted mean difference was −1.87 (95% CI, −4.64, 0.10; p = 0.061; I2 = 91%, Figure 2A), suggesting that children born to mothers with diabetes have significantly lower motor scores compared with control groups. We have performed a sensitivity analysis by including studies with only children born to mothers with pre-existing diabetes and found a pooled mean difference of −2.09 (95% CI, −0.45, −0.01; p = 0.037; I2 = 0%, Figure 2B), suggesting lower motor scores in children born to mothers with pre-existing diabetes. When the analysis was re-run, substituting mothers with pre-existing diabetes from the same Ornoy et al. cohort [32] with Ornoy et al.’s cohort of mothers with GDM [32] and other GDM groups [28,30], the pooled mean difference changed to −1.44 (95% CI, −1.32, 0.48; p = 0.15; I2 = 99%, Figure 2C). It should be noted that only two studies [21,30] found no significant differences in motor development between children born to mothers with and without diabetes.
3.4. Exposure to Maternal Diabetes and Gross Motor Development
Ten studies assessed gross motor development; however, only four could be pooled in the meta-analysis. Two studies used multiple regression data, which could not be pooled with the weighted mean differences extracted from the rest of the studies. Adane et al. [16] found that children born to mothers with diabetes had a higher risk of developmental delay, particularly gross motor skills, compared to the control group. This was consistent with Ghassabian et al. [35], who found that children born to mothers with GDM took longer to achieve major motor developmental milestones, such as sitting without support or walking.
Another two studies were not included as they assessed gross motor development by reporting the time the children started to walk [14] or the percentages of children with delayed gross motor development compared to children with typical development [13,14]. Biesenbach et al. [14] examined differences in the time children started to walk among two groups of children born to mothers with diabetes and nephropathy and those without nephropathy. There was no significant difference in the time children started to walk and these data could not be pooled as the control group was also diagnosed with diabetes and the study did not use a validated tool. Girchenko et al. [13] noted that overweight, obesity, and pre-eclampsia are also associated with motor developmental delay in children. The meta-analysis was performed using data from four studies. Ornoy et al. [32] included two cohorts of children born to mothers with pre-exiting diabetes and GDM, while Qiao et al. [29] included three different cohorts of children with neonatal hypoglycaemia and Torres-Espinola et al. [30] included cohorts of children aged 6 months and children aged 12 months. The pooled weighted mean difference was −2.71 (95% CI, −1.29, −0.21; p = 0.007; I2 = 24%, Figure 3), suggesting that children born to mothers with diabetes have significantly lower gross motor scores compared with control groups.
3.5. Exposure to Maternal Diabetes and Fine Motor Development
Ten studies reported on the association of maternal diabetes with fine motor development in children. Since Girchenko et al. [13] performed a stratified analysis by the mothers’ weight and presented data as percentages, this study could not be included in the meta-analysis. Stenninger et al. [21] used the manual dexterity subscale, which could not be pooled with other developmental scales used in the other studies. Stenninger et al. [21] found no significant differences in manual dexterity scores for children born to mothers with and without diabetes, however, children with hypoglycaemia showed lower motor scores compared to children without hypoglycaemia. The meta-analysis of six studies indicated a significant pooled mean difference of −4.57 (95% CI, −1.40, −0.56; p < 0.001; I2 = 84%, Figure 4).
4. Discussion
This meta-analysis found that children born to mothers with diabetes experience delayed motor development when compared to children born to mothers without diabetes, particularly for children born to mothers with pre-existing diabetes. There was also evidence of a low motor score in children born to mothers with GDM; however, there were insufficient studies and heterogeneity was too high to draw conclusions about these outcomes. Other gross and fine motor skills were also adversely affected among this population. We noted significantly lower pooled motor scores in children exposed to maternal diabetes compared with comparator groups with an overall effect of −2.71 motor points for gross motors skills and −4.54 motor points for fine motor skills.
It is worth noting that the pooled motor development score listed for motor development outcomes was unadjusted. Maternal obesity was a confounder in this meta-analysis as the majority of studies did not adjust findings by BMI, or other major confounders such as glycaemic control or gestational morbidities. Only one study adjusted data for weight gain during pregnancy [30], two studies adjusted data for birth weight and gestational age [15,19], and three studies adjusted for education level [12,20,28], thus, reported motor scores can be considered as independent of those confounders.
In this review, four studies matched in terms of some important confounders, such as age, socioeconomic status, and gestational age [30,31,32,33,34], yet residual confounding likely exists even after matching groups in those studies. This suggests that environmental influence on child motor development starts during intrauterine life and remains during growth.
Low socioeconomic alone or in combination with exposure to GDM increases the risk of neurobehavioral problems in children [28]. In addition, the influence of diabetes and other gestational comorbidities is also closely related to maternal overweight/obesity. For example, several studies reported that obesity is a strong predictor of maternal diabetes, including pre-existing diabetes and GDM [37,38]. Among all environmental factors, maternal pre-pregnancy overweight/obesity is the one that affects child motor development the most [13]. In a study performed in different diabetes cohorts with different means of BMI, children from diabetic and overweight mothers performed worse in the different motor development tests than those born to mothers with diabetes and normal weight [13]. However, when diabetes and control mothers came from a similar cohort, sharing the same parental obesity and glucose tolerance levels, the differences between their children’s neurodevelopment were not significant [17]. While those studies reported on the motor development of children exposed to maternal diabetes or not, they were not considered for this meta-analysis due to lack of standardisation among scores or our inability to reliably separate the statistical coefficient of motor development.
Our findings are congruent with earlier reports [13,28,39] that state that influences of other gestational comorbidities and environmental factors are closely related to a child’s development. Among all intrauterine factors, gestational comorbidities such as hypertension [13] and obesity [13,16,17,35] also affect child development and, therefore, among children of diabetic mothers, those with other gestational comorbidities were more likely to be at risk developmentally.
Another confounder in this review may be the type of maternal diabetes, which has been suggested previously to have a link with glycaemic control [40]. Five out of 18 studies did not differentiate the type of diabetes and considered all diabetic mothers as a single group [14,16,19,20,29]. When separated, our meta-analysis showed that children from mothers with pre-existing diabetes had worse motor development than those from GDM mothers. This may relate to the fact that GDM often involves a less severe form of hyperglycaemia of shorter duration. We noted that two studies were limited to women with pre-existing diabetes and had the largest difference in motor scores between groups [12,32], whereas studies limited to GDM [28,30] had the lowest difference between groups. In the study by Torres-Espinola et al. [30], children born to diabetic mothers had the lowest motor skill at 18 months, but this difference was lost in adjusted models.
5. Limitations
This study has several limitations. First, there are the small number of studies included in each analysis and the significant heterogeneity between studies calculated using I2 statistics. We noted significant heterogeneity for both general motor and fine motor skills that can be related to remarkable differences between studies in the type of measures used, time of assessment, and type of maternal diabetes. Therefore, our findings should be interpreted cautiously. Second, including only studies published in the English language raises a limitation by possibly missing out on studies published in other languages [41]. Third, the greatest limitation concerns the limited number of studies adjusted for important confounders, such as glycaemic control and other comorbidities. Some studies included in this review were minimally adjusted or unadjusted for important confounders and, therefore, the findings must be interpreted carefully. While some of the studies were adjusted to perinatal factors such as gestational age, birthweight, and neonatal hypoglycaemia, it remains unclear what the roles of other gestational comorbidities, such as maternal obesity, gestational hypertension, glycaemic control, and low socioeconomic status, are and to what degree those factors may have contributed to our findings.
6. Conclusions
Maternal diabetes during pregnancy was associated with reduced motor development in children. This study underscores the importance of the type of diabetes, particularly glycaemic levels, in reducing the disparities in motor development among children born to mothers with diabetes. For children born to mothers with diabetes, interventions should focus on maintaining glycaemic control and optimum weight during pregnancy in order to ensure children’s improved motor development. It is penitent also for policy makers to consider these variables when thinking about strategies to cope with the increased complexity and new challenges posed by the number of children appears to have developmental delay [42].
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/18/4/1699/s1, Text S1: Search strategy. Table S1: Excluded studies at full text; Table S2: Characteristics of case control included studies; Table S3: Characteristics of included cohort studies; Table S4: Case control study critical appraisal results; Table S5: Cohort study critical appraisal results.
Author Contributions
Conceptualisation, D.A., L.C.W., M.A.J., and G.A.; methodology, D.A., M.A.J., V.K., and M.J.; formal analysis and critical appraisal, M.A.J., M.J., and V.K. writing—original draft preparation, D.A.; review and editing, L.C.W. and G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to it being classified as meta-analysis and systematic review.
Informed Consent Statement
Patient consent was waived due to it being classified as systematic review and meta-analysis.
Data Availability Statement
The data presented in this review are available on request from the corresponding author.
Acknowledgments
We are grateful to the Centre of Nursing, Midwifery and Health Services Research at ECU, as well as to the JBI-affiliated group at the Centre for Evidence Informed Nursing, Midwifery and Healthcare Practice for the administrative and technical support provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pinero-Pinto, E.; Perez-Cabezas, V.; De-Hita-Cantalejo, C.; Ruiz-Molinero, C.; Gutiérrez-Sánchez, E.; Jimenez-Rejano, J.J.; Sánchez-González, J.; Sánchez-González, M.C. Vision Development Differences between Slow and Fast Motor Development in Typical Developing Toddlers: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 3597. [Google Scholar] [CrossRef]
- Edwards, S.L.; Sarwark, J.F. Infant and Child Motor Development. Clin. Orthop. Relat. Res. 2005, 434, 33–39. [Google Scholar] [CrossRef]
- Osorio-Valencia, E.; Torres-Sánchez, L.; López-Carrillo, L.; Rothenberg, S.J.; Schnaas, L. Early motor development and cognitive abilities among Mexican preschoolers. Child Neuropsychol. 2017, 24, 1–11. [Google Scholar] [CrossRef]
- Noritz, G.H.; Murphy, N.A.; Panel, N.S.E.; Hagan, J.F.; Lipkin, P.H.; Macias, M.M.; Navsaria, D.; Peacock, G.; Rosenbaum, P.L.; Saal, H.M.; et al. Motor Delays: Early Identification and Evaluation. Pediatrics 2013, 131, e2016–e2027. [Google Scholar] [CrossRef] [PubMed]
- Poulain, T.; Vogel, M.; Sobek, C.; Hilbert, A.; Körner, A.; Kiess, W. Associations Between Socio-Economic Status and Child Health: Findings of a Large German Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 677. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, R.; Fujioka, K.; Kyono, Y.; Yoshida, A.; Kido, T.; Suga, S.; Abe, S.; Ashina, M.; Nishida, K.; Tanimura, K.; et al. Neurodevelopmental Outcomes at 18 Months of Corrected Age for Late Preterm Infants Born at 34 and 35 Gestational Weeks. Int. J. Environ. Res. Public Health 2021, 18, 640. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.C.; Campoy, C.; Fernandez, L.G.; López-Pedrosa, J.M.; Rueda, R.; Martín, M.J. Maternal Diabetes and Cognitive Performance in the Offspring: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0142583. [Google Scholar] [CrossRef]
- Tong, S.-L.; Baghurst, P.; Vimpani, G.; McMichael, A. Socioeconomic Position, Maternal IQ, Home Environment, and Cognitive Development. J. Pediatr. 2007, 151, 284–288.e1. [Google Scholar] [CrossRef]
- Effects of socio-economic status and maternal education on gross motor development of preschool children. Dev. Med. Child Neurol. 2015, 57, 55–56. [CrossRef]
- Ahmed, R. Evolutionary interactions between diabetes and development. Diabetes Res. Clin. Pr. 2011, 92, 153–167. [Google Scholar] [CrossRef]
- Verner, A.M.; Manderson, J.; Lappin, T.R.J.; McCance, D.R.; Halliday, H.L.; Sweet, D.G. Influence of maternal diabetes mellitus on fetal iron status. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F399–F401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santa-Marina, L.; Lertxundi, N.; Andiarena, A.; Irizar, A.; Sunyer, J.; Molinuevo, A.; Llop, S.; Julvez, J.; Beneito, A.; Ibarluzea, J.; et al. Maternal Ferritin Levels during Pregnancy and ADHD Symptoms in 4-Year-Old Children: Results from the INMA–INfancia y Medio Ambiente (Environment and Childhood) Prospective Birth Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 7704. [Google Scholar] [CrossRef]
- Girchenko, P.; Tuovinen, S.; Lahti-Pulkkinen, M.; Lahti, J.; Savolainen, K.; Heinonen, K.; Pyhälä, R.; Reynolds, R.M.; Hämäläinen, E.; Villa, P.M. Maternal early pregnancy obesity and related pregnancy and pre-pregnancy disorders: Associations with child developmental milestones in the prospective PREDO Study. Int. J. Obes. 2018, 42, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Biesenbach, G.; Grafinger, P.; Zazgornik, J.; Stöger, H. Perinatal complications and three-year follow up of infants of diabetic mothers with diabetic nephropathy stage IV. Ren. Fail. 2000, 22, 573–580. [Google Scholar] [CrossRef]
- Hod, M.; Levy-Shiff, R.; Lerman, M.; Schindel, B.; Ben-Rafael, Ζ.; Bar, J. Developmental Outcome of Offspring of Pregestational Diabetic Mothers. J. Pediatr. Endocrinol. Metab. 1999, 12, 867–872. [Google Scholar] [CrossRef]
- Adane, A.A.; Mishra, G.D.; Tooth, L.R. Maternal preconception weight trajectories, pregnancy complications and offspring’s childhood physical and cognitive development. J. Dev. Orig. Health Dis. 2018, 9, 653–660. [Google Scholar] [CrossRef]
- Daraki, V.; Roumeliotaki, T.; Koutra, K.; Georgiou, V.; Kampouri, M.; Kyriklaki, A.; Vafeiadi, M.; Papavasiliou, S.; Kogevinas, M.; Chatzi, L. Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: The Rhea mother–child cohort, Crete, Greece. Eur. Child Adolesc. Psychiatry 2017, 26, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Alamolhoda, S.; Ahmadi Doulabi, M.; Afraz, F. The impact of gestational diabetes mellitus on motor development in 12-month-old children attending to Qazvin University of Medical Sciences, Iran. Int. J. Pediatrics 2020, 8, 12575–12583. [Google Scholar] [CrossRef]
- Churchill, J.A.; Berendes, H.W.; Nemore, J. Neuropsychological deficits in children of diabetic mothers: A report from the Collaborative Study of Cerebral Palsy. Am. J. Obstet. Gynecol. 1969, 105, 257–268. [Google Scholar] [CrossRef]
- Sells, C.J.; Robinson, N.M.; Brown, Z.; Knopp, R.H. Long-term developmental follow-up of infants of diabetic mothers. J. Pediatrics 1994, 125, S9–S17. [Google Scholar] [CrossRef]
- Stenninger, E.; Flink, R.; Eriksson, B.; Sahlen, C. Long term neurological dysfunction and neonatal hypoglycaemia after diabetic pregnancy. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 79, F174–F179. [Google Scholar] [CrossRef]
- Ojo, O.; Weldon, S.M.; Thompson, T.; Vargo, E.J. The Effect of Vitamin D Supplementation on Glycaemic Control in Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Environ. Res. Public Health 2019, 16, 1716. [Google Scholar] [CrossRef]
- Adane, A.A.; Mishra, G.D.; Tooth, L.R. Diabetes in Pregnancy and Childhood Cognitive Development: A Systematic Review. Pediatr. 2016, 137, e20154234. [Google Scholar] [CrossRef] [PubMed]
- Sesma, H.W.; Georgieff, M.K. The effect of adverse intrauterine and newborn environments on cognitive development: The experiences of premature delivery and diabetes during pregnancy. Dev. Psychopathol. 2003, 15, 991–1015. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Aromataris, E.; Tufanaru, C.; Stern, C.; Porritt, K.; Farrow, J.; Lockwood, C.; Stephenson, M.; Moola, S.; Lizarondo, L. The development of software to support multiple systematic review types: The Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI). Int. J. Evid.-Based Healthc. 2019, 17, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cohen, P.; Chen, S. How Big is a Big Odds Ratio? Interpreting the Magnitudes of Odds Ratios in Epidemiological Studies. Commun. Stat. Simul. Comput. 2010, 39, 860–864. [Google Scholar] [CrossRef]
- Nomura, Y.; Marks, D.J.; Grossman, B.; Yoon, M.; Loudon, H.; Stone, J.; Halperin, J.M. Exposure to gestational diabetes mellitus and low socioeconomic status: Effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch. Pediatrics Adolesc. Med. 2012, 166, 337–343. [Google Scholar]
- Qiao, L.-X.; Wang, J.; Yan, J.-H.; Xu, S.-X.; Wang, H.; Zhu, W.-Y.; Zhang, H.-Y.; Li, J.; Feng, X. Follow-up study of neurodevelopment in 2-year-old infants who had suffered from neonatal hypoglycemia. BMC Pediatr. 2019, 19, 133. [Google Scholar] [CrossRef]
- Torres-Espínola, F.J.; Berglund, S.K.; García-Valdés, L.M.; Moreno, M.T.S.; Jerez, A.; Campos, D.; Moreno-Torres, R.; Rueda, R.; Catena, A.; Pérez-García, M.; et al. Maternal Obesity, Overweight and Gestational Diabetes Affect the Offspring Neurodevelopment at 6 and 18 Months of Age—A Follow Up from the PREOBE Cohort. PLoS ONE 2015, 10, e0133010. [Google Scholar] [CrossRef]
- Ornoy, A.; Ratzon, N.; Greenbaum, C.; Peretz, E.; Soriano, D.; Dulitzky, M. Neurobehaviour of school age children born to diabetic mothers. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 79, F94–F99. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Ratzon, N.; Greenbaum, C.; Wolf, A.; Dulitzky, M. School-age Children Born to Diabetic Mothers and to Mothers with Gestational Diabetes Exhibit a High Rate of Inattention and Fine and Gross Motor Impairment. J. Pediatr. Endocrinol. Metab. 2001, 14, 681–690. [Google Scholar] [CrossRef]
- Ornoy, A.; Wolf, A.; Ratzon, N.; Greenbaum, C.; Dulitzky, M. Neurodevelopmental outcome at early school age of children born to mothers with gestational diabetes. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 81, F10–F14. [Google Scholar] [CrossRef] [PubMed]
- Ratzon, N.; Greenbaum, C.; Dulitzky, M.; Ornoy, A. Comparison of the motor development of school-age children born to mothers with and without diabetes mellitus. Phys. Occup. Ther. Pediatr. 2000, 20, 43–57. [Google Scholar] [CrossRef]
- Ghassabian, A.; Sundaram, R.; Wylie, A.; Bell, E.M.; Bello, S.C.; Yeung, E.H. Maternal medical conditions during pregnancy and gross motor development up to age 24 months in the Upstate KIDS study. Dev. Med. Child Neurol. 2016, 58, 728–734. [Google Scholar] [CrossRef]
- Bolaños, L.; Matute, E.; Ramírez-Dueñas, M.D.L.; Zarabozo, D.; Ramirez-Duenas, M.D.L. Neuropsychological Impairment in School-Aged Children Born to Mothers With Gestational Diabetes. J. Child Neurol. 2015, 30, 1616–1624. [Google Scholar] [CrossRef]
- Bianchi, C.; De Gennaro, G.; Romano, M.; Aragona, M.; Battini, L.; Del Prato, S.; Bertolotto, A. Pre-pregnancy obesity, gestational diabetes or gestational weight gain: Which is the strongest predictor of pregnancy outcomes? Diabetes Res. Clin. Pr. 2018, 144, 286–293. [Google Scholar] [CrossRef]
- Carbone, S.; Del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 2019, 62, 327–333. [Google Scholar] [CrossRef]
- Duffany, K.O.; McVeigh, K.H.; Lipkind, H.S.; Kershaw, T.; Ickovics, J.R. Large for Gestational Age and Risk for Academic Delays and Learning Disabilities: Assessing Modification by Maternal Obesity and Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 5473. [Google Scholar] [CrossRef]
- Shefali, A.K.; Kavitha, M.; Deepa, R.; Mohan, V. Pregnancy outcomes in pre-gestational and gestational diabetic women in comparison to non-diabetic women—A prospective study in Asian Indian mothers (CURES-35). J. Assoc. Physicians India 2006, 54, 613–618. [Google Scholar] [PubMed]
- Rasmussen, L.N.; Montgomery, P. The prevalence of and factors associated with inclusion of non-English language studies in Campbell systematic reviews: A survey and meta-epidemiological study. Syst. Rev. 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Arabiat, D.; Whitehead, L.; Al Jabery, M. The 12-year prevalence and trends of childhood disabilities in Australia: Findings from the Survey of Disability, Aging and Carers. Child Care Health Dev. 2018, 44, 697–703. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).