Unraveling the Role of Respiratory Muscle Metaboloreceptors under Inspiratory Training in Patients with Heart Failure
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Search Strategies
2.3. Study Selection
2.4. Data Extraction Processes
3. Results and Discussion
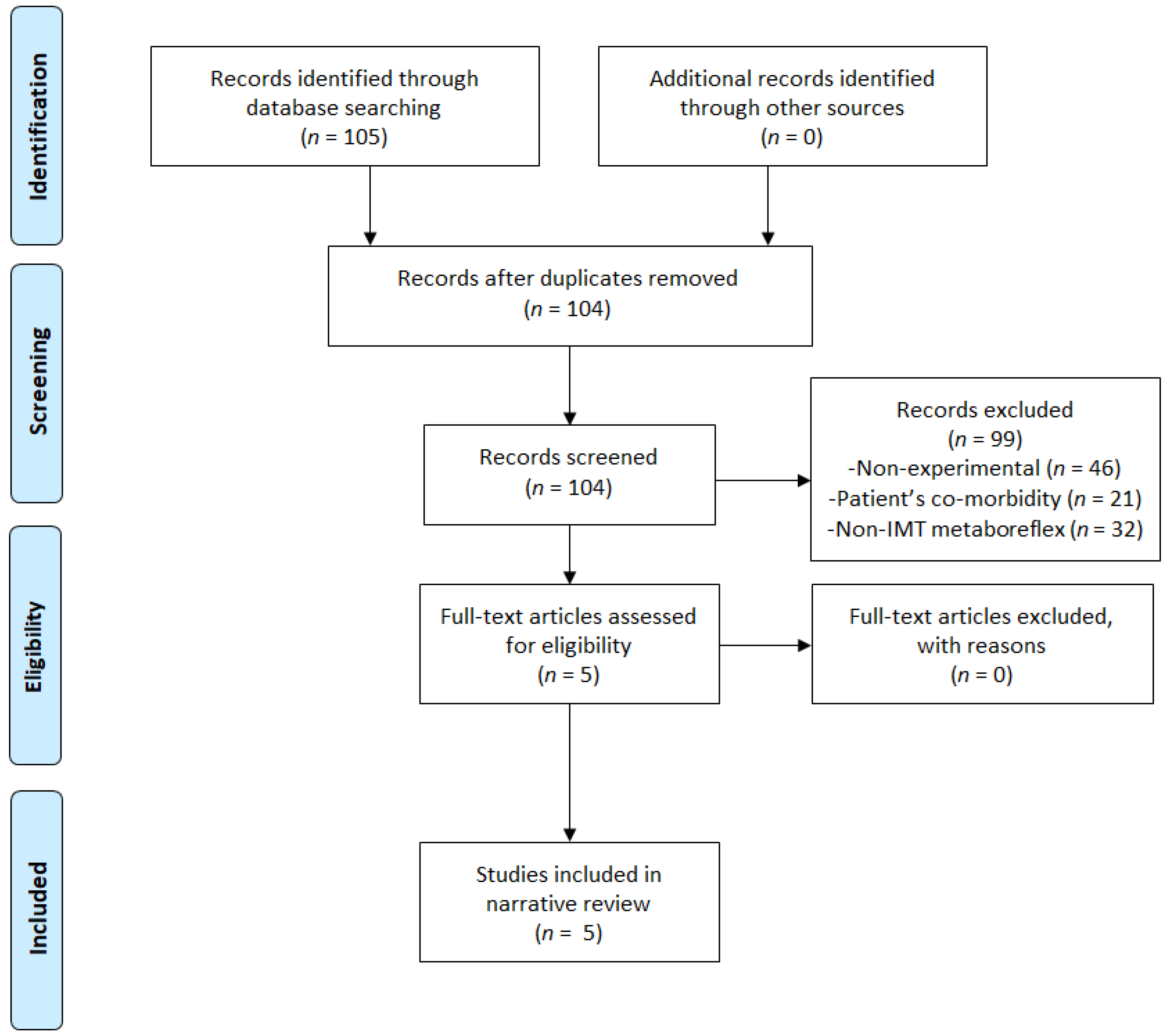
3.1. Flow Chart
3.2. IMT Effects on Metaboreflex in HF Patients
3.3. Future Research and Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhry, S.P.; Stewart, G.C. Advanced Heart Failure: Prevalence, Natural History, and Prognosis. Heart Fail. Clin. 2016, 12, 323–333. [Google Scholar] [CrossRef]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef]
- Poole, D.C.; Copp, S.W.; Hirai, D.M.; Musch, T.I. Dynamics of muscle microcirculatory and blood-myocyte O2 flux during contractions. Acta Physiol. 2010, 202, 293–310. [Google Scholar] [CrossRef]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Strömberg, A.; Van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Heart J. 2008, 29, 2388–2442. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Dimopoulos, K.; Concu, A.; Crisafulli, A. Cardiovascular and ventilatory control during exercise in chron-ic heart failure: Role of muscle reflexes. Int. J. Cardiol. 2008, 130, 3–10. [Google Scholar] [CrossRef]
- Warriner, D.; Sheridan, P.; Lawford, P. Heart failure: Not a single organ disease but a multisystem syndrome. Br. J. Hosp. Med. 2015, 76, 330–336. [Google Scholar] [CrossRef]
- Laoutaris, I.D. The ‘aerobic/resistance/inspiratory muscle training hypothesis in heart failure’. Eur. J. Prev. Cardiol. 2018, 25, 1257–1262. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive summary: Heart disease and stroke statistics-2010 update: A report from the american heart association. Circulation 2010, 121, 948–954. [Google Scholar]
- Fisher, J.D. New York Heart Association Classification. Arch. Intern. Med. 1972, 129, 836. [Google Scholar] [CrossRef]
- Lalande, S.; Cross, T.J.; Keller-Ross, M.L.; Morris, N.R.; Johnson, B.D.; Taylor, B.J. Exercise Intolerance in Heart Failure: Central Role for the Pulmonary System. Exerc. Sport Sci. Rev. 2020, 48, 11–19. [Google Scholar] [CrossRef]
- Nakagawa, N.K.; Diz, M.A.; Kawauchi, T.S.; De Andrade, G.N.; Umeda, I.I.K.; Murakami, F.M.; Oliveira-Maul, J.P.; Nascimento, J.A.; Nunes, N.; Takada, J.Y.; et al. Risk Factors for Inspiratory Muscle Weakness in Chronic Heart Failure. Respir. Care 2019, 65, 507–516. [Google Scholar] [CrossRef]
- Clark, A.L.; Poole-Wilson, P.A.; Coats, A.J. Exercise limitation in chronic heart failure: Central role of the periphery. J. Am. Coll. Cardiol. 1996, 28, 1092–1102. [Google Scholar] [CrossRef]
- Nilsson, K., Jr.; Duscha, B.D.; Hranitzky, P.M.; Kraus, W.E.; Nilsson, K.R. Chronic Heart Failure and Exercise Intolerance: The Hemodynamic Paradox. Curr. Cardiol. Rev. 2008, 4, 92–100. [Google Scholar] [CrossRef]
- Hammond, M.D.; Bauer, K.A.; Sharp, J.T.; Rocha, R.D. Respiratory Muscle Strength in Congestive Heart Failure. Chest 1990, 98, 1091–1094. [Google Scholar] [CrossRef]
- Walsh, J.T.; Andrews, R.; Johnson, P.; Phillips, L.; Cowley, A.J.; Kinnear, W.J. Inspiratory muscle endurance in patients with chronic heart failure. Heart 1996, 76, 332–336. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Green, H.J.; Cobb, F.R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 1990, 81, 518–527. [Google Scholar] [CrossRef]
- Mancini, N.M.; Henson, D.; LaManca, J.; Levine, S. Evidence of reduced respiratory muscle endurance in patients with heart failure. J. Am. Coll. Cardiol. 1994, 24, 972–981. [Google Scholar] [CrossRef]
- Piepoli, M.; Clark, A.L.; Volterrani, M.; Adamopoulos, S.; Sleight, P.; Coats, A.J. Contribution of Muscle Afferents to the Hemodynamic, Autonomic, and Ventilatory Responses to Exercise in Patients with Chronic Heart Failure. Circulation 1996, 93, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L. Origin of symptoms in chronic heart failure. Heart 2006, 92, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Boushel, R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol. 2010, 199, 367–383. [Google Scholar] [CrossRef]
- Katayama, K.; Saito, M. Muscle sympathetic nerve activity during exercise. J. Physiol. Sci. 2019, 69, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Autonomic Adjustments to Exercise in Humans. Compr. Physiol. 2015, 5, 475–512. [Google Scholar] [CrossRef] [PubMed]
- Grotle, A.K.; Macefield, V.G.; Farquhar, W.B.; O’Leary, D.S.; Stone, A.J. Recent advances in exercise pressor reflex function in health and disease. Auton. Neurosci. Basic Clin. 2020, 228, 102698. [Google Scholar] [CrossRef]
- Vissing, J.; Vissing, S.F.; MacLean, D.A.; Saltin, B.; Quistorff, B.; Haller, R.G. Sympathetic activation in exercise is not dependent on muscle acidosis. Direct evidence from studies in metabolic myopathies. J. Clin. Investig. 1998, 101, 1654–1660. [Google Scholar] [CrossRef]
- Smith, J.R.; Didier, K.D.; Hammer, S.M.; Alexander, A.M.; Kurti, S.P.; Copp, S.W.; Barstow, T.J.; Harms, C.A. Effect of cyclooxygenase inhibition on the inspiratory muscle metaboreflex-induced cardiovascular consequences in men. J. Appl. Physiol. 2017, 123, 197–204. [Google Scholar] [CrossRef]
- Amann, M.; Wan, H.-Y.; Thurston, T.S.; Georgescu, V.P.; Weavil, J.C. On the Influence of Group III/IV Muscle Afferent Feedback on Endurance Exercise Performance. Exerc. Sport Sci. Rev. 2020, 48, 209–216. [Google Scholar] [CrossRef]
- Romer, L.M.; Polkey, M.I. Exercise-induced respiratory muscle fatigue: Implications for performance. J. Appl. Physiol. 2008, 104, 879–888. [Google Scholar] [CrossRef]
- Moreno, A.M.; Toledo-Arruda, A.C.; Lima, J.S.; Duarte, C.S.; Villacorta, H.; Nóbrega, A.C. Inspiratory Muscle Training Improves Intercostal and Forearm Muscle Oxygenation in Patients with Chronic Heart Failure: Evidence of the Origin of the Respiratory Metaboreflex. J. Card. Fail. 2017, 23, 672–679. [Google Scholar] [CrossRef]
- Yamada, K.; Kinugasa, Y.; Sota, T.; Miyagi, M.; Sugihara, S.; Kato, M.; Yamamoto, K. Inspiratory Muscle Weakness Is Associated with Exercise Intolerance in Patients with Heart Failure With Preserved Ejection Fraction: A Preliminary Study. J. Card. Fail. 2016, 22, 38–47. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Chiappa, G.R.; Neder, J.A.; Frankenstein, L. Respiratory muscle function and exercise intolerance in heart failure. Curr. Heart Fail. Rep. 2009, 6, 95–101. [Google Scholar] [CrossRef]
- Sheel, A.W.; Boushel, R.C.; Dempsey, J.A. Competition for blood flow distribution between respiratory and locomotor muscles: Implications for muscle fatigue. J. Appl. Physiol. 2018, 125, 820–831. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Chiappa, G.R.; Callegaro, C.C. The contribution of inspiratory muscles function to exercise limitation in heart failure: Pathophysiological mechanisms Contribuição da musculatura inspiratória na limitação ao exercício na in-suficiência cardíaca: Mecanismos fisiopatológicos. Rev. Bras. Fisioter. 2012, 16, 261–268. [Google Scholar] [CrossRef]
- Taylor, B.J.; Bowen, T.S. Respiratory Muscle Weakness in Patients with Heart Failure: Time to Make It a Standard Clinical Marker and a Need for Novel Therapeutic Interventions? J. Card. Fail. 2018, 24, 217–218. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Romer, L.; Rodman, J.; Miller, J.; Smith, C. Consequences of exercise-induced respiratory muscle work. Respir. Physiol. Neurobiol. 2006, 151, 242–250. [Google Scholar] [CrossRef]
- Smith, J.R.; Joyner, M.J.; Curry, T.B.; Borlaug, B.A.; Keller-Ross, M.L.; Van Iterson, E.H.; Olson, T.P. Locomotor muscle group III/IV afferents constrain stroke volume and contribute to exercise intolerance in human heart failure. J. Physiol. 2020, 598, 5379–5390. [Google Scholar] [CrossRef]
- Scott, A.C.; Francis, D.P.; Davies, L.C.; Ponikowski, P.; Coats, A.J.S.; Piepoli, M.F. Contribution of skeletal muscle ‘ergoreceptors’ in the human leg to respiratory control in chronic heart failure. J. Physiol. 2000, 529, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Keller-Ross, M.L.; Johnson, B.D.; Carter, R.E.; Joyner, M.J.; Eisenach, J.H.; Curry, T.B.; Olson, T.P. Improved Ventilatory Efficiency with Locomotor Muscle Afferent Inhibition is Strongly Associated with Leg Composition in Heart Failure. Int. J. Cardiol. 2016, 202, 159–166. [Google Scholar] [CrossRef]
- Keller-Ross, M.L.; Johnson, B.D.; Joyner, M.J.; Olson, T.P. Influence of the metaboreflex on arterial blood pressure in heart failure patients. Am. Heart J. 2014, 167, 521–528. [Google Scholar] [CrossRef]
- Dall’Ago, P.; Chiappa, G.R.; Guths, H.; Stein, R.; Ribeiro, J.P. Inspiratory Muscle Training in Patients With Heart Failure and Inspiratory Muscle Weakness. J. Am. Coll. Cardiol. 2006, 47, 757–763. [Google Scholar] [CrossRef]
- Padula, C.A.; Yeaw, E.; Mistry, S. A home-based nurse-coached inspiratory muscle training intervention in heart failure. Appl. Nurs. Res. 2009, 22, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.J.; Borst, M.M.; Zugck, C.; Kirschke, A.; Schellberg, D.; Kübler, W.; Haass, M. Respiratory Muscle Dysfunction in Congestive Heart Failure. Circulation 2001, 103, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Montemezzo, D.; Fregonezi, G.A.; Pereira, D.A.; Britto, R.R.; Reid, W.D. Influence of Inspiratory Muscle Weakness on Inspiratory Muscle Training Responses in Chronic Heart Failure Patients: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2014, 95, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Plentz, R.D.M.; Sbruzzi, G.; Ribeiro, R.A.; Ferreira, J.B.; Dal Lago, P. Inspiratory muscle training in patients with heart failure: Meta-analysis of randomized trials. Arq. Bras. Cardiol. 2012, 99, 762–771. [Google Scholar] [CrossRef]
- Weiner, P.; Magadle, R.; Berar-Yanay, N.; Pelled, B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin. Cardiol. 1999, 22, 727–732. [Google Scholar] [CrossRef]
- Hamazaki, N.; Masuda, T.; Kamiya, K.; Matsuzawa, R.; Nozaki, K.; Maekawa, E.; Noda, C.; Yamaoka-Tojo, M.; Ako, J. Respiratory muscle weakness increases dead-space ventilation ratio aggravating ventilation-perfusion mismatch during exercise in patients with chronic heart failure. Respirology 2018, 24, 154–161. [Google Scholar] [CrossRef]
- Cahalin, L.P.; Arena, R.; Guazzi, M.; Myers, J.; Cipriano, G.; Chiappa, G.; Lavie, C.J.; Forman, D.E. Inspiratory muscle training in heart disease and heart failure: A review of the literature with a focus on method of training and outcomes. Expert Rev. Cardiovasc. Ther. 2013, 11, 161–177. [Google Scholar] [CrossRef]
- McParland, C.; Krishnan, B.; Wang, Y.; Gallagher, C.G. Inspiratory Muscle Weakness and Dyspnea in Chronic Heart Failure. Am. Rev. Respir. Dis. 1992, 146, 467–472. [Google Scholar] [CrossRef]
- Tikunov, B.; Levine, S.; Mancini, D. Chronic Congestive Heart Failure Elicits Adaptations of Endurance Exercise in Diaphragmatic Muscle. Circulation 1997, 95, 910–916. [Google Scholar] [CrossRef]
- Hart, N.; Kearney, M.T.; Pride, N.B.; Green, M.; Lofaso, F.; Shah, A.M.; Moxham, J.; Polkey, M.I. Inspiratory muscle load and capacity in chronic heart failure. Thorax 2004, 59, 477–482. [Google Scholar] [CrossRef]
- Giallauria, F.; Piccioli, L.; Vitale, G.; Sarullo, F.M.; Sarulli, F.M. Exercise training in patients with chronic heart failure: A new challenge for Cardiac Rehabilitation Community. Monaldi Arch. Chest Dis. 2018, 88, 38–44. [Google Scholar] [CrossRef]
- Cahalin, L.P.; Arena, R.A. Breathing Exercises and Inspiratory Muscle Training in Heart Failure. Heart Fail. Clin. 2015, 11, 149–172. [Google Scholar] [CrossRef]
- Olson, T.P.; Joyner, M.J.; Dietz, N.M.; Eisenach, J.H.; Curry, T.B.; Johnson, B.D. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J. Physiol. 2010, 588, 2487–2501. [Google Scholar] [CrossRef]
- Smith, J.R.; Berg, J.D.; Curry, T.B.; Joyner, M.J.; Olson, T.P. Respiratory muscle work influences locomotor convective and diffusive oxygen transport in human heart failure during exercise. Physiol. Rep. 2020, 8, e14484. [Google Scholar] [CrossRef]
- Borghi-Silva, A.; Carrascosa, C.; Oliveira, C.C.; Barroco, A.C.; Berton, D.C.; Vilaça, D.; Lira-Filho, E.B.; Ribeiro, D.; Nery, L.E.; Neder, J.A. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. Am. J. Physiol. Circ. Physiol. 2008, 294, H2465–H2472. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; D’Arsigny, C.; Raj, S.R.; Abdollah, H.; Webb, K.A. Ventilatory Assistance Improves Exercise Endurance in Stable Congestive Heart Failure. Am. J. Respir. Crit. Care Med. 1999, 160, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rubio, H.; Becerro-De-Bengoa-Vallejo, R.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Vicente-Campos, D.; Chicharro, J.L. Inspiratory Muscle Training in Patients with Heart Failure. J. Clin. Med. 2020, 9, 1710. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Smart, N.A.; Giallauria, F.; Dieberg, G. Efficacy of inspiratory muscle training in chronic heart failure patients: A systematic review and meta-analysis. Int. J. Cardiol. 2013, 167, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Selig, S.E.; Hare, D.L. Respiratory Muscle Dysfunction and Training in Chronic Heart Failure. Hear. Lung Circ. 2011, 20, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-J.; McElfresh, J.; Hall, B.; Bloom, R.; Farrell, K. Inspiratory Muscle Training in Patients with Heart Failure: A Systematic Review. Cardiopulm. Phys. Ther. J. 2012, 23, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, G.R.; Roseguini, B.T.; Vieira, P.J.; Alves, C.N.; Tavares, A.; Winkelmann, E.R.; Ferlin, E.L.; Stein, R.; Ribeiro, J.P. Inspiratory Muscle Training Improves Blood Flow to Resting and Exercising Limbs in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2008, 51, 1663–1671. [Google Scholar] [CrossRef]
- Stein, R.; Chiappa, G.R.; Güths, H.; Dall’Ago, P.; Ribeiro, J.P. Inspiratory Muscle Training Improves Oxygen Uptake Efficiency Slope in Patients with Chronic Heart Failure. J. Cardiopulm. Rehabil. Prev. 2009, 29, 392–395. [Google Scholar] [CrossRef]
- Winkelmann, E.R.; Chiappa, G.R.; Lima, C.O.; Viecili, P.R.; Stein, R.; Ribeiro, J.P. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am. Heart J. 2009, 158, 768.e1–768.e7. [Google Scholar] [CrossRef]
- Bosnak-Guclu, M.; Arikan, H.; Savci, S.; Inal-Ince, D.; Tulumen, E.; Aytemir, K.; Tokgözoglu, L. Effects of inspiratory muscle training in patients with heart failure. Respir. Med. 2011, 105, 1671–1681. [Google Scholar] [CrossRef]
- Laoutaris, I.D.; Adamopoulos, S.; Manginas, A.; Panagiotakos, D.B.; Kallistratos, M.S.; Doulaptsis, C.; Kouloubinis, A.; Voudris, V.; Pavlides, G.; Cokkinos, D.V.; et al. Benefits of combined aerobic/resistance/inspiratory training in patients with chronic heart failure. A complete exercise model? A prospective randomised study. Int. J. Cardiol. 2013, 167, 1967–1972. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Schmid, J.-P.; Dendale, P.; Poerschke, D.; Hansen, D.; Dritsas, A.; Kouloubinis, A.; Alders, T.; Gkouziouta, A.; Reyckers, I.; et al. Combined aerobic/inspiratory muscle training vs. aerobic training in patients with chronic heart failure. Eur. J. Hear. Fail. 2014, 16, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.; Ramírez-Sarmiento, A.L.; Coloma, A.; Sartor, M.; Comin-Colet, J.; Vila, J.; Enjuanes, C.; Bruguera, J.; Escalada, F.; Gea, J.; et al. High-intensity vs. sham inspiratory muscle training in patients with chronic heart failure: A prospective randomized trial. Eur. J. Heart Fail. 2013, 15, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Mello, P.R.; Guerra, G.M.; Borile, S.; Rondon, M.U.; Alves, M.J.; Negrão, C.E.; Lago, P.D.; Mostarda, C.; Irigoyen, M.C.; Consolim-Colombo, F.M. Inspiratory Muscle Training Reduces Sympathetic Nervous Activity and Improves Inspiratory Muscle Weakness and Quality of Life in Patients with Chronic Heart Failure. J. Cardiopulm. Rehabil. Prev. 2012, 32, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Palau, P.; Domínguez, E.; Núñez, E.; Schmid, J.-P.; Vergara, P.; Ramón, J.M.; Mascarell, B.; Sanchis, J.; Chorro, F.J.; Núñez, J. Effects of inspiratory muscle training in patients with heart failure with preserved ejection fraction. Eur. J. Prev. Cardiol. 2014, 21, 1465–1473. [Google Scholar] [CrossRef]
- Palau, P.; Domínguez, E.; López, L.; Ramón, J.M.; Heredia, R.; González, J.; Santas, E.; Bodi, V.; Miñana, G.; Valero, E.; et al. Inspiratory Muscle Training and Functional Electrical Stimulation for Treatment of Heart Failure with Preserved Ejection Fraction: The TRAINING-HF Trial. Rev. Española Cardiol. (Engl. Ed.) 2019, 72, 288–297. [Google Scholar] [CrossRef]
- Palau, P.; Domínguez, E.; Ramón, J.M.; López, L.; Briatore, A.E.; Tormo, J.P.; Ventura, B.; Chorro, F.J.; Núñez, J. Home-based inspiratory muscle training for management of older patients with heart failure with preserved ejection fraction: Does baseline inspiratory muscle pressure matter? Eur. J. Cardiovasc. Nurs. 2019, 18, 621–627. [Google Scholar] [CrossRef]
- Taya, M.; Amiya, E.; Hatano, M.; Maki, H.; Hosoya, Y.; Ishida, J.; Bujo, C.; Tsuji, M.; Konishi, Y.; Yokota, K.; et al. Inspiratory muscle training for advanced heart failure with lamin-related muscular dystrophy. J. Cardiol. Cases 2019, 20, 232–234. [Google Scholar] [CrossRef]
- Kawauchi, T.S.; Umeda, I.I.K.; Braga, L.M.; Mansur, A.; Rossi-Neto, J.M.; Sousa, A.G.D.M.R.; Hirata, M.H.; Cahalin, L.P.; Nakagawa, N.K. Is there any benefit using low-intensity inspiratory and peripheral muscle training in heart failure? A randomized clinical trial. Clin. Res. Cardiol. 2017, 106, 676–685. [Google Scholar] [CrossRef]
- Pour, A.H.H.; Gholami, M.; Saki, M.; Birjandi, M. The effect of inspiratory muscle training on fatigue and dyspnea in patients with heart failure: A randomized, controlled trial. Jpn. J. Nurs. Sci. 2019, 17, e12290. [Google Scholar] [CrossRef]
- Hornikx, M.; Buys, R.; Cornelissen, V.; Deroma, M.; Goetschalckx, K. Effectiveness of high intensity interval training supplemented with peripheral and inspiratory resistance training in chronic heart failure: A pilot study. Acta Cardiol. 2019, 75, 339–347. [Google Scholar] [CrossRef]
- Martínez, A.; Lisboa, C.; Jalil, J.; Muñoz, V.; Díaz, O.; Casanegra, P.; Corbalán, R.; Vásquez, A.M.; Leiva, A. Selective training of respiratory muscles in patients with chronic heart failure. Rev. Médica Chile 2001, 129, 133–139. [Google Scholar]
- Frota, A.X.; Mendes, F.D.S.N.S.; Vieira, M.C.; Saraiva, R.M.; Veloso, H.H.; Da Silva, P.S.; Da Silva, G.M.S.; De Sousa, A.S.; Mazzoli-Rocha, F.; Costa, H.S.; et al. Acute and subacute hemodynamic responses and perception of effort in subjects with chronic Chagas cardiomyopathy submitted to different protocols of inspiratory muscle training: A cross-over trial. Disabil. Rehabil. 2020, 1–8. [Google Scholar] [CrossRef]
- Marchese, L.D.D.; Chermont, S.; Warol, D.; De Oliveira, L.B.; Pereira, S.B.; Quintão, M.; Mesquita, E.T. Estudo Controlado das Alterações Hemodinâmicas Centrais de uma Sessão de Exercício Inspiratório com Diferentes Cargas na Insuficiência Cardíaca. Arq. Bras. Cardiol. 2020, 114, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Correa, L.M.; Trevizan, P.F.; Bacurau, A.V.; Ferreira-Santos, L.; Gomes, J.L.; Urias, U.; Oliveira, P.A.; Alves, M.J.N.; De Almeida, D.R.; Brum, P.C.; et al. Effects of aerobic and inspiratory training on skeletal muscle microRNA-1 and downstream-associated pathways in patients with heart failure. J. Cachex Sarcopenia Muscle 2019, 11, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Sadek, Z.; Salami, A.; Youness, M.; Awada, C.; Hamade, M.; Joumaa, W.H.; Ramadan, W.; Ahmaidi, S. A randomized controlled trial of high-intensity interval training and inspiratory muscle training for chronic heart failure patients with inspiratory muscle weakness. Chronic Illn. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mancini, N.M.; Henson, D.; La Manca, J.; Donchez, L.; Levine, S.; Manca, J.L. Benefit of Selective Respiratory Muscle Training on Exercise Capacity in Patients with Chronic Congestive Heart Failure. Circulation 1995, 91, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Cahalin, L.P.; Semigran, M.J.; Dec, G.W. Inspiratory muscle training in patients with chronic heart failure awaiting cardiac transplantation: Results of a pilot clinical trial. Phys. Ther. 1997, 77, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Cowley, A.; Kinnear, W. A randomized controlled trial of inspiratory muscle training in stable chronic heart failure. Eur. Hear. J. 1998, 19, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Laoutaris, I.; Dritsas, A.; Brown, M.D.; Manginas, A.; Alivizatos, P.A.; Cokkinos, D.V. Inspiratory muscle training using an incremental endurance test alleviates dyspnea and improves functional status in patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Laoutaris, I.D.; Dritsas, A.; Brown, M.D.; Manginas, A.; Kallistratos, M.S.; Degiannis, D.; Chaidaroglou, A.; Panagiotakos, D.B.; Alivizatos, P.A.; Cokkinos, D.V. Immune response to inspiratory muscle training in patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 679–686. [Google Scholar] [CrossRef]
- Laoutaris, I.D.; Dritsas, A.; Brown, M.D.; Manginas, A.; Kallistratos, M.S.; Chaidaroglou, A.; Degiannis, D.; Alivizatos, P.A.; Cokkinos, D.V. Effects of Inspiratory Muscle Training on Autonomic Activity, Endothelial Vasodilator Function, and N-Terminal Pro-brain Natriuretic Peptide Levels in Chronic Heart Failure. J. Cardiopulm. Rehabil. Prev. 2008, 28, 99–106. [Google Scholar] [CrossRef]
- Freeman, A.M.; Taub, P.R.; Lo, H.; Ornish, D. Intensive Cardiac Rehabilitation: An Underutilized Resource. Curr. Cardiol. Rep. 2019, 21, 19. [Google Scholar] [CrossRef]
- Guski, H.; Kogan, E.A.; Shvalev, V.N. Etiology and Pathogenesis of Sudden Cardiac Death. Diagn. Pathol. 2019, 5, 275–2364. [Google Scholar] [CrossRef]
- Kayser, G.; Kayser, K. Virtual Predictive Autopsy: From knowledge and understanding to education, research and com-munication in digital tissue—Based diagnosis. Diagn. Pathol. 2019, 5, 274–2364. [Google Scholar] [CrossRef]
| Year and Author | Group (n), Male/Female | Age (Years) | NYHA Class (II/III) | LVEF (%) | PImax (cmH2O) |
|---|---|---|---|---|---|
| 2008. Chiappa et al. [67] * | IMT: 18, 12/6 C: 10, 8/2 (Healthy subjects) | IMT: 57 ± 11 C: 38 ± 12 | IMT: 10/8 C: N/A | IMT: 24 ± 3 C: N/A | IMT: 60 ± 8 C: 153 ± 26 |
| 2008. Laoutaris et al. [66] | IMT: 14, 11/3 C: 9, 9/0 | IMT: 53.4 ± 2.1 C: 57.3 ± 4 | IMT: 9/5 C: 6/3 | IMT: 28.9 ± 2.4 C: 28.6 ± 1.9 | IMT: 78.5 ± 4.9 C: 84.6 ± 5.9 |
| 2012. Mello et al. [74] | IMT: 15, 9/6 C: 12, 5/7 | IMT: 54.3 ± 2 C: 53.3 ± 2 | IMT: 15/0 C: 12/0 | IMT: 33.6 ± 2.3 C: 37.6 ± 1.6 | IMT: 56.1 ± 2.3 C: 56.2 ± 2.1 |
| 2017. Moreno et al. [28] | IMT: 13, 8/5 C:13, 8/5 | IMT: 61 ± 14 C: 60 ± 13 | IMT: 6/7 C: 7/6 | IMT: 35 ± 9 C: 37 ± 6 | IMT: 60 ± 13 C: 60 ± 16 |
| 2020. Antunes Correa et al. [85] | IMT: 11, 3/8 C: 10, 6/4 AET: 12, 7/5 | IMT: 55 ± 3 C: 57 ± 3 AET: 57 ± 2 | IMT: 8/3 C: 9/1 AET: 9/3 | IMT: 31 ± 2 C: 25 ± 1 AET: 26 ± 2 | IMT: 86 ± 9 C: 85 ± 8 AET: 87 ± 10 |
| Year and Author | Type of IMT | Intensity of IMT | Duration per Session (min) | Frequency per Week (days) | Duration of Intervention (weeks) | Extra Commentary |
|---|---|---|---|---|---|---|
| 2008. Chiappa et al. [67] * | Threshold device | IMT: 30% PImax | IMT: 30 | IMT: 7 | 4 | Control group without intervention. In the experimental group, the intensity was readjusted weekly and one session weekly was supervised. |
| 2008. Laoutaris et al. [66] | Resistive load device | IMT: 60% SMIP C: 15% SMIP | IMT: N/A C: N/A | IMT: 3 C: 3 | 10 | All sessions were supervised. In the experimental group, the intensity was readjusted in each session; in the control group, it was fixed. The session had six efforts at each level: Level I: 60 s for rest in each inspiration effort Level II: 45 s for rest between series Level III: 30 s for rest between series Level IV: 15 s for rest between series Level V: 10 s for rest between series Level VI: 5 s for rest between series. After level VI, rest for 5 s maintained up to respiratory fatigue process. |
| 2012. Mello et al. [74] | Threshold device | IMT: 30% PImax | IMT: 10min x 3/day | IMT: 7 | 13 | Control group had usual care. In the experimental group, the intensity was readjusted weekly and one session weekly was supervised. |
| 2017. Moreno et al. [28] | Threshold or resistive load devices | IMT: 30% PImax | IMT: 30 min | IMT: 6 | 8 | Control group without intervention. In the experimental group, the intensity was readjusted weekly and one session weekly was supervised. |
| 2020. Antunes Correa et al. [85] | Resistive load device | IMT: 60% PImax | IMT: 30 min AET: 60 | IMT: 5 AET: 3 | 16 | Control group without intervention. In the experimental group, the intensity was readjusted weekly and one session weekly was supervised. In the AET group, all sessions were supervised. Sessions comprised 5 min for stretching exercise, 40 min for cycling, 10 min for local strengthening exercise and 5 min for cooldown. Aerobic exercise was performed under anaerobic thresholds up to 10% under respiratory compensation points. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Rubio, H.; Becerro-de-Bengoa-Vallejo, R.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Vicente-Campos, D.; Chicharro, J.L. Unraveling the Role of Respiratory Muscle Metaboloreceptors under Inspiratory Training in Patients with Heart Failure. Int. J. Environ. Res. Public Health 2021, 18, 1697. https://doi.org/10.3390/ijerph18041697
Fernández-Rubio H, Becerro-de-Bengoa-Vallejo R, Rodríguez-Sanz D, Calvo-Lobo C, Vicente-Campos D, Chicharro JL. Unraveling the Role of Respiratory Muscle Metaboloreceptors under Inspiratory Training in Patients with Heart Failure. International Journal of Environmental Research and Public Health. 2021; 18(4):1697. https://doi.org/10.3390/ijerph18041697
Chicago/Turabian StyleFernández-Rubio, Hugo, Ricardo Becerro-de-Bengoa-Vallejo, David Rodríguez-Sanz, César Calvo-Lobo, Davinia Vicente-Campos, and Jose López Chicharro. 2021. "Unraveling the Role of Respiratory Muscle Metaboloreceptors under Inspiratory Training in Patients with Heart Failure" International Journal of Environmental Research and Public Health 18, no. 4: 1697. https://doi.org/10.3390/ijerph18041697
APA StyleFernández-Rubio, H., Becerro-de-Bengoa-Vallejo, R., Rodríguez-Sanz, D., Calvo-Lobo, C., Vicente-Campos, D., & Chicharro, J. L. (2021). Unraveling the Role of Respiratory Muscle Metaboloreceptors under Inspiratory Training in Patients with Heart Failure. International Journal of Environmental Research and Public Health, 18(4), 1697. https://doi.org/10.3390/ijerph18041697







