Abstract
Lack of time is seen as a barrier to maintaining a physically active lifestyle. In this sense, interval training has been suggested as a time-efficient strategy for improving health, mainly due to its potential to increase cardiorespiratory fitness. Currently, the most discussed interval training protocols in the literature are the high-intensity interval training (HIIT) and the sprint interval training (SIT). Objective: We investigated, through a systematic review and meta-analysis, which interval training protocol, HIIT or SIT, promotes greater gain in cardiorespiratory fitness (O2max/peak). The studies were selected from the PubMed (MEDLINE), Scopus and Web of Science databases. From these searches, a screening was carried out, selecting studies that compared the effects of HIIT and SIT protocols on O2max/peak. A total of 19 studies were included in the final analysis. Due to the homogeneity between studies (I2 = 0%), fixed-effects analyses were performed. There was no significant difference in the O2max/peak gains between HIIT and SIT for the standardized mean difference (SMD = 0.150; 95% CI = −0.038 to 0.338; p = 0.119), including studies that presented both measurements in mL·kg−1·min−1 and l·min−1; and raw mean differences (RMD = 0.921 mL·kg−1·min−1; 95% CI = −0.185 to 2.028; p = 0.103) were calculated only with data presented in mL·kg−1·min−1. We conclude that the literature generates very consistent data to confirm that HIIT and SIT protocols promote similar gains in cardiorespiratory fitness. Thus, for this purpose, the choice of the protocol can be made for convenience.
1. Introduction
Lack of time has been acknowledged as one of the main barriers to maintaining a physically active lifestyle in humans [1]. Physical activity promotes and maintains health by improvement of cardiorespiratory fitness, for which the levels are inversely related to mortality from different causes [2]. International guidelines recommend the practice of at least 150 min per week for continuous moderate cardiorespiratory training or 75 min per week of vigorous intensity to promote health benefits [3].
In this sense, high-intensity interval training (HIIT) has been proposed as a time-efficient alternative strategy for maintaining a physically active lifestyle, since it promotes benefits equal to or even greater than traditional continuous aerobic training [4,5,6]. HIIT consists of performing repeated series of high-intensity efforts (usually between the second ventilatory threshold and the O2max/peak) interspersed with periods of low-intensity recovery/or pause [1,7,8,9]. In this perspective, variations of HIIT protocols have emerged, applying intensities and/or loads above the O2max/peak in very short periods of time. Here these short-bout protocols are referred to as sprint interval training (SIT) [1,10].
The prescription of either HIIT or SIT is complex and involves the manipulation of several variables, including duration of the effort and recovery phases, intensity of effort and recovery, and total number of efforts/recovery [9,10,11].
From the manipulation of these variables, a range of protocols may be designed, based on well-known protocols, such as Wingate, which consists of performing efforts lasting 30 s at all-out intensity, to protocols using intensities close to maximum (according to different forms of exercise prescription, e.g., O2max, maximum power output, Vmax, etc.) [10] and duration around to 4 min [4,12]. The differences in the training variables between HIIT and SIT include time of effort and intensity, which are expected to reflect changes in the metabolism and adaptations of organic systems [11]. When comparing these protocols, Matsuo et al. (2014) demonstrated a significant increase in O2max after 8 weeks of HIIT and SIT, evidencing that both programs are effective to improve cardiorespiratory fitness [13].
Previous studies seem to confirm that the manipulation of duration and intensity of the effort determines the energetic and metabolic stress of the exercise session, which constitute the driving force that triggers physiological and cellular processes that, in turn, lead to chronic adaptation to exercise training [14,15].
Higher gains in the O2max have been associated with training at longer-duration bouts close to that of the O2max [10,11,16]. This phenomenon is dependent on the increase of the exercise intensity, duration of the stimulus, recruitment/activation of types I and II muscle fibers and the use of energetic substrates [17]. Thus, different intensities and duration of effort have different impacts on the organic systems. For example, while bouts at maximum/supramaximal intensities with short duration should also affect the neuromuscular system, bouts at submaximal/near-to-maximum intensities mainly improve cardiovascular and cardiorespiratory fitness [10]. These findings led us to hypothesize that different durations in HIIT effort phase lead to different metabolic and organic system demands, thus leading to different adaptations in relation to O2max.
Therefore, the aim of this study was to investigate, through a systematic review and meta-analysis, which interval training protocol, HIIT or SIT, promotes greater gain in cardiorespiratory fitness (O2max/peak).
2. Materials and Methods
2.1. Search Strategy
Searches were conducted in October 2021, using articles from PubMed (MEDLINE), Scopus and Web of Science databases, with the following descriptors for protocols: high-intensity interval training, high-intensity interval exercise, high-intensity interval aerobic, interval training, sprint interval training, high-intensity intervals, repeat sprint training, high-intensity intermittent exercise, repeated sprint exercise, high-intensity intermittent exercise and aerobic interval training. The descriptors for duration of effort were exercise dose, short extent, long extent, long time, short time, long volume and short volume. The descriptors for the outcomes were maximal oxygen uptake, peak oxygen uptake, cardiorespiratory fitness and O2max/peak. For the combination of synonyms within the same category, “OR” was used between descriptors, while for combinations between the different groups of categories, “AND” was used.
The studies were screened by two research evaluators to select those containing both HIIT and SIT protocols. Quality assessment was performed by using the TESTEX scale (Table 1) that was also carried out by two evaluators [18]. To complement the number of studies included, a manual search was also carried out by consulting the list of references of the included studies. This study was reported by following the guidelines of the Preferred Reporting Items for Systematic and Meta-Analyses (PRISMA) [19].

Table 1.
TESTEX Scale.
2.2. Inclusion and Exclusion Criteria
2.2.1. Type of Studies
Studies written in English that compared HIIT to SIT training were included in this meta-analysis. Articles not containing data from both the pre- and post-O2max or peak (O2max/peak), conducted in animals or review studies were excluded. Studies were also excluded when HIIT and SIT protocols were not directly compared and they presented their data in a graphical mode that did not allow for data extraction via software.
2.2.2. Participants
No restrictions were applied to the different ages, sex, body composition, comorbidities and levels of physical activity of the subjects included in each survey. Studies involving subjects with spinal cord injuries or individuals with special health conditions were excluded.
2.2.3. Protocols
Studies with interventions lasting at least two weeks, with a weekly frequency of at least two days, were selected. Studies that did not present measurements of O2max/peak before and after the intervention period were excluded. To be included, studies should have subjects assigned to both the HIIT group and the SIT group. In the present study, for the purpose of comparing protocols, we made use of the classification suggested by Bucchet and Laursen (2013) [10] that considers efforts lasting less than 60 s as short duration, here called SIT (sprint interval training), while longer efforts (> 60 s) are considered long duration and are here named HIIT (high-intensity interval training). Thus, HIIT refers to protocols performed with effort lasting over 60 s up to 5 min, in an intensity close to the maximum, maximum or above the maximum (> 80% of the maximum intensity), according to the different forms of evaluation and prescription intensity (PPO, Vmax, O2max, HR, etc.).
2.3. Data-Extraction Strategy
The main outcomes data of each time-point and group and characteristics of the selected population, such as age, sex, height, weight, physical activity level, health conditions and the O2max or peak, were manually extracted for descriptive and statistical analysis purposes. Data were recorded as mean and standard deviation. In studies that did not present such data, the authors were contacted to provide the information.
2.4. Quality Assessment and Publication Bias
Egger’s test, together with the visual analysis of the funnel plot, was used to analyze the risk of publication bias [20].
2.5. Choice of Model and Analysis of Heterogeneity
The choice of the analysis model was made by observing the heterogeneity between the studies. For this, the I-square (I2) test and Cochran’s Q test and p-values were taken as parameters for this decision. Different I2 values above 25% were adopted as an indicator of significant heterogeneity between studies; however, the p-value > 0.05 of the Q test was analyzed primarily as an indicator of low heterogeneity [21].
2.6. Data Analysis
Data analysis was performed by using the Comprehensive Meta-Analysis software version 3 for Windows. The main results were presented as standardized mean difference (SMD), relativizing both the data in relative (mL·kg−1·min−1) or absolute (L·min−1) O2max/peak units according to their standard deviation. The SMD and raw mean differences (RMD) were based on the difference between the HIIT and the SIT intervention variation (post-training minus pre-training). In addition, a subgroup analysis of RMD was performed, including only studies providing relative O2max/peak data (mL·kg−1·min−1). The data were summarized by using the forest plot graph, representing the results of the SMD in the overall analysis and RMD for the subgroup analysis, and a 95% confidence interval was used for both (95% CI). Moderate correlation (r = 0.5) between pre- and post-training data and a fixed effect model were adopted for the analysis.
Subgroup analyses were performed to test differences between sexes (male vs. female, excluding studies with mixed samples), age and physical-activity levels (moderately trained vs. sedentary). Furthermore, a sensitivity analysis was performed for a subgroup of high-quality studies, in accordance with TESTEX (≥10).
3. Results
After we removed the replicated studies, those that did not include interval-training protocols and those involving animal models, 593 studies were selected for the writing of their abstract and title. After, in a second step, studies were selected based on inclusion and exclusion criteria. Studies that presented just one of the protocols and results in graphical mode that did not allow for data extraction by software were excluded [22,23,24,25]. Finally, 19 studies were meta-analyzed (Figure 1).
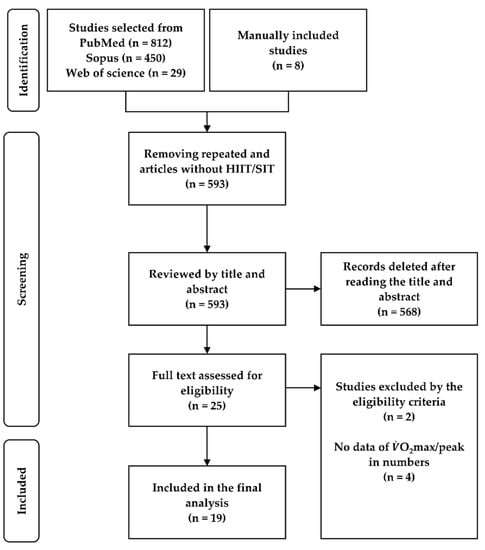
Figure 1.
Flowchart of the studies included.
The characteristics of the studies included are detailed in Table 2.

Table 2.
Study characteristics.
SIT vs. HIIT
The meta-analysis showed no significant SMD between SIT and HIIT training (SMD = 0.150; 95% CI = −0.038 to 0.338; p = 0.119) (Figure 2). There was also no significant difference between HIIT and SIT for the subgroup of studies presenting relative O2max/peak data (RMD = 0.921 mL·kg−1·min−1; 95% CI = −0.185 to 2.028; p = 0.103, Figure 3).
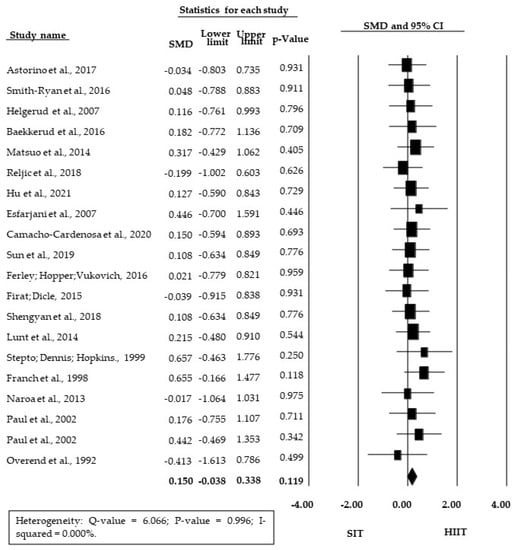
Figure 2.
Forest plot of standardized mean difference (SMD) between HIIT and SIT; 95% CI, 95% confidence interval; SIT, sprint interval training; HIIT, high-intensity interval training.
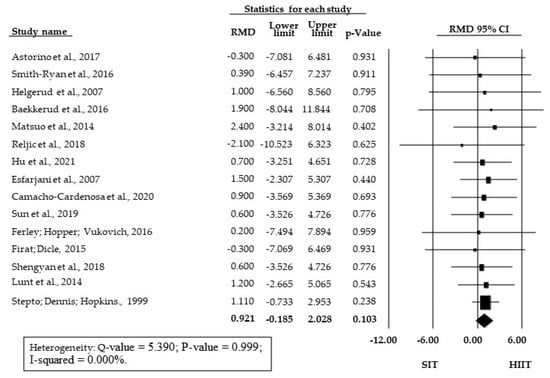
Figure 3.
Forest Plot of raw mean differences (RMD in mL·kg−1·min−1) between HIIT and SIT; 95% CI, 95% confidence interval; SIT, sprint interval training; HIIT, high-intensity interval training.
Since the analyses were significant homogeneous and considerably consistent across studies for both the outcomes measured (p = 0.999 for SMD and p = 1.000 for RMD and I2 = 0 for both), fixed effects were assumed for both analyses. There was no significant bias of publication (p = 0.828), so there was no asymmetry in the funnel plot (Figure 4).
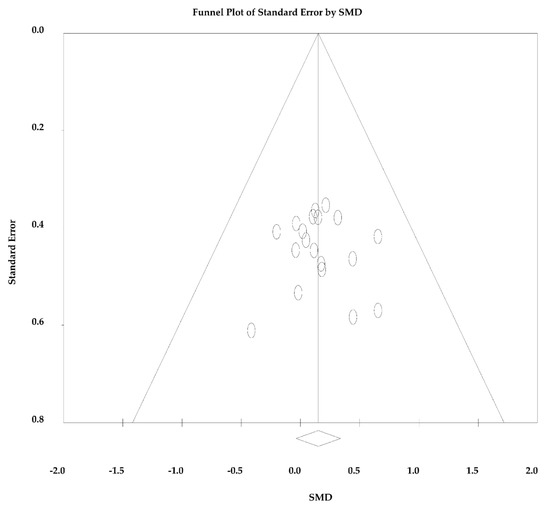
Figure 4.
Funnel plot standardized mean difference (SMD) in mean vs. standard error of O2max/peak.
Table 3 shows that there was no confounding factor in the analysis, and the same absence of difference between HIIT and SIT was seen for different sexes, intervention durations and physical activity levels. Moreover, the sensitivity analysis for the subgroup of five studies that achieved a TESTEX score equal to or above 10 showed the same results as the overall analysis (Table 3).

Table 3.
Subgroup analysis.
4. Discussion
No significant differences were found for the O2max/peak gains when comparing HIIT and SIT protocols. There was no significant heterogeneity between the studies (p > 0.05), and there was no significant risk of publication bias (analysis of the funnel plot (Figure 4) and Egger’s test (p = 0.828)). For these reasons, the results obtained were very consistent and suggest no need for further exploration of differences between the studies. The high consistency may be explained by the proximity of the experimental design of each study, as well as by the similarity of the tests used in each study to measure the O2max/peak [26].
Nonetheless, we explored the potential effect of some confounding factors by subgroup and sensitivity analysis. It reinforced that different sexes, level of physical activity and duration of intervention did not influence the main effects observed on O2max/peak. Furthermore, there was no difference between HIIT and SIT within the subgroup of high-quality studies (TESTEX ≥ 10).
The absence of significant differences for O2max/peak gains when comparing HIIT and SIT corroborates the findings of Rosenblat et al. (2020) [1]. Their meta-analysis included six studies and found no differences between short, medium and long HIIT protocols for the O2max. In the present study, only two categories were created for the interval training group (HIIT and SIT), according to the delimitation proposed by Bucchet and Laursen (2013) [10]. For them, efforts above 60 s should be considered as HIIT, and below this time, they should be considered SIT. The cutoff used here and the higher number of studies included (19) led to greater statistical power in our analysis. Despite the differences in experimental design between the previous meta-analysis and the present one, the lack of difference between protocols was a consensus. Likely, the manipulation of the remaining training variables contributed to the similar total workload between the HIIT and SIT.
Similar gains in HIIT and SIT on the O2max/peak may be explained by the fact that both current protocols are predominantly focused on oxidative metabolism, since they are constituted by successive repetitions of effort/short pause that, when combined, prolong the total duration of the oxidative predominance in an exercise session [27].
Although the aim of this study was not investigate potential physiological mechanism underlying the HIIT and SIT protocols, based on current evidence, we highlighted some mechanisms related to the obtained results. Both types of protocols lead to high physiological stress, the high recruitment pattern of type I and II fibers and the vigorous muscle contraction during physical activity, which unbalances the ATP/ADP relationship and increases the activation of the PGC1-α [13,15,28]. Additionally, there is a demonstrated increase in mRNA of PGC-1 α after a SIT session with cyclists. In a biopsy of the vastus lateralis, after a 16-week HIIT intervention, there was a 138% increase of PGC1-α and an increased O2max that were related [29].
Although a similar increase in O2max/peak was found for HIIT and SIT, these protocols may lead to different magnitudes of other adaptations related to aerobic fitness, such as neuromuscular, musculoskeletal tension, cardiovascular work, anaerobic glycolytic energy and cardiac autonomic stress, causing adaptations in other variables besides O2max [17,30,31]. Unfortunately, for these other outcomes, there are not enough studies to be meta-analyzed. It is noteworthy that Esfarjani et al. (2007) [32] found a significant increase in the speed of the test performed on 3000 m just for the training composed by longer intervals (HIIT), as compared to a control group. Astorino et al. (2016) [33] reported an increase in cardiac output after 10 and 20 weeks of SIT, while, for HIIT, there was a stagnation in adaptation after 10 weeks. Matsuo et al. (2013) [34] demonstrated a greater SMD for O2max and larger effect size for stroke volume for HIIT. On the other hand, only the SIT program led to a significant increase for hematocrit.
In humans and animal models, HIIT and SIT also improve body composition and the lipid profile by decreasing LDL cholesterol (low-density lipoprotein) and increasing HDL cholesterol (high-density lipoprotein) [13,27,35,36]. The LDL protein is inversely related to nitric oxide, which is important for vessel dilation and cardiovascular conditioning. LDL decrease has been shown at 4-min HIIT intervals with concomitant increase in nitric oxide [37]. Additionally, the highest intensities achieved in interval protocols (HIIT and SIT) have been shown to cause other adaptations beyond o O2max in organic systems of the body, hormonal tissue and cells [12,13]. Khalafi and Symonds (2020) [38] demonstrated a decrease in body fat mass. Protocols such as Wingate have shown an increase in epinephrine and norepinephrine levels after an acute session [37], and this increase may lead to an increase in lipolytic activity [6]. In longer protocols (HIIT), a decrease in triglyceride transport has been found that may lead to a decrease in fat deposition in adipocytes [39]. A meta-analysis carried out by Maillard, Pereira and Boisseau (2017) [40] found that HIIT protocols with intensities around 90% of HR peak are effective in reducing body visceral fat. In another meta-analysis, Keating et al. (2017) [41] demonstrated that, in both HIIT and SIT, there was a decrease in body fat. In addition, studies have shown beneficial changes in the body mass, percentage of body fat mass [39], total cholesterol [35,38] and inflammatory markers [38], as well as improved insulin sensitivity, fasting insulin, adiponectin levels and endothelial function after HIIT programs [13,37,42]. Moreover, interval protocols are expected to promoted cardiovascular autonomic and functional adaptations, leading to a decrease in rest HR, improvement in premature ventricular contraction [43], reduction of systolic and diastolic and mean arterial pressure [30,44,45].
A limitation found in the present meta-analysis is the format chosen individually by each study included in the meta-analysis to report the methods used. For some of these studies, it was not possible to calculate the amount of external workload performed and not even the caloric expenditure. Thus, it was not possible to infer the impact of the differences between the volumes of the protocols in the answers found for the O2max/peak in the protocol comparisons. However, given the similar improvements between HIIT and SIT, such a limitation has little relevance to the adaptations of O2max/peak.
5. Conclusions
HIIT and SIT are time-efficient protocols that lead to similar gains in cardiorespiratory fitness. Thus, the choice between these training protocols should be made according to the availability of time, aptitude to perform intense physical activity and specificity of the physical conditions of each individual to practice exercise.
Future studies are encouraged to compare the effect of manipulation of other training variables, such as recovery time, number of bouts and different types of HIIT exercises (running, cycling, rowing, boxing, swimming, etc.), using equalized caloric expenditure and/or the total work performed for a comprehensive comparison between HIIT and SIT protocols.
Author Contributions
S.G.d.O.-N. and A.C. contributed to the conception and design of the study; S.G.d.O.-N. and A.C. conducted the triage of the studies; S.G.d.O.-N., A.C. and A.V.S. performed the data analysis; S.G.d.O.-N. wrote the first draft of the manuscript; A.C., A.V.S., C.R.C. and M.P.T.C.-M. revised the original manuscript critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. AC was supported by the São Paulo Research Foundation (FAPESP) fellowship (#2020/13939-7).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosenblat, M.A.; Perrotta, A.S.; Thomas Scott, G. Effect of High-Intensity Interval Training Versus Sprint Interval Training on Time-Trial Performance: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 1145–1161. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [Green Version]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Helgerud, J.; Høydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic High-Intensity Intervals Improve VO2max More Than Moderate Training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Milanović, Z.; Sporis, G.; Weston, M. Effectiveness of High-Intensity Interval Training (HIT) and Continuous Endurance Training for VO2max Improvements: A Systematic Review and Meta-Analysis of Controlled Trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.; Coombes, J.S. The Impact of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Vascular Function: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 679–692. [Google Scholar] [CrossRef]
- Billat, V. Interval Training for Performance: A Scientific and Empirical Practice. Special recommendations for middle- and long-distance running. Part I: Aerobic interval training. Sports Med. 2001, 31, 13–31. [Google Scholar] [CrossRef]
- Fox, E.; Bartels RBillings, C.; Mathews, D.; Bason, R.; Webb, W. Intensity and distance of interval training programs and changes in aerobic power. Med. Sci. Sports 1973, 5, 18–22. [Google Scholar] [PubMed]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [Green Version]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef] [PubMed]
- Laursen, P.B.; Shing, C.M.; Peake, J.M.; Coombes, J.; Jenkins, D. Interval training program optimization in highly trained endurance cyclists. Med. Sci. Sports Exerc. 2002, 34, 1801–1807. [Google Scholar] [CrossRef] [Green Version]
- Gibala, M.J.; McGee, S.L. Metabolic adaptations to short-term high-intensity interval training: A little pain for a lot of gain? Exerc. Sport Sci. Rev. 2008, 36, 58–63. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2011, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Baar, K. The signaling underlying. Appl. Physiol. Nutr. Metab. 2009, 3, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Tjønna, A.E.; Rognmo, Ø.; Stølen, T.; Bye, A.; Haram, P.M.; Loennechen, J.P.; Slørdahl, S.A.; Wisløff, U.; Lee, S.J.; Al-Share, Q.Y.; et al. Response to Letter Regarding Article, “Aerobic Interval Training Versus Continuous Moderate Exercise as a Treatment for the Metabolic Syndrome: A Pilot Study”. Circulation 2009, 119, 226. [Google Scholar] [CrossRef]
- Midgley, A.W.; McNaugton, L.R.; Wilkinson, M. Is there an optimal training intensity for enhancing the maximal oxygen uptake of distance runners? Sports Med. 2006, 36, 117–132. [Google Scholar] [CrossRef]
- Krustrup, P.; Mohr, M.; Bangsbo, J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflüger’s Archiv. Gesamte Physiol. Menschen Tiere 2004, 447, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.A.; Waldron, M.; Ismail, H.; Giallauria, F.; Vigorito, C.; Cornelissen, V.; Dieberg, G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. JBI Evid. Implement. 2015, 13, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; Bieuzen, F.; Billaut, F. The effect of HIIT vs. SIT on muscle oxygenation in trained sprint kayakers. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 121, 2743–2759. [Google Scholar] [CrossRef]
- Vaccari, F.; Giovanelli, N.; Lazzer, S. High-intensity decreasing interval training (HIDIT) increases time above 90% VO2peak. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 120, 2397–2405. [Google Scholar] [CrossRef]
- Rønnestad, B.R.; Hansen, J.; Vegge, G.; Tønnessen, E.; Slettaløkken, G. Short intervals induce superior training adaptations compared with long intervals in cyclists—An effort-matched approach. Scand. J. Med. Sci. Sports 2015, 25, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Munoz, I.; Seiler, S.; Alcocer, A.; Carr, N.; Esteve-Lanao, J. Specific Intensity for Peaking: Is Race Pace the Best Option? Asian J. Sports Med. 2015, 6, e24900. [Google Scholar] [CrossRef] [Green Version]
- Huedo-Medina, T.B.; Sanchez-Meca, J.; Marín-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Parolin, M.L.; Chesley, A.; Matsos, M.P.; Spriet, L.L.; Jones, N.L.; Heigenhauser, G.J.F. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol. Metab. 1999, 277, E890–E900. [Google Scholar] [CrossRef] [PubMed]
- Niklas, P.; Li, W.; Jens, W.; Michail, T.; Kent, S. Mitochondrial gene expression in elite cyclists: Effects of high-intensity interval exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 110, 597–606. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Kong, Z.; Shi, Q.; Tong, T.K.; Nie, J. Twelve weeks of low volume sprint interval training improves cardio-metabolic health outcomes in overweight females. J. Sports Sci. 2018, 37, 1257–1264. [Google Scholar] [CrossRef]
- Batacan, R.B., Jr.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017, 51, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Poon, E.T.-C.; Little, J.P.; Sit, C.H.-P.; Wong, S.H.-S. The effect of low-volume high-intensity interval training on cardiometabolic health and psychological responses in overweight/obese middle-aged men. J. Sports Sci. 2020, 38, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Esfarjani, F.; Laursen, P.B. Manipulating high-intensity interval training: Effects on VO2max, the lactate threshold and 3000 m running performance in moderately trained males. J. Sci. Med. Sport 2007, 10, 27–35. [Google Scholar] [CrossRef]
- Astorino, T.A.; Edmunds, R.M.; Clark, A.; King, L.; Gallant, R.A.; Namm, S.; Fischer, A.; Wood, K.M. High-intensity interval training increases cardiac output and VO2max. Med. Sci. Sports Exerc. 2017, 49, 265–273. [Google Scholar] [CrossRef]
- Matsuo, T.; Saotome, K.; Seino, S.; Shimojo, N.; Matsushita, A.; Iemitsu, M.; Ohshima, H.; Tanaka, K.; Mukai, C. Effects of a low-volume aerobic-type interval exercise on VO2max and cardiac mass. Med. Sci. Sports Exerc. 2014, 46, 42–50. [Google Scholar] [CrossRef]
- Aktaş, H.Ş.; Uzun, Y.E.; Kutlu, O.; Pençe, H.H.; Özçelik, F.; Çil, E.Ö.; Irak, L.; Altun, Ö.; Özcan, M.; Özsoy, N.; et al. The effects of high intensity-interval training on vaspin, adiponectin and leptin levels in women with polycystic ovary syndrome. Arch. Physiol. Biochem. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Coll-Risco, I.; Aparicio, V.A.; Nebot, E.; Camiletti-Moirón, D.; Martínez, R.; Kapravelou, G.; López-Jurado, M.; Porres, J.M.; Aranda, P. Effects of interval aerobic training combined with strength exercise on body composition, glycaemic and lipid profile and aerobic capacity of obese rats. J. Sports Sci. 2015, 34, 1452–1460. [Google Scholar] [CrossRef]
- Vincent, S.; Berthon, P.; Zouhal, H.; Moussa, E.; Catheline, M.; Bentu-Ferrer, D.; Gratas-Delamarche, A.; Vincent, S.; Berthon, P. Plasma Glucose, Insulin and Catecholamine Responses to a Wingate Test in Physically Active Women and Men. Eur. J. Sport Sci. 2004, 91, 15–21. [Google Scholar] [CrossRef]
- Khalafi, M.; Symonds, M.E. The impact of high-intensity interval training on inflammatory markers in metabolic disorders: A meta-analysis. Scand. J. Med. Sci. Sports 2020, 30, 2020–2036. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Fu, J.; Sun, S.; Zhao, G.; Cheng, W.; Dou, C.; Quan, M. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: A meta-analysis. PLoS ONE 2019, 14, e0210644. [Google Scholar] [CrossRef]
- Maillard, F.; Pereira, B.; Boisseau, N. Effect of high-intensity interval training on total, abdominal and visceral fat mass: A meta-analysis. Sports Med. 2018, 48, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Johnson, N.; Mielke, G.; Coombes, J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes. Rev. 2017, 18, 943–964. [Google Scholar] [CrossRef] [PubMed]
- Martin-Smith, R.; Cox, A.; Buchan, D.S.; Baker, J.S.; Grace, F.; Sculthorpe, N. High Intensity Interval Training (HIIT) Improves Cardiorespiratory Fitness (CRF) in Healthy, Overweight and Obese Adolescents: A Systematic Review and Meta-Analysis of Controlled Studies. Int. J. Environ. Res. Public Health 2020, 17, 2955. [Google Scholar] [CrossRef]
- Guiraud, T.; Labrunee, M.; Gaucher-Cazalis, K.; Despas, F.; Meyer, P.; Bosquet, L.; Gales, C.; Vaccaro, A.; Bousquet, M.; Galinier, M.; et al. High-Intensity Interval Exercise Improves Vagal Tone and Decreases Arrhythmias in Chronic Heart Failure. Med. Sci. Sports Exerc. 2013, 45, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Grace, F.; Herbert, P.; Elliott, A.D.; Richards, J.; Beaumont, A.; Sculthorpe, N. High intensity interval training (HIIT) improves resting blood pressure, metabolic (MET) capacity and heart rate reserve without compromising cardiac function in sedentary aging men. Exp. Gerontol. 2018, 109, 75–81. [Google Scholar] [CrossRef]
- Leal, J.M.; Galliano, L.M.; Del Vecchio, F.B. Effectiveness of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Hypertensive Patients: A Systematic Review and Meta-Analysis. Curr. Hypertens. Rep. 2020, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).