Abstract
Characterizing the breeding sites of Culex pipiens complex is of major importance for the control of West Nile disease and other related diseases. However, little information is available about the characteristics and associated factors of the breeding sites of the Cx. pipiens complex in Lhasa, a representative high-altitude region in Southwestern China. In this study, a cross-sectional study concerning the breeding site characteristics and associated factors of the Cx. pipiens complex was carried out in Lhasa, Tibet from 2013–2016. Chi-square analysis and binary logistic regression analysis were applied to identify the key factors associated with the presence of Cx. pipiens complex larvae. Using a standard dipping method, 184 water bodies were examined and Cx. pipiens complex larvae were observed in 36 (19.57%) of them. There were significant differences in the composition of Cx. pipiens complex larvae among the breeding site stability (χ2 = 19.08, p = 0.00) and presence or absence of predators (χ2 = 6.986, p = 0.008). Binary logistic regression analysis indicated that breeding site stability and presence or absence of predators were significantly associated with the presence of Cx. pipiens complex larvae in Chengguan District, Lhasa. Relatively permanent water bodies such as water bodies along river fringes, ponds and puddles, and water bodies with no predators should be paid more attention for future Cx. pipiens complex larvae abatement campaigns in Lhasa, China.
1. Introduction
Culex pipiens complex mosquitoes have a global distribution and are primary vectors of pathogens with public health significance including West Nile disease [1,2], St. Louis encephalitis [3], Sindbis [4], Rift Valley fever viruses [5,6], and periodic filariasis and encephalitis [7]. In China, the Cx. pipiens complex consists of four subspecies, including: Cx. pipiens pipiens, Cx. pipiens quinquefasciatus, Cx. pipiens pallens, and Cx. pipiens molestus [8].
Lhasa, the capital of the Tibet Autonomous Region (TAR) of China, is an international tourist city with plateau and national characteristics. It is known as one of the highest cities in the world, having an elevation of about 3650 meters, and lies in the center of the Tibetan Plateau. Evidence has shown that mosquitoes in the Cx. pipiens complex have already settled in urban Lhasa, TAR, and the local Cx. pipiens complex comprises the subspecies Cx. pipiens pipiens, Cx. pipiens pallens, Cx. pipiens quinquefasciatus, and hybrids of these subspecies. In addition, climate change may have played a role in the establishment of mosquitoes in Lhasa [9]. At present, the transmission of diseases by the Cx. pipiens complex has not been reported in Lhasa. However, further warming raises the risk of the outbreak of mosquito-borne diseases in the future [10,11]. This has already constituted a potential public health threat to the locals [12].
Each species of mosquito has its preferred breeding site for oviposition [13], depending on climate conditions, physical geography, and human activity [14]. Breeding sites can be natural or artificial, shaded or sunny, permanent or temporary, of various sizes, and found in running or stagnant water bodies, among others. Globally, many studies have displayed that the breeding habits of the Cx. pipiens complex are similar, and the main breeding sites are water bodies that are not seriously polluted, such as sinkholes, sewer ditches, cesspits with clear water, stagnant water in low-lying land, and so on [15]. The Cx. pipiens complex has better capacity of adaptation towards diverse breeding sites. In the Wroclaw area of Poland, evidence has shown that Cx. pipiens s.l. (L.) was well adapted to various breeding site types including ditches, catch basins, flowerpots, and buckets with diverse water quality [16].
Informed larval interventions that target more profuse breeding sites have enormous potential in combating Cx.-pipiens-complex-related diseases, especially at a regional scale. Though some studies have examined the types of Culex breeding sites and their related characterization in high-altitude regions [17], little information is available concerning the high altitude regions in China. This poses a serious challenge for the prevention and control of potential mosquito-borne diseases in the future. Therefore, this study aims to explore the breeding site characteristics of the Cx. pipiens complex and related environmental and physico-chemical parameters in urban Lhasa, to determine which breeding site characteristics can better explain the presence of Cx. pipiens complex larvae. The results of this study could provide first-hand scientific assessment of Cx. pipiens complex breeding sites and provide implications for developing intervention measures to control mosquito-borne diseases in Lhasa in the future.
2. Materials and Methods
2.1. Study Area
Lhasa City is an international tourist city with plateau and ethnic characteristics and is the administrative capital of the Tibet Autonomous Region of the People’s Republic of China, consisting of one municipal district (Chengguan District) and seven counties (Linzhou county, Dangxiong county, Nimu county, Qushui county, Duilongdeqing county, Dazi county, and Mozhugongka county).
This study was conducted in selected sites of Chengguan District, Lhasa from 2013–2016 (Figure 1). Chengguan District was the only municipal district in Lhasa city during the study period, with a population of 279,074 in 2013. By 2012, Chengguan District covered an area of 523 square km, but the municipal district only accounts for about 10% of the total area of Chengguan District. These sites were selected mainly according to the geographic and socio-economic characteristics of urban Lhasa. The selected research sites from 2013–2016 mentioned above are summarized in Table 1.
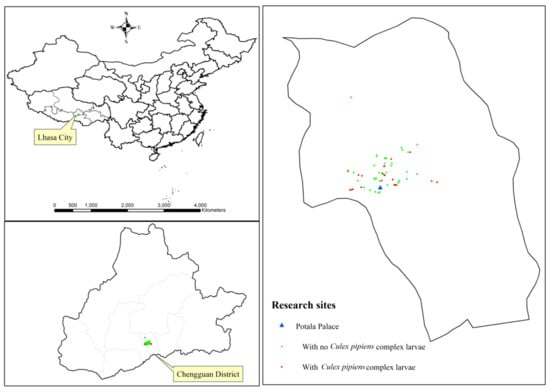
Figure 1.
The research sites of this study.

Table 1.
Locations of positive larvae breeding sites in Lhasa, 2013–2016, Tibet.
2.2. Mosquito Larvae Sampling and Identification
Based on our previous research [9], mosquito species of Chengguan District, Lhasa belong to the subspecies of the Cx. pipiens complex. Evidence has shown that the breeding sites of mosquito of the Cx. pipiens complex mainly include sinkholes, sewer ditches, cesspits, low-lying land, and so on, which generally exist in outdoor environments [15]. Therefore, the selection of potential breeding sites in this study mainly focused on outdoor surroundings.
The larval sampling was conducted using a standard dipping method [18]. In the outdoor surroundings, all the potential breeding sites were located and inspected. When mosquito larvae were present, 10 dips were taken with a dipper in each breeding site. When a breeding site was too small to make 10 dips, water was dipped as many times as possible. In large water bodies, dipping was carried out 100 m apart [14].
To further identify the species of the collected mosquito larvae in Lhasa, the late instars of mosquito larvae were immediately preserved in 90% absolute ethanol and then taken to the laboratory of the National Institute for Communicable Disease Control and Prevention (ICDC), the Chinese Center for Disease Control and Prevention (China CDC) [19]. A multiplex PCR protocol was adopted to identify the subspecies of mosquitoes using polymorphisms in the second intron of the acetylcholinesterase-2 (ace-2) locus, developed by Smith and Fonseca [20]. For the polymerase chain reaction (PCR) identification, the method used in this study was the same as in our previous research [9].
2.3. Breeding Site Characterization
Prior to the survey of potential breeding sites, information about the research sites was recorded, including geographic location, population, economic development level, water bodies, park, housing conditions, and land utilization. The larval breeding sites were characterized either visually or using hand-held equipment.
Some key breeding site characteristics, such as breeding site types, the location of water bodies, distance to the nearest household, perimeter of water body, breeding site stability, substrate types, predators, vegetation, nature (artificial or natural), water flow or static water, shade, water depth, pH, water temperature, dissolved oxygen, turbidity, soluble solids, conductivity, salinity, and resistance, were recorded or tested in this study.
Identified water bodies were classified according to their nature, classified as: river fringes (breeding sites formed along riverbanks when the water level drops), ponds (water area larger than 50 m2), puddles (water area less than 50 m2), irrigation or drainage ditches, and ground pools [21]. The perimeter of each breeding site was categorized by estimation as shorter than 1 m, 1–10 m, or longer than 10 m. Substrate types were classified into cement or concrete, soil, metal, and others. Distance to the nearest house was measured by GPS and classified as less than 10 m, 10–100 m, and greater than 100 m. Water depth was classified into greater than or equal to 0.5 m and less than 0.5 m. The stability of mosquito larval breeding sites was classified as either temporary or permanent. Temporary breeding sites held water for a short period of time (approximately two weeks after the rainy season ended) and stemmed mainly from rain showers. When rain ceased, these breeding sites dried out. On the other hand, the permanent breeding sites held water for a longer period of time (approximately two to three months after the rain ended or fed by natural underground sources) and hence were more stable.
pH, water temperature, dissolved oxygen, turbidity, soluble solids, conductivity, salinity, and resistance were recorded by handheld equipment. pH was recorded by a Waterproof pHTestr 30 (OAKTON Instruments, Vernon Hills, IL USA) [22]. Dissolved oxygen was recorded by portable dissolved oxygen meter (SG6-FK2 CN, Mettler-Toledo, LLC, Columbus, OH USA). To measure turbidity, turbidity meter was adopted in this study. Some indices, such as soluble solids, conductivity, salinity, and resistance were recorded by a portable multiparameter tester (SG23-FK-CN, Mettler-Toledo, LLC, Columbus, OH, USA).
Temperature (°C) and relative humidity (%) data were obtained from the China Weather Website (http://www.weather.com.cn). During collections, ambient outdoor air temperature and relative humidity were recorded by portable weather station (Davis Weather Link 6.0.3, Davis, CA, USA).
2.4. Ethics Statement
This study was approved by the Ethics Committee of China CDC (No. 201214). Ethical approvals were also obtained from the Lhasa Health Bureau, Chengguan District CDC and Tibet CDC respectively in the Tibet Autonomous Region.
2.5. Statistical Analysis
Chi-square test was applied to determine the importance of factors for explaining the presence or absence of Cx. pipiens complex larvae. Some factors with statistical significance in the chi-square analysis were selected to do further binary logistic regression analysis to calculate the odds ratio (OR) and 95% Wald confidence intervals. Presence of larvae was categorized as one, while the absence of larvae was categorized as zero in the logistic regression model. Statistical analysis was carried out using SPSS software (Version 19.0 for windows, SPSS Inc., Chicago, IL, USA). p < 0.05 was considered as statistically significant, and all tests were two-tailed.
3. Results
3.1. The Potential Mosquito Breeding Sites in Lhasa, 2013–2016
In this study, 184 potential mosquito breeding sites were examined from the sampled locations from 2013–2016. Representative water bodies in Lhasa are shown in Figure 2.
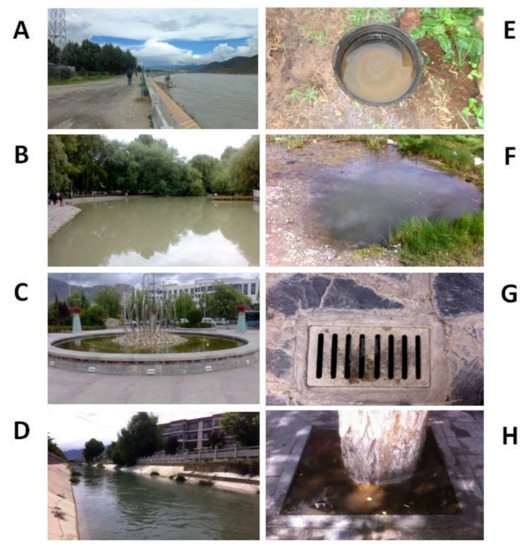
Figure 2.
Representative water bodies in Lhasa City, Tibet, P. R. China. (A) River fringe; (B) Pond (≥50 m2); (C) Puddle (<50 m2); (D) Irrigation/drainage ditch; (E) Containers; (F) Temporary ground pool; (G) Sewer/tube well; (H) Other.
3.2. The Positive Breeding Sites and Species of Mosquito Larvae in Lhasa
Among potential mosquito breeding sites, Cx. pipiens complex larvae were observed in 37 water bodies, accounting for 20.1% of overall water bodies (Table 1). In total, 180 Culex larvae collected in 2013–2016 from 36 water bodies as mentioned above were further identified to subspecies according to a multiplex PCR identification. It was demonstrated that all the identified mosquito larvae belonged to subspecies of the Cx. Pipiens complex, including 63 pure mosquitoes (35%) and 117 hybrids (65%). The pure mosquitoes included 22 Cx. Pipiens pipiens, 11 Cx. Pipiens quinquefasciatus, and 30 Cx. pipiens pallens. Possible hybrids consisted of 80 Cx. pipiens pipiens × Cx. pipiens pallens, 26 Cx. pipiens pallens × Cx. pipiens quinquefasciatus, and 11 Cx. pipiens pipiens × Cx. pipiens quinquefasciatus. Sequence analysis confirmed the accuracy of multiplex PCR in this study.
3.3. The Main Characteristics of 184 Potential Mosquito Breeding Sites in Lhasa
Among 184 sites which contained water, 36 (19.57%) were productive for Cx. pipiens complex larvae, including 12 puddles, 6 sewer or tube wells, 5 ponds, 4 temporary ground pools, 3 river fringes, 3 irritation or drainage ditches, and 3 other water bodies. The frequency and percent composition of the main characteristics of 184 potential mosquito breeding sites in Lhasa, Tibet are shown in Table 2.

Table 2.
The frequency and percent composition of the main characteristics of 184 potential mosquito breeding sites in Lhasa, Tibet.
3.4. Positive Breeding Sites and Key Factors Associated with the Presence of Mosquito Larvae
There were significant differences in the composition of Cx. pipiens complex larvae among the breeding site stability (χ2 = 19.08, p = 0.00), and the presence or absence of predators (χ2 = 6.986, p = 0.008, Table 3). Based on the results of chi-square analysis, we found that no significant differences were observed in the presence of Cx. pipiens complex larvae among breeding site type, distance to the nearest house, artificial or natural, flow or static, perimeter, pH, dissolved oxygen, soluble solid, salinity, substrate type, presence or absence of predators, vegetation, shade, water depth, water temperature, turbidity, conductivity, or resistance.

Table 3.
Positive parameters determining the presence of Culex pipiens complex larvae in water bodies in Lhasa by chi-square analysis.
3.5. The Findings of Binary Logistic Regression Analysis
To further exclude the confounding factors of the results of chi-square analysis, binary logistic regression analysis was adopted. Breeding site stability and the presence or absence of predators were found to be the key factors which determined the presence of mosquito larvae (Table 4).

Table 4.
Positive parameters determining the presence of Cx. pipiens complex larvae in water bodies in Lhasa by logistic regression analysis.
4. Discussion
The precise identification of mosquito larvae species in Lhasa is of great importance not only for the study of the mosquito ecology but also for prevention and control of mosquito-borne diseases in future. In this study, a multiplex PCR method revealed that all identified mosquito larvae belonged to subspecies of the Cx. pipiens complex. This is consistent with the results from previous reports in Tibet [9].
This study found that the larvae of the Cx. pipiens complex mainly existed in river fringes, puddles, sewers, and temporary ground pools, although there were no significant differences of mosquito larvae among these water bodies. Except for the breeding sites along the river fringe, other water bodies were mainly artificial and represented major types of breeding sites in Lhasa. This indicated the possibly important infection locations of mosquito-borne diseases such as filariasis, West Nile disease, and other potential diseases in the future. The findings of this study are similar to the studies of Savage and Miller and Hribar in the USA [15,23,24]. Considering their findings, members of the Cx. pipiens complex readily breed in storm sewer catch basins, clean and polluted ground pools, ditches, animal waste lagoons, effluent from sewage treatment plants, and other sites that are slightly to very eutrophic or polluted with organic wastes. Generally, Cx. pipiens quinquefasciatus is associated with more eutrophic water than Cx. pipiens. Habitats along river fringes were more important during the dry season when the water levels reduced, and stagnant pools of water suitable for mosquito breeding were created [25].
In this study, among the 184 breeding sites which contained water, nearly one-fifth of water bodies were productive for mosquito larvae. Based on the literature review and our previous findings, the subspecies of the Cx. pipiens complex settled their populations in Lhasa in only a short time. Furthermore, evidence has shown that adult mosquito density in Lhasa has been low in recent years, with 1.47 mosquitoes per trap per hour in 2012 and 0.20 mosquitoes per trap per hour in 2013 (light traps), 3.06 mosquitoes per net per hour in 2012 (bed net traps), and 7.90 mosquitoes per person per hour in 2012 (labor hour method) [26]. Therefore, this may be the main reason for the low proportion of mosquito larvae in the examined water bodies during the study period.
Regarding the subspecies of the Cx. pipiens complex, we found that both pure Culex mosquitoes (35%) and their hybrids (65%) existed in the study sites of Lhasa city. These included pure Culex mosquitoes (Cx. pipiens pipiens, Cx. pipiens quinquefasciatus, and Cx. pipiens pallens) and their hybrids (Cx. pipiens pipiens × Cx. pipiens pallens, Cx. pipiens pallens × Cx. pipiens quinquefasciatus, and Cx. pipiens pipiens × Cx. pipiens quinquefasciatus). The findings mentioned above were identical to those in the previous research in this region [9,27].
In the present study, the stability of mosquito larval breeding sites was identified to be the key reason associated with the presence of Cx. pipiens complex larvae in Lhasa. We discovered that the majority of Cx. pipiens complex larvae were observed in permanent breeding sites, including along river fringes, ponds, and puddles. The results of the study are similar to the findings of two studies in western Kenya. Fillinger et al. found that semi-permanent and permanent habitats were suitable for the proliferation of Culicines and Anopheles gambiae sensu lato [28]. Fort Ternan et al. found that permanent habitats held water for a long period of time, and that after the rain these habitats were more preferred by the Culicines and Anopheline mosquitoes [25].
Predator presence/absence was another key factor associated with the presence of Cx. pipiens complex larvae. Predators of mosquito larvae may include larvivorous fish [29,30,31], tadpoles, frogs [32], water bugs, cyclopoids, dragonflies, and Chironomus larvae [33,34]. If they were found in breeding sites, mosquito larvae were generally absent [35,36]. Tranchida et al. found that native Argentinean cyclopoids (Crustacea: Copepoda) were predators of Cx. pipiens (Diptera: Culicidae) mosquitoes in La Plata, Argentina [37].
Other factors potentially affecting the presence of Cx. pipiens complex larvae included distance to the nearest house, artificial or natural, flow or static, perimeter, pH, dissolved oxygen, soluble solid, salinity, substrate types, vegetation, shade, water depth, water temperature, turbidity [38], conductivity, and resistance [39]. However, there were no marked differences in the presence of Cx. pipiens complex larvae among these variables mentioned above, and further study needs to be carried out in the future.
To date, many studies have found that some biological and physicochemical characteristics of larval habitats such as pH, water temperature, dissolved oxygen, turbidity, soluble solids, conductivity, salinity, and resistance were correlated with the presence of mosquito larvae [14,17,32,40,41,42]. One study in Egypt examined the effects of environmental parameters on larval population density [43], including pH, biological and chemical oxygen demands, daytime water temperature, plant growth, salinity, total organic matter, and concentrations of heavy metals. They found that Cx. pipiens larvae displayed high tolerance to elevated levels of heavy metals in sewage water and sewage or domestic waste. Besides, these breeding sites had compensatory effects, probably caused by their high nutrient levels. Muturi et al. found that Cx. quinquefasciatus was associated with turbid water in U.S.A. [44]. However, no significant association was detected between the presence of Cx. pipiens complex larvae and habitat related variables in this study. The potential reasons still need to be investigated in further studies.
This study mainly studied the potential breeding sites in outdoor environments, however, a small amount of discard containers in rooms and drain pits of tap water in the courtyard pose a potential threat under suitable conditions. Further study could also focus on the variations of the breeding habit of the Cx. pipiens complex along with the change of the ecological environment caused by the urbanization in Lhasa in recent years. Other factors such as heavy metallic elements and their compounds [45], orthophosphates, biochemical oxygen demand (BOD), radioactive substances, the contents of minerals and their compounds [46], and some microbial contents were not detected in the current study, and similar studies could focus on these.
Furthermore, we could not ignore the possible error from identifying subspecies of the Cx. pipiens complex using multiplex PCR method in itself. There has been some research undertaken using DNA barcoding [47] and protein profiling [48] to distinguish the subspecies of the complex mentioned above. Since the adopted primers in this study only focused on three forward primers (ACEquin, ACEpall, and ACEpip) and one backward primer (B1246s), plus the limitation of sampling to some extent, Cx. pipiens molestus was not detected as larvae in this study in Lhasa [49].
5. Conclusions
The present study found that breeding site stability and presence or absence of predators were two key influencing factors which were significantly related to the presence of Cx. pipiens complex larvae. Mosquito larvae of subspecies of the Cx. pipiens complex mainly bred in permanent water bodies, and the absence of predators may increase the probability of finding them. Therefore, permanent water bodies with no predators should be highly emphasized for future Cx. pipiens complex control campaigns in Lhasa.
Author Contributions
Conceptualization, X.L. and Q.L.; methodology, Y.Y. and X.L.; investigation, Baimaciwang., J.L., C. (Cirendunzhu), H.W., Pengcuociren, Y.G., C. (Cirenwangla), D.R., D. (Dazhen), J.Y., C. (Cideji), J.L., Z. (Zhaxisangmu), N.Z., J.W.; writing—original draft preparation, X.L.; data curation, X.L., Y.Y. and J.S.; funding acquisition, Q.L. and X.L.
Funding
This study was funded by the National Basic Research Program of China (973 Program) (Grant No.2012CB955504), National Natural Science Foundation of China (81703280) and National Science and Technology Major Project (2017ZX10303404005001).
Acknowledgments
We would like to acknowledge Jianjun Xiang from Adelaide University, Australia, for his revision of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andreadis, T.G. The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile virus in North America. J. Am. Mosq. Control Assoc. 2012, 28, 137–151. [Google Scholar] [CrossRef]
- Korba, R.A.; Alayat, M.S.; Bouiba, L.; Boudrissa, A.; Bouslama, Z.; Boukraa, S.; Francis, F.; Failloux, A.B.; Boubidi, S.C. Ecological differentiation of members of the Culex pipiens complex, potential vectors of West Nile virus and Rift Valley fever virus in Algeria. Parasit. Vectors 2016, 9, 455. [Google Scholar]
- Pawelek, K.A.; Niehaus, P.; Salmeron, C.; Hager, E.J.; Hunt, G.J. Modeling dynamics of Culex pipiens complex populations and assessing abatement strategies for West Nile Virus. PLoS ONE 2014, 9, e108452. [Google Scholar] [CrossRef]
- Hesson, J.C.; Verner-Carlsson, J.; Larsson, A.; Ahmed, R.; Lundkvist, A.; Lundstrom, J.O. Culex torrentium Mosquito Role as Major Enzootic Vector Defined by Rate of Sindbis Virus Infection, Sweden, 2009. Emerg. Infect. Dis. 2015, 21, 875–878. [Google Scholar] [CrossRef]
- Turell, M.J.; Dohm, D.J.; Fonseca, D.M. Comparison of the Potential for Different Genetic Forms in the Culex pipiens Complex in North America to Transmit Rift Valley Fever Virus. J. Am. Mosq. Control Assoc. 2014, 30, 253–259. [Google Scholar] [CrossRef]
- Turell, M.J. Members of the Culex pipiens complex as vectors of viruses. J. Am. Mosq. Control Assoc. 2012, 28, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Chin, P.A.; Kramer, L.D. The effect of hybridization of Culex pipiens complex mosquitoes on transmission of West Nile virus. Parasit. Vectors 2013, 6, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Li, C.X.; Xing, D.; Yu, Y.H.; Liu, N.; Xue, R.D.; Dong, Y.D.; Zhao, T.Y. Detection and widespread distribution of sodium channel alleles characteristic of insecticide resistance in Culex pipiens complex mosquitoes in China. Med. Vet. Entomol. 2012, 26, 228–232. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Cirendunzhu; Woodward, A.; Pengcuociren; Bai, L.; Baimaciwang; Sang, S.; Dazhen; Wan, F.; et al. Mosquitoes established in Lhasa city, Tibet, China. Parasit. Vectors 2013, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Nye, E.R. Global warming and possums: contributors in the future to new mosquito-borne human diseases in New Zealand? N. Z. Med. J. 2007, 120, U2839. [Google Scholar] [PubMed]
- Benitez, M.A. Climate change could affect mosquito-borne diseases in Asia. Lancet 2009, 373, 1070. [Google Scholar] [CrossRef]
- Liu, X.; Wan, F.; Cirendunzhu; Cirenwangla; Bai, L.; Pengcuociren; Zhou, L.; Baimaciwang; Guo, Y.; Dazhen; et al. Community knowledge and experience of mosquitoes and personal prevention and control practices in Lhasa, Tibet. Int. J. Environ. Res. Public Health 2014, 11, 9919–9937. [Google Scholar] [CrossRef]
- Muhammad-Aidil, R.; Imelda, A.; Jeffery, J.; Ngui, R.; Wan Yusoff, W.S.; Aziz, S.; Lim, Y.A.; Rohela, M. Distribution of mosquito larvae in various breeding sites in National Zoo Malaysia. Trop. Biomed. 2015, 32, 183–186. [Google Scholar]
- Liu, X.B.; Liu, Q.Y.; Guo, Y.H.; Jiang, J.Y.; Ren, D.S.; Zhou, G.C.; Zheng, C.J.; Liu, J.L.; Chen, Y.; Li, H.S.; et al. Random repeated cross sectional study on breeding site characterization of Anopheles sinensis larvae in distinct villages of Yongcheng City, People’s Republic of China. Parasit. Vectors 2012, 5, 58. [Google Scholar] [CrossRef]
- Shaman, J.; Day, J.F.; Komar, N. Hydrologic conditions describe West Nile virus risk in Colorado. Int. J. Environ. Res. Public Health 2010, 7, 494–508. [Google Scholar] [CrossRef]
- Weitzel, T.; Jawien, P.; Rydzanicz, K.; Lonc, E.; Becker, N. Culex pipiens s.l. and Culex torrentium (Culicidae) in Wroclaw area (Poland): Occurrence and breeding site preferences of mosquito vectors. Parasitol. Res. 2015, 114, 289–295. [Google Scholar] [CrossRef]
- Dejenie, T.; Yohannes, M.; Assmelash, T. Characterization of mosquito breeding sites in and in the vicinity of tigray microdams. Ethiop. J. Health Sci. 2011, 21, 57–66. [Google Scholar] [CrossRef]
- Vargas, M.; Vargas, J.V. Male and mosquito larvae survey at the Arenal-Tempisque irrigation project, Guanacaste, Costa Rica. Rev. Biol. Trop. 2003, 51, 759–762. [Google Scholar] [PubMed]
- Lu, B.L. Fauna Sinica, Insecta, Diptera: Culicidae II; Science Press: Beijing, China, 1997; Volume 9. [Google Scholar]
- Smith, J.L.; Fonseca, D.M. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: culicidae). Am. J. Trop. Med. Hyg. 2004, 70, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, N.; Mutero, C.M.; Githure, J.I.; Beier, J.C.; Yan, G. Spatial distribution and habitat characterization of anopheline mosquito larvae in Western Kenya. Am. J. Trop. Med. Hyg. 1999, 61, 1010–1016. [Google Scholar] [CrossRef]
- Ndungu, J.; Augustijn, D.M.C.; Hulscher, S.J.M.H.; Fulanda, B.; Kitaka, N.; Mathooko, J.M. A multivariate analysis of water quality in Lake Naivasha, Kenya. Mar. Freshw. Res. 2014, 66, 177–186. [Google Scholar] [CrossRef]
- Hribar, L.J. Larval habitats of potential mosquito vectors of West Nile virus in the Florida Keys. J. Water Health 2007, 5, 97–100. [Google Scholar] [CrossRef]
- Hribar, L.J.; Smith, J.M.; Vlach, J.J.; Verna, T.N. Survey of container-breeding mosquitoes from the Florida Keys, Monroe County, Florida. J. Am. Mosq. Control. Assoc. 2001, 17, 245–248. [Google Scholar]
- Imbahale, S.S.; Paaijmans, K.P.; Mukabana, W.R.; van Lammeren, R.; Githeko, A.K.; Takken, W. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malar. J. 2011, 10, 81. [Google Scholar] [CrossRef]
- Liu, X.B.; Cirendunzhu; Guo, Y.H.; Pengcuociren; Bai, L.; Sang, S.W.; Baimaciwang; Gu, S.H.; Dazhen; Chen, B.; et al. A survey of species composition and population dynamics of mosquitoes in Lhasa, Tibet, China from 2009–2013. Chin. J. Vector. Biol. Contr. 2014, 25, 200–204. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, J.L.; Li, M.H.; Fu, S.H.; Wang, H.Y.; Wang, Z.Y.; Jiang, S.Y.; Wang, X.W.; Guo, P.; Zhao, S.C.; et al. Mosquitoes and mosquito-borne arboviruses in the Qinghai-Tibet Plateau-focused on the Qinghai area, China. Am. J. Trop. Med. Hyg. 2010, 82, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, U.; Sonye, G.; Killeen, G.F.; Knols, B.G.; Becker, N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: Operational observations from a rural town in western Kenya. Trop. Med. Int. Health 2004, 9, 1274–1289. [Google Scholar] [CrossRef]
- Manna, B.; Aditya, G.; Banerjee, S. Vulnerability of the mosquito larvae to the guppies (Poecilia reticulata) in the presence of alternative preys. J. Vector Borne Dis. 2008, 45, 200–206. [Google Scholar]
- Haq, S.; Yadav, R.S. Geographical distribution and evaluation of mosquito larvivorous potential of Aphanius dispar (Ruppell), a native fish of Gujarat, India. J. Vector Borne Dis. 2011, 48, 236–240. [Google Scholar]
- Chandra, G.; Bhattacharjee, I.; Chatterjee, S.N.; Ghosh, A. Mosquito control by larvivorous fish. Indian J. Med. Res. 2008, 127, 13–27. [Google Scholar] [PubMed]
- Raghavendra, K.; Sharma, P.; Dash, A.P. Biological control of mosquito populations through frogs: Opportunities & constrains. Indian J. Med. Res. 2008, 128, 22–25. [Google Scholar] [PubMed]
- Dida, G.O.; Gelder, F.B.; Anyona, D.N.; Abuom, P.O.; Onyuka, J.O.; Matano, A.S.; Adoka, S.O.; Kanangire, C.K.; Owuor, P.O.; Ouma, C.; et al. Presence and distribution of mosquito larvae predators and factors influencing their abundance along the Mara River, Kenya and Tanzania. SpringerPlus 2015, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Aditya, G.; Bhattacharyya, S.; Kundu, N.; Saha, G.K.; Raut, S.K. Predatory efficiency of the water bug Sphaerodema annulatum on mosquito larvae (Culex quinquefasciatus) and its effect on the adult emergence. Bioresour. Technol. 2004, 95, 169–172. [Google Scholar] [CrossRef]
- Wanji, S.; Mafo, F.F.; Tendongfor, N.; Tanga, M.C.; Tchuente, E.; Bilong Bilong, C.E.; Njine, T. Spatial distribution, environmental and physicochemical characterization of Anopheles breeding sites in the Mount Cameroon region. J. Vector Borne Dis. 2009, 46, 75–80. [Google Scholar] [PubMed]
- Service, M.W. Mortalities of the immature stages of species B of the Anopheles gambiae complex in Kenya: Comparison between rice fields and temporary pools, identification of predators, and effects of insecticidal spraying. J. Med. Entomol. 1977, 13, 535–545. [Google Scholar] [CrossRef]
- Tranchida, M.C.; Micieli, M.V.; Macia, A.; Garcia, J.J. Native Argentinean cyclopoids (Crustacea: Copepoda) as predators of Aedes aegypti and Culex pipiens (Diptera: Culicidae) mosquitoes. Rev. Biol. Trop. 2009, 57, 1059–1068. [Google Scholar] [CrossRef]
- Paaijmans, K.P.; Takken, W.; Githeko, A.K.; Jacobs, A.F. The effect of water turbidity on the near-surface water temperature of larval habitats of the malaria mosquito Anopheles gambiae. Int. J. Biometeorol. 2008, 52, 747–753. [Google Scholar] [CrossRef]
- Gardner, A.M.; Anderson, T.K.; Hamer, G.L.; Johnson, D.E.; Varela, K.E.; Walker, E.D.; Ruiz, M.O. Terrestrial vegetation and aquatic chemistry influence larval mosquito abundance in catch basins, Chicago, USA. Parasit. Vectors 2013, 6, 9. [Google Scholar] [CrossRef]
- Banerjee, A.; Chandra, G. Role of some factors on the breeding of JE vector Culex vishnui group. J. Commun. Dis. 2004, 36, 260–263. [Google Scholar] [PubMed]
- Mereta, S.T.; Yewhalaw, D.; Boets, P.; Ahmed, A.; Duchateau, L.; Speybroeck, N.; Vanwambeke, S.O.; Legesse, W.; De Meester, L.; Goethals, P.L. Physico-chemical and biological characterization of anopheline mosquito larval habitats (Diptera: Culicidae): Implications for malaria control. Parasit. Vectors 2013, 6, 320. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M. Biological vector control of mosquito-borne diseases. Lancet Infect. Dis. 2011, 11, 84–85. [Google Scholar] [CrossRef]
- Bahgat, I.M. Impact of physical and chemical characteristics of breeding sites on mosquito larval abundance at Ismailia Governorate, Egypt. J. Egypt. Soc. Parasitol. 2013, 43, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Shililu, J.I.; Gu, W.; Jacob, B.G.; Githure, J.I.; Novak, R.J. Larval habitat dynamics and diversity of Culex mosquitoes in rice agro-ecosystem in Mwea, Kenya. Am. J. Trop. Med. Hyg. 2007, 76, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mireji, P.O.; Keating, J.; Hassanali, A.; Mbogo, C.M.; Nyambaka, H.; Kahindi, S.; Beier, J.C. Heavy metals in mosquito larval habitats in urban Kisumu and Malindi, Kenya, and their impact. Ecotox. Environ. Saf. 2008, 70, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, V.G. The effect of mineral fertilizers on mosquito larvae (Culicidae). Parazitologiia 1989, 23, 193–199. [Google Scholar]
- Batovska, J.; Blacket, M.J.; Brown, K.; Lynch, S. Molecular identification of mosquitoes (Diptera: Culicidae) in southeastern Australia. Ecol. Evol. 2016, 6, 3001–3011. [Google Scholar] [CrossRef]
- Lawrence, A.; Batovska, J.; Webb, C.E.; Lynch, S.E.; Blacke, M.J.; Šlapeta, J.; Parol, P.; Laroche, M. Accurate identification of Australian mosquitoes using protein profiling. Parasitol 2019, 146, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Kassim, N.F.A.; Webb, C.E.; Wang, Q.; Russell, R.C. Australian distribution, genetic status and seasonal abundance of the exotic mosquito Culex molestus (Forskal) (Diptera: Culicidae). Aust. J.Entomol. 2013, 52, 185–198. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).