Liver δ-Aminolevulinate Dehydratase Activity is Inhibited by Neonicotinoids and Restored by Antioxidant Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Tissue Preparation
2.4. Protein
2.5. δ-ALA-D Activity
2.6. Inhibitory Effect of Imidacloprid to δ-ALA-D Activity and IC50 Determination
2.7. Effect of Dithiothreitol (DTT) and Zinc Chloride (ZnCl2)
2.8. Effect of Resveratrol, Curcumin, Ascorbic Acid and Reduced Glutathione on Liver δ-ALA-D Activity in the Presence of Imidacloprid
2.9. Quantification of Chemical Elements in Imidacloprid
2.10. Statistical Analysis
3. Results
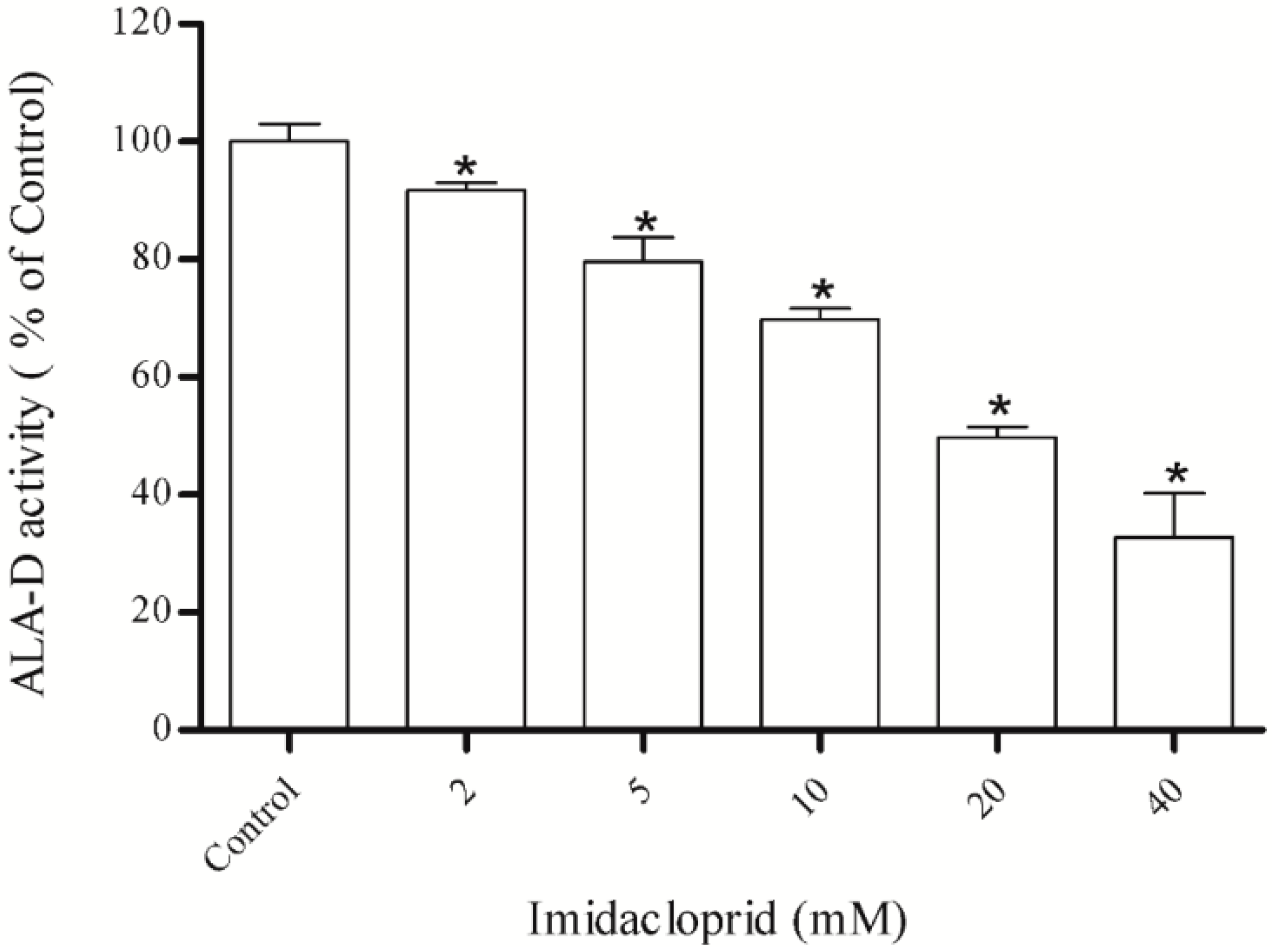
3.1. Inhibitory Effect of Imidacloprid on δ-ALA-D Activity and Determination of IC50
3.2. Effect of Dithiothreitol (DTT) and Zinc Chloride (ZnCl2)

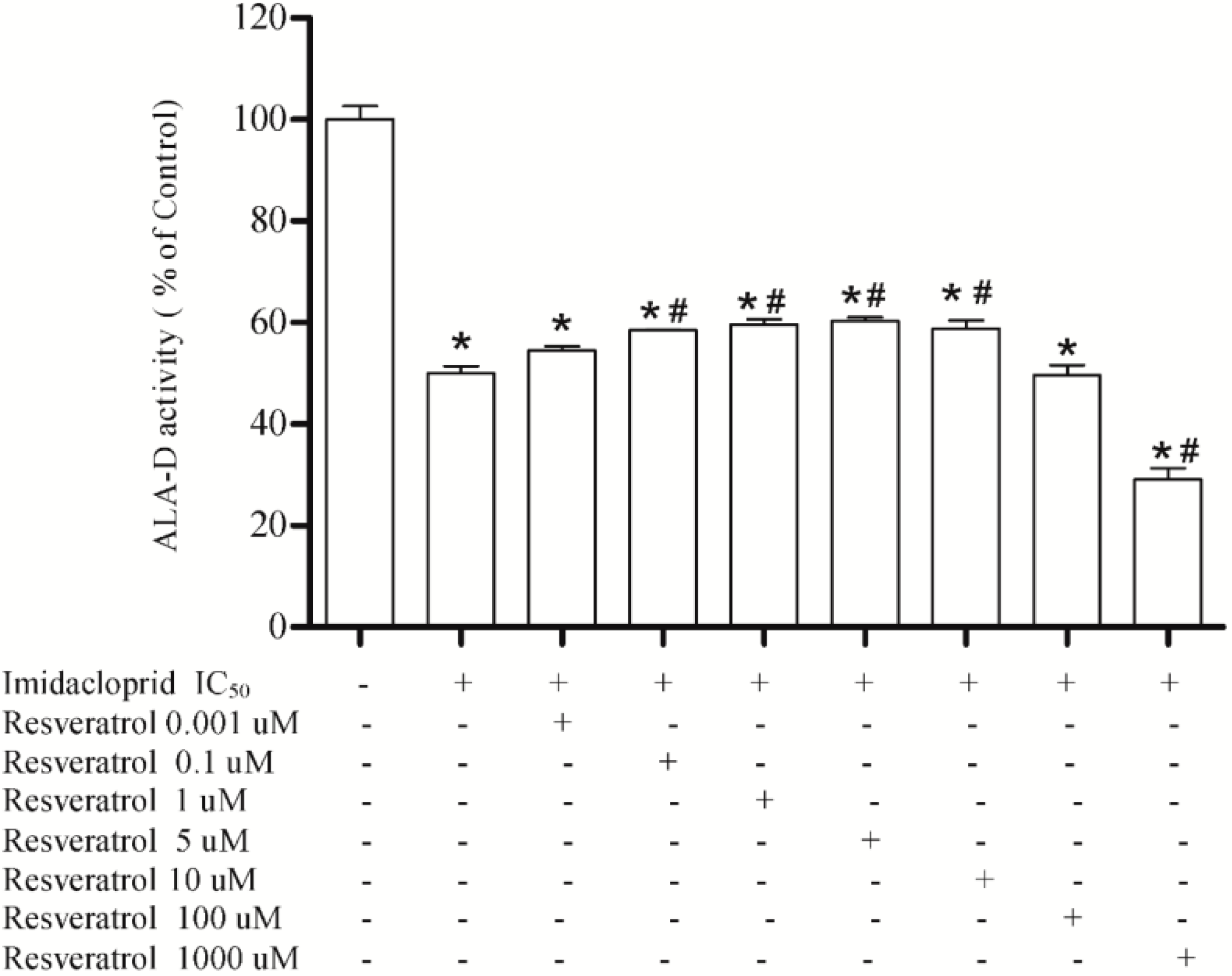
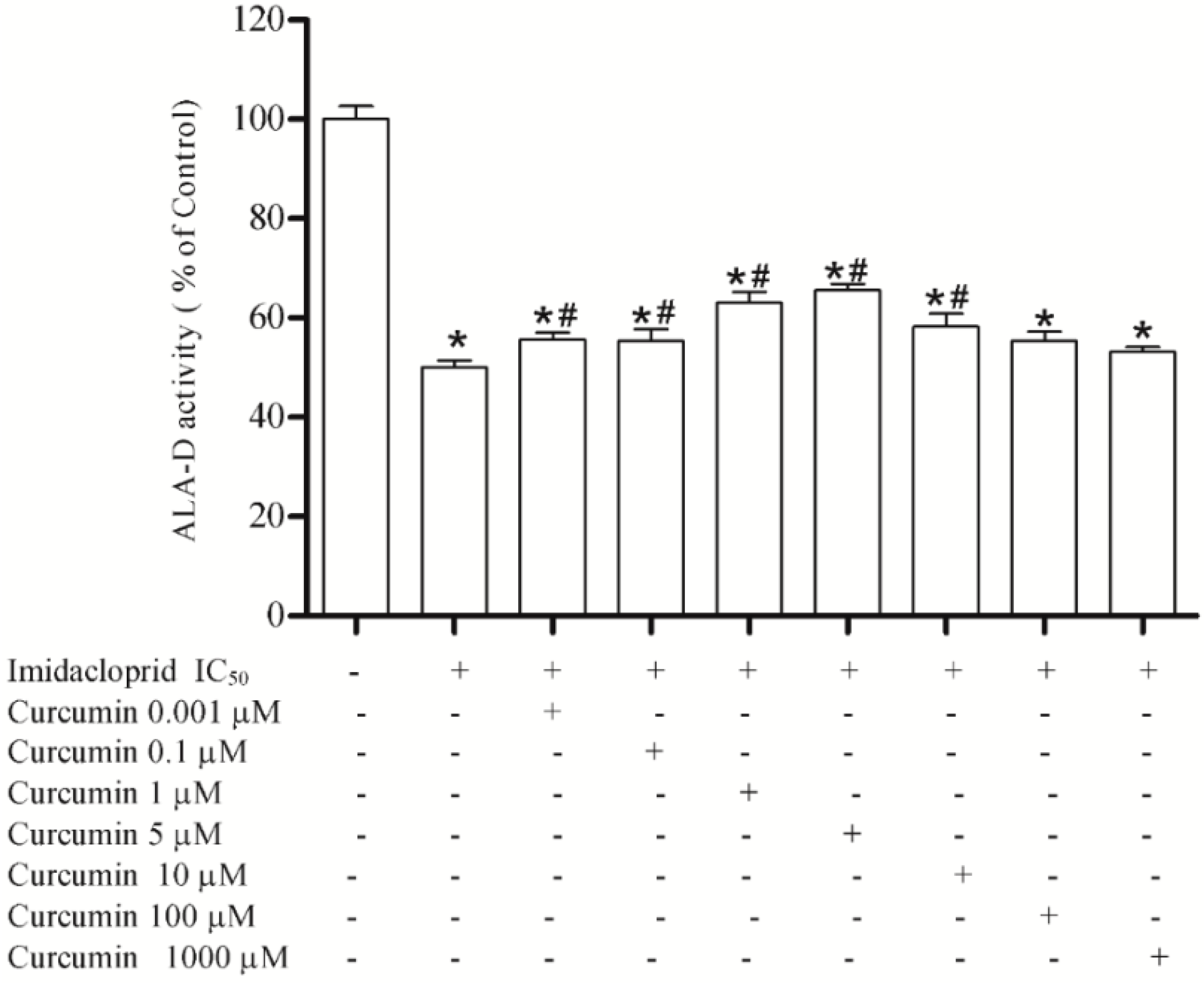
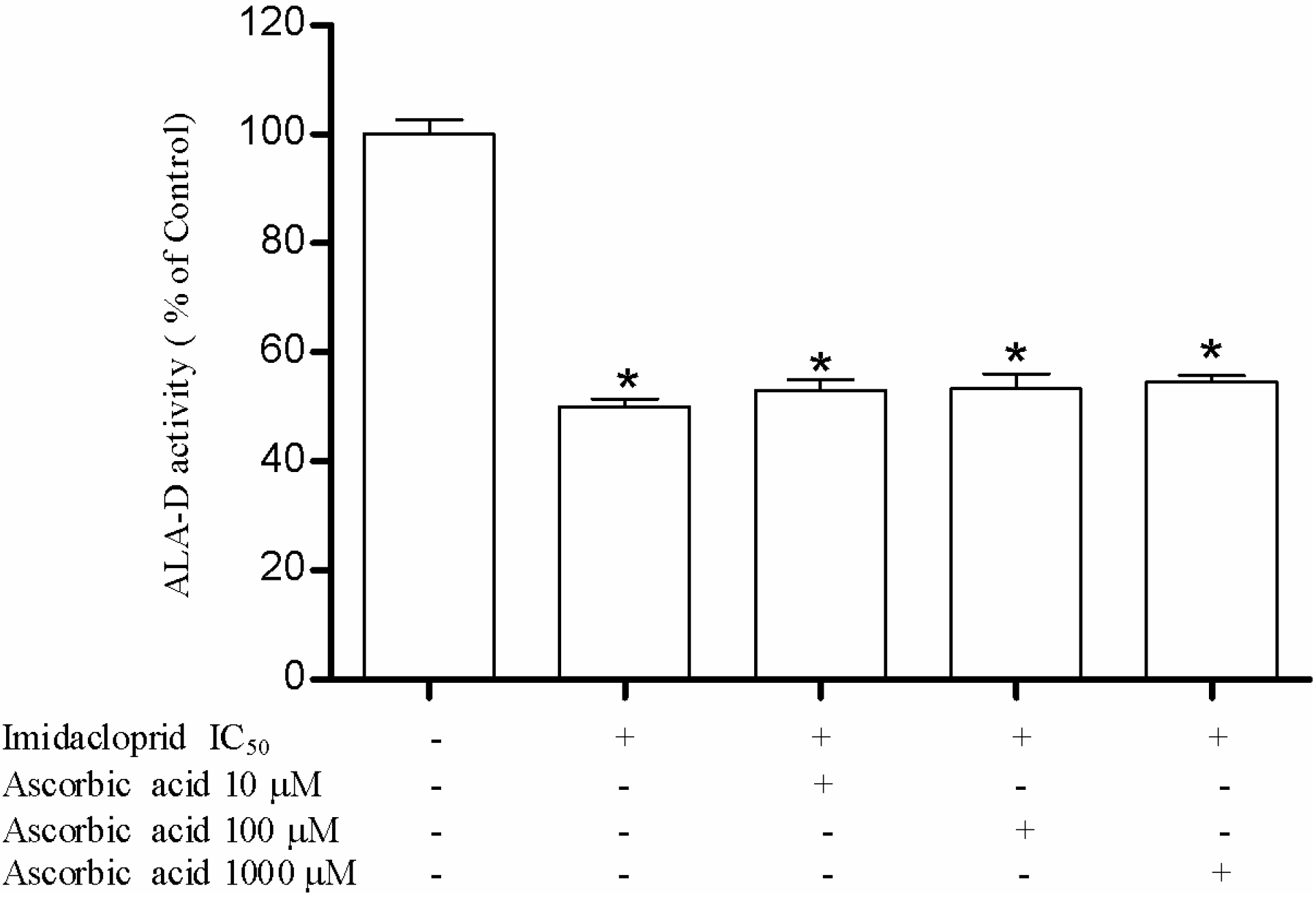
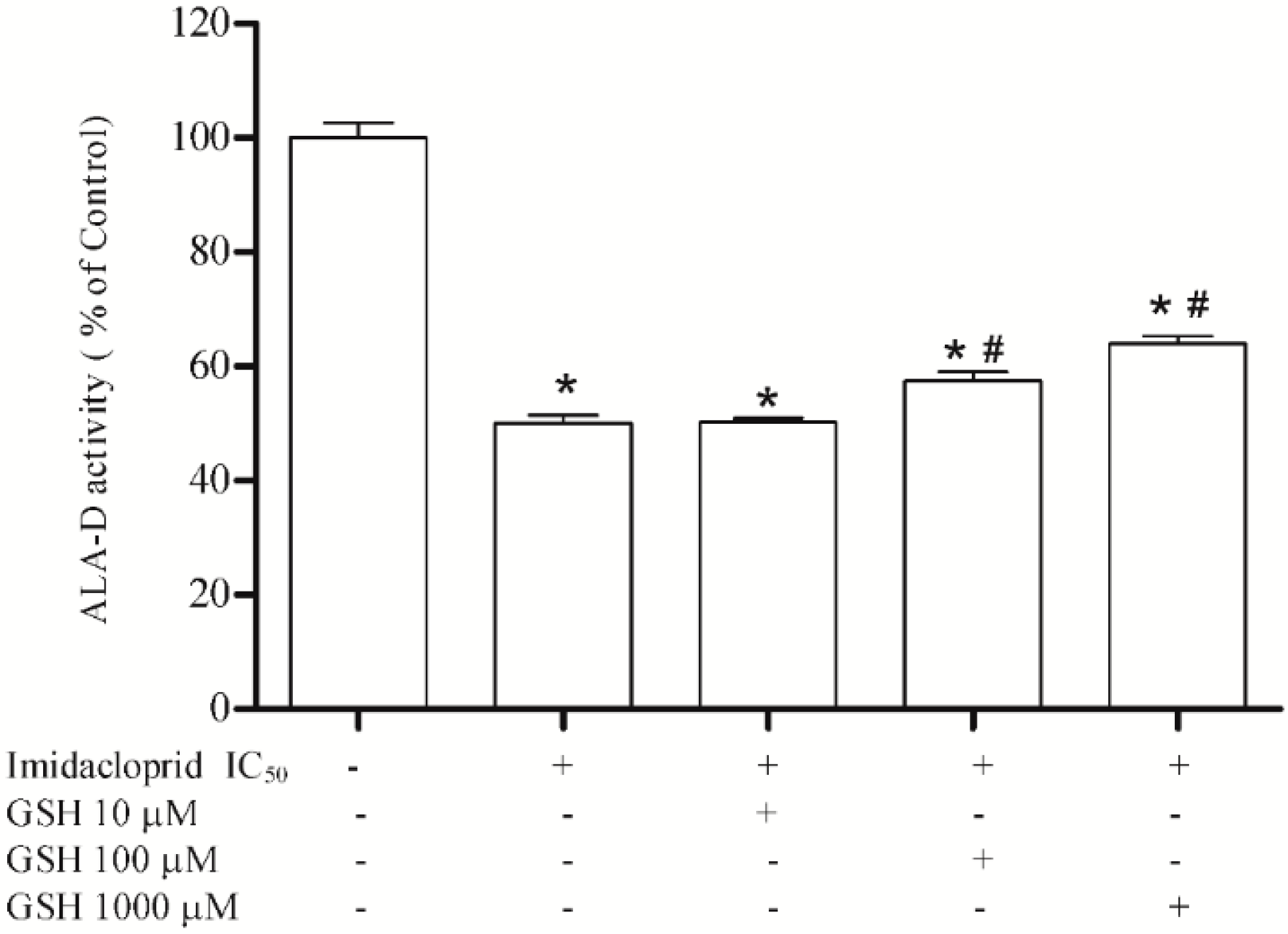
3.3. Effect of Resveratrol, Curcumin, Ascorbic Acid and Reduced Glutathione on Liver δ-AlA-D Activity in the Presence of Imidacloprid
3.4. Determination of Chemical Elements in Imidacloprid
| Chemical elements | Concentration (mg∙L−1) |
|---|---|
| Aluminum | 8.43 |
| Arsenic | 0.28 |
| Cadmium | 0.02 |
| Cobalt | 0.05 |
| Copper | 2.98 |
| Chromium | 2.81 |
| Lead | 0.11 |
| Manganese | 77.62 |
| Mercury | 0.11 |
| Nickel | 0.51 |
| Strontium | 3.27 |
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, RA141–RA147. [Google Scholar] [PubMed]
- El-Gendy, K.S.; Aly, N.M.; Mahmoud, F.H.; Kenawy, A.; El-Sebae, A.K.H. The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem. Toxicol. 2010, 48, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Segura, M.E.; Gómez-Arroyo, S.; Villalobos-Pietrini, R.; Martínez-Valenzuela, C.; Carbajal-López, Y.; Calderón-Ezquerro, M.D.C.; Cortés-Eslava, J.; García-Martínez, R.; Flores-Ramírez, D.; Rodríguez-Romero, M.I. Evaluation of genotoxic and cytotoxic effects in human peripheral blood lymphocytes exposed in vitro to neonicotinoid insecticides news. J. Toxicol. 2012, 2012. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2010, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Duzguner, V.; Erdogan, S. Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver and central nervous system of rats. Pestic. Biochem. Physiol. 2012, 104, 58–64. [Google Scholar] [CrossRef]
- Dwivedi, P.; Das, M.; Khanna, S. Role of cytochrome P450 in quinalphos toxicity: Effect on hepatic and brain antioxidant enzymes in RatsITRC communication No. 1965. Food Chem. Toxicol. 1998, 36, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, Z.; Ju, B.; Wang, Y.; Wang, J.; Bai, D. Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Exp. Toxicol. Pathol. 2008, 59, 415–423. [Google Scholar]
- Jaffe, E.K. The porphobilinogen synthase family of metalloenzymes. Acta Crystallogr. Sect. D 2000, 56, 115–128. [Google Scholar] [CrossRef]
- Rocha, J.B.; Saraiva, R.A.; Garcia, S.C.; Gravina, F.S.; Nogueira, C.W. Aminolevulinate dehydratase (δ ALA-D) as marker protein of intoxication with metals and other pro-oxidant situations. Toxicol. Res. 2012, 1, 85–102. [Google Scholar] [CrossRef]
- Hinderer, R.K.; Menzer, R.E. Comparative enzyme activities and cytochrome P-450 levels of some rat tissues with respect to their metabolism of several pesticides. Pestic. Biochem. Physiol. 1976, 6, 148–160. [Google Scholar] [CrossRef]
- Olfert, E.; Cross, B.; McWilliam, A. Canadian Council on Animal Care—Guide to the Care and Use of Experimental Animals; Brada Printing Services: Ottawa, ON, Canada, 1993; Volume 1. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sassa, S. Delta-aminolevulinic acid dehydratase assay. Enzyme 1981, 28, 133–145. [Google Scholar]
- Meister, R.T. Farm Chemicals Handbook; Meister Publishing Company: Willoughby, OH, USA, 1995. [Google Scholar]
- Mohamed, F.; Gawarammana, I.; Robertson, T.A.; Roberts, M.S.; Palangasinghe, C.; Zawahir, S.; Jayamanne, S.; Kandasamy, J.; Eddleston, M.; Buckley, N.A. Acute human self-poisoning with imidacloprid compound: A neonicotinoid insecticide. PLoS One 2009, 4. [Google Scholar] [CrossRef]
- Imamura, T.; Yanagawa, Y.; Nishikawa, K.; Matsumoto, N.; Sakamoto, T. Two cases of acute poisoning with acetamiprid in humans. Clin. Toxicol. 2010, 48, 851–853. [Google Scholar] [CrossRef]
- David, D.; George, I.A.; Peter, J.V. Toxicology of the newer neonicotinoid insecticides: Imidacloprid poisoning in a human. Clin. Toxicol. 2007, 45, 485–486. [Google Scholar] [CrossRef]
- Aydin, B. Effects of thiacloprid, deltamethrin and their combination on oxidative stress in lymphoid organs, polymorphonuclear leukocytes and plasma of rats. Pestic. Biochem. Physiol. 2011, 100, 165–171. [Google Scholar] [CrossRef]
- Markham, G.D.; Myers, C.B.; Harris, K.A.; Volin, M.; Jaffe, E.K. Spatial proximity and sequence localization of the reactive sulfhydryls of porphobilinogen synthase. Protein. Sci. 1993, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Barbosa, N.; Nogueira, C.; Folmer, V.; Zeni, G.; Andrade, L.; Braga, A.; Rocha, J. Reaction of diphenyl diselenide with hydrogen peroxide and inhibition of delta-aminolevulinate dehydratase from rat liver and cucumber leaves. Braz. J. Med. Biol. Res. 2002, 35, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Needleman, H. Lead poisoning. Annu. Rev. Med. 2004, 55, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, T.; Pagel, F.W.; Alves, L.C.B.; Regner, A.; Souza, D.O. Inhibition of adenylate cyclase activity by 5-aminolevulinic acid in rat and human brain. Neurochem. Int. 2001, 38, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Valentini, J.; Grotto, D.; Paniz, C.; Roehrs, M.; Burg, G.; Garcia, S.C. The influence of the hemodialysis treatment time under oxidative stress biomarkers in chronic renal failure patients. Biomed. Pharmacother. 2008, 62, 378–382. [Google Scholar] [CrossRef]
- Roehrs, M.; Valentini, J.; Bulcão, R.; Moreira, J.C.; Biesalski, H.; Limberger, R.P.; Grune, T.; Garcia, S.C. The plasma retinol levels as pro-oxidant/oxidant agents in haemodialysis patients. Nephrol. Dial. Transplant. 2009, 24, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.L.; Benvegnú, D.M.; Bonfanti, G.; Frediani, A.V.; Rocha, J.B. δ-ALA-D activity is a reliable marker for oxidative stress in bone marrow transplant patients. BMC Cancer 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cuartero, B.; Rebollar, J.; Batlle, A.; Enriquez de Salamanca, R. Delta aminolevulinate dehydratase (ALA-D) activity in human and experimental diabetes mellitus. Int. J. Biochem. Cell Biol. 1999, 31, 479–488. [Google Scholar] [CrossRef]
- Rocha, J.B.; Heinzmann Bulow, N.M.; Correa, E.F.; Scholze, C.; Nogueira, C.W.; Barbosa, N.B. Dexmedetomidine protects blood delta-aminolevulinate dehydratase from inactivation caused by hyperoxygenation in total intravenous anesthesia. Hum. Exp. Toxicol. 2011, 30, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.M.; Charão, M.; Brucker, N.; Bulcão, R.; Freitas, F.; Guerreiro, G.; Baierle, M.; Nascimento, S.; Waechter, F.; Hirakata, V. Effects of low-level exposure to xenobiotics present in paints on oxidative stress in workers. Sci. Total Environ. 2010, 408, 4461–4467. [Google Scholar] [CrossRef]
- Folmer, V.; Bolzan, R.C.; Farina, M.; Zeni, G.; Nogueira, C.W.; Emanuelli, T.; Rocha, J.B.T. Mechanism of delta-aminolevulinate dehydratase inhibition by phenyl selenoacetylene involves its conversion to diphenyl diselenide. Toxicology 2005, 206, 403–411. [Google Scholar] [CrossRef]
- Erikson, K.M.; Syversen, T.; Aschner, J.L.; Aschner, M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ. Toxicol. Pharmacol. 2005, 19, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Guilarte, T.R.; Schneider, J.S.; Zheng, W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007, 221, 131–147. [Google Scholar] [CrossRef]
- Josephs, K.; Ahlskog, J.; Klos, K.; Kumar, N.; Fealey, R.; Trenerry, M.; Cowl, C. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology 2005, 64, 2033–2039. [Google Scholar] [CrossRef]
- Sebastià, N.; Montoro, A.; Montoro, A.; Almonacid, M.; Villaescusa, J.I.; Cervera, J.; Such, E.; Silla, M.A.; Soriano, J.M. Assessment in vitro of radioprotective efficacy of curcumin and resveratrol. Radiat. Meas. 2011, 46, 962–966. [Google Scholar] [CrossRef]
- El-Azab, M.; Hishe, H.; Moustafa, Y.; El-Awady, E.-S. Anti-angiogenic effect of resveratrol or curcumin in Ehrlich ascites carcinoma-bearing mice. Eur. J. Pharmacol. 2011, 652, 7–14. [Google Scholar]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative study of natural antioxidants-curcumin, resveratrol and melatonin—In cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Stocco, B.; Toledo, K.; Salvador, M.; Paulo, M.; Koyama, N.; Torqueti Toloi, M.R. Dose-dependent effect of resveratrol on bladder cancer cells: Chemoprevention and oxidative stress. Maturitas 2012, 72, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Bendich, A. Antioxidant micronutrients and immune responses. Ann. N.Y. Acad. Sci. 1990, 587, 168–180. [Google Scholar] [PubMed]
- Bindhumol, V.; Chitra, K.; Mathur, P. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 2003, 188, 117–124. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Tsai, H.-L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food. Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Meister, A. Selective modification of glutathione metabolism. Science 1983, 220, 472–477. [Google Scholar] [CrossRef]
- Borok, Z.; Buhl, R.; Hubbard, R.; Holroyd, K.; Roum, J.; Czerski, D.; Crystal, R.; Grimes, G.; Bokser, A.; Cantin, A. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet 1991, 338, 215–216. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Mori, A. Immobilization stress-induced antioxidant defense changes in rat plasma: Effect of treatment with reduced glutathione. Int. J. Biochem. 1994, 26, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska-Ślebodzińska, E.; Ślebodziński, A.; Pietras, B.; Wieczorek, G. Antioxidant effect of vitamin E and glutathione on lipid peroxidation in boar semen plasma. Biol. Trace Element. 1995, 47, 69–74. [Google Scholar] [CrossRef]
- Cascinu, S.; Catalano, V.; Cordella, L.; Labianca, R.; Giordani, P.; Baldelli, A.M.; Beretta, G.D.; Ubiali, E.; Catalano, G. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: A randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2002, 20, 3478–3483. [Google Scholar] [CrossRef]
- Kimura-Kuroda, J.; Komuta, Y.; Kuroda, Y.; Hayashi, M.; Kawano, H. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauer, E.; Moro, A.M.; Brucker, N.; Nascimento, S.; Gauer, B.; Fracasso, R.; Gioda, A.; Beck, R.; Moreira, J.C.F.; Eifler-Lima, V.L.; et al. Liver δ-Aminolevulinate Dehydratase Activity is Inhibited by Neonicotinoids and Restored by Antioxidant Agents. Int. J. Environ. Res. Public Health 2014, 11, 11676-11690. https://doi.org/10.3390/ijerph111111676
Sauer E, Moro AM, Brucker N, Nascimento S, Gauer B, Fracasso R, Gioda A, Beck R, Moreira JCF, Eifler-Lima VL, et al. Liver δ-Aminolevulinate Dehydratase Activity is Inhibited by Neonicotinoids and Restored by Antioxidant Agents. International Journal of Environmental Research and Public Health. 2014; 11(11):11676-11690. https://doi.org/10.3390/ijerph111111676
Chicago/Turabian StyleSauer, Elisa, Angela M. Moro, Natália Brucker, Sabrina Nascimento, Bruna Gauer, Rafael Fracasso, Adriana Gioda, Ruy Beck, José C. F. Moreira, Vera Lucia Eifler-Lima, and et al. 2014. "Liver δ-Aminolevulinate Dehydratase Activity is Inhibited by Neonicotinoids and Restored by Antioxidant Agents" International Journal of Environmental Research and Public Health 11, no. 11: 11676-11690. https://doi.org/10.3390/ijerph111111676




