Abstract
Tetrodotoxin (TTX) and its deoxy analogs, 5-deoxyTTX, 11-deoxyTTX, 6,11-dideoxyTTX, and 5,6,11-trideoxyTTX, were quantified in the tissues of three female and three male specimens of the marine puffer fish, Fugu niphobles, from the southern coast of Korea, and in the whole body of the brackishwater puffer fishes, Tetraodon nigroviridis (12 specimens) and Tetrodon biocellatus (three specimens) from Southeast Asia using LC/MS in single ion mode (SIM). Identification of these four deoxy analogs in the ovarian tissue of F. niphobles were further confirmed by LC/MS/MS. TTX and 5,6,11-trideoxyTTX were detected in all three puffer fish species as the major TTX analogs, similar to Japanese Fugu pardalis. While 6,11-dideoxyTTX was also found to be a major analog in almost all tissues of Korean F. niphobles, this analog was minor in the two Tetraodon species and Japanese F. pardalis. Among the tissues of F. niphobles, the concentrations of TTXs were highest in the ovaries (female) and skin (female and male).
1. Introduction
Tetrodotoxin (TTX), the primary chemical toxin of puffer fishes, exists as a mixture of its analogs (Figure 1). Among them, 4-epiTTX and 4,9-anhydroTTX are the chemical equilibrium analogs of TTX [1,2]. Four deoxy analogs, such as 5-deoxyTTX [3], 11-deoxyTTX [4], 6,11-dideoxyTTX [5] and 5,6,11-trideoxyTTX [6], were also isolated from puffer fishes and/or newts as the chemical non-equilibrium analogs. The values of LD50 (50% lethal dose, mice, intraperitoneal injection) of TTX, 11-deoxyTTX, and 6,11-dideoxyTTX were determined as 10 μg/kg [7], 70 μg/kg [4], and 420 μg/kg [5], respectively, and the value of LD99 of 5,6,11-trideoxyTTX was 750 μg/kg [6]. The LD value of 5-deoxyTTX has not been determined yet.
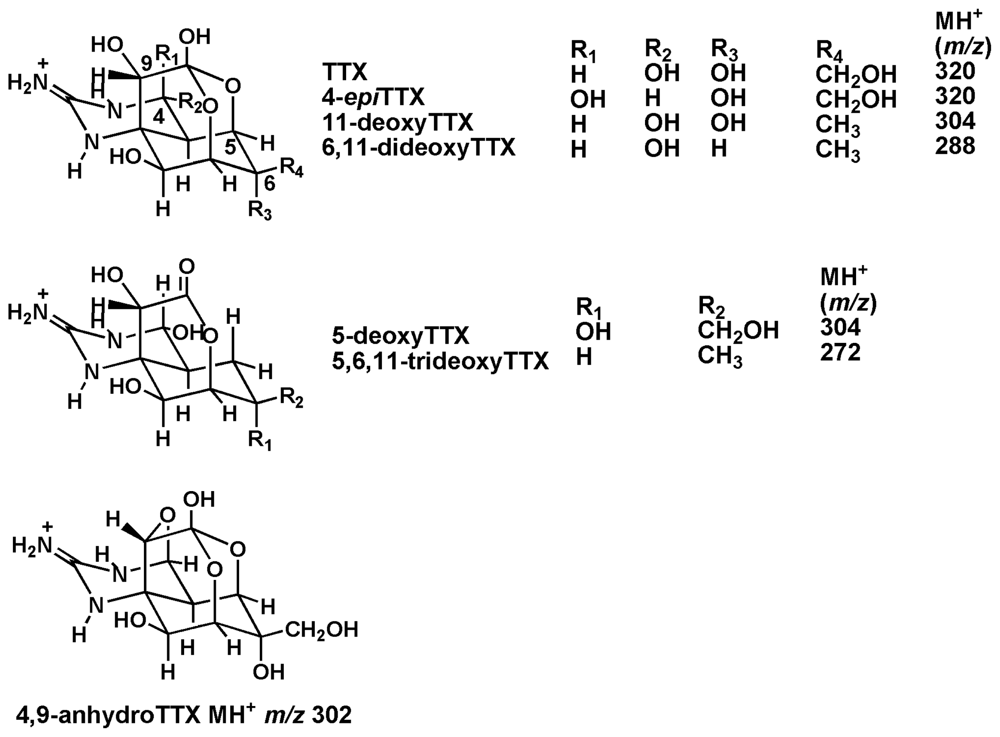
Figure 1.
The chemical structures of TTX and its analogs found in puffer fishes.
We previously established the hydrophilic interaction liquid chromatography-electrospray ionization/mass spectrometry (HILIC-ESI/MS) system for TTXs [8,9]. This system has enabled us to quantify the four deoxy analogs of TTX which were not detectable with liquid chromatography fluorescent detection (LC-FLD) [8,11–12]. Using this LC/MS, distribution of TTX analogs among tissues of Japanese marine puffer fish Fugu pardalis was revealed, and 5,6,11-trideoxyTTX was found to be the major analog in all tissues [10]. Rodriguez et al. [13] also reported the presence of TTX and 5,6,11-trideoxyTTX as the major TTX analogs in the European trumpet shell, Charonia lampas lampas, using LC/MS/MS. Contrary to the marine animals, 5,6,11-trideoxyTTX was not detected in the newt, Cynops ensicauda [9]. It has not been clarified whether these deoxy analogs and TTX are chemically modified with each other in puffer fishes, or if they are all produced by the TTX producing bacteria [14,15]. However, these analogs can be speculated to be precursors or metabolites of TTX [16,17] because of their structural similarity. To support this assumption, we need to confirm that these deoxy analogs are commonly present in a range of species of puffer fishes.
Herein we examined the contents of TTX and its deoxy analogs in the tissues of three female and three male specimens of the marine puffer fish F. niphobles collected at the southern coast of Korea, and in two brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia, purchased in a pet market in Japan [18], by LC/MS in HILIC mode.
2. Results and Discussion
TTXs were quantified by LC/MS in single ion monitoring (SIM) mode, because the ratios of signal to noise of the peaks of TTXs were higher than those obtained by LC/MS/MS in multiple reaction monitoring (MRM) mode, probably due to some specific reasons related to our MS spectrometer. Tetrodonic acid was not analyzed in this study. In Figure 2, LC/MS separation in SIM mode of (B) the ovary from female no. 3 specimen of Korean F. niphobles, (C) whole body of no. 5 specimen of T. nigroviridis, and (D) whole body of no. 2 specimen of T. biocellatus from Southeast Asia are illustrated as representatives. Based on the peak area on the mass chromatograms, the concentrations of TTXs in the ovary from female no. 3 F. niphobles are estimated at TTX (263 nmol/g), 4-epiTTX (26 nmol/g), 4,9-anhydroTTX (83 nmol/g), 5-deoxyTTX (22 nmol/g), 11-deoxyTTX (39 nmol/g), 6,11-dideoxyTTX (310 nmol/g), and 5,6,11-trideoxyTTX (603 nmol/g). Similarly, the toxin contents in each tissue of three female and three male specimens of F. niphobles, and those in whole body of twelve specimens of T. nigroviridis and three specimens of T. biocellatus are determined, and summarized in Table 1 and Table 2, respectively.
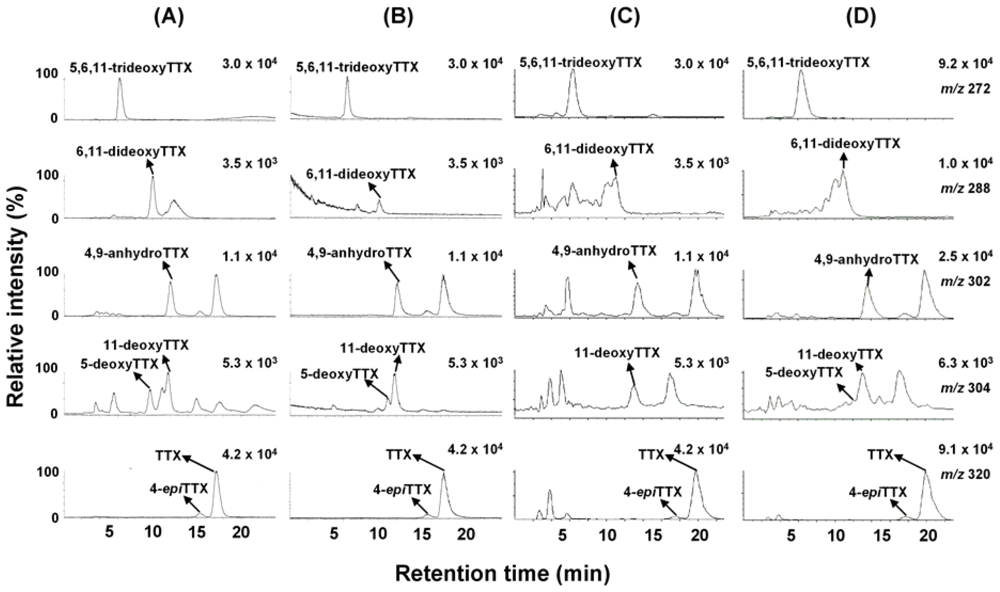
Figure 2.
The mass chromatograms of LC/MS in SIM mode. (A) Volume of 4 μl of the semi-purified TTXs mixture containing TTX (53 pmol), 4-epiTTX (5.6 pmol), 4,9-anhydroTTX (7.4 pmol), 5-deoxyTTX (1.8 pmol), 11-deoxtyTTX (6.8 pmol), 6,11-dideoxyTTX (1.5 pmol), and 5,6,11-trideoxyTTX (38 pmol) prepared from ovary of F. poecilonotus as standard [10], (B) Sample solution (1 μl) of the ovary from female no. 3 F. niphobles, (C) Sample solution (1 μl) of whole body of no. 5 specimen of T. nigroviridis, (D) Sample solution (1 μl) of whole body of no. 2 specimen of T. biocellatus.

Table 1.
Anatomical distribution of TTX and its analogs in F. niphobles (n = 3).

Table 2.
The contents of TTX and its analogs in the whole body extracts of T. nigroviridis (n = 12) and T. biocellatus (n = 3).
We previously reported characteristic fragment ions, including a major ion at m/z 162, from [M+H]+ ions of TTX, 4-epiTTX, 4,9-anhydroTTX, 5-deoxyTTX, 11-deoxyTTX, and 5,6,11-trideoxyTTX by MS/MS (product ion) scan, and applied them to detection of TTXs by LC/MS/MS in MRM mode [8] as listed in Table 3. In the present study, we determined the major fragment ions by product ion scan from [M+H]+ m/z 288 for 6,11-dideoxyTTX for the first time (Figure 3). Distinctively from other TTX analogs [8], 6,11-dideoxyTTX produced the major fragment ion at m/z 224 instead at m/z162. Therefore, m/z 288–224 fragment was set up for detection of 6,11-dideoxyTTX by LC/MS/MS. Identification of TTX analogs by LC/MS in SIM mode was confirmed by LC/MS/MS in MRM mode, only for the ovary from female no. 3 Korean F. niphobles (Figure 4B).

Table 3.
MRM conditions for TTX analogs in positive ESI-mode. Collision Energy: 42 eV.
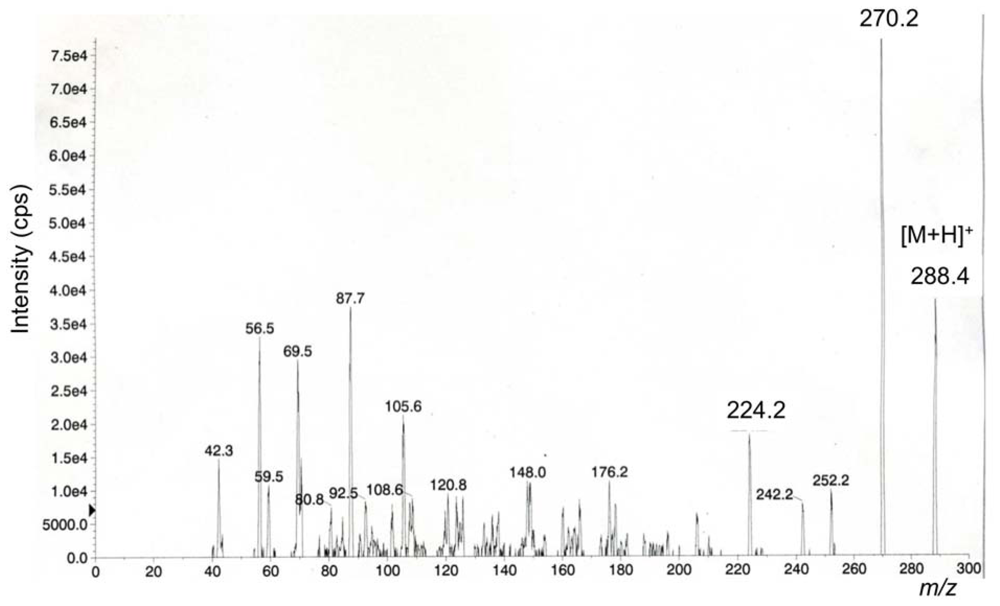
Figure 3.
The fragment ion spectrum from [M+H]+ m/z 288 of 6,11-dideoxyTTX (150 pmol) injected into 0.2 mL/min of methanol. Collision energy: 42 eV.
Figure 4.
The mass chromatograms of the LC/MS/MS obtained under MRM operation (m/z 320–162 for TTX and 4-epiTTX, m/z 302–162 for 4,9-anhydroTTX, m/z 304–162 for 5-deoxyTTX and 11-deoxyTTX, 288–224 for 6,11-dideoxyTTX, and m/z 272–162 for 5,6,11-tridexyTTX). (A) Volume of 4 μl of the semi-purified TTXs mixture containing TTX (53 pmol), 4-epiTTX (5.6 pmol), 4,9-anhydroTTX (7.4 pmol), 5-deoxyTTX (1.8 pmol), 11-deoxtyTTX (6.8 pmol), 6,11-dideoxyTTX (1.5 pmol), and 5,6,11-trideoxyTTX (38 pmol) prepared from ovary of F. poecilonotus as standard [10], (B) Volume of 1 μl of ovary sample solution from female no. 3 F. niphobles.
As shown in Table 1 and Table 2, TTX and 5,6,11-trideoxyTTX are commonly detected as the major toxins in all tissues of Korean F. niphobles and also in the two Tetraodon species, similar to Japanese Fugu pardalis as we reported previously [10]. Interestingly, 6,11-dideoxyTTX, which was previously discovered in the ovaries of Japanese F. pardalis as a minor analog [5], is detected as another major TTX analog in almost all tissues in Korean F. niphobles with exception of the skin from females. Also, the concentration of 6,11-dideoxyTTX is higher than that of TTX in the intestine and liver from female no. 3 F. niphobles. This analog is also detected in some specimens of T. nigroviridis and T. biocellatus as a minor analog. 5-DeoxyTTX and 11-deoxyTTX are contained in low concentrations in almost all tissues of Korean F. niphobles, but they are less than detection limit (<0.5 nmol/g) in the muscle and testicle. 11-DeoxyTTX is a minor analog also in the two Tetraodon species, while 5-deoxyTTX is slightly detected only in T. biocellatus and it is less than detection limit (<0.5 nmol/g) in T. nigroviridis. Except for 5-deoxyTTX in T. nigroviridis, these data suggest that these four deoxy analogs of TTX are commonly present in puffer fishes, although the ratios of them are specific to the species of puffer fishes or to the regions where the fishes were collected.
Among the tissues of Korean F. niphobles, the concentration of TTXs is highest in the ovary and skin. This is in agreement with the report by Ryu et al. [19] that skin was highly toxic (10–674 mouse unit/g = 7–465 nmol/g TTX) in Korean F. niphobles by mouse assay method (1 mouse unit is equivalent to 220 ng of TTX). Also, this is consistent with the data reported by Kono et al. [20] that dietarily administrated TTX to Japanese cultured juvenile F. niphobles was first accumulated in liver and then transferred to skin. According to Ikeda et al. [21], the high toxicity in the ovaries of Takifugu (=Fugu) poecilonotus might account for the TTXs transfer from liver, skin, intestine, and muscle into the ovaries during spawning season. Unlike Korean F. niphobles, Japanese F. pardalis contained the highest concentration of TTXs in ovary and liver, and medium level TTXs in skin [10]. Edible parts except intestine and ovary of F. niphobles, which contained fewer than 10 mouse units per gram, are used in a favorite fish soup or other food materials in Korea and cause TTX poisoning sporadically. More careful precautions need to be taken to remove toxic body parts before cooking. Concerning the toxicity of the puffer fishes genus Tetraodon, Shin-Jung et al. [22] examined the toxicity of 42 and 12 specimens of T. ocellatus and T. nigroviridis, respectively, collected in Taiwan by using mouse bioassay, and identified TTX and 4,9-anhydroTTX in both species by LC. The authors reported the highest toxicity score of T. nigroviridis to be 124 mouse unit/g (=85 nmol/g TTX), rather lower than those found in our present study (Table 2).
3. Experimental Section
3.1. Puffer fishes specimens
Specimens of three female and three male each of F. niphobles (body weight 12–37 g) were collected on May 12th, 2008 at Tongyeong bay, South Korea. The twelve specimens of T. nigroviridis (body weight 0.8–3.8 g, sex not identified) and three specimens of T. biocellatus (body weight 1.6–3.7g, sex not identified) collected from Southeast Asia were purchased in a pet market of Miyagi Prefecture, Japan. Samples were immediately kept on ice, transported to the laboratory and kept frozen below −20 °C for no longer than a month until use.
3.2. Preparation of sample solutions
Sample solutions were prepared as we reported previously [8,9] with minor modification. F. niphobles were dissected to obtain organs of ovary/testis, liver, intestine, dorsal skin and dorsal muscle, while T. nigroviridis and T. biocellatus were used as whole body. The toxins were extracted from a part (1 g) of homogenized tissues with 5 mL of 0.05 M acetic acid (v/v) by heating in boiling water for 5 min. The obtained suspensions were centrifuged at 4,000 rpm for 15 min at 4 °C, then the supernatants were filled up to 5 mL with 0.05 M acetic acid. An aliquot (2 mL) was applied to the reverse phase cartridge column (Sep-Pak C18, Waters) which was equilibrated with water after washing with MeOH before use. The first passing solution (1.5 mL) was discarded, and the following 0.5 mL was collected and defatted with CHCl3 (0.5 mL). After removal of the remaining CHCl3 under the stream of N2 gas, the aqueous phase was lyophilized and filled up to 5 mL with water, and then loaded on an activated charcoal (0.5 mL), which was packed in a glass pipette. After washing the column with water (0.5 mL), the toxins were eluted with acetic acid/EtOH/H2O (1:50:49, 1.5 mL), and the volatile was evaporated from the eluate. The remaining residue was dissolved in 0.5 mL of 0.05 M acetic acid, and then ultrafiltered (Ultrafree-MC, 10,000 NMWL, Millipore, Bedford, MA) by centrifugation at 8,000 rpm for 15 min at 4 °C. This filtrate was used as the sample solution for LC/MS and LC/MS/MS analysis.
3.3. Preparation of semi-purified TTXs mixture as the standard for LC/MS
Semi-purified TTXs mixture was prepared from the ovary of Fugu poecilonotus, collected in Shimonoseki, Japan, by extraction of toxins with hot 0.05 M acetic acid, followed by partition with ethyl acetate and purification on a charcoal column [6]. TTX, 4-epiTTX, 4,9-anhydroTTX, 5-deoxyTTX, 11-deoxyTTX, 6,11-dideoxyTTX, and 5,6,11-trideoxyTTX in the obtained mixture were quantified using LC/MS in SIM mode on the basis of the standard curve for each analog drawn using highly purified analogs [8–10]. Because highly purified TTX analogs are very limited, we used this semi-purified TTXs mixture as the standard for LC/MS.
3.4. LC/MS, MS/MS, and LC/MS/MS
LC/MS was performed based on HILIC as we reported previously [9, 10]. Briefly, LC system consisted of a Shimadzu LC-10AD pump (Japan) and a TSKgel Amide-80 column (2.0-i.d. x 150 mm, 5 μm, Toso, Tokyo, Japan). LC was performed using an aqueous solution containing 16 mM ammonium formate buffer (pH 5.5) and acetonitrile (3:7, v/v) as a mobile phase at a flow rate of 0.2 mL/min at 25 °C. LC/MS, LC/MS/MS and flow-injection MS/MS experiments were recorded on API2000 mass spectrometer (Applied Bio-systems MDS SCIEX, Foster City, CA) equipped with an ESI source in the positive-ion mode. ESI was evoked by a spray voltage of +5.5 kV and heated capillary temperature was maintained at 500 °C. Six ions at m/z 272, 288, 302, 304 and 320 corresponding to the [M+H]+ ions of TTX analogs were detected in SIM mode, respectively. The dwell time was set at 200 ms per Da. The MS/MS measurements of 6,11-dideoxyTTX was based on collision induced dissociation (CID) occurring in the collision cell (Q2) of the triple quadrupole at a collision energy of 42 eV. Nitrogen was used as the target gas. 150 pmol of authentic 6,11-dideoxyTTX [5] (4 μl in 0.05M acetic acid) was introduced into the mass spectrometer by flow injection with methanol at a flow rate of 0.2 mL/min. The LC/MS/MS was performed under the same conditions as those used for the LC/MS and MS/MS experiments. The ions m/z 320–162 for TTX and 4-epiTTX, m/z 302–162 for 4,9-anhydroTTX, m/z 304–162 for 5-deoxyTTX and 11-deoxyTTX, m/z 288–224 for 6,11-dideoxyTTX, and m/z 272–162 for 5,6,11-tridexyTTX were detected in MRM mode [8]. The minimum TTX and its analogs to be detected were 0.5 nmol/g tissue in SIM mode.
4. Conclusions
In this study, four deoxy analogs of TTX were all detected in three species of puffer fishes tested with exception of 5-deoxyTTX in T. nigroviridis. We also found these deoxy analogs in ovary from Japanese Fugu poecilonotus when we prepared standard mixture of TTXs for LC/MS as described in the Experimental section. These results indicate that the deoxy analogs of TTX are common analogs in a range of puffer fishes. For the next work, we should investigate whether these deoxy analogs are chemically modified with each other in puffer fishes, or if they are provided from external origin, such as bacteria [14,15] and then accumulated in puffer fishes. The ability of puffer fishes to accumulate TTX was confirmed by Yamamori et al.[23] and Kono et al.[20] by the fact that cultured juvenile non-toxic Japanese Takifugu (Fugu) niphobles become toxic when TTX and its analogs were dietarily administered. Kono et al.[24] also reported that cultured juvenile F. niphobles administered TTX by intramuscular injection did not transform TTX to deoxy analogs or to 11-oxoTTX (which has CHO in the position of R4 in Figure 1 instead of CH2OH of TTX). However, transformation from deoxy analogs or 11-oxoTTX to TTX has not been tested, and dietary administration might be important for such experiments. We will further examine whether these deoxy analogs are the products of transformation or metabolites in puffer fishes.
Acknowledgements
The authors acknowledge financial support from the Japan Society for the Promotion of Science (JSPS), Research Fellow No. 200805892 to J. H. J., and Grants-in-Aid for Scientific Research No. 21380070 to M. Y. Y.
References and Notes
- Goto, T; Kishi, Y; Takahashi, S; Hirata, Y. Tetrodotoxin. Tetrahedron 1965, 21, 2059–2088. [Google Scholar]
- Nakamura, M; Yasumoto, T. Tetrodotoxin derivatives in puffer fish. Toxicon 1985, 23, 271–276. [Google Scholar]
- Yotsu-Yamashita, M; Schimmele, B; Yasumoto, T. Isolation and structural assignment of 5-deoxytetrodotoxin from the puffer fish Fugu poecilonotus. Biosci Biotechnol Biochem 1999, 63, 961–963. [Google Scholar]
- Yasumoto, T; Yotsu, M; Murata, M; Naoki, H. New tetrodotoxin analogue from the newt Cynops ensicauda. J Am Chem Soc 1988, 110, 2344–2345. [Google Scholar]
- Jang, JH; Yotsu-Yamashita, M. 6,11-DideoxyTTX from the puffer fish, Fugu pardalis. Toxicon 2007, 50, 947–951. [Google Scholar]
- Yotsu-Yamashita, M; Yamagishi, Y; Yasumoto, T. 5,6,11-Trideoxytetrodotoxin from the puffer fish, Fugu poecilonotus. Tetrahedron Lett 1995, 36, 9329–9332. [Google Scholar]
- Kao, CY; Furman, F. A Pharmacological studies on tetrodotoxin, a potent neurotoxin. J Pharmacol Exp Ther 1963, 140, 31–40. [Google Scholar]
- Shoji, Y; Yotsu-Yamashita, M; Miyazawa, T; Yasumoto, T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: liquid mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal Biochem 2001, 290, 10–17. [Google Scholar]
- Nakagawa, T; Jang, J; Yotsu-Yamashita, M. Hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal Biochem 2006, 352, 142–144. [Google Scholar]
- Jang, J; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987. [Google Scholar]
- Yasumoto, T; Michishita, T. Fluorometric determination of tetrodotoxin by high performance liquid chromatography. Agric Biol Chem 1985, 49, 3077–3080. [Google Scholar]
- Yotsu, M; Endo, A; Yasumoto, T. An improved tetrodotoxin analyzer. Agric Biol Chem 1989, 53, 895–898. [Google Scholar]
- Rodriguez, P; Alfonso, A; Vale, C; Alfonso, C; Vale, P; Tellez, A; Botana, LM. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal Chem 2008, 80, 5622–5629. [Google Scholar]
- Yasumoto, T; Yasumura, D; Yotsu, M; Michishita, T; Endo, A; Kotaki, Y. Bacterial production of tetrodotoxin and anhydrotetrodotoxin. Agric Biol Chem 1986, 50, 793–795. [Google Scholar]
- Noguchi, T; Jeo, J; Arakawa, O; Sugita, H; Deguchi, Y; Shida, Y; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. Isolated from the intestines of a xanthid crab, Atergatis floridus. J Biochem 1986, 99, 311–314. [Google Scholar]
- Yasumoto, T; Yotsu-Yamashita, M. Chemical and etiological studies on tetrodotoxin and its analogs. J Toxicol Toxin Rev 1996, 15, 81–90. [Google Scholar]
- Yotsu-Yamashita, M. Chemistry of puffer fish toxin. J Toxicol Toxin Rev 2001, 20, 51–66. [Google Scholar]
- Jang, JH; Yotsu-Yamashita, M. Tetrodotoxin and 5,6,11-trideoxytetrodotoxin in Tetraodon nigroviridis and T. biocellatus collected from Southeast Asia. Proceedings of the 5th World Fisheries Congress, Preliminary data of Tetraodon nigroviridis and Tetraodon biocellatus has been reported in the proceedings, Yokohama, Japan, 20–24 October 2008.
- Ryu, CH; Kim, DG; Kim, JH; Jang, JH; Lee, JS. Toxicity of the Grass puffer, Takifugu niphobles (Bogseom). J Korean Soc Food Sci Nutr 2003, 32, 986–990. (in Korean). [Google Scholar]
- Kono, M; Matsui, T; Furukawa, K; Yotsu-Yamashita, M; Yamamori, K. Accumulation of tetrodotoxin and 4,9-anhydrotetrodotoxin in cultured juvenile kusafugu Fugu niphobles by dietary administration of natural toxic komonfugu Fugu poecilonotus liver. Toxicon 2008, 51, 1269–1273. [Google Scholar]
- Ikeda, K; Emoto, Y; Tatsuno, R; Wang, JJ; Ngy, L; Taniyama, S; Takatani, T; Arakawa, O. Maturation-associated changes in toxicity of the pufferfish Takifugu poecilonotus. Toxicon 2010, 55, 289–297. [Google Scholar]
- Shin-Jung, L; Liao, CF; Arakawa, O; Noguchi, T; Hwang, DF. Toxicities of two freshwater puffers in Taiwan. J Nat Toxins 2002, 11, 103–110. [Google Scholar]
- Yamamori, K; Furukawa, K; Kono, M; Matsui, T. The toxification of juvenile cultured kusafugu Takifugu niphobles by oral administration of crystalline tetrodotoxin. J Food Hyg Soc Jpn 2004, 45, 73–75. (in Japanese). [Google Scholar]
- Kono, M; Matsui, T; Furukawa, K; Takase, T; Yamamori, K; Kaneda, H; Aoki, D; Jang, JH; Yotsu-Yamashita, M. Examination of transformation among tetrodotoxin and its analogs in the living cultured juvenile puffer fish, kusafugu, Fugu niphobles by intramuscular administration. Toxicon 2008, 52, 714–720. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).