Abstract
Bioassay-guided isolation from the ethanol extract of a marine sponge Theonella sp. collected in Palau yielded bistheonellide A, which strongly inhibited the colony formation of Chinese hamster V79 cells (EC50 = 6.8 nM). Bistheonellide A is an actin-polymerization inhibitor and was suggested to control cytokine production. Therefore, we attempted to detect an effect of this compound on IL-8 production in PMA-stimulated HL-60 cells. Interestingly, bistheonellide A did not modulate the production of IL-8 under cytotoxic concentrations as determined by LDH analysis. Although the correlation between the inhibition of microtubule assembly and the stimulation of IL-8 production has been observed for several compounds, the polymerization of actin was not related to an IL-8 production in the case of bistheonellide A. It will be suggested that the actin polymerization is not involved in the IL-8 production system.
1. Introduction
Interleukin-8 (IL-8) is a member of the superfamily of C-X-C chemokines and a chemotactic factor for T cells, neutrophils and basophils [1]. The expression of IL-8 has been observed in a variety of human cancers and is suggested to be a factor in tumor progression and metastasis [2–5]. Therefore, the regulation of IL-8 production is an important event for medical treatment.
In the course of our studies on biologically active metabolites of marine organisms, we have examined the extracts of marine sponges and ascidians collected in Palau and Indonesia for inhibitory activity against the colony formation of Chinese hamster V79 cells and found that the ethanol extract of a Palauan Theonella sp. revealed strong activity. Bioassay-guided isolation from the extract afforded bistheonellide A as the responsible component for the inhibitory activity.
Bistheonellide A has been isolated from Theonella sp. collected from Okinawa and Hachijo-jima in Japan [6] and inhibits the polymerization of actin, by which the toxic effects of this compound on viable cells are caused [7,8]. The progression of the cell cycle was apparently slowed down or completely inhibited at the G1 phase by treatment with bistheonellide A [8]. Therefore, we observed the effect of bistheonellide A on IL-8 production in PMA-stimulated HL-60 cells. This system has been established to reveal the relationship between the inhibition of microtubule polymerization and IL-8 production [in preparation].
We describe here the inhibitory activity towards the colony formation of V79 cells and the effects against PMA-stimulated HL-60 cells on IL-8 production, cell proliferation and the cytotoxicity of bistheonelide A.
2. Materials and Methods
2.1. Materials
Bistheonellide A was isolated from the ethanol extract of a marine sponge Theonella sp. collected in Palau, and the structure was assigned on the basis of its spectral data and comparison with the reported data [6]. The structure of bistheonellide A is shown in Figure 1. Dimethylsulfoxide (DMSO) was purchased from Pierce Chemical Co. (Rockfield, IL) and fetal bovine serum (FBS) was obtained from GIBCO after checking of the lot. All other reagents and chemicals used were of the highest commercial grade available.
Figure 1.
Structure of bistheonellide A.
2.2. Cell lines and culture conditions
The human promyelocytic cell line, HL-60, was obtained from the Japanese Cancer Research Resources Bank (JCRB, Kamiyoga, Tokyo, Japan). This cell line was maintained in tissue culture dishes in RPMI 1640 medium (Nissui Seiyaku, Tokyo, Japan) supplemented with 10% heat-inactivated fetal calf serum (FBS), 2 mM glutamine, 100 U/ml penicillin G and 100 μg/ml streptomycin.
Chinese hamster V79 cells were grown as a monolayer culture in Eagle’s MEM (Nissui Seiyaku Co., Ltd., Tokyo, Japan) with 10% heat-inactivated FBS.
2.3. Relative Plating Efficiency
The relative plating efficiencies against V79 cells in the presence of different concentrations of drugs were determined as the ratio of the number of colonies at a given drug concentration to that obtained in the control culture in the absence of any drug, as described in the previous paper [9]. Two hundred cells were seeded onto a 60/15-mm Petri dish in 4 ml MEM with 10% FBS and incubated overnight at 37°C, after which a sample in DMSO (4 μl) was added and further cultured for four days. The numbers of colonies in the sample dishes were counted and compared with those in the control cultures.
2.4. Detection of human IL-8 by ELISA
The IL-8 concentrations of the culture supernatants under control and various test conditions were measured by ELISA using a combination of monoclonal and polyclonal antibodies [10]. All samples were assayed at least in duplicate. Data are presented as the means ± SE of three independent experiments (Figure 2).
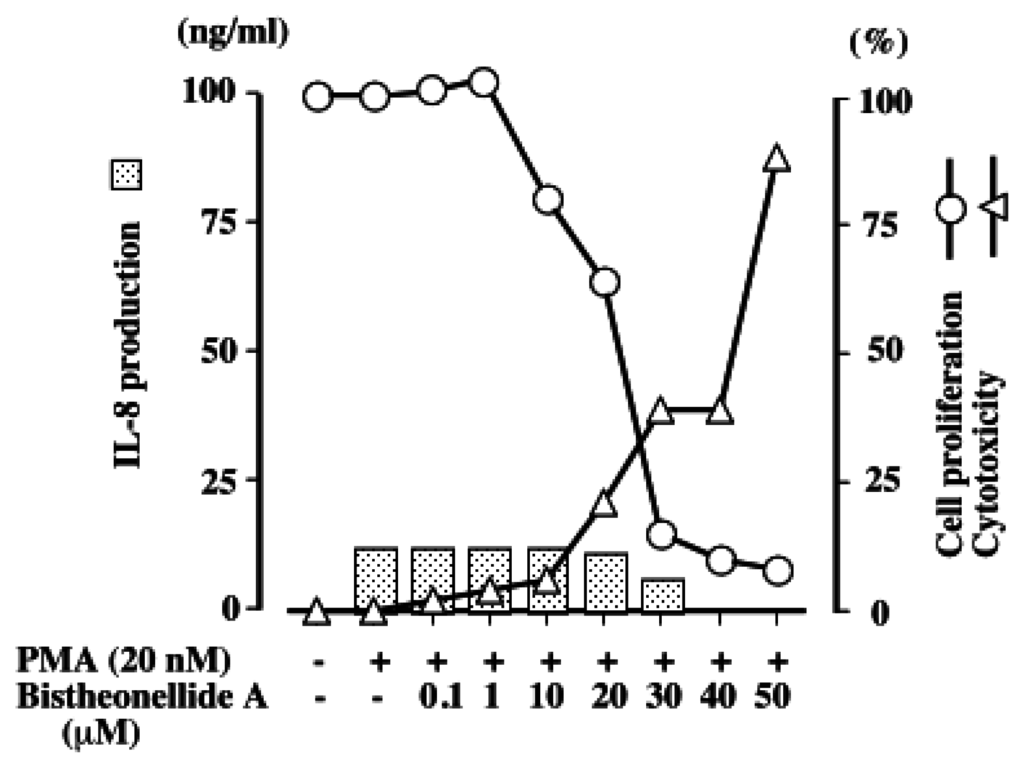
Figure 2.
Effects of bistheonellide A on IL-8 production, cell proliferation, and cytotoxicity in PMA-stimulated HL-60 cells. HL-60 cells (1 × 106 cells/ml) were treated for 24 h with PMA (20 nM) and the indicated concentration of the compound. The IL-8 concentration in the culture supernatant under each condition was determined by ELISA. The inhibitory activity on cell proliferation and cytotoxicity were determined as described in the Materials and Methods.
2.5. Determination of cell proliferation
Cell proliferation was evaluated by enumerating the viable cells using the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) formazan production method [11]. HL-60 cells (1 × 106 cells/ml) were treated with 20 nM of PMA (with or without test compounds), then transferred to 96-well microtiter plates. After a 24 h incubation, 20 μl of MTT reagent (5 mg/ml in PBS) was added to each well. After incubation for 3 h, the formazan production was assessed by measuring the optical density (OD570 nm). Data were obtained as values relative (%) to each PMA-stimulated optical density and are presented as the means ± SE of two independent experiments (Figure 2).
2.6. Determination of cytotoxicity
The lethality of HL-60 was estimated by measuring the lactate dehydrogenase (LDH) release [12]. Data were yielded as values relative (%) to the release of LDH from all cells at each optical density and are shown as the means ± SE of two independent experiments (Figure 2).
3. Results and Discussion
The inhibitor of the colony formation of V79 cells isolated from the Palauan Theonella sp. was bistheonellide A, which showed an EC50 value of 6.8 nM against V79 cells. This is the first time to report the inhibitory activity on the colony formation of V79 cells for this compound. Bistheonellide A has been reported to inhibit the polymerization of G-actin and also to depolymerize F-actin in a dose-dependent manner [13]. The biochemical studies on the mechanisms of cell cycle inhibition by bistheonellide A suggested that the treatment inhibits cytokinesis by controling cytokine production. Therefore, we investigated the effect of this compound on IL-8 production in PMA-stimulated HL-60 cells.
Essentially, IL-8 production is markedly induced by IL-1 and tumor necrosis factor (TNF) stimulation in inflammation, and information about its signal pathway has been clarified [14,15]. In HL-60 cells, PMA stimulation induces IL-8 production, although the amount is not high [16]. We have recently revealed that an inhibitor of the polymerization of microtubules, which form the actin cytoskeleton, induced marked IL-8 production in this condition [in preparation].
Figure 2 shows the IL-8 levels released from the PMA-stimulated HL-60 cells by the treatment with 0–50 μM of bistheonellide A together with the effects on cell proliferation and cytotoxicity. The left vertical axis in Figure 2 represents the results from the experiment of IL-8 production, and the right vertical axis shows the viable cell rate detected by the MTT method and cytotoxicity measured by the LDH method. The cell proliferation of HL-60 cells was inhibited in a dose-dependent manner by the actin polymerization inhibited dose (>1 μM). The cytotoxicity markedly increased from 20 μM. The IL-8 production induced by PMA stimulation was not affected by bistheonellide A at the concentrations up to 20 μM. The compound did not show apparent cytotoxicity at these concentrations. If bistheonellide A has the ability to modulate IL-8 production, the IL-8 level should have increased or decreased at 10 μM, the level at which the compound did not show cytotoxicity, but inhibited the cell proliferation.
Inhibitors of microtubule polymerization induced IL-8 production in the experimental system we used [in preparation]. However the actin-polymerization inhibitor, bistheonellide A, did not show such an effect. We have also examined an another actin-inhibitor, phenochalasin B [17], isolated from several strains of the marine sponge-derived fungi, Phomopsis spp., collected in Pohnpei, and found that IL-8 production was not affected by this compound (unpublished data). Recently, Etil et al. revealed that actin polymerization was not related to IL-8 production in the NF-κB system utilizing the YadA-promoted cell entry [18]. We have elucidated that the AP-1 transcription factor, but not NF-κB, is strongly involved in the IL-8 production by PMA-stimulating HL-60 cells [in preparation]. Therefore, it will be suggested that the actin polymerization is not involved in the induction of IL-8 production.
Acknowledgements
We thank Prof. Tadashi Kasahara for providing the IL-8 monoclonal antibody. This work was supported in part by Grant-in-Aid for Scientific Research on Priority Areas 17035029 from The Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan to M. N.
References
- Graves, D. T.; Jiang, Y. Molecular cloning and functional analysis of the promoter of the human squalene synthase gene. Crit. Rev. Oral Biol. Med 1995, 6, 109–118. [Google Scholar]
- Di Celle, P. F.; Carbone, A.; Marchis, D.; Zhou, D.; Sozzani, S.; Zupo, S.; Pini, M.; Mantovani, A.; Foa, R. Cytokine gene expression in B-cell chronic lymphocytic leukemia: evidence of constitutive interleukin-8 (IL-8) mRNA expression and secretion of biologically active IL-8 protein. Blood 1994, 84, 220–228. [Google Scholar]
- Green, A. R.; Green, V. L.; White, M. C.; Speirs, V. Expression of Cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int. J. Cancer 1997, 72, 937–941. [Google Scholar]
- Konig, B.; Steinbach, F.; Janocha, B.; Drynda, A.; Stumm, M.; Philipp, C.; Allhoff, E. P.; Konig, W. The differential expression of proinflammatory cytokines IL-6, IL-8 and TNF-alpha in renal cell carcinoma. Anticancer Res 1999, 19, 1519–1524. [Google Scholar]
- Galffy, G.; Mohammed, K. A.; Dowling, P. A.; Nasreen, N.; Ward, M. J.; Antony, V. B. Interleukin 8: an autocrine growth factor for malignant mesothelioma. Cancer Res 1999, 59, 367–371. [Google Scholar]
- Kato, Y.; Fusetani, N.; Matsunaga, S.; Hashimoto, K.; Sakai, R.; Higa, T.; Kashman, Y. Antitumor macrolides isolated from a marine sponge Theonella sp.: structure revision of misakinolide A. Tetrahedron Lett 1987, 28, 6225–6228. [Google Scholar]
- Watabe, S.; Wada, S.; Saito, S.; Matsunaga, S.; Fusetani, N.; Ozaki, H.; Karaki, H. Cellular changes of rat embryonic fibroblasts by an actin-polymerization inhibitor, bistheonellide A, from a marine sponge. Cell Struct. Funct 1996, 21, 199–212. [Google Scholar]
- Saito, S.; Karaki, H. A family of novel actin-inhibiting marine toxins. Clin. Exp. Pharmcol. Physiol 1996, 23, 743–746. [Google Scholar]
- Sato, Y.; Sakakibara, Y.; Oda, T.; Aizu-Yokota, E.; Ichinoseki, I. Effects of estradiol and ethynylestradiol on microtubule distribution in Chinese hamster V79 cells. Chem. Pharm. Bull 1992, 40, 182–184. [Google Scholar]
- Kasahara, T.; Oda, T.; Hatake, K.; Akiyama, M.; Mukaida, N.; Matsushima, K. Interleukin-8 and monocyte chemotactic protein-1 production by a human glioblastoma cell line, T98G in coculture with monocytes: involvement of monocyte-derived interleukin-1alpha. Eur. Cytokine Netw 1998, 9, 47–55. [Google Scholar]
- Carmichael, J.; DeGraff, W. G.; Gazdar, A. F.; Minna, J. D.; Mitchell, J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 1987, 47, 939–942. [Google Scholar]
- Shimizu, S.; Nomoto, M.; Naito, S.; Yamamoto, T.; Momose, K. Stimulation of nitric oxide synthase during oxidative endothelial cell injury. Biochem. Pharmacol. 1998, 55, 77–83. [Google Scholar]
- Saito, S. Y.; Watanabe, S.; Ozaki, H.; Kobayashi, M.; Suzuki, T.; Kobayashi, H.; Fusetani, N.; Karaki, H. Actin-depolymerizing effect of dimeric macrolides, bistheonellide A and swinholide A. J. Biochem 1998, 123, 571–578. [Google Scholar]
- Kasahara, T.; Mukaida, N.; Yamashita, K.; Yagisawa, H.; Akahoshi, T.; Matsushima, K. IL-1 and TNF-α induction of IL-8 and monocyte chemotactie and activating factor (MCAF) mRNA espression in a human astrocytoma cell line. Immunology 1991, 74, 60–67. [Google Scholar]
- Brasier, A. R.; Jamaluddin, R. M.; Casola, A.; Duan, W.; Shen, Q.; Garofalo, R. P. A promotor recruitment mechanism for tumor necrosis factor-a-induced interleukin-8 transcription in type II pulmonary epithelial cells. J. Biol. Chem 1996, 273, 3551–3561. [Google Scholar]
- Meier, R. W.; Niklaus, G.; Dewald, B.; Fey, M. F.; Tobler, A. Inhibition of the arachidonic acid pathway prevents induction of IL-8 mRNA by phorbol ester and changes the release of IL-8 from HL-60 cells: differential inhibition of induced expression of IL-8, TNF-alpha, IL-1 alpha, and IL-1 beta. J. Cell Physiol 1995, 165, 62–70. [Google Scholar]
- Tomoda, H.; Namatame, I.; Si, S.; Kawaguchi, K.; Masuma, R.; Namikoshi, M.; Omura, S. Phenochalasins, inhibitors of lipid droplet formation in mouse macrophages, produced by Phomopsis sp. FT-0211. J. Antibiot 1999, 52, 851–856. [Google Scholar]
- Etil, J.; Heise, T.; Dersch, P. Cell invasion and IL-8 production pathways initiated by YadA of Yersinia pseudotuberculosis require common signalling molecules (FAK, c-Src, Ras) and distinct cell factors. Cellular Micribiol 2005, 7, 63–77. [Google Scholar]
- Sample availability: not available.
© 2006 by MDPI Reproduction is permitted for noncommercial purposes.