Abstract
Conventional methods for extracting bioactive compounds from microalgae rely on organic solvents that are both polluting and potentially harmful to human health. In recent years, a noticeable shift has emerged toward greener extraction alternatives that are more environmentally friendly and sustainable. This review highlights various green extraction techniques, compounds, and yields obtained from different microalgal species for a range of applications and provides a comparison between the yields of conventional and green extraction methods. Green extraction methods have shown yields that are comparable to, or even exceed, those of conventional techniques, although they are predominantly studied for the extraction of lipids and pigments. This review aims to provide an overview of the current state of green extraction applied to microalgae, and to outline future research perspectives in this emerging field.
1. Introduction
Microalgae represent the most diverse group of photosynthetic eukaryotic organisms, thriving across a wide range of ecosystems, including freshwater, brackish, and marine environments [1]. Their cultivation is relatively simple compared to marine plants and other macroorganisms, and they exhibit rapid growth rates [2]. Microalgae are characterized by their remarkable biodiversity and their ability to grow under extreme environmental conditions, such as variations in temperature, salinity, pH, and light intensity [2]. This adaptability makes them excellent producers of high-value compounds [3]. Among microalgae, diatoms are a particularly notable group, comprising over 100,000 species [3]. As a major component of phytoplankton, diatoms contribute to approximately 50% of global oceanic primary production [4]. The diverse applications of diatoms span sectors such as biofuels, nutrition, biomolecular materials, and nanotechnology [5]. In addition, other groups of microalgae, such as green algae, flagellates, and dinoflagellates, are being explored for their biotechnological potential [6,7,8,9].
Microalgae have recently gained attention as potential sources of bioactive compounds derived from their primary and secondary metabolism [10]. These metabolites present promising applications across various sectors, including nutrition, biofuels, cosmetics, and pharmaceuticals [3,10].
The nutritional sector represents the foremost area for the commercialization of microalgae, given their richness in proteins, polyunsaturated fatty acids (PUFAs), pigments, vitamins, and minerals [11]. Microalgae can be consumed either in their raw form or as processed products [12]. Notably, species such as Limnospira (formerly Spirulina or Arthrospira) and Chlorella exemplify the nutritional potential of microalgae, contributing to a global market worth hundreds of millions of dollars [13]. Microalgae are most consumed in their raw form as dietary supplements, either in capsule form (e.g., Dunaliella by Terra&Vita, Organic Chlorella Tablets by Bulk, or Spirulina by Nature’s Way) or as powders (e.g., Chlorelle Poudre by Sol Semilla, Spirulina Powder by Bulk, Polvere di Alga Kelp Bio, or a kelp powder by ErbaVoglio).
Over the past two decades, the use of microalgae as a biodiesel source has gained significant interest [14]. Unlike terrestrial crops, which pose challenges such as inefficient land use, low lipid yield, and high water consumption, microalgae offer a more sustainable alternative [15]. Certain microalgal strains are capable of accumulating substantial lipid content within their cells, which can be converted into biodiesel [16] by thermochemical or biochemical conversion [17]. The microalgae Chlorella, Nannochloropsis, and Phaeodactylum contain between 20–50% lipids (as a % of dry weight biomass) and exhibit significantly higher productivity compared to lipid-rich species such as Botryococcus braunii [18]. However, to fully replace petroleum-derived liquid fuels with oil extracted from microalgae, advancements are required in algal biology through genetic and metabolic engineering, as well as in photobioreactor technology [16]. Photobioreactors are a critical tool for optimizing algal cultivation and enhancing biofuel production [19].
Numerous studies have demonstrated the antioxidant potential of bioactive compounds such as pigments, polyphenols, and vitamins derived from microalgae, making them a promising source for novel bioproducts, particularly in the cosmetic industry [20]. Due to their exposure to environmental stresses, such as UV radiation and high salinity, microalgae produce active compounds like chlorophyll and carotenoids, which are potential candidates for developing bioproducts to protect the skin from sun-induced damage [20]. For example, Chlorella microalgae extract has been shown to stimulate collagen synthesis, providing essential support for skin tissue and regeneration [21]. Additionally, compounds such as polysaccharides, hydroxy acids, and exopolysaccharides extracted from microalgae can act as effective moisturizers [20]. While the use of these bioactive compounds as active ingredients in cosmetics is becoming more widespread, their applications in the industry often remain limited to roles such as thickening, gelling, and antioxidant agents [22,23]. Various bioactive compounds derived from microalgae are found in cosmetic products, such as phycocyanin from Arthrospira platensis (Spiruderm® by AlgoSource), and exopolysaccharides (EPS) from Porphyridium cruentum (Exopolysaccharides–EPS de Porphyridium by AlgoSource). Additionally, Yves Rocher France offers a range of six products under the Pure Algue line by Cosmétique Végétale®, which utilizes raw extracts of Tetraselmis. Expanding the utilization of microalgae-derived compounds in cosmetics offers an opportunity to develop multifunctional and sustainable skincare products.
The use of microalgae in the pharmaceutical sector is still in its early stages but shows significant promise, particularly as most microalgae are recognized under the Generally Recognized as Safe (GRAS) status [24]. Species such as Dunaliella, Chlorella, and Spirulina are notable for their production of polyunsaturated fatty acids (PUFAs), which have demonstrated efficacy in the prevention or treatment of cardiovascular diseases [25]. For example, Arthrospira, due to its high γ-linolenic acid (GLA) content, reduces lipid levels in patients with hyperlipidemic nephrotic syndrome, thereby potentially contributing to an indirect reduction in cardiovascular risk [26]. Carotenoids and sterols from microalgae also demonstrated an ability in the prevention of cardiovascular diseases [27]. PUFAs derivatives, eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and (GLA) also show potential in managing type 2 diabetes, skin disorders, and asthma [25]. The following bioactive compounds—phenols, flavonoids, and sterols—extracted from Nannochloropsis sp. have demonstrated antidiabetic properties for type 2 diabetes by restricting α-glucosidase and α-amylase enzyme [28]. Pigments recovered from Porphyridium sp. showed potential for α-glucosidase inhibition and, hence, for antidiabetic activities [29]. The pigments potentially responsible for this inhibition include phycoerythrins, phycocyanins and allophycocyanins, which have been shown to exhibit enhanced production in media containing Fe3+ and Co2+, highlighting the need to optimize culture parameters [29]. Furthermore, polysaccharides derived from Chlorella vulgaris have exhibited significant antiviral activity [30] and immunomodulatory effects [31]. Triglycerides rich fraction of Skeletonema costatum and nucleosides rich fraction of S. dohrnii exhibited immunostimulant activity on human peripheral blood mononuclear cells (PBMCs) [32]. Bioactive compounds from microalgae are also being explored as potential anti-Alzheimer’s disease agents by inhibiting glutaminyl cyclase (QC), a key enzyme involved in the disease’s onset [33], astaxanthin microencapsulated in Spirulina has demonstrated neuroprotective benefits in rats [34]. Microalgae also possess antiproliferative properties, inducing cell cycle arrest and apoptosis, positioning them as promising candidates for anticancer drug development [35]. Cancer, which is not a single disease but rather a conglomerate of over 100 different types, remains one of the leading causes of mortality worldwide, claiming millions of lives annually [36]. Recent studies have highlighted the anticancer potential of Skeletonema marinoi, a eukaryotic photosynthetic diatom, with effects on lung cancer A549 and colon cancer COLO 205 tumor cells [3]. Similarly, an investigation of 32 microalgae species, including diatoms, dinoflagellates, and flagellates, identified diatoms as the only group exhibiting bioactivity, underscoring their capacity to produce bioactive compounds for therapeutic applications [3]. These findings reinforce the potential of microalgae as a rich source of bioactive compounds for pharmaceutical applications, particularly in the development of novel treatments for cancer and other diseases.
All the listed sectors above, among many others, rely on the extraction of bioactive compounds from microalgae. Currently, conventional extraction methods remain predominant. However, these traditional methods are often designed to isolate only a single class of molecules [37], which limits the diversity of compounds extracted and consequently reduces their potential applications. Conventional extraction methods have several drawbacks, including being time-consuming, labor-intensive, and exhibiting low selectivity and/or extraction yields [38]. Additionally, they typically require large quantities of organic solvents [38], and thus many hazardous residues [39]. In 2014, a total of 25.45 billion pounds of toxic chemical waste related to production activities was reported in the United States according to the Toxics Release Inventory [40]. These methods, such as Soxhlet extraction, Folch extraction, the Bligh and Dyer method, and maceration, not only pose significant environmental and health risks but also have considerable limitations in terms of sustainability [37]. The Folch and Bligh and Dyer methods are commonly used for lipid extraction, with Bligh and Dyer being less exhaustive, and therefore one of the most recommended methods for determining total lipid content [37,41]. Furthermore, at an industrial scale, conventional extraction processes can consume up to 50% of the total energy required for the entire production chain, further highlighting their low efficiency and environmental impact [42].
New extraction techniques are emerging that require little to no toxic solvents, thereby minimizing environmental impact [37,42]. These methods can not only reduce extraction time but also enhance the yield of bioactive compounds [39]. Among various industries, the cosmetic sector has been at the forefront of adopting greener alternatives. The solvents used in this field must comply with strict regulatory standards, leading to the gradual phase-out of hazardous solvents such as chloroform and phenol, which were previously employed for extract enrichment or metabolite isolation. These are now being replaced by green solvents, such as ethanol and water, particularly in cosmetics [43]. Green extraction, as defined by Chemat et al. [42] (p. 2), refers to “the discovery and design of extraction processes that reduce energy consumption, allow the use of alternative solvents and renewable natural products, and ensure a safe and high-quality extract/product” and contains six principles: (i) innovation by selection of varieties and use of renewable plant resources; (ii) use of alternative solvents and principally water or agro-solvents; (iii) reduce energy consumption by energy recovery and using innovative technologies; (iv) production of co-products instead of waste to include the bio- and agro-refining industry; (v) reduce unit operations and favor safe, robust, and controlled processes; and (vi) aim for a non-denatured and biodegradable extract without contaminants [42].
Previous reviews in the field considered only some of the techniques reported here, or only one technique, or they did not focus on the extracted compounds [37,39,42]. For this review, the keywords “green extraction” and “microalgae” were used to search publications on PubMed and Google Scholar. All publications from our literature research mentioning green techniques were included, without any time restriction. The current review focuses on green extraction techniques used for microalgae, comparing their efficiency and bioactive compound yields with those obtained through conventional extraction methods and critically discussing their strengths, weaknesses, opportunities, and threats.
2. Green Extraction Techniques
2.1. Supercritical Fluid Extraction (SFE)
Supercritical fluid extraction (SFE) using carbon dioxide (CO2) is a non-conventional extraction method that has demonstrated its efficiency in recovering high-value compounds from microalgae [44] since the 1970s [42]. SFE can enable higher extraction selectivity and shorter processing times at optimized conditions compared to conventional extraction methods [45]. Indeed, according to Nobre et al. [46], the extraction of lipids from Nannochloropsis sp. using Soxhlet reached 40.7% lipid recovery and 25.3% for the Bligh and Dyer method against 45% of lipid recovery using SFE-CO2 with the addition of ethanol as a co-solvent. The thermodynamic properties and heat transfer characteristics of CO2 make it the most widely used solvent for SFE. Additionally, the extraction of thermolabile molecules via SFE-CO2 prevents degradation or changes in the chemical composition of metabolites [45,47]. Moreover, CO2 is non-flammable, has low toxicity, is cost-effective compared to organic solvents [48], and is recognized as a GRAS solvent [49]. However, a major limitation of this technique is the non-polarity of CO2, which restricts its ability to extract polar compounds. To overcome this limitation, a co-solvent with opposite polarity, such as water, ethanol, or methanol, can be added [45]. Another limitation is the high initial investment cost, estimated at approximately USD 170,000 for an extraction vessel with a 500 mL capacity (Waters, Milford, MA, USA) [39]. Nevertheless, SFE is economically advantageous due to its closed-system design, allowing for CO2 and co-solvent recovery while preventing solvent residues in the final extract [45]. This technique is widely applied in various industries, including food, cosmetics, and pharmaceuticals [42].
SFE is based on the use of an extraction fluid maintained at supercritical conditions by applying pressure and temperature above its critical point (for CO2: 31.1 °C, 73.8 bar) [45,49]. The process typically begins with biomass pretreatment, either chemically or mechanically, to enhance metabolite recovery, such as fatty acids [50]. According to Esquivel-Hernández et al. [39] (p. 217) the general steps of the SFE process are as follows: (i) the biomass is loaded and absorbs the supercritical solvent; (ii) soluble compounds dissolve into the solvent; (iii) the dissolved compounds diffuse to the solid surface; (iv) the solvent transports the compounds and are removed from the extractor. Operating conditions, such as CO2 flow rate (g/min), extraction time (min), temperature (°C), and pressure (bar), vary depending on the target metabolites and biomass type [51]. Temperature and pressure play a crucial role in the SFE-CO2 process due to their combined effect on solvent density and solute vapor pressure [52].
SFE-CO2 enables the extraction of a wide range of bioactive compounds from freshwater and marine microalgae, generally of low molecular weight [42]. These include fatty acids such as saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), including eicosapentaenoic acid (EPA) docosahexaenoic acid (DHA), and γ-linolenic acid (GLA), diolefins, carotenoids such as β-carotene, fucoxanthin, and astaxanthin, phenolic compounds from various food waste and microalgae/cyanobacteria sources, terpenes (mono- and diterpenes), proteins, carbohydrates, and sterols [39,44,49,52,53,54,55].
2.2. Microwave-Assisted Extraction
Microwave-Assisted Extraction (MAE) is a non-conventional green extraction technique first introduced in 1986, which has gained significant interest in recent years due to its automation capability, short extraction times, and reduced use of organic solvents—requiring up to ten times less solvent compared to conventional methods. This reduction mitigates pollution, lowers production costs, and increases both the yield and quality of the extract [56,57,58,59]. According to Krishnan et al. [60], the extraction of lipids from Chlorella vulgaris using MAE, with a solution of ionic liquids as solvent, yielded 19.2% of lipids while with the conventional Bligh and Dyer method only 10.9% of lipids were yielded. MAE utilizes microwave energy to heat the solvent in contact with the sample of interest through ionic conduction and dipole rotation, facilitating the separation of bioactive compounds into the solvent phase [56,57]. The initial investment cost for a MAE vessel is lower than SFE or Pressurized Liquid Extraction (PLE), with an estimated cost of approximately USD 50,000 (CEM, Matthews, NC, USA) [39]. One of the main limitations of this technique is the dependency of microwave absorption on the solvent used, as heat generation by MAE varies according to the dielectric properties of the solvent [57]. Several solvent mechanisms can be employed in MAE: (i) a single solvent with strong microwave absorption, (ii) a combination of solvents with high and low dielectric losses (a measure of efficiency in converting microwave energy into heat), or (iii) a microwave-transparent solvent (which cannot be heated by microwaves) when extracting compounds from samples with high dielectric loss [57]. The extraction of microalgal compounds by MAE aligns with four of the six green extraction principles outlined by Chemat et al. and Bermúdez Menéndez et al. [42,61]. This technique is widely applied in the extraction of bioactive and nutraceutical compounds from microalgae, biodiesel recovery, and the isolation of compounds for the food industry [49,59,62].
According to Sparr Eskilsson and Björklund [57] (p. 232), the extraction process follows these steps: the sample is placed in the extraction vessel, and the solvent is added. Microwave radiation is then applied, initiating a pre-extraction phase to heat the solvent to the desired conditions. The sample is subsequently irradiated and extracted at the target temperature for a set duration (static extraction). Key parameters influencing MAE include the solvent/sample ratio, which directly affects the transfer of target metabolites (e.g., microalgal compounds) between the sample and the solvent. Temperature is another crucial factor, as it is directly influenced by microwave power, highlighting the importance of optimizing process parameters to minimize energy waste [39].
MAE enables the extraction of various bioactive compounds from freshwater and marine microalgae, particularly lipids such as essential oils and fatty acids (including PUFAs such as EPA and DHA), terpenoids (phytol and fucosterol), pigments (phycobiliproteins, phycoerythrin, carotenoids, and chlorophyll), phenolic compounds from soils, sediments, food by-products, and microalgae, as well as sulfated carbohydrates like fucoidans [39,49,57,63,64,65,66].
Although MAE is considered a green extraction technique, it still relies on small amounts of organic solvents such as hexane or chloroform. Future research should focus on the use of green solvents in MAE [39]. Additionally, while lipid extraction using MAE is well-documented, the extraction of other high-value compounds remains underexplored [67].
2.3. Pressurized Liquid Extraction (PLE)
Pressurized Liquid Extraction (PLE) is a more recent green extraction technique compared to SFE and MAE, emerging in 1995 [68]. In the literature, PLE is also referred to as Pressurized Solvent Extraction (PSE), Accelerated Solvent Extraction (ASE), or Enhanced Solvent Extraction (ESA) [39]. The principle of PLE involves applying high pressure (34 bar–204 bar) to maintain the solvent in its liquid state even at temperatures (20 °C–200 °C) above its boiling point [68,69]. PLE is characterized by short extraction times due to automation and the ability to process multiple samples simultaneously. A key advantage of this technique is its reduced consumption of organic solvents, as well as the possibility of using green solvents [70]. Additionally, the controlled solvent properties allow for higher extraction yields compared to conventional extraction [71]. A study from Cha et al. [71], showed promising results in the extraction of pigments from Chlorella vulgaris using PLE compared with Soxhlet and maceration extraction techniques. PLE with ethanol as a co-solvent yielded 3.78 mg/g of lutein and 9.63 mg/g of chlorophyll a, while Soxhlet yielded 3.42 mg/g of lutein and 3.32 mg/g of chlorophyll a, and 2.97 mg/g of lutein and 4.26 mg/g of chlorophyll a using maceration extraction. However, two main limitations of PLE include its tendency to produce non-selective extractions, highlighting the importance of solvent choice [72], and its high initial investment cost, estimated at approximately USD 140,000 (Thermo-Fisher, Waltham, MA, USA) [39]. PLE is primarily applied in the food, cosmetic, and pharmaceutical industries [68]. This technique is compatible with various solvents, making it a suitable option for green solvents such as limonene, which has been proposed as a replacement for hexane—a solvent with known environmental and toxicological concerns, particularly related to neuropathies [73]. Limonene has a dielectric constant similar to hexane [74] and is a by-product of the citrus fruit industry [75].
According to Waldebäck [68] (pp. 26–27) and Esquivel-Hernández et al. [39] (p. 223), the PLE process follows these steps: the matrix (e.g., microalgal dry biomass) is loaded into the extraction cell and filled with solvent. Temperature and pressure are then applied, with a pre-heating step necessary to desorb the target compounds from the matrix. This is followed by a static extraction phase, during which the compounds of interest diffuse into the matrix pores before being extracted. Multiple static extraction steps (one to five cycles) can be performed sequentially. After extraction, the cell is washed with fresh solvent, and pressurized nitrogen is injected to purge the solvent, allowing for the recovery of the extract. A crucial parameter in PLE is solvent selection, as extraction selectivity can only be achieved by adjusting the solvent composition or solvent mixtures according to the target compounds [68]. Additionally, temperature and extraction time are key factors influencing efficiency [76].
PLE enables the extraction of a wide range of bioactive compounds from freshwater and marine microalgae, including essential oils, pigments such as carotenoids (xanthophylls, β-carotene, lutein), chlorophyll a and b, phycobiliproteins, fatty acids such as saturated fatty acids, GLA, MUFAs, PUFAs such as EPA, polyphenols, and proteins [39,77,78,79,80,81,82,83,84].
2.4. Ultrasound-Assisted Extraction (UAE)
Ultrasound-Assisted Extraction (UAE) is a widely developed green extraction technique in the food industry [85], and is increasingly being adopted in other sectors. This method provides high purity of the final product and high extraction yields, making it more economical and eco-friendlier than conventional techniques. In the study of Zhao et al. [86], the use of UAE as a green extraction technique with cultivation media as a solvent yielded 36.85 g/100 g of carbohydrates while only 9.06 g/100 g of carbohydrates were yielded by solvent extraction from Chlorella sp. UAE significantly reduces solvent consumption—in some cases, no solvent was used—and shortens extraction time [81,85]. This technique appears to be well-suited for industrial-scale up, given its low investment cost and the possibility of modifying existing ultrasound extraction reactors already used in the food and chemical industries [85]. The principle of UAE relies on the propagation of ultrasound waves through the solvent, creating cavitation phenomena. This phenomenon disrupts cell walls, allowing the extraction of target compounds [87], depending on their solubility in the solvent [88]. Ultrasound application can also be used as a pretreatment, enhancing cell membrane permeability, either alone or in combination with other green extraction techniques, such as MAE [89].
According to Usman et al. [90] (p. 10) UAE process consists of the following steps: the biological matrix is suspended in a solvent, and a probe transmits ultrasound waves at a selected frequency (>20 kHz) and power for a defined extraction time. Several studies indicate that lower temperatures are preferable to prevent degradation of thermolabile bioactive compounds and improving yield recovery [88,89].
One of the limiting factors of this technique is the choice of solvent. The use of toxic solvents does not necessarily comply with green chemistry principles, and further research is needed to explore the application of UAE with green solvents [91]. However, commonly used solvents include acidified water, alcohols, acetone, hexane, and water [81,90]. While acetone is flammable and irritating, hexane has negative environmental and toxicological effects [83]. The selection of a solvent depends on the solubility of the target bioactive compounds as well as viscosity, surface tension, and vapor pressure of the solvent [88], physical parameters that can be affected by the applied temperature, and thereby the cavitation phenomena.
UAE technique enables the extraction of high-value compounds from marine and freshwater microalgae, including polyphenols, sterols, chlorophylls, carotenoids such as lutein, lipids such as PUFAs, MUFAs, and SFAs, and polysaccharides [81,85,91,92,93].
2.5. Subcritical Water Extraction (SWE)
Subcritical water extraction (SWE) is one of the green extraction techniques developed for the recovery of high-value bioactive compounds. SWE is applied in the food, pharmaceutical and bioenergy sectors for compounds extraction [94,95] and is well documented for plant extraction (excluding microalgae). This technique offers higher selectivity, shorter extraction times, and eliminates the need for toxic organic solvents, as it uses water as solvent [48]. Consequently, SWE has gained increasing attention due to its safe utilization, and eco-friendly nature [94]. In the literature, SWE can be found under different names, such as Pressurized Hot Water Extraction (PHWE) or SuperHeated Water Extraction (SHWE) [96]. This extraction technique uses water as a solvent and applies temperature and pressure, following a principle like PLE (Section 2.3). The water is maintained at a temperature between its boiling point and critical point (100 °C–374 °C) under pressure (10 bar–221 bar), ensuring it remains in a liquid state while enhancing the solubility of target compounds for extraction [76,94]. SWE offers several advantages but also has limitations. Due to the use of high temperatures, this technique may not be suitable for thermolabile compounds [94]. Additionally, subcritical water can induce oxidation of certain compounds. To mitigate this issue, water degassing through sonication or purging with dinitrogen (N2) can be implemented [94].
The SWE process consists of the following steps: (i) The matrix and solvent are either loaded into the extraction cell (static extraction) or pumped into the extraction cell (dynamic extraction). (ii) Extraction begins once the oven reaches the desired temperature and pressure is applied. (iii) After the extraction time, the extract is collected [48,76,94,95,96]. The metabolites are diffused to the surface of the matrix (e.g., microalgae), are dissolved in the water solvent and are eluted through the extraction cell [96]. The main factor influencing SWE is temperature. Subcritical water exhibits high diffusivity, low viscosity, and low surface tension, which reduces its dielectric constant and enhances the solubility of target bioactive compounds in water [76,94]. Therefore, it is essential to optimize the applied temperature based on the metabolites of interest. In contrast, pressure does not directly affect metabolite recovery but is necessary to maintain water in its liquid state during the extraction process [76].
Compounds from microalgae extracted by SWE are phenols, proteins, polysaccharides, amino acids such as glutamic acid, glucose, carbohydrates breakdown such as (di-D)-fructose anhydrides (DFAs) and lipids, and EPA from marine and freshwater microalgae [97,98,99,100,101,102].
2.6. Enzyme-Assisted Extraction (EAE)
The composition of the microalgal cell wall depends on the taxonomic group to which they belong. Consequently, the recovery of bioactive compounds is highly dependent on cell wall disruption. Techniques such as microwave or ultrasonic waves are commonly used for this purpose, but they involve high costs [103]. The use of enzymatic hydrolysis for cell wall disruption and subsequent extraction of bioactive compounds presents an economically attractive alternative, enabling high product yields and high-quality recovery [103,104] at laboratory scale [105]. The choice of enzyme or enzymes mixture—leading to higher yields [106]—depends on the enzyme’s specificity and region-selectivity towards the target compounds while preserving their properties [104,107]. EAE, when combined with other green extraction techniques, could further enhance the extraction efficiency of compounds from the microalgal matrix [108,109]. Depending on the composition of the microalgal cell wall and the target compounds, different enzymes can be used. Table 1 summarizes the various enzymes employed based on the microalgal species and the extracted compounds.

Table 1.
Enzymes used in EAE on microalgae.
The EAE process follows these steps: a microalgal paste is mixed with a specific enzyme or enzyme mixture in a solution at an active pH, placed in a centrifuge tube, and incubated in a water bath or a thermostated system while being magnetically stirred for the required extraction time [103,106,110]. EAE offers several advantages, including operation at low temperatures, the possibility of using green solvents, and a short extraction time. However, it also presents limitations, as it remains underexplored in microalgal research, particularly regarding the use of enzymes mixture in EAE [113], while in terrestrial plant research the use of enzymes mixtures for EAE is well documented [113].
Additionally, EAE requires precise control over parameters such as pH and temperature, which can affect the extraction efficiency of bioactive compounds. Therefore, optimization of these parameters is crucial [104,108].
EAE is widely used for lipid extraction from marine, brackish, and fresh water microalgae [103,106,110,113]. It is also applied in protein hydrolysis to produce peptides [114], and in the recovery of carotenoids [111].
2.7. Green Solvents Extraction
In addition to green extraction techniques, green solvents can be used for the extraction of bioactive compounds. These include agro- or bio-solvents, which are potential alternatives to conventional organic solvents [42]. Green solvents are biodegradable, non-toxic, and non-flammable, sometimes derived from natural sources [42]. They can be used either alone or in combination with extraction techniques, although research in the field of solvent screening remains limited [115]. Extraction using a green solvent can be performed at room temperature by soaking the matrix for the desired extraction time [116,117,118,119]. In Prat et al. [120], all the solvents presented in this section are well classified and recommended as green solvents.
Ethanol is considered a bio-solvent when derived from the fermentation of natural sugar-rich materials, despite its flammability [42]. However, ethanol can also be synthesized petrochemically, in which case it should no longer be qualified as a bio-solvent. Ethanol is inexpensive and has a high affinity for lipid extraction, often achieving yields comparable to or higher than those obtained using organic solvents [118]. Ethyl acetate (EtOAc) is another green solvent with minimal impact on human health [115]. 2-Methyltetrahydrofuran (2-MeTHF), produced from renewable resources, exhibits extraction efficiency nearly identical to that of chloroform [115]. Terpenes, such as limonene, offer a promising alternative to organic solvents due to their natural origin (citrus fruits), low cost, and low toxicity [121]. Additionally, its extraction yields are very similar to those obtained with hexane, as its dielectric constant is close to that of hexane [83]. Water, at ambient temperature and pressure, is a highly polar solvent. However, by modifying its temperature and pressure, its dielectric constant can be reduced to facilitate the extraction of water-soluble compounds such as proteins, sugars, and organic acids [42]. Its use is well-documented in subcritical water extraction (SWE), as discussed in Section 2.5, and it is considered the safest, least expensive, and non-toxic solvent [79]. Ionic liquids (ILs) are also considered an alternative to organic solvents due to their non-volatile and non-flammable nature, classifying them as green solvents [122]. However, in some cases, ILs exhibit a greater life cycle environmental impact compared to conventional organic solvents [37,123]. Another emerging alternative to both ILs and organic solvents is switchable solvents (SSs). These are non-volatile liquids that can transition from a hydrophobic to a hydrophilic state in response to changes in temperature, pH, or the addition/removal of a gas [37,124], making SSs particularly promising for the extraction of both polar and non-polar compounds. Deep eutectic solvents (DESs) have emerged as alternatives to conventional organic solvents, exhibiting simple preparation, low toxicity and high biodegradability, but studies have mostly focused on the extraction of lipids and carotenoids from microalgae [125]. DESs are also promising green alternatives for the pretreatments of microalgal biomass, which is a crucial step in the extraction of bioactive compounds by rupturing the cell wall [125]. DESs consist of a mixture of two or more solid components (hydrogen band acceptors such as choline chloride and hydrogen band donors such as urea) allowing a melting point at least 100 °C lower than the starting materials. DESs are found to be cheaper than ILs, which emphasize their use in the industry and the interest by the scientific community [126]. Natural deep eutectic solvents (NaDESs) are also highly regarded due to their profusely availability in nature, and renewable properties, consisting of cholinium chloride, natural carboxylic acids, sugars, and amino acids [126,127]. The main limitation of DESs and NaDESs is the choice of the pretreatment that needs to maximize the recovery of the bioactive compounds [127]. A fluorous solvent (FS) such as perfluorocyclohexane is a green solvent mostly used in the extraction of lipids from microalgal biomass. FSs exhibit several advantages: FSs are inert in nature, non-toxic, and facilitate phase separation as they consist of an aqueous phase and a non-aqueous phase (rich in fluor). However, their main limitations are the dependance to temperature, with which their density and viscosity can change [59]. Thermomorphic systems (TMSs) consist of different components (e.g., water, buffers, ILs, DESs) that can be altered via the temperature, therefore changing the miscibility of the solution and allowing the recovery of polar bioactive compounds in the aqueous phase and non-polar compounds in the non-aqueous phase [128]. However, to our knowledge, TMSs have not yet been tested to extract compounds from microalgal biomass.
These solvents enable the extraction of lipids and carotenoids from marine and freshwater microalgae species [59,83,115,117,118,121,125]. However, further research is needed to better characterize the extracted compounds and expand the range of target compounds.
3. Green Extraction Techniques Compared with Conventional Techniques
Conventional extraction methods rely on organic solvents such as methanol, chloroform, and hexane, which pose several challenges. At the pilot or industrial scale, these solvents require high energy consumption, are flammable, environmentally unfriendly, and can be hazardous to human health [37]. In Prat et al. [120], hexane and chloroform are classified as highly hazardous, with concerning environmental and health scores. Nevertheless, they are still widely used for extracting bioactive compounds from microalgae across various application sectors.
Among conventional extraction techniques, the Folch method is the most used for lipid extraction from microalgae, followed closely by the Bligh and Dyer method [37]. Both techniques involve simple procedures but rely on hazardous solvent mixtures (chloroform/methanol and chloroform/methanol/water, respectively). Soxhlet extraction, on the other hand, requires a long extraction time, leading to high energy consumption, and is often used with organic solvents for the extraction of lipids and carotenoids [37,129].
In Table 2, green extraction methods, compared to conventional techniques, show promising potential, often achieving higher yields. The mass of microalgae used varies, whether dry or wet (column biomass in Table 2), further complicating an effective comparison. The yields were calculated based on the biomass used, then reported in mg/g of dry biomass, in g/100 g of dry biomass, or % of dry biomass, complicating an efficient comparison due to a lack of standardization in the report of results.

Table 2.
Comparison of yields obtained by conventional extraction techniques and green extraction techniques.
Green extraction techniques are emerging as sustainable methods in the recovery of bioactive compounds, prioritizing sustainability (use of renewable biomass, alternative solvents, production of co-product), safety (final extract without contaminants), and efficiency, aligning with the six principles of green extraction of natural products. Figure 1 aims to highlight the positioning of green extraction techniques. The SWOT (strengths, weaknesses, opportunities, and threats) assessment highlights the potential of green extraction techniques as eco-friendly and sustainable alternatives to conventional methods. Their strengths in sustainability, bioactivity improvement, and global initiatives are offset by economic and scalability challenges. However, technological innovations and rising demand for sustainable products by consumers are driving their adoption. Addressing the weaknesses in scalability and market applications could accelerate their integration at industrial scale by reducing operation costs through the optimization of the green extraction processes and training more professionals.
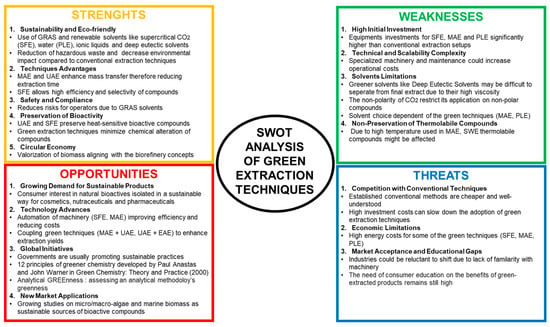
Figure 1.
SWOT (strengths, weaknesses, opportunities, and threats) of green extraction techniques.
4. Compounds Extracted from Microalgae with Green Extraction Techniques
Studies on microalgae extraction by using green extraction techniques have been reported for both freshwater and marine species, with a total of 19 species of microalgae studied, of which 12 marine and 7 freshwaters. Main data are available on the marine species Chlorella sp. and Nannochloropsis sp., and the freshwater species Arthrospira platensis.
Primary bioactive compounds extracted were lipids and pigments, respectively extracted for biodiesel production and the sectors of food and pharmaceutics. The three most studied species, i.e., Chlorella sp., Nannochloropsis sp., and Arthrospira platensis, have all been subjected to each of the green extraction techniques discussed in this review. Depending on the extraction technique and the microalgal species, the compounds extracted may vary. Lipids are predominantly extracted across all extraction methods for Nannochloropsis sp., whereas for Chlorella sp., both pigments and lipids are extracted in roughly equal proportions, depending on the technique used, highlighting the selective nature of certain green extraction methods regarding the compounds targeted. For Arthrospira platensis, pigments are the most commonly extracted compounds across all techniques.
Even if the yield units reported are sometimes different (e.g., % or grams), it seems that higher yields of lipids and pigments have been obtained by SFE-CO2 and PLE extraction. Moreover, yield from PLE can be obtained by combining the technique with green solvents, making it a highly promising technique. SWE is primarily used for the extraction of proteins and carbohydrates, for which it provides better yields compared to other green extraction techniques. Detailed information is reported in Table 3.

Table 3.
Compounds extracted from microalgae with green extraction techniques.
5. Discussion
The extraction methods reviewed in this paper present specific advantages and limitations. Conventional extraction techniques remain widely used within the scientific community due to their simplicity, relatively high yields, and established practices. However, growing environmental concerns are driving a shift towards alternative methods that minimize or eliminate the use of organic solvents. Among these, SFE-CO2 is a promising green alternative that enables selective extractions but requires a high initial investment. MAE, UAE, and PLE significantly reduce solvent consumption, but their efficiency is dependent on solvent choice, which varies depending on the target bioactive compounds. SWE has gained considerable attention due to its environmentally friendly approach, using water as a solvent, but it is not recommended for thermolabile molecules. Conversely, EAE is a suitable alternative for thermolabile compounds but relies on the effective disruption of the microalgal cell wall.
In the process of discovering marine natural product drugs, once the bioactive compounds are identified and their structure characterized, the material can either be used as-is or modified. In either case, the compound must be obtained in larger quantities, requiring a scale-up process to potentially supply enough material for clinical trials [148]. Few, if any, studies have been conducted on the scale-up of green extraction methods for microalgae due to non-availability of proper optimized production systems, proper microalgal strains, and optimal growth parameters for enhanced biomass yields [25,149], though this has been widely documented for terrestrial plants [150,151,152,153,154,155]. Large-scale investigations for terrestrial plants have shown that UAE is one of the best non-thermal green extraction techniques, and MAE shows promising results, though few studies have been conducted [152]. SFE-CO2 is commonly used in laboratory-scale extraction and optimization of compounds from terrestrial plants, and it demonstrates higher to similar yields in oil extraction at pilot-scales [152]; for example, the extraction of oil from seed rape reached 100.3 g per kWh at pilot-scale (extractor of 16 L against less than 1 L at laboratory scale) close to the oil recovery reached at laboratory scale, 97.4 g per kWh. While these findings could be applicable to microalgae, a major limitation is the amount of biomass required for these extractions. Working with unicellular organisms requires greater biomass production than working with terrestrial plants. Moreover, the utilization of SFE, particularly in the production of biodiesel from microalgal biomass, requires high investment costs which can cool down industries from investing and large amounts of biomass to potentially be able to be marketed. According to Tabernero et al., 2012 [156], the production of 10,000 ton/year of biodiesel from heterotrophic microalgae would not be viable due to the excessive number of photobioreactors required and the loss of oil in the extraction process, emphasizing the need for the optimization of green extraction techniques and microalgal biomass production to reach fully commercialization. Another limitation in the production of bioactive compounds from microalgae is the cost of the total production process. According to Guedes et al. [149], natural astaxanthin from Haematococcus is more expensive to produce than synthetic astaxanthin (USD 1000/kg). To be as competitive as synthetic astaxanthin, natural astaxanthin should be produced at less than USD 30/kg, which is not the case even with the current technologies.
One of the major limitations in comparing the yields of bioactive compounds from microalgae across different extraction techniques is the non-standardized use of units for reporting results. In this review, yields are expressed in various ways, such as percentage of dry weight, grams of extract per 100 g of dry biomass, and percentage of recovery, among others, making effective comparison challenging. Additionally, the amount of microalgal biomass used per extraction technique is another factor influencing yield outcomes. This parameter varies significantly between studies, ranging from a few milligrams to several grams, and is not standardized for each microalgal species. Not only does biomass quantity and moisture content affect yield, but it is also influenced by the microalgal species, cultivation conditions, and pretreatment methods often applied to enhance extraction efficiency, and thus biological activity. For instance, diatoms are frequently subjected to pretreatments due to their frustule, a silica shell encasing the cell, to optimize the extraction of bioactive compounds [157,158]. However, the use of pretreatments (e.g., ultrasound, freeze-drying, enzymatic digestion) may introduce additional economic and environmental costs, such as increased energy consumption and the use of organic solvents.
Green extractions of bioactive compounds from microalgae are influenced by upstream conditioning and biomass stabilization. Indeed, the optimization of upstream conditions (growth optimization, harvesting, and pretreatment) and biomass stabilization will influence the yield and efficiency of the bioactive compounds [159,160].
The type of bioactive compounds and their yield is influenced by the growth conditions [161]. Microalgae can be classified as autotrophs, mixotrophs and heterotrophs according to the source of carbon they require therefore influencing the bioactive compounds produced. Indeed, in heterotrophic condition, literature showed a higher lipid production (four times higher) compared to autotroph conditions [162]. Other growth factors, such as light, nutrient, salinity, and temperature, influence the growth of the microalgae, and therefore the production of bioactive compounds [163].
The harvesting part of the upstream conditions counts for 20–30% of the microalgal biomass cost, emphasizing the need to understand which harvesting technique is more suitable for high-valued metabolites [164]. Biomass harvesting is also dependent of the microalgal species; for example, diatoms are usually harvested at the end of their stationary phase where it has been shown to yield more secondary metabolites [161,165]. For biodiesel production from microalgae, coagulation and flocculation seem to be the more suitable methods [159,164], while for high-valued products from microalgae (e.g., pigments), centrifugation followed by coagulation and flocculation are the more suitable harvesting techniques [164]. The pretreatment of the algal biomass (e.g., ultrasound, bead milling, hydrolysis) is crucial in the disturbance of the cell wall for an effective release of the compounds and is dependent on the robustness of the specific cell wall of microalgae species [127,166,167]. The pretreatments often use before the extraction with NaDESs are MAE, UAE, and EAE due to their economical, efficient, and fast advantages, achieving up to 90% of recovery [127]. However, according to the extraction use, some techniques do not necessarily need pretreatment, such as UAE or MAE (Table 3), because the microwave and ultrasound energy are enough to disturb the cell wall and achieve an efficient recovery of compounds.
To prevent deterioration and the quality of the bioactive compounds, stabilization is a crucial step. However, the stabilization methods employed can affect the extraction yields, by being compound-specific, and properties of the compounds highlighting the importance to understand the effect of stabilization method on the extraction techniques [160]. The stabilization of microalgae biomass can be classified in three methods: physical, chemical, and biological. Physical stabilization are diverse such as drying methods lowering the enzymatic activities of the biomass and preventing microbial growth [160]. Ultrasound is also known to be a non-thermal physical stabilization method by damaging the microbial cell walls therefore preventing microbial growth [160]. Chemical stabilization methods also prevent microbial growth and limit enzymatic activities by adding acid/base solutions to the microalgae biomass to lower or increase its pH [160]. Biological stabilization such as fermentation or the addition of enzymes are also techniques used in the stabilization of the biomass [160].
Despite the difficulty in comparing the extracted compounds, particularly in Table 3, pigments and lipids are the most extracted compounds. Pigments are primarily extracted for applications in the cosmetic, nutrition, and pharmaceutical sectors, while lipids are mainly used for biofuel production. Chlorella sp., Nannochloropsis sp., and Arthrospira platensis are the most studied microalgae in this review. Indeed Chlorella sp., Nannochloropsis sp., and Arthrospira sp. are the most studied species in literature for the production of bioactive compounds [25]. While over 200,000 microalgae species have been discovered, only a few are used for industrial applications [168]. The main reason is safety regulations, as compounds extracted from untested species have to overcome socio-ethnological and toxicity-related issues before possible market applications [25]. Chlorella has been extensively studied for the extraction of both lipids and pigments. This is largely due to its exceptionally high chlorophyll content, one of the highest found in nature [169,170], as well as its classification among unicellular green microalgae with a high lipid content [171,172], supporting its frequent evaluation for lipid and pigment extraction in this review. Chlorella is valued for its versatility in cultivation, as it can grow under both autotrophic and heterotrophic conditions, similar to Arthrospira platensis, making it an ideal candidate for large-scale production with reduced contamination risks and higher productivity [173]. UAE is one of the most commonly used methods for Chlorella, primarily due to its multi-layered cell wall, which requires efficient disruption to release intracellular compounds [166,174] while preserving potentially thermolabile bioactive compounds. Nannochloropsis has been extensively studied for its high lipid content, which ranges from 30% to 60% of its dry weight, as well as for its fatty acid composition, which is particularly relevant for biodiesel production [175]. It is important to highlight that the use of microalgae in biodiesel production must be optimized to ensure both economic and environmental sustainability, with particular emphasis on the green extraction techniques employed for lipid recovery from Nannochloropsis sp. In this review, various green extraction methods have been applied, showing promising results in terms of lipid and fatty acid yields. However, comparison between green and conventional techniques and yield among green techniques remains challenging due to inconsistencies in reporting units and the biomass loading used in different studies. Furthermore, pretreatment is commonly required for lipid extraction from Nannochloropsis due to its robust cell wall. The coupling of green extraction techniques with environmentally friendly cell disruption methods, such as ultrasound, has demonstrated increased lipid yields, supporting the development of more sustainable processes for biodiesel production from Nannochloropsis sp. [167]. Finally, Arthrospira platensis is primarily studied for its applications in the food and pharmaceutical sectors due to its production of secondary metabolites such as phycocyanin, a blue pigment, which has demonstrated nutritional value when included in the diet, along with a wide range of medical effects including antioxidant, antidiabetic, neuroprotective, and antimicrobial properties [176]. This review highlights the extraction of primarily pigments from Arthrospira platensis. As pigments are known to be thermolabile, green extraction techniques that avoid high temperatures are preferred, such UAE, PLE, or EAE, which have shown to be efficient for pigment recovery in Table 3 [177]. Since Arthrospira platensis is a microalga that produces several valuable metabolites, it is also worth exploring techniques that allow for the efficient extraction of lipids, pigments, and vitamins at the same time, such as SFE-CO2 and MAE [138].
Initiatives in the use of greenness assessment criteria to determine whether an analytical method is more or less sustainable have been developed, such as the Analytical GREEnness calculator [178]. This tool takes the 12 criteria of green analytical chemistry and transforms them in order to give a score between 0–1 to minimize environmental impact in laboratory processes. Analytical GREEnness is a metric system that is comprehensive, flexible (possibility to assign weights), and easy to interpret and perform.
6. Conclusions
In this review, green extraction techniques generally yield higher results compared to conventional methods. In rare cases, where conventional extraction achieves slightly better yields, the difference does not exceed 3% differences (Table 2). This highlights the potential of green extraction methods for recovering bioactive compounds for applications across various sectors. Among the studies analyzed, the most extracted compounds using green extraction techniques are lipids, which are predominantly obtained through SFE-CO2 for biofuel production, and many works focus on comparing green and conventional methods for lipid extraction from microalgae. While green extraction methods for pigments, carbohydrates, and proteins are under development and are relatively well-documented, they remain less extensively studied compared to lipid extraction for biofuel production from microalgae. Further investigation is needed to compare the extraction of other bioactive compounds such as vitamins, carbohydrates, and proteins. Depending on the green extraction technique employed, both the yield and the composition of bioactive compounds of the extracts vary. In this review, lipids and pigments are the most frequently extracted bioactive compounds across all the techniques discussed. The highest yields of both lipids and pigments are obtained using SFE-CO2, MAE, and PLE. These yields, however, are highly dependent on the quantity and characteristics of the microalgal biomass used. The choice of extraction techniques will always be dependent on the objectives and conditions: biomass cell specificity, biomass availability, choice of solvent, targeted compounds, type of industry application, and economic valorization goal. For future research perspectives, the optimization of green extraction methods is necessary, particularly in the use of green solvents in already green techniques to maximize the yields of bioactive compounds. Green extraction methods show promising results at the laboratory scale; however, scaling up to an industrial level remains limited due to high investment and production costs. Compared to conventional extraction methods, green extractions exhibit a lower environmental impact. However, further research on the overall production process, including analyses of carbon footprint, water and energy consumption, and waste management, is an important research perspective to ensure the sustainability of these emerging green technologies. This review highlights the need to further explore green extraction techniques of bioactive compounds from microalgae trying to reduce the production costs since they promise more advantageous results in terms of yield but also in terms of sustainability than conventional methods.
Author Contributions
Conceptualization, G.K. and C.L.; writing—original draft preparation, G.K. and C.L.; writing—review and editing, G.K. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was conducted as part of the thesis IMBRSea Master’s program (UGent). Some graphical elements used in the graphical abstract were retrieved and adapted from Servier Medical Art (https://smart.servier.com/; accessed on 14 April 2025), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), and from Jane Thomas and Jane Hawkey, from Integration and Application Network (ian.umces.edu/media-library; accessed on 25 June 2025). The IAN/UMCES and Image Libraries are licensed under Attribution-ShareAlike 4.0 International (CC BY-SA 4.0). The graphical abstract was created using Canva (https://www.canva.com/; accessed on 14 April 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fu, W.; Nelson, D.R.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive Compounds from Microalgae: Current Development and Prospects. Stud. Nat. Prod. Chem. 2017, 54, 199–225. [Google Scholar]
- Saide, A.; Riccio, G.; Ianora, A.; Lauritano, C. The Diatom Cylindrotheca closterium and the Chlorophyll Breakdown Product Pheophorbide a for Photodynamic Therapy Applications. Appl. Sci. 2023, 13, 2590. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Nelson, D.M.; Tréguer, P.; Brzezinski, M.A.; Leynaert, A.; Quéguiner, B. Production and Dissolution of Biogenic Silica in the Ocean: Revised Global Estimates, Comparison with Regional Data and Relationship to Biogenic Sedimentation. Glob. Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Bozarth, A.; Maier, U.-G.; Zauner, S. Diatoms in Biotechnology: Modern Tools and Applications. Appl. Microbiol. Biotechnol. 2009, 82, 195–201. [Google Scholar] [CrossRef]
- MacKinnon, S.L.; Cembella, A.D.; Burton, I.W.; Lewis, N.; LeBlanc, P.; Walter, J.A. Biosynthesis of 13-Desmethyl Spirolide C by the Dinoflagellate Alexandrium ostenfeldii. J. Org. Chem. 2006, 71, 8724–8731. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, G.M.H.; Prinsep, R.M. Marine Natural Products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; Ko, J.-Y.; Shah, M.M.R.; Lee, J.-H.; Kang, M.-C.; Kwon, O.-N.; Lee, J.-B.; Jeon, Y.-J. In Vitro Studies of Anti-Inflammatory and Anticancer Activities of Organic Solvent Extracts from Cultured Marine Microalgae. ALGAE 2013, 28, 111–119. [Google Scholar] [CrossRef]
- Kobayashi, J. Amphidinolides and Its Related Macrolides from Marine Dinoflagellates. J. Antibiot. 2008, 61, 271–284. [Google Scholar] [CrossRef]
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the Health Potential of Microalgae as Sustainable Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 4383. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, S.; Bellon-Fontaine, M.-N. Chimie Verte et Industries Agroalimentaires: Vers une Bioéconomie Durable; Tec & Doc: Paris, France, 2020; ISBN 978-2-7430-7513-2. [Google Scholar]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic Potentials and Applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ye, X.; Bi, H.; Shen, Z. Microalgae Biofuels: Illuminating the Path to a Sustainable Future amidst Challenges and Opportunities. Biotechnol. Biofuels 2024, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.; Mientus, M.; Frey, D.; Amini-Hajibashi, A.; Ozturk, S.; Shaikh, F.; Sengupta, D.; El-Halwagi, M.M. A Review of Biodiesel Production from Microalgae. Clean. Techn. Environ. Policy 2017, 19, 637–668. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Amin, S. Review on Biofuel Oil and Gas Production Processes from Microalgae. Energy Convers. Manag. 2009, 50, 1834–1840. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Delavar, M.A.; Wang, J. Advanced Methods and Mathematical Modeling of Biofilms: Applications in Health Care, Medicine, Food, Aquaculture, Environment, and Industry; Academic Press: Cambridge, MA, USA, 2022; ISBN 978-0-323-90374-5. [Google Scholar]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of Microalgal Compounds in Trending Natural Cosmetics: A Review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially Important Bioproducts from Microalgae and Their Current Applications—A Review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Xia, D.; Qiu, W.; Wang, X.; Liu, J. Recent Advancements and Future Perspectives of Microalgae-Derived Pharmaceuticals. Mar. Drugs 2021, 19, 703. [Google Scholar] [CrossRef]
- Jha, D.; Jain, V.; Sharma, B.; Kant, A.; Garlapati, V.K. Microalgae-Based Pharmaceuticals and Nutraceuticals: An Emerging Field with Immense Market Potential. ChemBioEng Rev. 2017, 4, 257–272. [Google Scholar] [CrossRef]
- Samuels, R.; Mani, U.V.; Iyer, U.M.; Nayak, U.S. Hypocholesterolemic Effect of Spirulina in Patients with Hyperlipidemic Nephrotic Syndrome. J. Med. Food 2002, 5, 91–96. [Google Scholar] [CrossRef]
- Raposo, M.F.; Morais, A.M.M.B. Microalgae for the Prevention of Cardiovascular Disease and Stroke. Life Sci. 2015, 125, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Deepa, P.K.; Subramanian, A.; Manjusha, W.A. Phytochemical Screening and Evaluation of Antidiabetic Activity of the Marine Microalgae: Nannochloropsis sp. Int. J. Life Sci. Pharm. Res. 2022, 10, L36–L40. [Google Scholar] [CrossRef]
- Priatni, S.; Ratnaningrum, D.; Kosasih, W. The Screening of Antidiabetic Activity and The Cultivation Study of Local Marine Microalgae. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012066. [Google Scholar] [CrossRef]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibañez, E.; Reglero, G.; Señorans, F.J. Pressurized Liquid Extraction as an Alternative Process To Obtain Antiviral Agents from the Edible Microalga Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 8522–8527. [Google Scholar] [CrossRef]
- Tabarsa, M.; Shin, I.-S.; Lee, J.H.; Surayot, U.; Park, W.; You, S. An Immune-Enhancing Water-Soluble α-Glucan from Chlorella vulgaris and Structural Characteristics. Food Sci. Biotechnol. 2015, 24, 1933–1941. [Google Scholar] [CrossRef]
- Cutignano, A.; Nuzzo, G.; Ianora, A.; Luongo, E.; Romano, G.; Gallo, C.; Sansone, C.; Aprea, S.; Mancini, F.; D’Oro, U.; et al. Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts. Mar. Drugs 2015, 13, 5736–5749. [Google Scholar] [CrossRef]
- Krause-Hielscher, S.; Demuth, H.-U.; Wessjohann, L.; Arnold, N.; Griehl, C. Microalgae as Source for Potential Anti-Alzheimer’s Disease Directed Compounds—Screening for Glutaminyl Cyclase (QC) Inhibiting Metabolites. Int. J. Pharm. Biol. Sci. 2015, 5, 164–170. [Google Scholar]
- Martin, M.; Pusceddu, M.M.; Teichenné, J.; Negra, T.; Connolly, A.; Escoté, X.; Torrell Galceran, H.; Cereto Massagué, A.; Samarra Mestre, I.; del Pino Rius, A.; et al. Preventive Treatment with Astaxanthin Microencapsulated with Spirulina Powder, Administered in a Dose Range Equivalent to Human Consumption, Prevents LPS-Induced Cognitive Impairment in Rats. Nutrients 2023, 15, 2854. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Raj, A.; Tiwari, A.; Saxena, A.; Raj, A.; Tiwari, A. Exploring the Anti-Cancer Potential of Microalgae. In Progress in Microalgae Research—A Path for Shaping Sustainable Futures; IntechOpen: London, UK, 2022; ISBN 978-1-80356-024-3. [Google Scholar]
- Martínez, K.A.; Saide, A.; Crespo, G.; Martín, J.; Romano, G.; Reyes, F.; Lauritano, C.; Ianora, A. Promising Antiproliferative Compound from the Green Microalga Dunaliella tertiolecta Against Human Cancer Cells. Front. Mar. Sci. 2022, 9, 778108. [Google Scholar] [CrossRef]
- Imbimbo, P.; D’Elia, L.; Liberti, D.; Olivieri, G.; Monti, D.M. Towards Green Extraction Methods from Microalgae Learning from the Classics. Appl. Microbiol. Biotechnol. 2020, 104, 9067–9077. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Martín-Álvarez, P.J.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of Accelerated Solvent Extraction of Antioxidants from Spirulina platensis Microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Ibarra-Garza, I.P.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.; Alemán-Nava, G.S.; García-Pérez, J.S.; Parra-Saldívar, R. Green Extraction Technologies for High-Value Metabolites from Algae: A Review. Biofuels Bioprod. Biorefining 2017, 11, 215–231. [Google Scholar] [CrossRef]
- TRI National Analysis: Introduction. 2014. Available online: https://www.epa.gov/sites/default/files/2017-01/documents/tri_na_2014_complete_english.pdf (accessed on 25 June 2025).
- Iverson, S.J.; Lang, S.L.C.; Cooper, M.H. Comparison of the Bligh and Dyer and Folch Methods for Total Lipid Determination in a Broad Range of Marine Tissue. Lipids 2001, 36, 1283–1287. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Filaire, E.; Gerbore, J.; Guyo, J.B.; Dreux-Zigha, A.; Terrisse, F.; Ranouille, E.; Berthon, J.Y. Chapter 7: Biotechnologie Au Service de La Cosmétique. In Matières Premières Cosmétiques—Actifs Naturels, 2nd ed.; Cosmetic Valleys: Paris, France, 2020; pp. 103–120. [Google Scholar]
- Marino, T.; Leone, G.P.; Casella, P.; Iovine, A.; Musmarra, D.; Zoani, C.; Balducchi, R.; Molino, A. Green Extraction of Microalgae Components for Incorporation in Food and Feed Supplements. Chem. Eng. Trans. 2021, 87, 457–462. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent Developments in Supercritical Fluid Extraction of Bioactive Compounds from Microalgae: Role of Key Parameters, Technological Achievements and Challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Nobre, B.P.; Villalobos, F.; Barragán, B.E.; Oliveira, A.C.; Batista, A.P.; Marques, P.A.S.S.; Mendes, R.L.; Sovová, H.; Palavra, A.F.; Gouveia, L. A Biorefinery from Nannochloropsis sp. Microalga—Extraction of Oils and Pigments. Prod. Production Biohydrogen from the Leftover Biomass. Bioresour. Technol. 2013, 135, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Sarker, Z.I.; Ferdosh, S.; Akanda, J.H.; Easmin, S.; Shamsudin, S.H.; Yunus, K.B. Phytosterols and Their Extraction from Various Plant Matrices Using Supercritical Carbon Dioxide: A Review. J. Sci. Food Agric. 2015, 95, 1385–1394. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and Supercritical Fluid Extraction of Functional Ingredients from Different Natural Sources: Plants, Food-by-Products, Algae and Microalgae: A Review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- López-Salas, L.; Expósito-Almellón, X.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Design of Experiments for Green and GRAS Solvent Extraction of Phenolic Compounds from Food Industry By-Products—A Systematic Review. TrAC Trends Anal. Chem. 2024, 171, 117536. [Google Scholar] [CrossRef]
- Molino, A.; Martino, M.; Larocca, V.; Di Sanzo, G.; Spagnoletta, A.; Marino, T.; Karatza, D.; Iovine, A.; Mehariya, S.; Musmarra, D. Eicosapentaenoic Acid Extraction from Nannochloropsis gaditana Using Carbon Dioxide at Supercritical Conditions. Mar. Drugs 2019, 17, 132. [Google Scholar] [CrossRef]
- Sanzo, G.D.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical Carbon Dioxide Extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.P.; Balducchi, R.; Mehariya, S.; Martino, M.; Larocca, V.; Di Sanzo, G.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; et al. Selective Extraction of ω-3 Fatty Acids from Nannochloropsis sp. Using Supercritical CO2 Extraction. Molecules 2019, 24, 2406. [Google Scholar] [CrossRef] [PubMed]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Microwave–Hydrothermal Extraction and Degradation of Fucoidan from Supercritical Carbon Dioxide Deoiled Undaria pinnatifida. Ind. Eng. Chem. Res. 2013, 52, 7940–7946. [Google Scholar] [CrossRef]
- Pan, J.-L.; Wang, H.-M.; Chen, C.-Y.; Chang, J.-S. Extraction of Astaxanthin from Haematococcus pluvialis by Supercritical Carbon Dioxide Fluid with Ethanol Modifier. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Becerra, M.; Boutefnouchet, S.; Córdoba, O.; Vitorino, G.P.; Brehu, L.; Lamour, I.; Laimay, F.; Efstathiou, A.; Smirlis, D.; Michel, S.; et al. Antileishmanial Activity of Fucosterol Recovered from Lessonia vadosa Searles (Lessoniaceae) by SFE, PSE and CPC. Phytochem. Lett. 2015, 11, 418–423. [Google Scholar] [CrossRef]
- Tsevdou, M.; Ntzimani, A.; Katsouli, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Comparative Study of Microwave, Pulsed Electric Fields, and High Pressure Processing on the Extraction of Antioxidants from Olive Pomace. Molecules 2024, 29, 2303. [Google Scholar] [CrossRef] [PubMed]
- Sparr Eskilsson, C.; Björklund, E. Analytical-Scale Microwave-Assisted Extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Ganzler, K.; Salgó, A.; Valkó, K. Microwave Extraction: A Novel Sample Preparation Method for Chromatography. J. Chromatogr. A 1986, 371, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Vijay Kumar, G.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable Green Solvents and Techniques for Lipid Extraction from Microalgae: A Review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Krishnan, S.; Ghani, N.A.; Aminuddin, N.F.; Quraishi, K.S.; Azman, N.S.; Cravotto, G.; Leveque, J.-M. Microwave-Assisted Lipid Extraction from Chlorella vulgaris in Water with 0.5%–2.5% of Imidazolium Based Ionic Liquid as Additive. Renew. Energy 2020, 149, 244–252. [Google Scholar] [CrossRef]
- Bermúdez Menéndez, J.M.; Arenillas, A.; Menéndez Díaz, J.Á.; Boffa, L.; Mantegna, S.; Binello, A.; Cravotto, G. Optimization of Microalgae Oil Extraction under Ultrasound and Microwave Irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1779–1784. [Google Scholar] [CrossRef]
- Chaari, M.; Akermi, S.; Elhadef, K.; Said-Al Ahl, H.A.H.; Hikal, W.M.; Mellouli, L.; Smaoui, S. Microwave-Assisted Extraction of Bioactive and Nutraceuticals. In Bioactive Extraction and Application in Food and Nutraceutical Industries; Sarkar, T., Pati, S., Eds.; Springer: New York, NY, USA, 2024; pp. 79–102. ISBN 978-1-0716-3601-5. [Google Scholar]
- Juin, C.; Chérouvrier, J.-R.; Thiéry, V.; Gagez, A.-L.; Bérard, J.-B.; Joguet, N.; Kaas, R.; Cadoret, J.-P.; Picot, L. Microwave-Assisted Extraction of Phycobiliproteins from Porphyridium purpureum. Appl. Biochem. Biotechnol. 2015, 175, 1–15. [Google Scholar] [CrossRef]
- Xiao, X.-H.; Yuan, Z.-Q.; Li, G.-K. Preparation of Phytosterols and Phytol from Edible Marine Algae by Microwave-Assisted Extraction and High-Speed Counter-Current Chromatography. Sep. Purif. Technol. 2013, 104, 284–289. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the Microalgal Pigments Extraction Process: Performance of Microwave Assisted Extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-Assisted Extraction of Sulfated Polysaccharides (Fucoidan) from Brown Seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Waldebäck, M. Pressurized Fluid Extraction: A Sustainable Technique with Added Values. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2005. [Google Scholar]
- Nickerson, B.; Colón, I. Liquid–Liquid and Solid-Phase Extraction Techniques. In Sample Preparation of Pharmaceutical Dosage Forms: Challenges and Strategies for Sample Preparation and Extraction; Nickerson, B., Ed.; Springer: Boston, MA, USA, 2011; pp. 63–92. ISBN 978-1-4419-9631-2. [Google Scholar]
- Turner, C.; Waldebäck, M. 2—Principles of Pressurized Fluid Extraction and Environmental, Food and Agricultural Applications. In Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Rizvi, S.S.H., Ed.; Woodhead Publishing Series in Food Science; Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2013; pp. 39–70. ISBN 978-1-84569-645-0. [Google Scholar]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.-G.; Lee, D.-U.; Pan, C.-H. Optimization of Pressurized Liquid Extraction of Carotenoids and Chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Reighard, T.S.; Olesik, S.V. Bridging the Gap Between Supercritical Fluid Extraction and Liquid Extraction Techniques: Alternative Approaches to the Extraction of Solid and Liquid Environmental Matrices. Crit. Rev. Anal. Chem. 1996, 26, 61–99. [Google Scholar] [CrossRef]
- Misirli, H.; Domaç, F.M.; Somay, G.; Araal, O.; Ozer, B.; Adigüzel, T. N-Hexane Induced Polyneuropathy: A Clinical and Electrophysiological Follow Up. Electromyogr. Clin. Neurophysiol. 2008, 48, 103–108. [Google Scholar]
- Virot, M.; Tomao, V.; Ginies, C.; Visinoni, F.; Chemat, F. Green Procedure with a Green Solvent for Fats and Oils’ Determination: Microwave-Integrated Soxhlet Using Limonene Followed by Microwave Clevenger Distillation. J. Chromatogr. 2008, 1196–1197, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Cháfer, A.; Muñoz, R.; Burguet, M.C.; Berna, A. The Influence of the Temperature on the Liquid–Liquid Equlibria of the Mixture Limonene + Ethanol + H2O. Fluid. Phase Equilibria 2004, 224, 251–256. [Google Scholar] [CrossRef]
- Ramos, L.; Kristenson, E.M.; Brinkman, U.A.T. Current Use of Pressurised Liquid Extraction and Subcritical Water Extraction in Environmental Analysis. J. Chromatogr. 2002, 975, 3–29. [Google Scholar] [CrossRef]
- Wang, M.; Morón-Ortiz, Á.; Zhou, J.; Benítez-González, A.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Barba, F.J. Effects of Pressurized Liquid Extraction with Dimethyl Sulfoxide on the Recovery of Carotenoids and Other Dietary Valuable Compounds from the Microalgae Spirulina, Chlorella and Phaeodactylum tricornutum. Food Chem. 2023, 405, 134885. [Google Scholar] [CrossRef]
- Jaime, L.; Rodríguez-Meizoso, I.; Cifuentes, A.; Santoyo, S.; Suarez, S.; Ibáñez, E.; Señorans, F.J. Pressurized Liquids as an Alternative Process to Antioxidant Carotenoids’ Extraction from Haematococcus pluvialis Microalgae. LWT Food Sci. Technol. 2010, 43, 105–112. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Urreta, I.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E.; Suárez-Alvarez, S. Optimization of Clean Extraction Methods to Isolate Carotenoids from the Microalga Neochloris oleoabundans and Subsequent Chemical Characterization Using Liquid Chromatography Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2013, 405, 4607–4616. [Google Scholar] [CrossRef]
- Herrero, M.; Jaime, L.; Martín-Álvarez, P.J.; Cifuentes, A.; Ibáñez, E. Optimization of the Extraction of Antioxidants from Dunaliella salina Microalga by Pressurized Liquids. J. Agric. Food Chem. 2006, 54, 5597–5603. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Santoyo, S.; Jaime, L.; Avalo, B.; Cifuentes, A.; Reglero, G.; García-Blairsy Reina, G.; Señoráns, F.J.; Ibáñez, E. Comprehensive Characterization of the Functional Activities of Pressurized Liquid and Ultrasound-Assisted Extracts from Chlorella vulgaris. LWT Food Sci. Technol. 2012, 46, 245–253. [Google Scholar] [CrossRef]
- Herrero, M.; Simó, C.; Ibáñez, E.; Cifuentes, A. Capillary Electrophoresis-Mass Spectrometry of Proteins Obtained by Pressurized Liquid Extraction. Electrophoresis 2005, 26, 4215–4224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Golmakani, M.-T.; Mendiola, J.A.; Rezaei, K.; Ibáñez, E. Pressurized Limonene as an Alternative Bio-Solvent for the Extraction of Lipids from Marine Microorganisms. J. Supercrit. Fluids 2014, 92, 1–7. [Google Scholar] [CrossRef]
- Pieber, S.; Schober, S.; Mittelbach, M. Pressurized Fluid Extraction of Polyunsaturated Fatty Acids from the Microalga Nannochloropsis oculata. Biomass Bioenergy 2012, 47, 474–482. [Google Scholar] [CrossRef]
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-Free” Ultrasound-Assisted Extraction of Lipids from Fresh Microalgae Cells: A Green, Clean and Scalable Process. Bioresour. Technol. 2012, 114, 457–465. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, X.; Wang, L.; Zhou, S.; Feng, H.; Chen, W.N.; Lau, R. Ultrasound Assisted Extraction of Carbohydrates from Microalgae as Feedstock for Yeast Fermentation. Bioresour. Technol. 2013, 128, 337–344. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of Extraction of Natural Products Using Ultrasonic Irradiations—A Review of Current Status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-Assisted Green Solvent Extraction of High-Added Value Compounds from Microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Deenu, A.; Naruenartwongsakul, S.; Kim, S.M. Optimization and Economic Evaluation of Ultrasound Extraction of Lutein from Chlorella vulgaris. Biotechnol. Bioproc. Eng. 2013, 18, 1151–1162. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean Recovery of Antioxidant Compounds from Plant Foods, by-Products and Algae Assisted by Ultrasounds Processing. Modeling Approaches to Optimize Processing Conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent Advances in the Extraction of Bioactive Compounds with Subcritical Water: A Review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Thiruvenkadam, S.; Izhar, S.; Yoshida, H.; Danquah, M.K.; Harun, R. Process Application of Subcritical Water Extraction (SWE) for Algal Bio-Products and Biofuels Production. Appl. Energy 2015, 154, 815–828. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2016, 8, 23–34. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the Formation of New Antioxidants in Natural Samples after Subcritical Water Extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Subcritical Water Extraction and Characterization of Bioactive Compounds from Haematococcus pluvialis Microalga. J. Pharm. Biomed. Anal. 2010, 51, 456–463. [Google Scholar] [CrossRef]
- Ho, B.C.H.; Kamal, S.M.M.; Danquah, M.K.; Harun, R. Optimization of Subcritical Water Extraction (SWE) of Lipid and Eicosapentaenoic Acid (EPA) from Nannochloropsis gaditana. BioMed Res. Int. 2018, 2018, 8273581. [Google Scholar] [CrossRef]
- Reddy, H.K.; Muppaneni, T.; Sun, Y.; Li, Y.; Ponnusamy, S.; Patil, P.D.; Dailey, P.; Schaub, T.; Holguin, F.O.; Dungan, B.; et al. Subcritical Water Extraction of Lipids from Wet Algae for Biodiesel Production. Fuel 2014, 133, 73–81. [Google Scholar] [CrossRef]
- Zainan, N.H.; Thiruvenkadam, S.; Danquah, M.K.; Harun, R. Biochemical Analysis and Potential Applications of Aqueous and Solid Products Generated from Subcritical Water Extraction of Microalgae Chlorella pyrenoidosa Biomass. J. Appl. Phycol. 2020, 32, 111–126. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Rodríguez-Seoane, P.; Flórez-Fernández, N.; Torres, M.D.; Díaz-Reinoso, B.; Moure, A.; Domínguez, H. Subcritical Water for the Extraction and Hydrolysis of Protein and Other Fractions in Biorefineries from Agro-Food Wastes and Algae: A Review. Food Bioprocess. Technol. 2021, 14, 373–387. [Google Scholar] [CrossRef]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.; Dou, C. Disruption of Chlorella vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae Biomolecules: Extraction, Separation and Purification Methods. Processes 2021, 9, 10. [Google Scholar] [CrossRef]
- Zuorro, A.; Miglietta, S.; Familiari, G.; Lavecchia, R. Enhanced Lipid Recovery from Nannochloropsis Microalgae by Treatment with Optimized Cell Wall Degrading Enzyme Mixtures. Bioresour. Technol. 2016, 212, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-Assisted Extraction of Bioactives from Plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Kim, Y.-S.; Jeon, Y.-J.; Kim, S.-K. Enzyme-Assisted Extraction of Bioactive Compounds from Seaweeds and Microalgae. TrAC Trends Anal. Chem. 2023, 167, 117266. [Google Scholar] [CrossRef]
- Marsol-Vall, A.; Aitta, E.; Guo, Z.; Yang, B. Green Technologies for Production of Oils Rich in N-3 Polyunsaturated Fatty Acids from Aquatic Sources. Crit. Rev. Food Sci. Nutr. 2022, 62, 2942–2962. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, Q.; Cong, W. Enzyme-Assisted Aqueous Extraction of Lipid from Microalgae. J. Agric. Food Chem. 2012, 60, 11771–11776. [Google Scholar] [CrossRef] [PubMed]
- Tavanandi, H.A.; Vanjari, P.; Raghavarao, K.S.M.S. Synergistic Method for Extraction of High Purity Allophycocyanin from Dry Biomass of Arthrospira platensis and Utilization of Spent Biomass for Recovery of Carotenoids. Sep. Purif. Technol. 2019, 225, 97–111. [Google Scholar] [CrossRef]
- Law, S.Q.K.; Halim, R.; Scales, P.J.; Martin, G.J.O. Conversion and Recovery of Saponifiable Lipids from Microalgae Using a Nonpolar Solvent via Lipase-Assisted Extraction. Bioresour. Technol. 2018, 260, 338–347. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Z.; Cui, F.; Zou, B.; Zhao, P.; Yuan, Z. Enzyme-Assisted Extraction of Oil from Wet Microalgae Scenedesmus sp. G4. Energies 2015, 8, 8165–8174. [Google Scholar] [CrossRef]
- Chaminda Lakmal, H.H.; Samarakoon, K.W.; Jeon, Y.-J. Enzyme-Assisted Extraction to Prepare Bioactive Peptides from Microalgae. In Marine Algae Extracts; John Wiley & Sons: Weinheim, Germany, 2015; pp. 305–318. ISBN 978-3-527-67957-7. [Google Scholar]
- Angles, E.; Jaouen, P.; Pruvost, J.; Marchal, L. Wet Lipid Extraction from the Microalga Nannochloropsis sp.: Disruption, Physiological Effects and Solvent Screening. Algal Res. 2017, 21, 27–34. [Google Scholar] [CrossRef]
- Yang, F.; Xiang, W.; Sun, X.; Wu, H.; Li, T.; Long, L. A Novel Lipid Extraction Method from Wet Microalga Picochlorum sp. Room Temperature. Mar. Drugs 2014, 12, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Rehan, S.S.; Aftab, D.S. Evaluation of Antioxidant and Anti-Cancer Potential of Ethanolic and n-Hexane Extracts of Fresh Water Algae from Lahore. Preprint 2023. [Google Scholar] [CrossRef]
- Yang, F.; Cheng, C.; Long, L.; Hu, Q.; Jia, Q.; Wu, H.; Xiang, W. Extracting Lipids from Several Species of Wet Microalgae Using Ethanol at Room Temperature. Energy Fuels 2015, 29, 2380–2386. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Liu, T.; Zhang, W. Subcritical Ethanol Extraction of Lipid from Wet Microalgae Paste of Nannochloropsis sp. J. Biobased Mater. Bioenergy 2011, 5, 385–389. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; Robert McElroy, C.; Abou-Shehada, S.; Dunn, J.P. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green. Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Dejoye Tanzi, C.; Abert Vian, M.; Ginies, C.; Elmaataoui, M.; Chemat, F. Terpenes as Green Solvents for Extraction of Oil from Microalgae. Molecules 2012, 17, 8196–8205. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green. Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Bakshi, B.R.; Demessie, E.S. Life Cycle Assessment of an Ionic Liquid versus Molecular Solvents and Their Applications. Environ. Sci. Technol. 2008, 42, 1724–1730. [Google Scholar] [CrossRef]
- Al-Ameri, M.; Al-Zuhair, S. Using Switchable Solvents for Enhanced, Simultaneous Microalgae Oil Extraction-Reaction for Biodiesel Production. Biochem. Eng. J. 2019, 141, 217–224. [Google Scholar] [CrossRef]
- Asevedo, E.A.; das Chagas, B.M.E.; de Oliveira Júnior, S.D.; dos Santos, E.S. Recovery of Lipids and Carotenoids from Dunaliella salina Microalgae Using Deep Eutectic Solvents. Algal Res. 2023, 69, 102940. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green Extraction of Value-Added Compounds Form Microalgae: A Short Review on Natural Deep Eutectic Solvents (NaDES) and Related Pre-Treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Patrice Didion, Y.; Gijsbert Tjalsma, T.; Su, Z.; Malankowska, M.; Pinelo, M. What Is next? The Greener Future of Solid Liquid Extraction of Biobased Compounds: Novel Techniques and Solvents Overpower Traditional Ones. Sep. Purif. Technol. 2023, 320, 124147. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of Lipids from Microalgae Using Classical and Innovative Approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef]
- Samorì, C.; Barreiro, D.L.; Vet, R.; Pezzolesi, L.; Brilman, D.W.F.; Galletti, P.; Tagliavini, E. Effective Lipid Extraction from Algae Cultures Using Switchable Solvents. Green. Chem. 2013, 15, 353–356. [Google Scholar] [CrossRef]
- Zullaikah, S.; Jessinia, M.C.P.; Rinaldi, R.; Yasmin, M.; Rachimoellah, M.; Wu, D.W. Lipids Extraction from Wet and Unbroken Microalgae Chlorella vulgaris Using Subcritical Water. Mater. Sci. Forum 2019, 964, 103–108. [Google Scholar] [CrossRef]
- Imbimbo, P.; Bueno, M.; D’Elia, L.; Pollio, A.; Ibañez, E.; Olivieri, G.; Monti, D.M. Green Compressed Fluid Technologies To Extract Antioxidants and Lipids from Galdieria phlegrea in a Biorefinery Approach. ACS Sustain. Chem. Eng. 2020, 8, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Zghaibi, N.; Omar, R.; Mustapa Kamal, S.M.; Awang Biak, D.R.; Harun, R. Microwave-Assisted Brine Extraction for Enhancement of the Quantity and Quality of Lipid Production from Microalgae Nannochloropsis sp. Molecules 2019, 24, 3581. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Schuur, B.; Brilman, D.W.F. Maximizing Lipid Yield in Neochloris oleoabundans Algae Extraction by Stressing and Using Multiple Extraction Stages with N-Ethylbutylamine as Switchable Solvent. Ind. Eng. Chem. Res. 2017, 56, 8073–8080. [Google Scholar] [CrossRef]
- Zimmerer, J.; Pingen, D.; Hess, S.K.; Koengeter, T.; Mecking, S. Integrated Extraction and Catalytic Upgrading of Microalgae Lipids in Supercritical Carbon Dioxide. Green. Chem. 2019, 21, 2428–2435. [Google Scholar] [CrossRef]
- Kalsum, U.; Kusuma, H.S.; Roesyadi, A.; Mahfud, M. Lipid Extraction from Spirulina platensis Using Microwave for Biodiesel Production. Korean Chem. Eng. Res. 2019, 57, 301–304. [Google Scholar] [CrossRef]
- Hadiyanto, H. Suttrisnorhadi Response Surface Optimization of Ultrasound Assisted Extraction (UAE) of Phycocyanin from Microalgae Spirulina platensis|EBSCOhost. Available online: https://openurl.ebsco.com/contentitem/doi:10.9755%2Fejfa.2015-05-193?sid=ebsco:plink:crawler&id=ebsco:doi:10.9755%2Fejfa.2015-05-193 (accessed on 13 March 2025).
- Esquivel-Hernández, D.A.; López, V.H.; Rodríguez-Rodríguez, J.; Alemán-Nava, G.S.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.; Parra-Saldívar, R. Supercritical Carbon Dioxide and Microwave-Assisted Extraction of Functional Lipophilic Compounds from Arthrospira platensis. Int. J. Mol. Sci. 2016, 17, 658. [Google Scholar] [CrossRef]
- Du, L.; Kruse, A. Cell Disruption and Value-Added Substances Extraction from Arthrospira platensis Using Subcritical Water. J. Supercrit. Fluids 2021, 171, 105193. [Google Scholar] [CrossRef]
- Goto, M.; Kanda, H.; Wahyudiono; Machmudah, S. Extraction of Carotenoids and Lipids from Algae by Supercritical CO2 and Subcritical Dimethyl Ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of Astaxanthin from Haematococcus pluvialis Using Supercritical CO2 and Ethanol as Entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of Extraction Methods for Recovery of Astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010, 46, 64–70. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Salinas, F.; Medina, E.; Rincón, B.; Martín, M.Á.; Gutiérrez, M.C.; Cerezal-Mezquita, P. Supercritical Fluid Extraction of Fucoxanthin from the Diatom Phaeodactylum tricornutum and Biogas Production through Anaerobic Digestion. Mar. Drugs 2022, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-López, B.; Barranco, A.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Development of New Green Processes for the Recovery of Bioactives from Phaeodactylum tricornutum. Food Res. Int. 2017, 99, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Vintila, A.C.N.; Vlaicu, A.; Radu, E.; Ciltea-Udrescu, M.; Enascuta, E.C.; Banu, I.; Oprescu, E.-E. Evaluation of Ultrasound Assisted Extraction of Bioactive Compounds from Microalgae. Food Meas. 2022, 16, 2518–2526. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M.; Tariq, S. Supercritical Carbon Dioxide Extraction of Microalgae Lipid: Process Optimization and Laboratory Scale-Up. J. Supercrit. Fluids 2014, 86, 57–66. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Rawat, I.; Ramluckan, K.; Bux, F. Efficacy of Drying and Cell Disruption Techniques on Lipid Recovery from Microalgae for Biodiesel Production. Fuel 2014, 128, 46–52. [Google Scholar] [CrossRef]
- Francesch, A.M.; Glaser, K.B.; Jaspars, M.; Jiménez, C.; Luesch, H.; Mayer, A.M.; Newman, D.J.; Pierce, M.L.; Jaspars, O.T.-S. Author M. Pharmaceuticals from Marine Sources: Past, Present and Future. Available online: https://pharmaceutical-journal.com/article/research/pharmaceuticals-from-marine-sources-past-present-and-future (accessed on 31 March 2025).
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of High Added-Value Compounds—A Brief Review of Recent Work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Cauduro, V.H.; Gohlke, G.; da Silva, N.W.; Cruz, A.G.; Flores, E.M. A Review on Scale-up Approaches for Ultrasound-Assisted Extraction of Natural Products. Curr. Opin. Chem. Eng. 2025, 48, 101120. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Parjikolaei, B.R.; Lari, H.N.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Pilot-Plant Scale Extraction of Phenolic Compounds from Mango Leaves Using Different Green Techniques: Kinetic and Scale up Study. Chem. Eng. J. 2016, 299, 420–430. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent Advances in Scaling-up of Non-Conventional Extraction Techniques: Learning from Successes and Failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Ibañez, E. Green Extraction Processes, Biorefineries and Sustainability: Recovery of High Added-Value Products from Natural Sources. J. Supercrit. Fluids 2018, 134, 252–259. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green Extraction Processes of Natural Products as Tools for Biorefinery. Biofuels Bioprod. Biorefining 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Tabernero, A.; Martín del Valle, E.M.; Galán, M.A. Evaluating the Industrial Potential of Biodiesel from a Microalgae Heterotrophic Culture: Scale-up and Economics. Biochem. Eng. J. 2012, 63, 104–115. [Google Scholar] [CrossRef]
- Hogan, P.; Otero, P.; Murray, P.; Saha, S.K. Effect of Biomass Pre-Treatment on Supercritical CO2 Extraction of Lipids from Marine Diatom Amphora sp. and Its Biomass Evaluation as Bioethanol Feedstock. Heliyon 2021, 7, e05995. [Google Scholar] [CrossRef]
- Eynde, E.V.; Lenaerts, B.; Tytgat, T.; Verbruggen, S.W.; Hauchecorne, B.; Blust, R.; Lenaerts, S. Effect of Pretreatment and Temperature on the Properties of Pinnularia Biosilica Frustules. RSC Adv. 2014, 4, 56200–56206. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S.; Joshi, G. Exploration of Upstream and Downstream Process for Microwave Assisted Sustainable Biodiesel Production from Microalgae Chlorella vulgaris. Bioresour. Technol. 2016, 216, 793–800. [Google Scholar] [CrossRef]
- Vanleenhove, B.; Xu, L.; De Meester, S.; Raes, K. Impact of Stabilization Technology on the Extraction Yield and Functionality of Macroconstituents from Biomass: A Systematic Review. J. Agric. Food Chem. 2023, 71, 9628–9643. [Google Scholar] [CrossRef]
- Mashhadinejad, A. Effect of Growth Conditions and Extraction Solvents on Enhancement of Antimicrobial Activity of the Microalgae Chlorella vulgaris. Pharm. Biomed. Res. 2016, 2, 65–73. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel Production from Heterotrophic Microalgal Oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Microalgae Cultivation and Metabolites Production: A Comprehensive Review. Biofuels Bioprod. Biorefining 2018, 12, 304–324. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae Harvesting Techniques: A Review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. Age and Nutrient Limitation Enhance Polyunsaturated Aldehyde Production in Marine Diatoms. Phytochemistry 2007, 68, 2059–2067. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Vijayan, D.; Praveenkumar, R.; Han, J.-I.; Lee, K.; Park, J.-Y.; Chang, W.-S.; Lee, J.-S.; Oh, Y.-K. Cell-Wall Disruption and Lipid/Astaxanthin Extraction from Microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef]
- Sousa, S.C.; Freitas, A.C.; Gomes, A.M.; Carvalho, A.P. Extraction of Nannochloropsis Fatty Acids Using Different Green Technologies: The Current Path. Mar. Drugs 2023, 21, 365. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Jacob, A.; Nader, C.; Oliveira, C.D.L.; Matos, Â.P.; Araújo, E.S.; Shabnam, N.; Ashok, B.; Gálvez, A.O. An Overview on Microalgae as Renewable Resources for Meeting Sustainable Development Goals. J. Environ. Manag. 2022, 320, 115897. [Google Scholar] [CrossRef] [PubMed]
- Seyfabadi, J.; Ramezanpour, Z.; Amini Khoeyi, Z. Protein, Fatty Acid, and Pigment Content of Chlorella vulgaris under Different Light Regimes. J. Appl. Phycol. 2011, 23, 721–726. [Google Scholar] [CrossRef]
- Burlew, J.S. Algal Culture from Laboratory to Pilot Plant. Available online: https://library.wur.nl/WebQuery/titel/533590 (accessed on 1 April 2025).
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the US Department of Energy’s Aquatic Species Program: Biodiesel from Algae; National Renewable Energy Laboratory: Golden, CO, USA, 1998.
- Widjaja, A.; Chien, C.-C.; Ju, Y.-H. Study of Increasing Lipid Production from Fresh Water Microalgae Chlorella vulgaris. J. Taiwan. Inst. Chem. Eng. 2009, 40, 13–20. [Google Scholar] [CrossRef]
- Abreu, A.P.; Martins, R.; Nunes, J. Emerging Applications of Chlorella sp. Spirulina (Arthrospira) sp. Bioengineering 2023, 10, 955. [Google Scholar] [CrossRef]
- Hui, G.T.; Meng, T.K.; Kassim, M.A. Green Ultrasonication-Assisted Extraction of Microalgae Chlorella sp. for Polysaturated Fatty Acid (PUFA) Rich Lipid Extract Using Alternative Solvent Mixture. Bioprocess. Biosyst. Eng. 2023, 46, 1499–1512. [Google Scholar] [CrossRef]
- Ma, X.-N.; Chen, T.-P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Tzima, S.; Georgiopoulou, I.; Louli, V.; Magoulas, K. Recent Advances in Supercritical CO2 Extraction of Pigments, Lipids and Bioactive Compounds from Microalgae. Molecules 2023, 28, 1410. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).