Selective Antiproliferative Effects of Marine Oils on Neuroblastoma Cells in 3D Cultures
Abstract
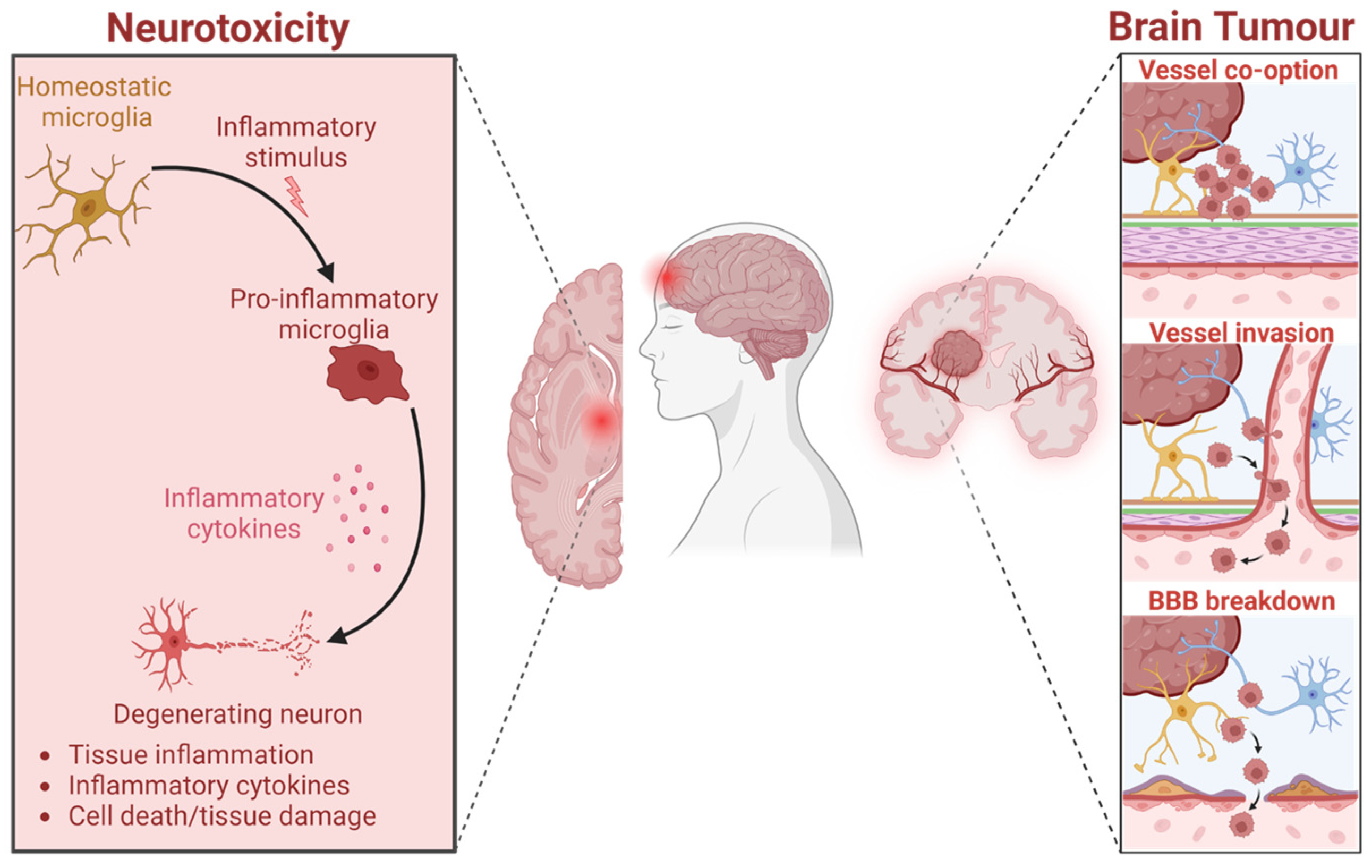
1. Introduction
2. Results and Discussion
2.1. Polar and Non-Polar Marine Oils
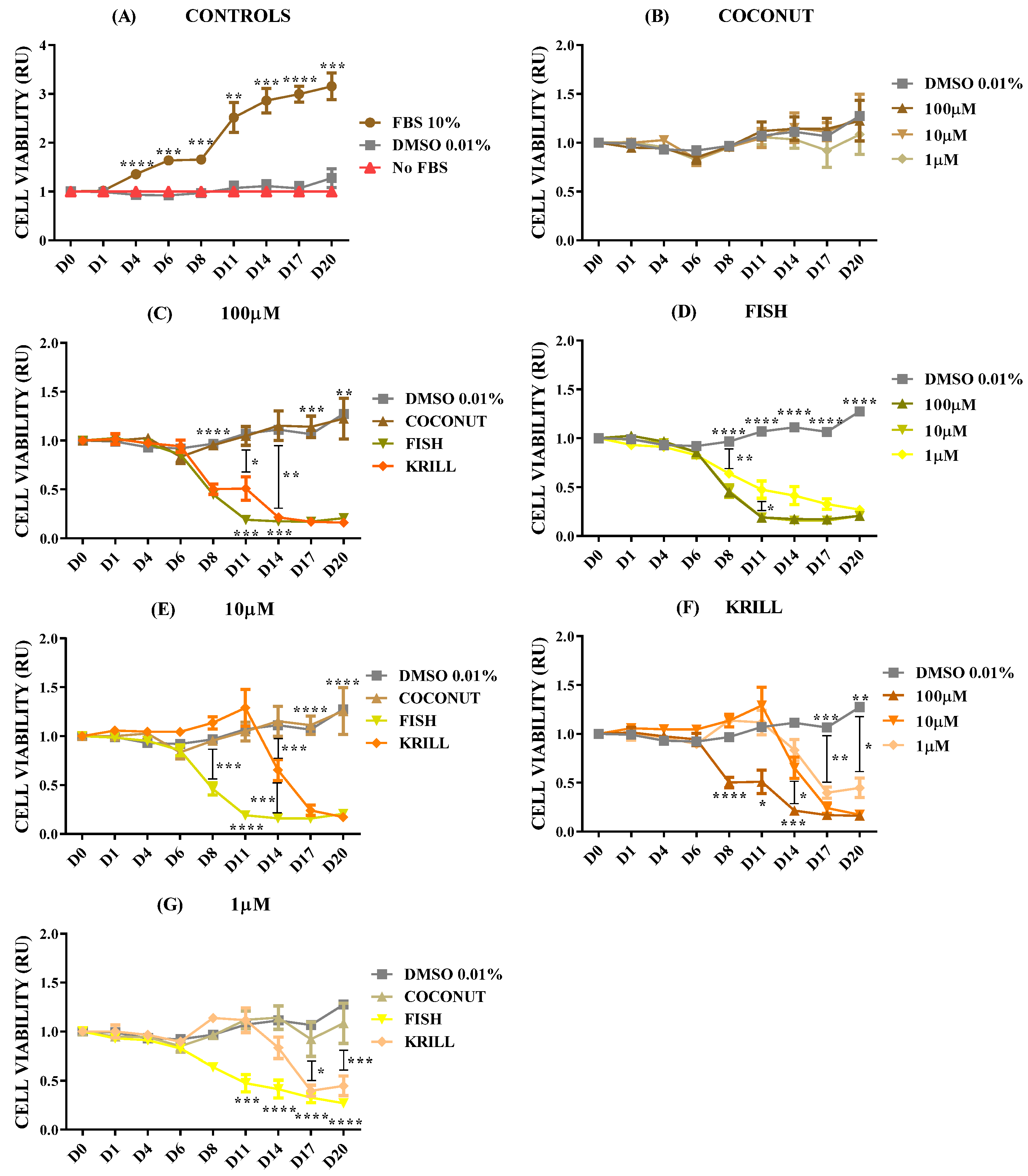
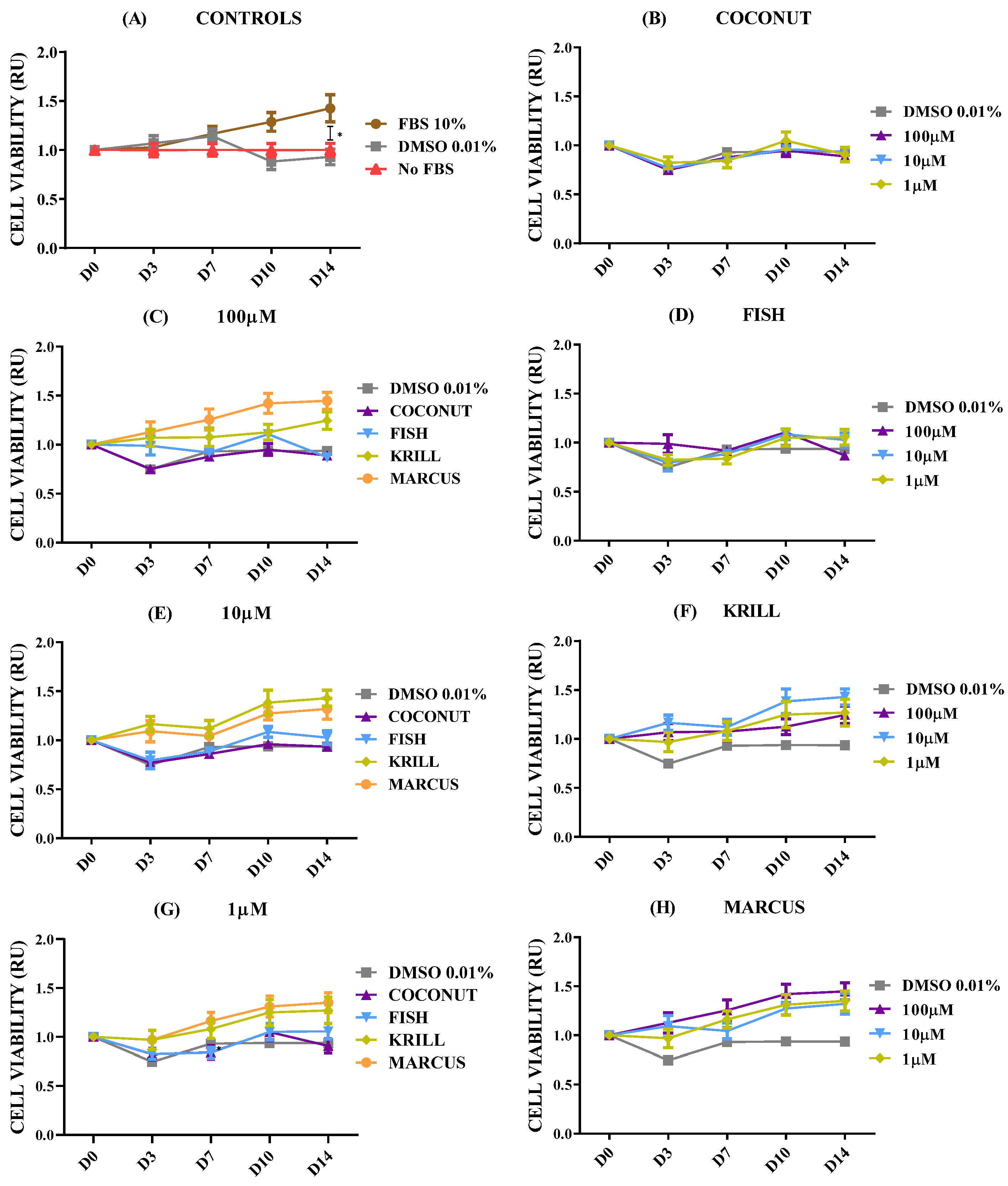
2.2. Effect of Polar Marine Oils on NB Cell Viability
2.3. Effect of Polar Marine Oils on GB Cell Viability
2.4. Effect of Polar Marine Oils on Non-Neural Cell Line Viability
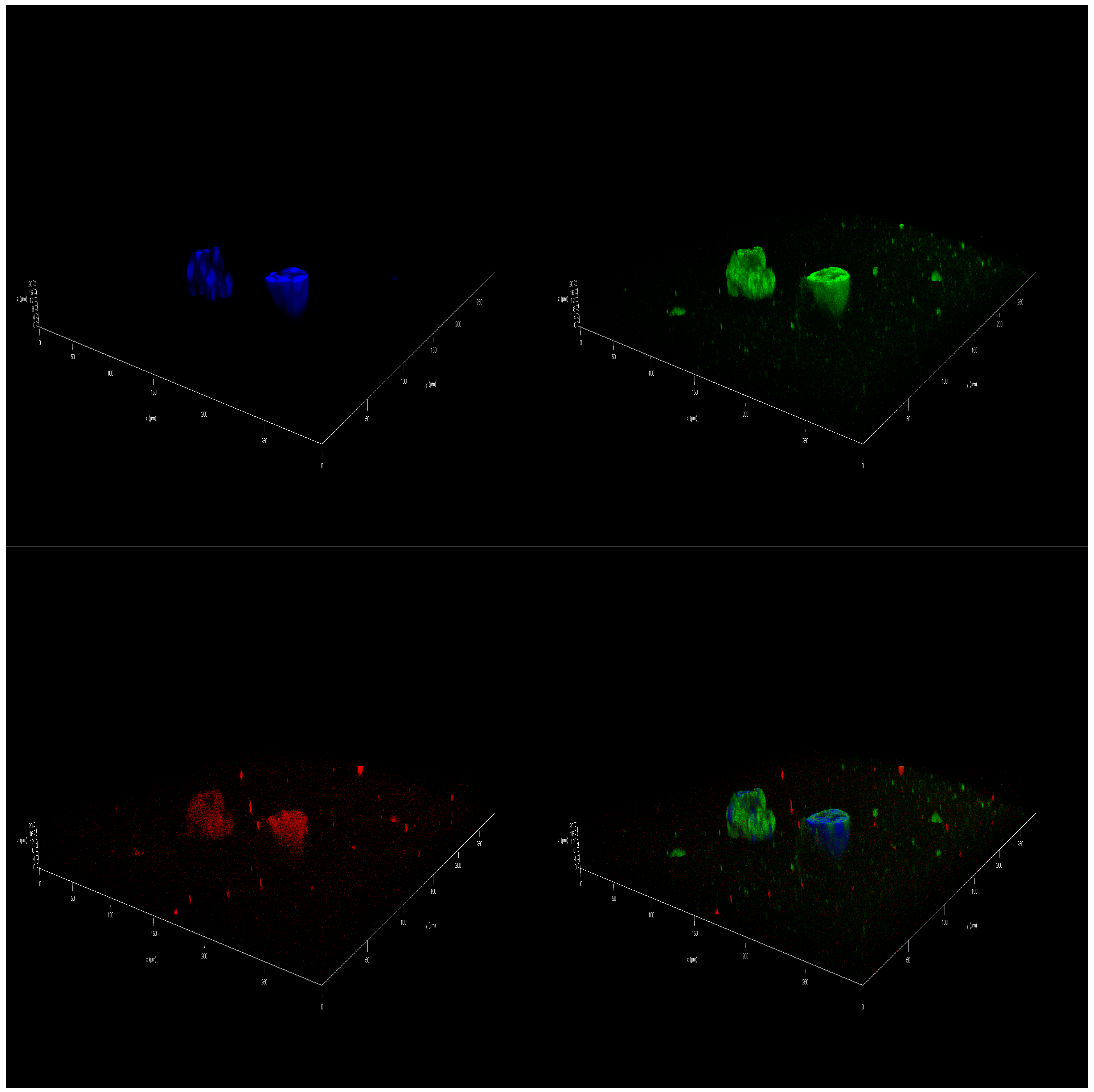
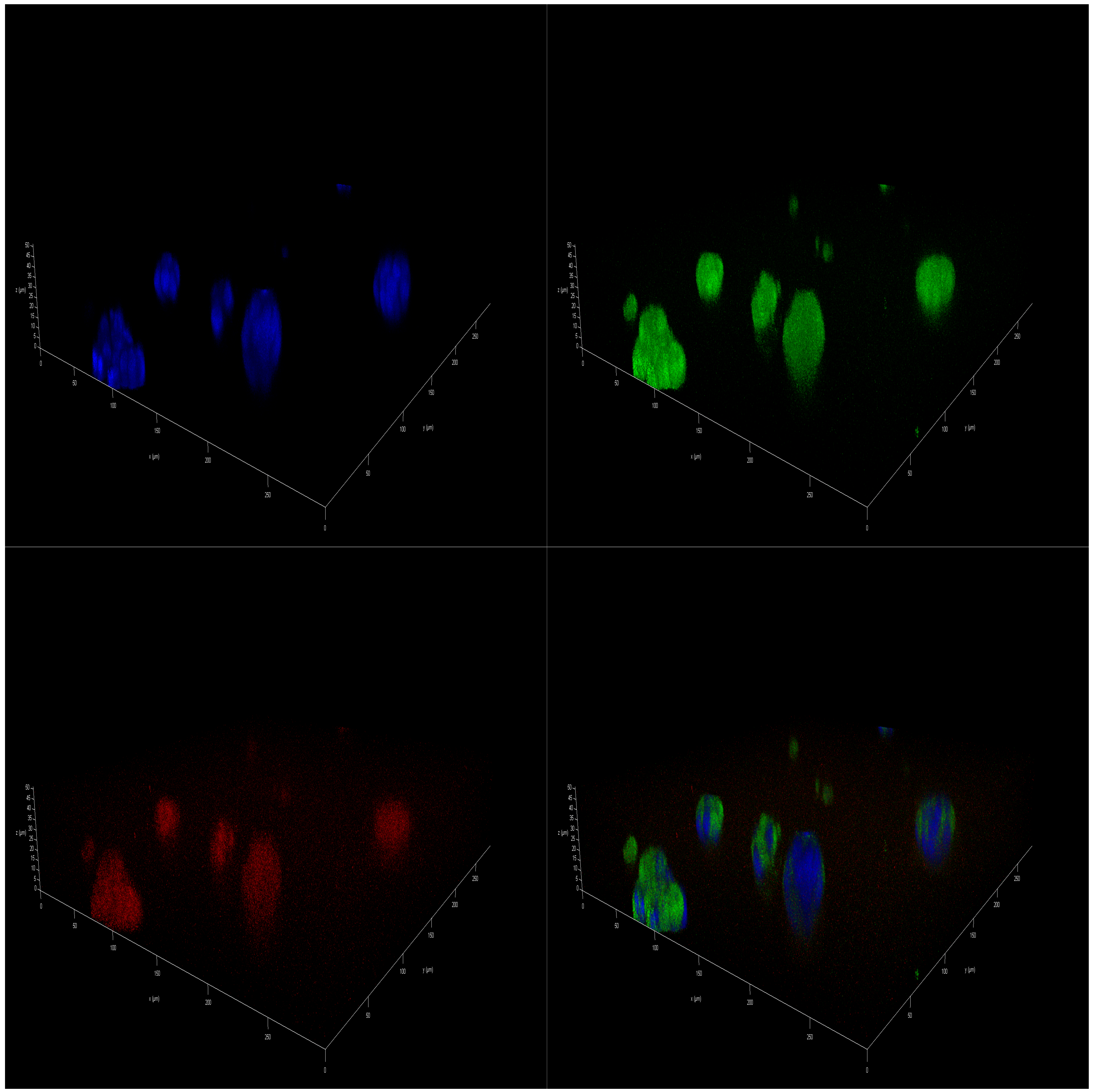
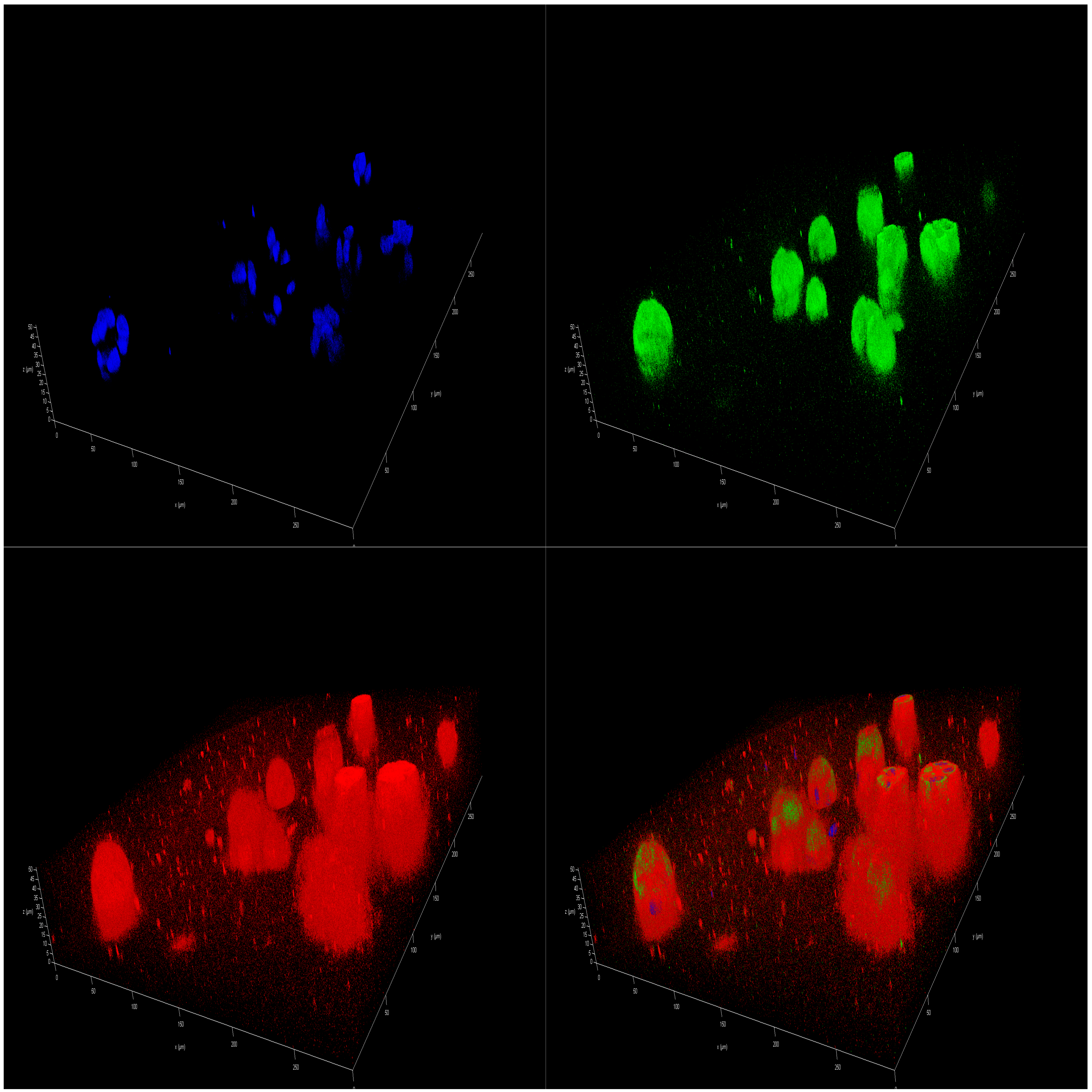
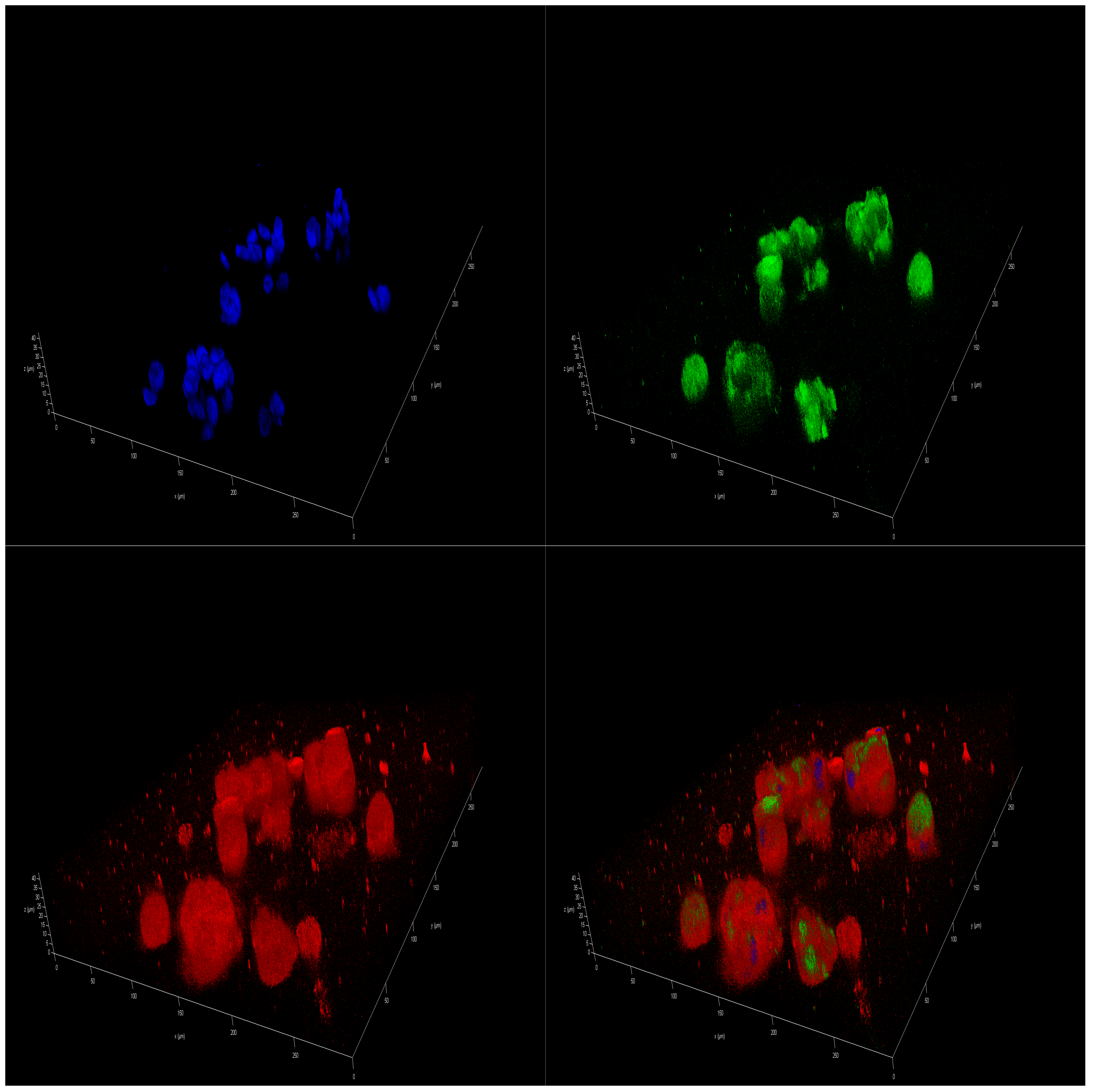
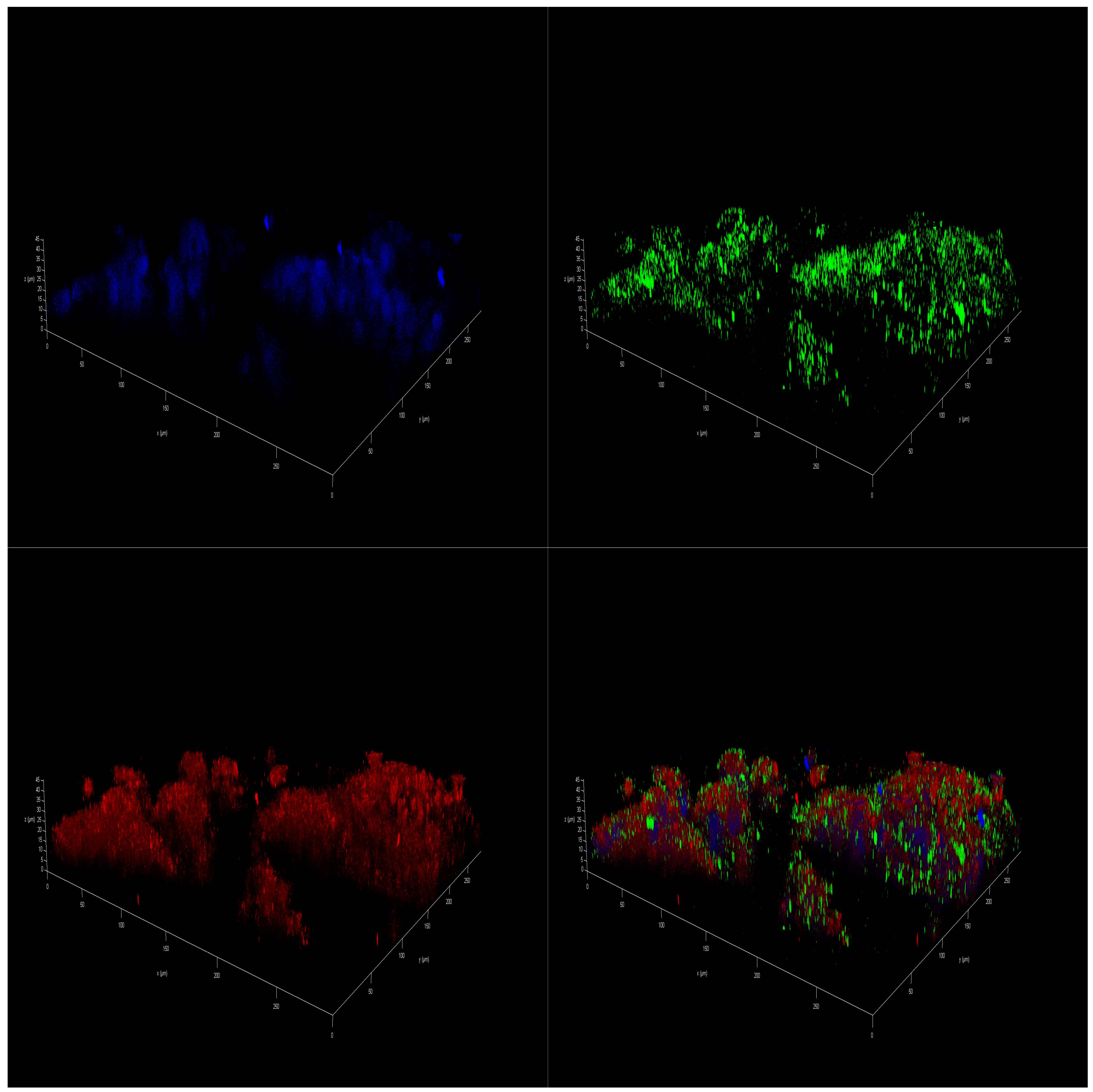
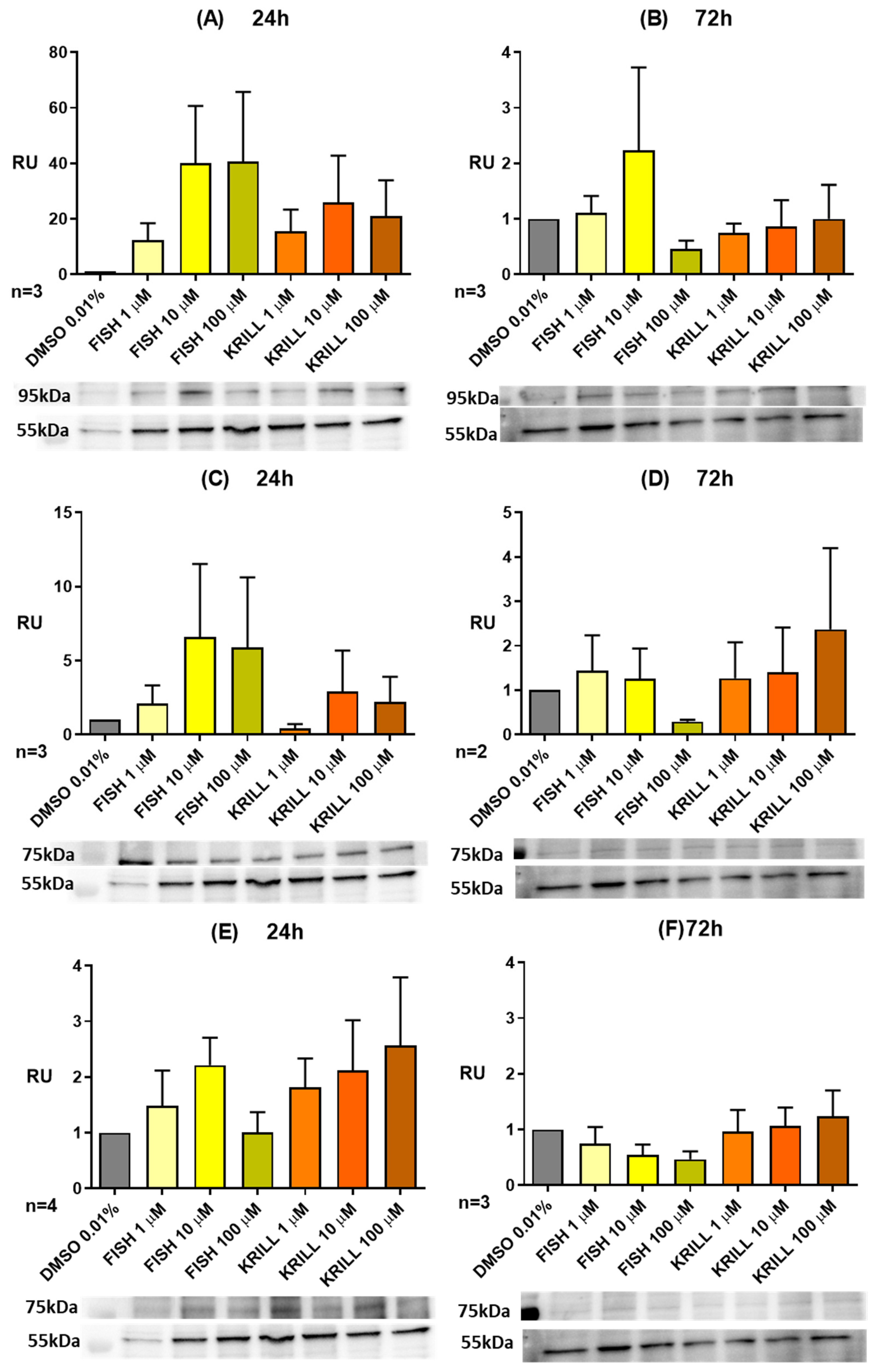
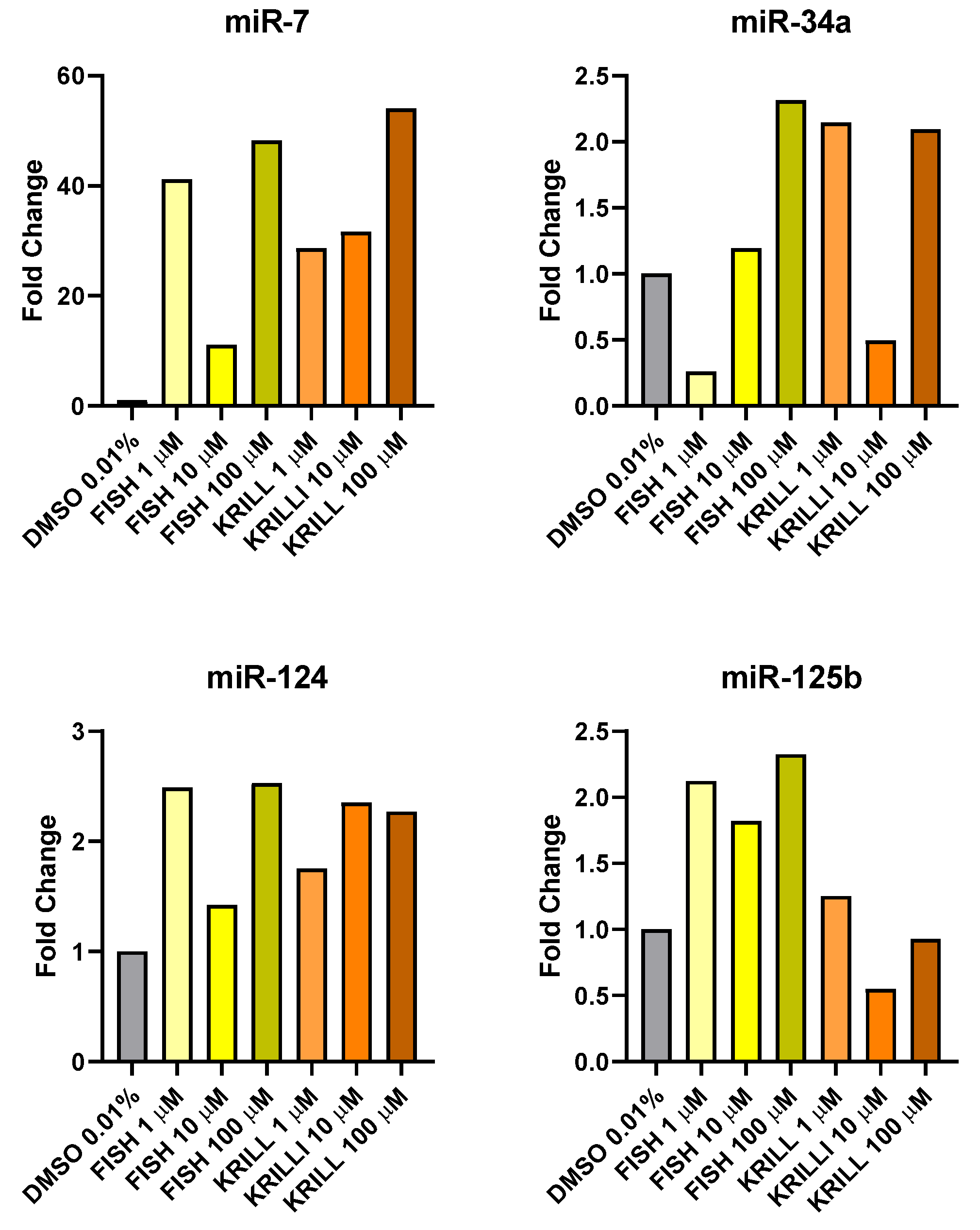
2.5. Effect of Polar Marine Oils on the Expression of NB Signaling Proteins and miRNAs
3. Materials and Methods
3.1. Fatty Acid Composition, Fractional Characterization, and Use for Cell Culture of the Tested Oils
3.2. Cell Lines Culture
3.3. Alginate–Carboxymethyl Chitosan Spheroids
3.4. Cell Viability and Metabolism Assay
3.5. Immunofluorescence Staining
3.6. Western Blot
3.7. RNA Extraction
3.8. Reverse Transcription and Real Time-qPCR
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berquin, I.M.; Edwards, I.J.; Chen, Y.Q. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008, 269, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Gleissman, H.; Yang, R.; Martinod, K.; Lindskog, M.; Serhan, C.N.; Johnsen, J.I.; Kogner, P. Docosahexaenoic acid metabolome in neural tumors: Identification of cytotoxic intermediates. FASEB J. 2010, 24, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Cancer and inflammation: Implications for pharmacology and therapeutics. Clin. Pharmacol. Ther. 2010, 87, 401–406. [Google Scholar] [CrossRef]
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigraphy, D. Regulation of Inflammation in Carcer by Eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef]
- Roesler, R.; Dini, S.A.; Isolan, G.R. Neuroinflammation and immunoregulation in glioblastoma and brain metastases: Recent developments in imaging approaches. Clin. Exp. Immunol. 2021, 206, 314–324. [Google Scholar] [CrossRef]
- Dharmajaya, R.; Sari, D.K. Role and value of inflammatory markers in brain tumors: A case controlled study. Ann. Med. Surg. 2021, 63, 102107. [Google Scholar] [CrossRef]
- Blalock, E.M.; Chen, K.C.; Stromberg, A.J.; Norris, C.M.; Kadish, I.; Kraner, S.D.; Porter, N.M.; Landfield, P.W. Harnessing the power of gene microarrays for the study of brain aging and Alzheimer’s disease: Statistical reliability and functional correlation. Ageing Res. Rev. 2005, 4, 481–512. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Y.; Wang, Y.; Wu, J.; Song, L.; Xian, W.; Yuan, S.; Pei, L.; Shang, Y. Resolvin D1 promotes the interleukin-4-induced alternative activation in BV-2 microglial cells. J. Neuroinflamm. 2014, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, L.X.; Cao, D.L.; Gao, Y.J. Spinal injection of docosahexaenoic acid attenuates carrageenan-induced inflammatory pain through inhibition of microglia-mediated neuroinflammation in the spinal cord. Neuroscience 2013, 241, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Diau, G.-Y. The Influence of Dietary Docosahexaenoic Acid and Arachidonic Acid on Central Nervous System Polyunsaturated Fatty Acid Composition. Prostaglandins Leukot. Essent. Fat. Acids Essent Fat. Acids 2007, 77, 247–250. [Google Scholar] [CrossRef]
- Glomset, J.A. Role of docosahexaenoic acid in neuronal plasma membranes. Sci. STKE 2006, 2006, pe6. [Google Scholar] [CrossRef]
- Lo Van, A.; Fourmaux, B.; Picq, M.; Guichardant, M.; Lagarde, M.; Bernoud-Hubac, N. Synthesis and Identification of AceDoxyPC, a Protectin-Containing Structured Phospholipid, Using Liquid Chromatography/Mass Spectrometry. Lipids 2017, 52, 751–761. [Google Scholar] [CrossRef]
- Reynolds, L.M.; Dalton, C.F.; Reynolds, G.P. Phospholipid fatty acids and neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 2001, 309, 193–196. [Google Scholar] [CrossRef]
- Serhan, C.; Chiang, N.; Van Dyke, T. Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef]
- Gleissman, H.; Segerström, L.; Hamberg, M.; Ponthan, F.; Lindskog, M.; Johnsen, J.I.; Kogner, P. Omega-3 fatty acid supplementation delays the progression of neuroblastoma in vivo. Int. J. Cancer 2011, 128, 1703–1711. [Google Scholar] [CrossRef]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.Y.; Lee, K.S.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Stahl, A.; Chen, J.; Seaward, M.R.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Connor, K.M.; Aderman, C.M.; Liclican, E.; et al. 5-Lipoxygenase Metabolite 4-HDHA Is a Mediator of the Antiangiogenic Effect of ω-3 Polyunsaturated Fatty Acids. Sci. Transl. Med. 2011, 3, 69ra12. [Google Scholar] [CrossRef]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Tong, Z.; Vega, O.M.; Morales-Martínez, M.; Abkenari, S.; Pedraza-Chaverri, J.; Huerta-Yepez, S. Importance of the role of ω-3 and ω-6 polyunsaturated fatty acids in the progression of brain cancer. Brain Sci. 2020, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Cantin, A.M.; Rousseau, É.; Sirois, M.; Sirois, C.; Rizcallah, E.; Fortin, S. Proresolving action of docosahexaenoic acid monoglyceride in lung inflammatory models related to cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Khaddaj-Mallat, R.; Morin, C.; Rousseau, É. Novel n-3 PUFA monoacylglycerides of pharmacological and medicinal interest: Anti-inflammatory and anti-proliferative effects. Eur. J. Pharmacol. 2016, 792, 70–77. [Google Scholar] [CrossRef]
- Bell, H.S.; Wharton, S.B.; Leaver, H.A.; Whittle, I.R. Effects of N-6 essential fatty acids on glioma invasion and growth: Experimental studies with glioma spheroids in collagen gels. J. Neurosurg. 1999, 91, 989–996. [Google Scholar] [CrossRef]
- Leaver, H.A.; Bell, H.S.; Rizzo, M.T.; Ironside, J.W.; Gregor, A.; Wharton, S.B.; Whittle, I.R. Antitumour and pro-apoptotic actions of highly unsaturated fatty acids in glioma. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 19–29. [Google Scholar] [CrossRef][Green Version]
- Morin, S.; Simard, M.; Flamand, N.; Pouliot, R. Biological action of docosahexaenoic acid in a 3D tissue-engineered psoriatic skin model: Focus on the PPAR signaling pathway. Biochim. Biophys. Acta- Mol. Cell Biol. Lipids 2021, 1866, 159032. [Google Scholar] [CrossRef]
- Gevariya, N.; Lachance, G.; Robitaille, K.; Beauparlant, C.J.; Beaudoin, L.; Fournier, E.; Fradet, Y.; Droit, A.; Julien, P.; Marette, A.; et al. Omega-3 eicosapentaenoic acid reduces prostate tumor vascularity. Mol. Cancer Res. 2021, 19, 516–527. [Google Scholar] [CrossRef]
- Guesnet, P.; Alessandri, J.M. Docosahexaenoic acid (DHA) and the developing central nervous system (CNS)—Implications for dietary recommendations. Biochimie 2011, 93, 7–12. [Google Scholar] [CrossRef]
- Moore, S.A. Polyunsaturated Fatty Acid Synthesis and Release. J. Mol. Neurosci. 2001, 16, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Shafat, I.; Jones, P.J.H. Supplementation of krill oil with high phospholipid content increases sum of EPA and DHA in erythrocytes compared with low phospholipid krill oil. Lipids Health Dis. 2015, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Xu, J.; Wang, Y.M.; Xue, C.H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Ezquerra-Brauer, J.M.; Aubourg, S.P. Recent trends for the employment of jumbo squid (Dosidicus gigas) by-products as a source of bioactive compounds with nutritional, functional and preservative applications: A review. Int. J. Food Sci. Technol. 2019, 54, 987–998. [Google Scholar] [CrossRef]
- Ferraro, V.; Cruz, I.B.; Jorge, R.F.; Malcata, F.X.; Pintado, M.E.; Castro, P.M.L. Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Res. Int. 2010, 43, 2221–2233. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; Volume 35. [Google Scholar]
- Méndez, L.; Rodríguez, A.; Aubourg, S.P.; Medina, I. Low-Toxicity Solvents for the Extraction of Valuable Lipid Compounds from Octopus (Octopus vulgaris) Waste. Foods 2023, 12, 3631. [Google Scholar] [CrossRef]
- Rodríguez, A.; Trigo, M.; Aubourg, S.P.; Medina, I. Optimisation of Low-Toxicity Solvent Employment for Total Lipid and Tocopherol Compound Extraction from Patagonian Squid By-Products. Foods 2023, 12, 504. [Google Scholar] [CrossRef]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M.; et al. Three-dimensional Tissue Culture Based on Magnetic Cell Levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.R.; Raphael, R.M.; Kilian, T.C. 3D Cell Culturing with the Bio-AssemblerTM from Nano3D Biosciences; A Nano3D Biosciences White Paper; Nano3D Biosciences, Inc.: Houston, TX, USA, 2013; pp. 1–11. [Google Scholar]
- Aubourg, S.P.; Trigo, M.; González, M.J.; Lois, S.; Medina, I. Comparative Study of Bioactive Lipid Extraction from Squid (Doryteuthis gahi) by-Products by Green Solvents. Foods 2022, 11, 2188. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Eggert, A.; Caron, H. Neuroblastoma: Biology, Prognosis, and Treatment. Hematol. Oncol. Clin. N. Am. 2010, 24, 65–86. [Google Scholar] [CrossRef]
- Maris, J.M. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr. Opin. Pediatr. 2005, 17, 7–13. [Google Scholar] [CrossRef]
- So, W.W.; Liu, W.N.; Leung, K.N. Omega-3 polyunsaturated fatty acids trigger cell cycle arrest and induce apoptosis in human neuroblastoma LA-N-1 cells. Nutrients 2015, 7, 6956–6973. [Google Scholar] [CrossRef]
- Barnés, C.M.; Prox, D.; Christison-lagay, E.A.; Le, H.D.; Short, S.; Cassiola, F.; Panigrahy, D.; Chaponis, D.; Butterfield, C.; Nehra, D.; et al. Inhibition of neuroblastoma cell proliferation with omega-3 fatty acids and treatment of a murine model of human neuroblastoma using a diet enriched with omega-3 fatty acids in combination with sunitinib. Transl. Investig. 2012, 71, 168–178. [Google Scholar] [CrossRef]
- Rajasekharan Nair, J.; Pillai, D.; Joseph, S.M.; Gomathi, P.; Senan, P.V.; Sherief, P.M. Cephalopod research and bioactive substances. Indian J. Mar. Sci. 2011, 40, 13–27. [Google Scholar]
- Aubourg, S.P.; Trigo, M.; Prego, R.; Cobelo-García, A.; Medina, I. Nutritional and healthy value of chemical constituents obtained from Patagonian squid (Doryteuthis gahi) by-products captured at different seasons. Foods 2021, 10, 2144. [Google Scholar] [CrossRef]
- Saito, H.; Sakai, M.; Wakabayashi, T. Characteristics of the lipid and fatty acid compositions of the Humboldt squid, Dosidicus gigas: The trophic relationship between the squid and its prey. Eur. J. Lipid Sci. Technol. 2014, 116, 360–366. [Google Scholar] [CrossRef]
- Hari, A.D.; Naidu, V.G.M.; Das, U.N. n-6 and n-3 Fatty acids and their metabolites augment inhibitory action of doxorubicin on the proliferation of human neuroblastoma (IMR-32) cells by enhancing lipid peroxidation and suppressing Ras, Myc, and Fos. BioFactors 2018, 44, 387–401. [Google Scholar] [CrossRef]
- Lindskog, M.; Gleissman, H.; Ponthan, F.; Castro, J.; Kogner, P.; Johnsen, J.I. Neuroblastoma cell death in response to docosahexaenoic acid: Sensitization to chemotherapy and arsenic-induced oxidative stress. Int. J. Cancer 2006, 118, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H.; Clark, G.O.; Scherer, P.E.; Orci, L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2010, 1801, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Louie, S.M.; Daniele, J.R.; Tran, Q.; Dillin, A.; Zoncu, R.; Nomura, D.K.; Olzmann, J.A. DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev. Cell 2017, 42, 9–21.e5. [Google Scholar] [CrossRef]
- Mitrousis, N.; Tropepe, V.; Hermanson, O. Post-translational modifications of histones in vertebrate neurogenesis. Front. Neurosci. 2015, 9, 483. [Google Scholar] [CrossRef]
- Lindberg, N.; Kastemar, M.; Olofsson, T.; Smits, A.; Uhrbom, L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene 2009, 28, 2266–2275. [Google Scholar] [CrossRef]
- Faragó, N.; Fehér, L.Z.; Kitajka, K.; Das, U.N.; Puskás, L.G. MicroRNA profile of polyunsaturated fatty acid treated glioma cells reveal apoptosis-specific expression changes. Lipids Health Dis. 2011, 10, 173. [Google Scholar] [CrossRef]
- Kim, S.; Jing, K.; Shin, S.; Jeong, S.; Han, S.H.; Oh, H.; Yoo, Y.S.; Han, J.; Jeon, Y.J.; Heo, J.Y.; et al. ω3-polyunsaturated fatty acids induce cell death through apoptosis and autophagy in glioblastoma cells: In vitro and in vivo. Oncol. Rep. 2018, 39, 239–246. [Google Scholar] [CrossRef]
- Huerta-Yépez, S.; Tirado-Rodriguez, A.B.; Hankinson, O. Papel de las dietas ricas en omega-3 y omega-6 en el desarrollo del cáncer. Bol. Med. Hosp. Infant. Mex. 2016, 73, 446–456. [Google Scholar] [CrossRef]
- Das, U.N. From bench to the clinic: γ-linolenic acid therapy of human gliomas. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 539–552. [Google Scholar] [CrossRef]
- Seydi, E.; Sadeghi, H.; Ramezani, M.; Mehrpouya, L.; Pourahmad, J. Selective Toxicity Effect of Fatty Acids Omega-3, 6 and 9 Combination on Glioblastoma Neurons through their Mitochondria. Drug Res 2021, 72, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Preusser, M.; De Ribaupierre, S.; Wöhrer, A.; Erridge, S.C.; Hegi, M.; Weller, M.; Stupp, R. Current concepts and management of glioblastoma. Ann. Neurol. 2011, 70, 9–21. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.H.; Chang, C.L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef]
- Cleland, N.R.W.; Bruce, K.D. Fatty acid sensing in the brain: The role of glial-neuronal metabolic crosstalk and horizontal lipid flux. Biochimie 2024, 223, 166–178. [Google Scholar] [CrossRef]
- Alaarg, A.; Jordan, N.Y.; Verhoef, J.J.F.; Metselaar, J.M.; Storm, G.; Kok, R.J. Docosahexaenoic acid liposomes for targeting chronic inflammatory diseases and cancer: An in vitro assessment. Int. J. Nanomed. 2016, 11, 5027–5040. [Google Scholar] [CrossRef]
- Mendanha, D.; Gimondi, S.; Costa, B.M.; Ferreira, H.; Neves, N.M. Microfluidic-derived docosahexaenoic acid liposomes for glioblastoma therapy. Nanomed. Nanotechnol. Biol. Med. 2023, 53, 102704. [Google Scholar] [CrossRef]
- Albino, A.P.; Juan, G.; Traganos, F.; Reinhart, L.; Connolly, J.; Rose, D.P.; Darzynkiewicz, Z. Cell cycle arrest and apoptosis of melanoma cells by docosahexaenoic acid: Association with decreased pRb phosphorylation. Cancer Res. 2000, 60, 4139–4145. [Google Scholar]
- Zhang, C.; Yu, H.; Shen, Y.; Ni, X.; Shen, S.; Das, U.N. Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch. Med. Sci. 2015, 11, 1081–1094. [Google Scholar]
- Hawkins, R.A.; Sangster, K.; Arends, M.J. Apoptotic death of pancreatic cancer cells induced by polyunsaturated fatty acids varies with double bond number and involves an oxidative mechanism. J. Pathol. 1998, 185, 61–70. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Y.; Fang, M.-S.; Li, Y.; Li, W.; Mahaman, Y.A.R.; Zeng, K.; Xia, Y.; Ke, D.; Liu, R.; et al. ω-3PUFAs Improve Cognitive Impairments Through Ser133 Phosphorylation of CREB Upregulating BDNF/TrkB Signal in Schizophrenia. Neurotherapeutics 2020, 17, 1271–1286. [Google Scholar] [CrossRef]
- Bianchini, F.; Giannoni, E.; Serni, S.; Chiarugi, P.; Calorini, L. 22: 6n-3 DHA inhibits differentiation of prostate fibroblasts into myofibroblasts and tumorigenesis. Br. J. Nutr. 2012, 108, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, I.A.A.; Brown, I.; Wahle, K.W.J.; Heys, S.D. Enhancing cytotoxic therapies for breast and prostate cancers with polyunsaturated fatty acids. Nutr. Cancer 2010, 62, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jia, X.; Hou, L.; Liu, X.; Gao, Q. Involvement of apoptotic pathways in docosahexaenoic acid-induced benefit in prostate cancer: Pathway-focused gene expression analysis using RT2 Profile PCR Array System. Lipids Health Dis. 2017, 16, 59. [Google Scholar] [CrossRef]
- Sottejeau, Y.; Patel, A.M.; Gerber, G.; Pierre, S.V.; Maixent, J.M. Effect of a novel Omegacoeur®/Doluperine® nutritional combination on human embryonic kidney cell viability. Cell. Mol. Biol. 2010, 56, 1400–1409. [Google Scholar] [CrossRef]
- Crupi, R.; Marino, A.; Cuzzocrea, S. n-3 Fatty Acids: Role in Neurogenesis and Neuroplasticity. Curr. Med. Chem. 2013, 20, 2953–2963. [Google Scholar] [CrossRef]
- Aryal, S.; Hussain, S.; Drevon, C.A.; Nagelhus, E.; Hvalby, Ø.; Jensen, V.; Walaas, S.I.; Davanger, S. Omega-3 fatty acids regulate plasticity in distinct hippocampal glutamatergic synapses. Eur. J. Neurosci. 2018, 49, 40–50. [Google Scholar] [CrossRef]
- Bie, N.; Feng, X.; Li, C.; Meng, M.; Wang, C. The Protective Effect of Docosahexaenoic Acid on PC12 Cells in Oxidative Stress Induced by H2O2 through the TrkB-Erk1/2-CREB Pathway. ACS Chem. Neurosci. 2021, 12, 3433–3444. [Google Scholar] [CrossRef]
- Akerele, O.A.; Cheema, S.K. Maternal diet high in Omega-3 fatty acids upregulate genes involved in neurotrophin signalling in fetal brain during pregnancy in C57BL/6 mice. Neurochem. Int. 2020, 138, 104778. [Google Scholar] [CrossRef]
- Kraemer, B.R.; Snow, J.P.; Vollbrecht, P.; Pathak, A.; Valentine, W.M.; Deutch, A.Y.; Carter, B.D. A role for the p75 neurotrophin receptor in axonal degeneration and apoptosis induced by oxidative stress. J. Biol. Chem. 2014, 289, 21205–21216. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Kalueff, A.V.; Song, C. Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates BDNF function in a rodent model of neuroinflammation induced by central interleukin-1β administration. Eur. J. Nutr. 2018, 57, 1781–1791. [Google Scholar] [CrossRef]
- Song, C.; Xiang, Y.Z.; Manku, M. Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: Effects of chronic ethyl-eicosapentaenoate treatment. J. Neurosci. 2009, 29, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shieh, C.H.; Wu, Y.S.; Kalueff, A.; Gaikwad, S.; Su, K.P. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: Acting separately or synergistically? Prog. Lipid Res. 2016, 62, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Ai, W.; Banik, N.L.; Ray, S.K. Overexpression of miR-7-1 increases efficacy of green tea polyphenols for induction of apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-DZ cells. Neurochem. Res. 2013, 38, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.E.L.; Tang, B.L. miR-34a in Neurophysiology and Neuropathology. J. Mol. Neurosci. 2019, 67, 235–246. [Google Scholar] [CrossRef]
- Le, M.T.N.; Xie, H.; Zhou, B.; Chia, P.H.; Rizk, P.; Um, M.; Udolph, G.; Yang, H.; Lim, B.; Lodish, H.F. MicroRNA-125b Promotes Neuronal Differentiation in Human Cells by Repressing Multiple Targets. Mol. Cell. Biol. 2009, 29, 5290–5305. [Google Scholar] [CrossRef]
- Sharif, S.; Ghahremani, M.H.; Soleimani, M. Induction of morphological and functional differentiation of human neuroblastoma cells by miR-124. J. Biosci. 2017, 42, 555–563. [Google Scholar] [CrossRef]
- Dai, X.; Li, M.; Geng, F. Omega-3 polyunsaturated fatty acids eicosapentaenoic acid and docosahexaenoic acid enhance dexamethasone sensitivity in multiple myeloma cells by the p53/miR-34a/Bcl-2 axis. Biochemistry 2017, 82, 826–833. [Google Scholar] [CrossRef]
- Yip, P.K.; Bowes, A.L.; Hall, J.C.E.; Burguillos, M.A.; Richard Ip, T.H.; Baskerville, T.; Liu, Z.H.; Mohamed, M.A.E.K.; Getachew, F.; Lindsay, A.D.; et al. Docosahexaenoic acid reduces microglia phagocytic activity via miR-124 and induces neuroprotection in rodent models of spinal cord contusion injury. Hum. Mol. Genet. 2019, 28, 2427–2448. [Google Scholar] [CrossRef]
- Ding, M.; Ma, H.; Du, H.; Yang, Y.; Yu, M.; Zhang, C. Omega-3 fatty acid normalizes postsynaptic density proteins via miRNAs regulation in hippocampus and prevents DEHP-induced impairment of learning and memory in mice. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chakraborty, N.; Muhie, S.; Kumar, R.; Gautam, A.; Srinivasan, S.; Sowe, B.; Dimitrov, G.; Miller, S.A.; Jett, M.; Hammamieh, R. Contributions of polyunsaturated fatty acids (PUFA) on cerebral neurobiology: An integrated omics approach with epigenomic focus. J. Nutr. Biochem. 2017, 42, 84–94. [Google Scholar] [CrossRef]
- Crespo, M.C.; Tomé-Carneiro, J.; Gómez-Coronado, D.; Burgos-Ramos, E.; Garciá-Serrano, A.; Martín-Hernández, R.; Baliyan, S.; Fontecha, J.; Venero, C.; Dávalos, A.; et al. Modulation of miRNA expression in aged rat hippocampus by buttermilk and krill oil. Sci. Rep. 2018, 8, 3993. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
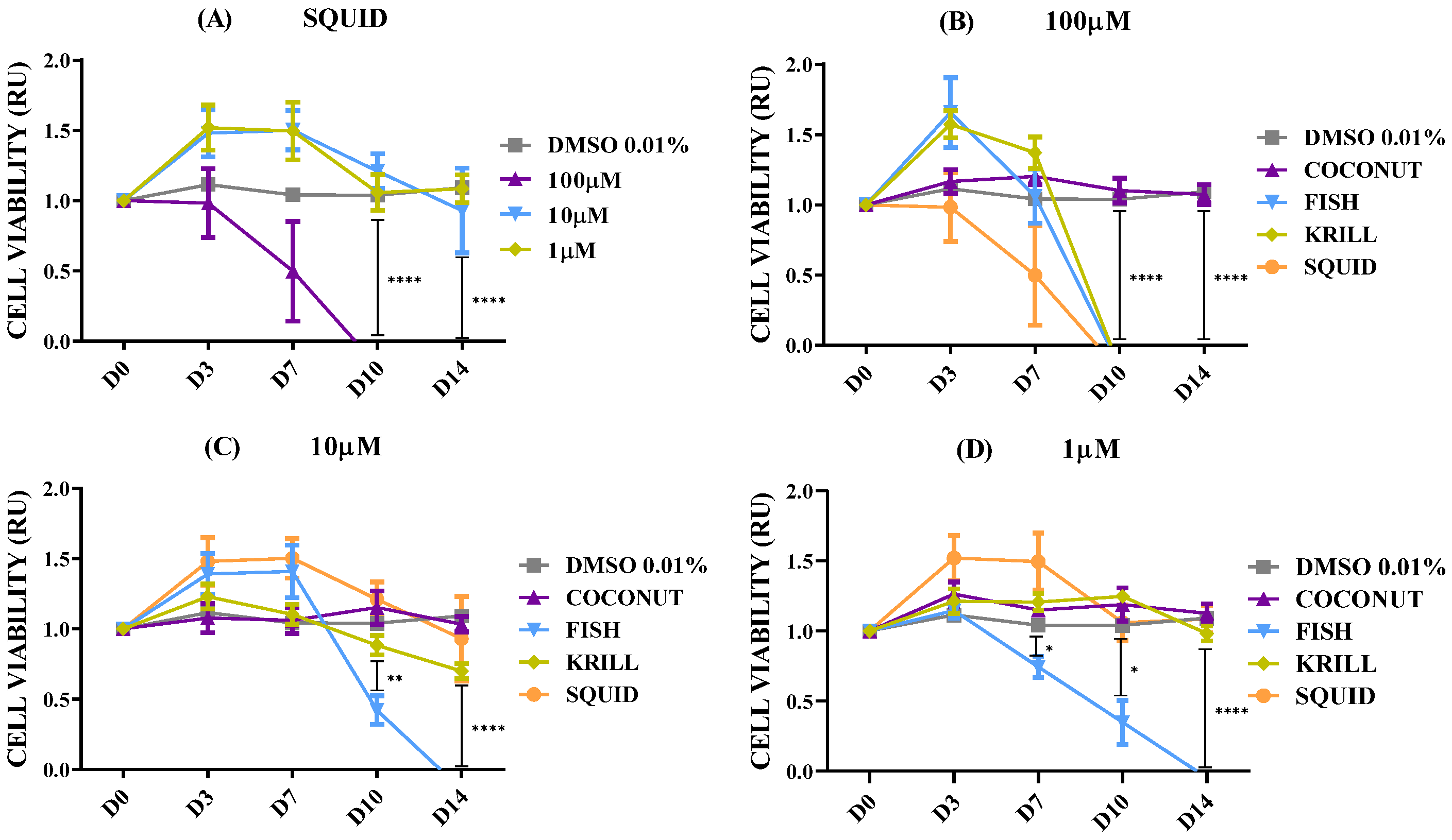
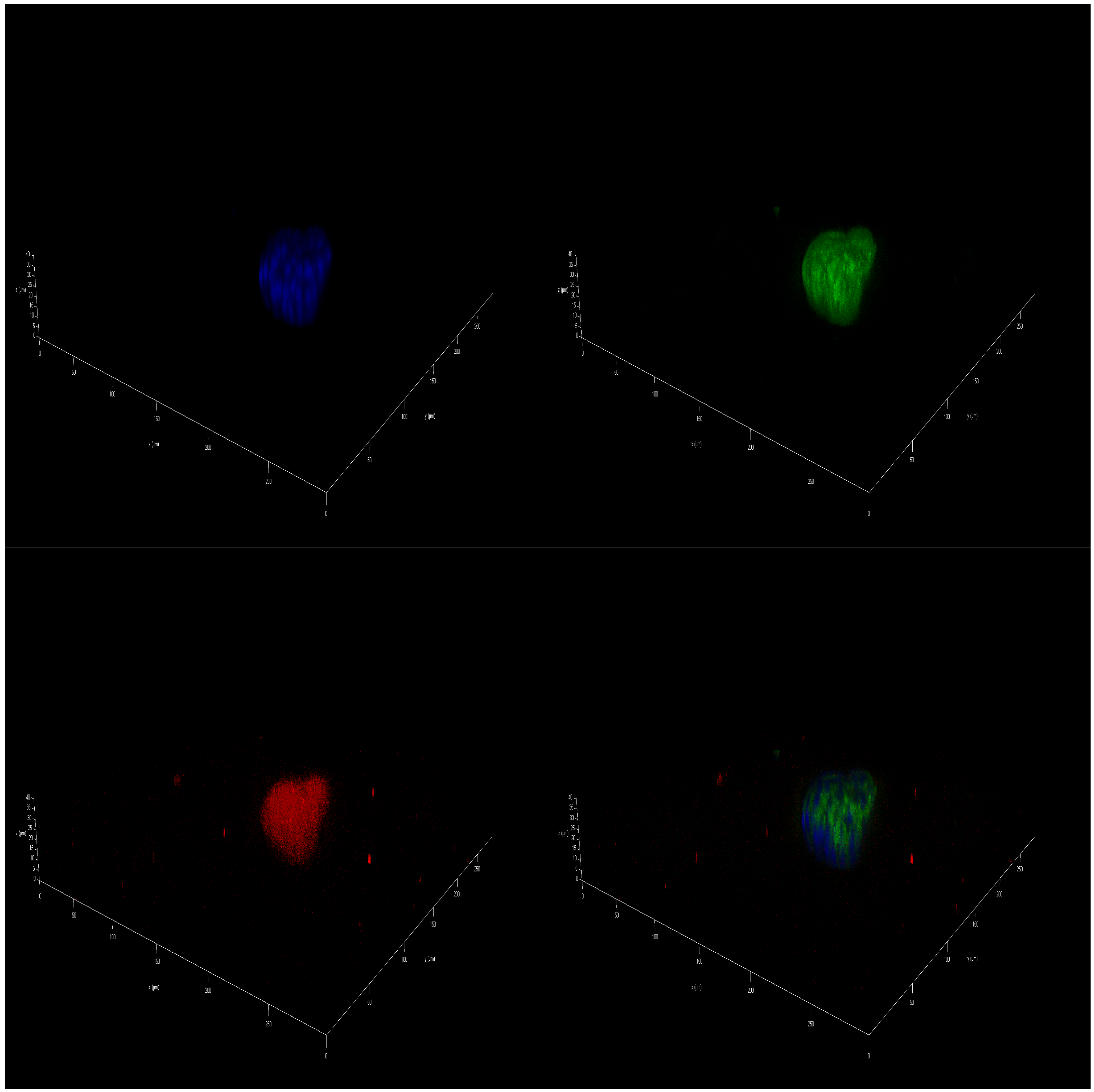
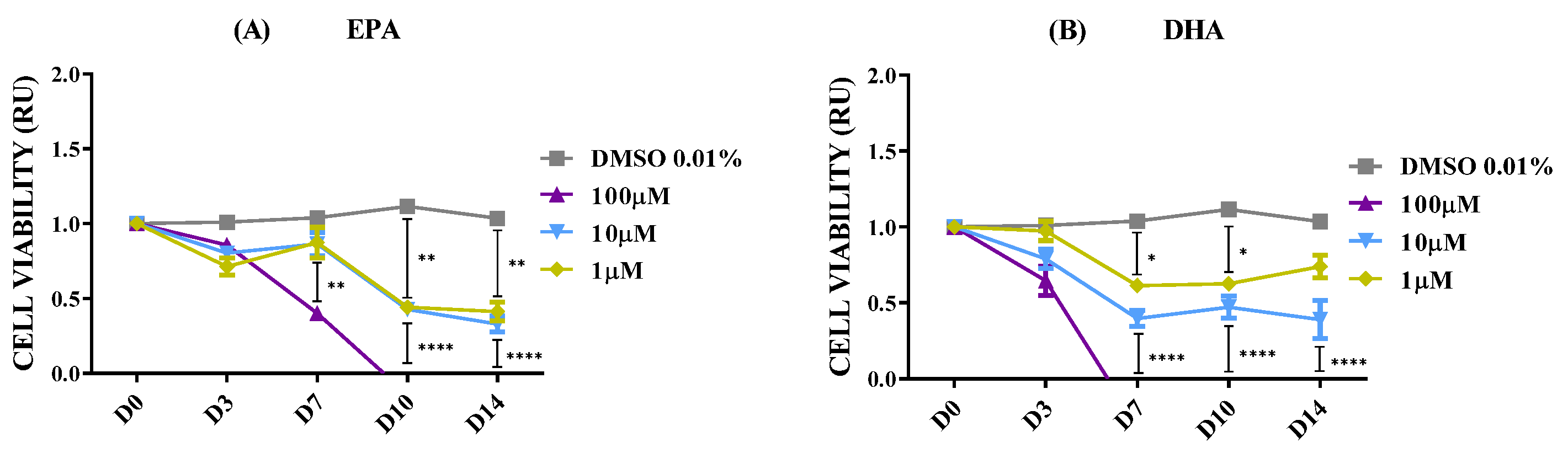
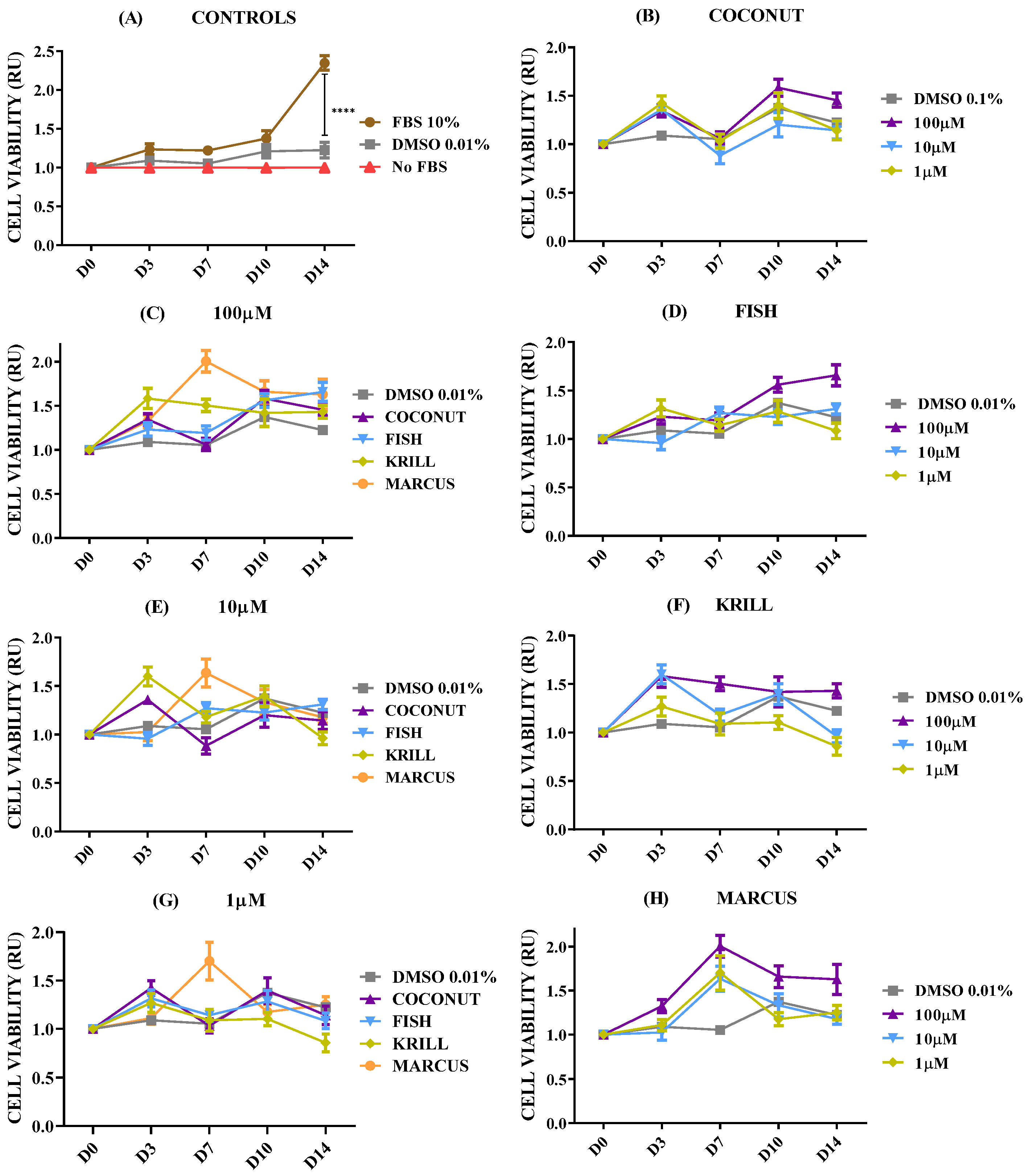
| Fatty Acid | Coconut | Fish | Krill | Squid |
|---|---|---|---|---|
| 8:0 | 30.59 | - | - | - |
| 10:0 | 55.95 | - | - | - |
| 11:0 | 44.29 | - | - | - |
| 12:0 | 270.03 | - | - | - |
| 14:0 | 87.59 | 26.69 | 36.56 | 5.07 |
| 15:0 | - | 1.92 | 1.38 | 0.70 |
| 16:0 | 34.02 | 61.85 | 57.59 | 28.98 |
| 16:1ω7 | - | 28.35 | 16.50 | 2.62 |
| 17:0 | - | 2.70 | 5.49 | 1.10 |
| 18:0 | 11.11 | 15.13 | 3.71 | 4.42 |
| 18:1ω9 | 17.86 | 33.32 | 27.69 | 5.47 |
| 18:1ω7 | - | 10.39 | 15.96 | 3.15 |
| 18:2ω6 | 2.59 | 4.05 | 4.66 | 0.88 |
| 20:0 | - | 1.81 | 0.25 | 0.22 |
| 18:3ω3 | - | 1.72 | 3.95 | 0.67 |
| 20:1ω9 | - | 2.48 | 1.66 | 5.33 |
| 18:4ω3 | - | 5.39 | 10.69 | 2.13 |
| 20:2ω6 | - | 0.68 | 0.29 | 0.50 |
| 20:3ω6 | - | 0.45 | 0.21 | 0.00 |
| 20:4ω6 | - | 3.25 | 0.99 | 2.43 |
| 22:1ω11 | - | 1.25 | 0.34 | 0.89 |
| 22:1ω9 | - | 0.42 | 1.20 | 0.66 |
| 20:4ω3 | - | 1.83 | 0.95 | 0.46 |
| 20:5ω3 (EPA) | - | 45.27 | 36.24 | 19.54 |
| 22:4ω6 | - | 1.98 | 1.15 | 0.31 |
| 24:1ω9 | - | 0.64 | 0.25 | 0.78 |
| 22:5ω6 | - | 1.14 | 0.13 | 0.36 |
| 22:5ω3 | - | 4.52 | 0.90 | 0.52 |
| 22:6ω3 (DHA) | - | 24.30 | 19.58 | 32.78 |
| total mmol/100 g | 554.03 | 281.54 | 248.31 | 119.95 |
| total mmol/g | 5.54 | 2.82 | 2.48 | 1.20 |
| Lipid Classes | Coconut | Fish | Krill | Squid |
|---|---|---|---|---|
| PL 1 | - | - | 32.31 | 43.36 |
| O-A-DAG 2 | - | 1.26 | 1.19 | 0.84 |
| MG 3 | - | - | - | 3.43 |
| DG 4 | - | t 9 | t 9 | - |
| CH 5 | - | t 9 | 6.96 | 11.13 |
| FFA 6 | - | - | 19.18 | 31.97 |
| TG 7 | 99 | 95 | 40.37 | t 9 |
| CH-ES 8 | - | - | - | 9.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freiría-Martínez, L.; Oliva-Montero, J.M.; Rodríguez-Tébar, A.; Hermanson, O.; Aubourg, S.P.; Spuch, C.; Medina, I. Selective Antiproliferative Effects of Marine Oils on Neuroblastoma Cells in 3D Cultures. Mar. Drugs 2025, 23, 268. https://doi.org/10.3390/md23070268
Freiría-Martínez L, Oliva-Montero JM, Rodríguez-Tébar A, Hermanson O, Aubourg SP, Spuch C, Medina I. Selective Antiproliferative Effects of Marine Oils on Neuroblastoma Cells in 3D Cultures. Marine Drugs. 2025; 23(7):268. https://doi.org/10.3390/md23070268
Chicago/Turabian StyleFreiría-Martínez, Luís, Jose María Oliva-Montero, Ainhoa Rodríguez-Tébar, Ola Hermanson, Santiago P. Aubourg, Carlos Spuch, and Isabel Medina. 2025. "Selective Antiproliferative Effects of Marine Oils on Neuroblastoma Cells in 3D Cultures" Marine Drugs 23, no. 7: 268. https://doi.org/10.3390/md23070268
APA StyleFreiría-Martínez, L., Oliva-Montero, J. M., Rodríguez-Tébar, A., Hermanson, O., Aubourg, S. P., Spuch, C., & Medina, I. (2025). Selective Antiproliferative Effects of Marine Oils on Neuroblastoma Cells in 3D Cultures. Marine Drugs, 23(7), 268. https://doi.org/10.3390/md23070268









