Dehydration, Rehydration and Thermal Treatment: Effect on Bioactive Compounds of Red Seaweeds Porphyra umbilicalis and Porphyra linearis
Abstract
1. Introduction
2. Results and Discussion
2.1. Carotenoid Content
2.2. Phycobiliproteins
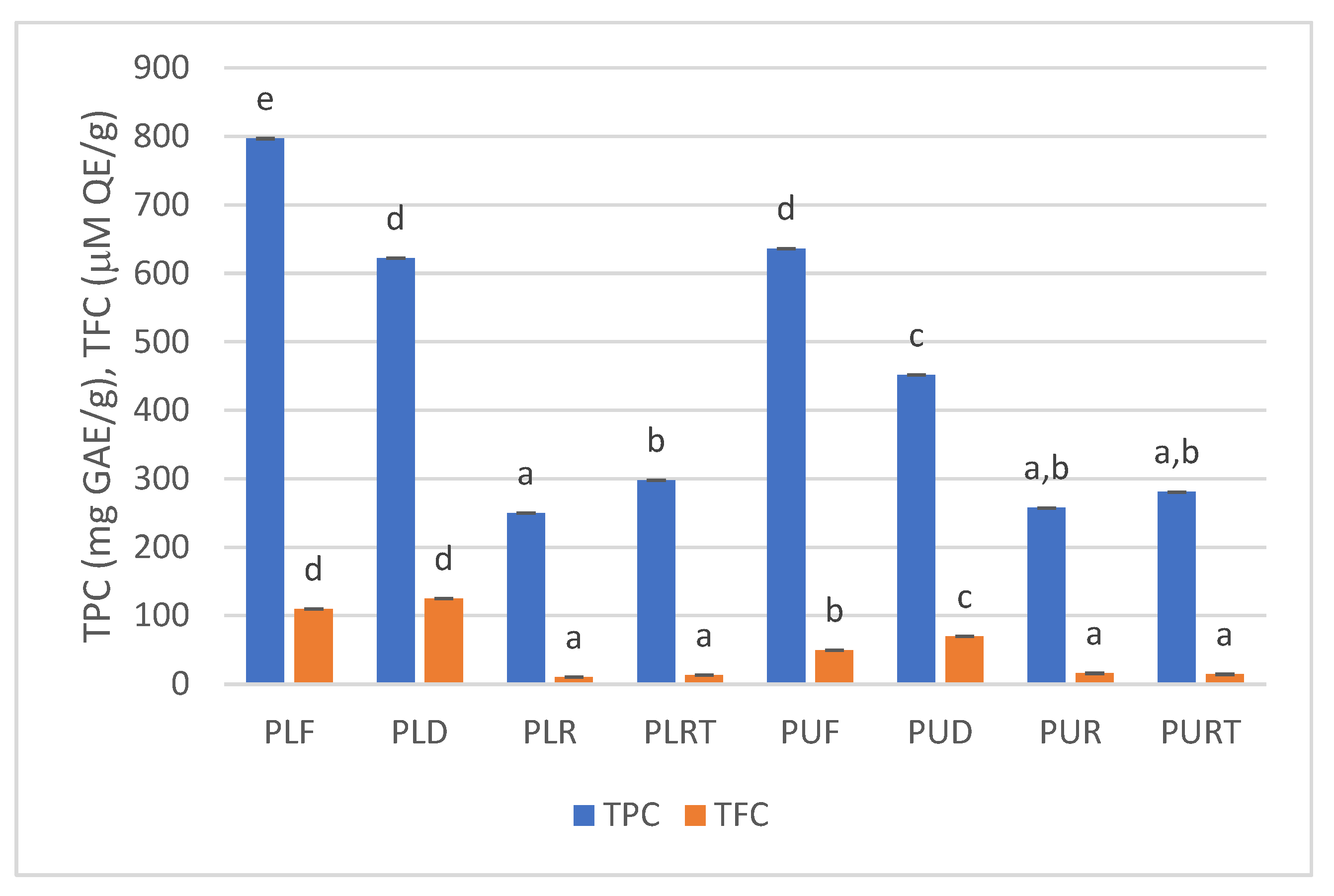
2.3. TPC and TFC
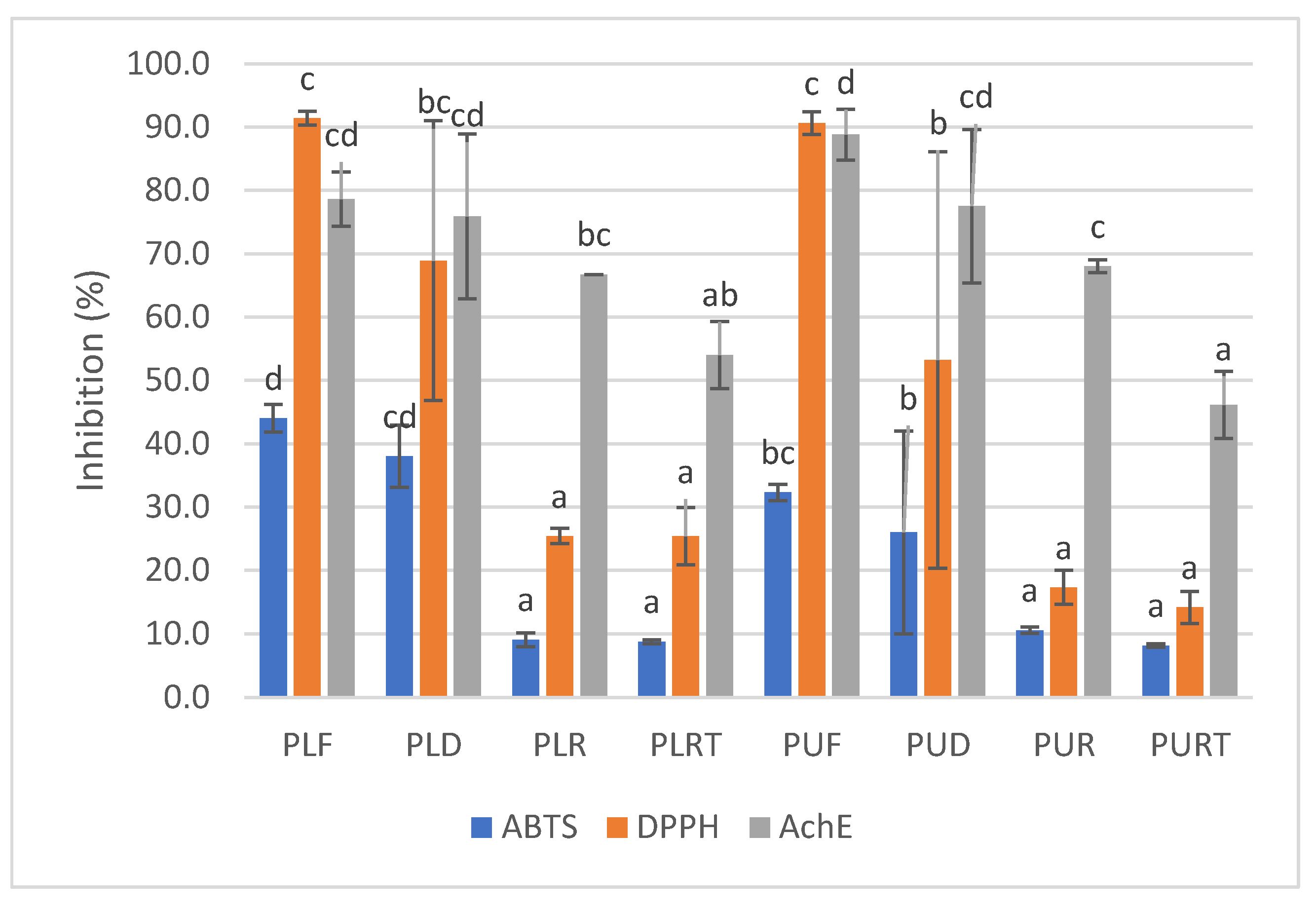
2.4. DPPH and ABTS Radical Scavenging Activity and AchE Inhibitory Activity
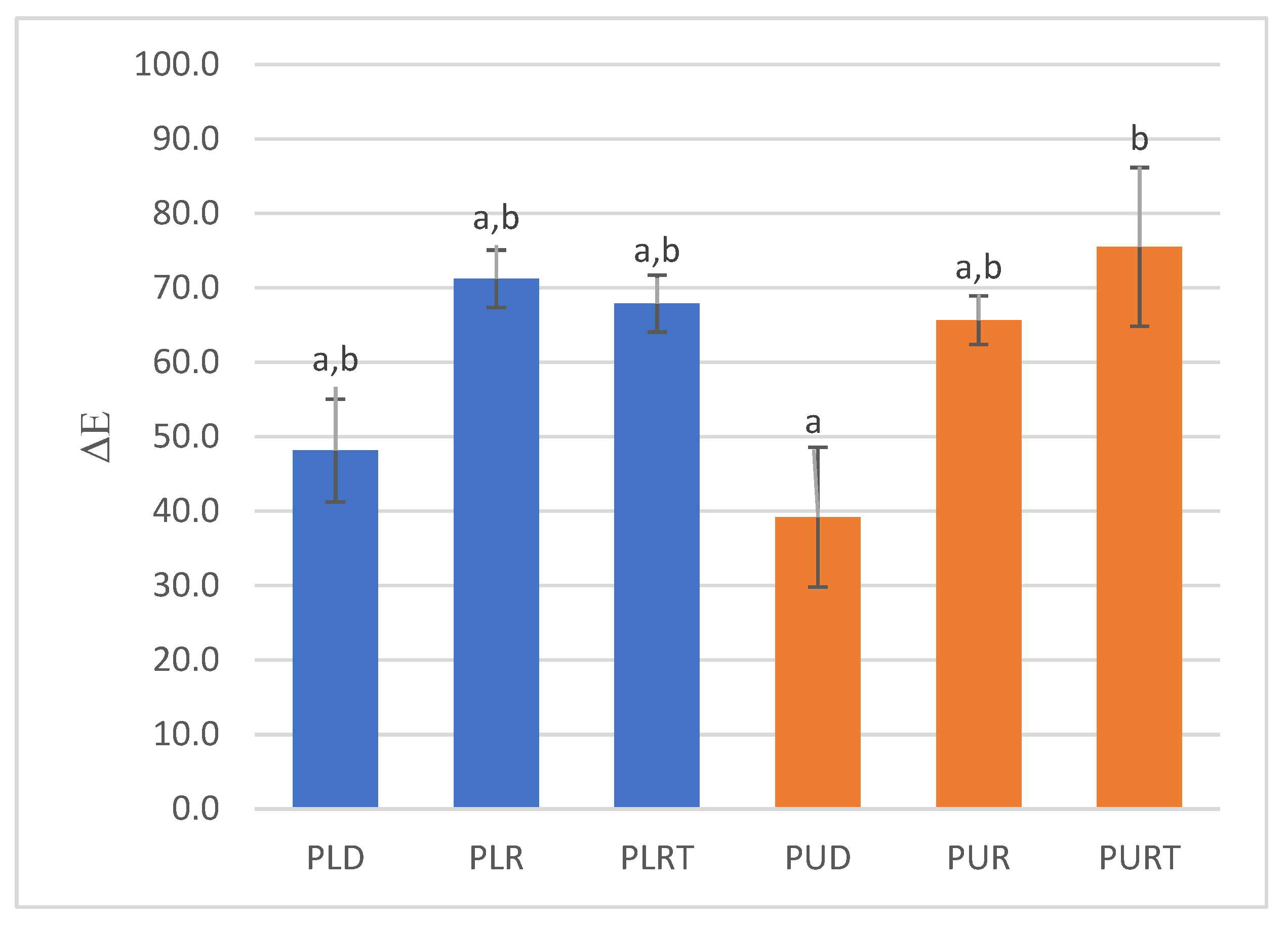
2.5. Color Analysis
3. Materials and Methods
3.1. Seaweed Species
3.2. Seaweed Treatments
3.2.1. Dehydration
3.2.2. Rehydration
3.2.3. Thermal Treatment
3.3. Seaweeds Analysis
3.3.1. Carotenoid Content
| Carotenoid | λ (nm) | MM (g mol−1) | ε (L mol−1 cm−1) | Reference |
|---|---|---|---|---|
| Neurosporene | 440 | 538.90 | 157,000 | [26] |
| α-Carotene | 445 | 536.88 | 145,000 | [26] |
| Lutein | 450 | 568.87 | 145,000 | [27] |
| Zeaxanthin | 450 | 568.88 | 134,000 | [26] |
| Fucoxanthin | 453 | 658.92 | 109,000 | [28] |
| β-Carotene | 453 | 536.88 | 139,000 | [28] |
| Astaxanthin | 470 | 596.85 | 125,000 | [26] |
3.3.2. Phycobiliprotein Content
3.3.3. Seaweed Extracts
3.3.4. Total Phenolic Content (TPC)
3.3.5. Total Flavonoid Content (TFC)
3.3.6. Antioxidant Activity
DPPH Radical Scavenging Activity
ABTS Radical Scavenging Activity
Acetylcholinesterase (AChE) Inhibitory Activity
3.3.7. Color Analysis
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; No. 1229; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Pereira, L. A Review of the Nutrient Composition of Selected Edible Seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses, 1st ed.; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 15–47. [Google Scholar]
- Kraan, S. Pigments and minor compounds in algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 205–251. [Google Scholar] [CrossRef]
- Kovaleski, G.; Kholany, M.; Dias, L.M.S.; Correia, S.F.H.; Ferreira, R.A.S.; Coutinho, J.A.P.; Ventura, S.P.M. Extraction and purification of phycobiliproteins from algae and their applications. Front. Chem. 2022, 10, 1065355. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Badmus, U.O.; Taggart, M.; Boyd, K.G. The effect of different drying methods on certain nutritionally important chemical constituents in edible brown seaweeds. J. Appl. Phycol. 2019, 31, 3883–3897. [Google Scholar] [CrossRef]
- Santhoshkumar, P.; Yoha, K.; Moses, J. Drying of seaweed: Approaches, challenges and research needs. Trends Food Sci. Technol. 2023, 138, 153–163. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Effect of drying and extraction conditions on the recovery of bioactive compounds from Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 2947–2960. [Google Scholar] [CrossRef]
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red Seaweed Pigments from a Biotechnological Perspective. Phycology 2021, 2, 1–29. [Google Scholar] [CrossRef]
- Pina, A.; Costa, A.; Lage-Yusty, M.; López-Hernández, J. An evaluation of edible red seaweed (Chondrus crispus) components and their modification during the cooking process. LWT Food Sci. Technol. 2014, 56, 175–180. [Google Scholar] [CrossRef]
- Amorim-Carrilho, K.; Lage-Yusty, M.A.; López-Hernández, J. Variation of bioactive compounds in dried seaweed Himanthalia elongata subjected to different culinary processes. CyTA J. Food 2014, 12, 336–339. [Google Scholar] [CrossRef]
- Amorim, K.; Lage-Yusty, M.-A.; López-Hernández, J. Changes in bioactive compounds content and antioxidant activity of seaweed after cooking processing. CyTA J. Food 2012, 10, 321–324. [Google Scholar] [CrossRef]
- Bayomy, H.M. Effects of culinary treatments on the physicochemical properties of Ulva lactuca collected from Tabuk coast of Red sea in Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 2355–2362. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, M.; Wang, Y.; Yin, W.; Jiang, P.; Qiu, B.; Qi, H. Traditional Cooking Methods Affect Color, Texture and Bioactive Nutrients of Undaria pinnatifida. Foods 2022, 11, 1078. [Google Scholar] [CrossRef]
- Susanto, E.; Fahmi, A.S.; Agustini, T.W.; Rosyadi, S.; Wardani, A.D. Effects of Different Heat Processing on Fucoxanthin, Antioxidant Activity and Colour of Indonesian Brown Seaweeds. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 012063. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible Irish brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 2485–2493. [Google Scholar] [CrossRef]
- Li, B.; Smith, B.; Hossain, M. Extraction of phenolics from citrus peels. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a Valuable Source of Naturally Occurring Bioactive Compounds for the Treatment of Alzheimer’s Disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.R.; Amoo, S.O.; Aremu, A.O.; Stirk, W.A.; Gruz, J.; Šubrtová, M.; Dolezal, K.; Van Staden, J. Phenolic profiles, antioxidant capacity, and acetylcholinesterase inhibitory activity of eight South African seaweeds. J. Appl. Phycol. 2015, 27, 1599–1605. [Google Scholar] [CrossRef]
- Son, H.J.; Um, M.Y.; Kim, I.; Cho, S.; Han, D.; Lee, C. In Vitro Screening for Anti-Dementia Activities of Seaweed Extracts. J. Korean Soc. Food Sci. Nutr. 2016, 45, 966–972. [Google Scholar] [CrossRef]
- Pires, C.; Marques, C.; Carvalho, M.; Batista, I. Chemical Characterization of Cancer Pagurus, Maja Squinado, Necora Puber and Carcinus Maenas Shells. Poult. Fish. Wildl. Sci. 2017, 5, 1000181. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Food; ILSI Press: Washington, DC, USA, 2001. [Google Scholar]
- Craft, N. Laboratory Procedure Manual—Fat Soluble Micronutrients (Vitamins A, E and Carotenoids); CDC: Wilson, NC, USA, 2008. [Google Scholar]
- Jeffrey, S.W.; Mantoura, R.F.C.; Wright, S.W. Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; PANGAEA: Bremerhaven, Germany, 1997. [Google Scholar]
- Pan-utai, W.; Iamtham, S. Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem. 2019, 82, 189–198. [Google Scholar] [CrossRef]
- Monteiro, M.; Santos, R.A.; Iglesias, P.; Couto, A.; Serra, C.R.; Gouvinhas, I.; Barros, A.; Oliva-Teles, A.; Enes, P.; Díaz-Rosales, P. Effect of extraction method and solvent system on the phenolic content and antioxidant activity of selected macro- and microalgae extracts. J. Appl. Phycol. 2020, 32, 349–362. [Google Scholar] [CrossRef]
- Sapatinha, M.; Oliveira, A.; Costa, S.; Pedro, S.; Gonçalves, A.; Mendes, R.; Bandarra, N.; Pires, C. Red and brown seaweeds extracts: A source of biologically active compounds. Food Chem. 2022, 393, 133453. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Lee, K.-H.; Je, J.-Y. Enzymatic production of bioactive protein hydrolysates from tuna liver: Effects of enzymes and molecular weight on bioactivity. Int. J. Food Sci. Technol. 2010, 45, 562–568. [Google Scholar] [CrossRef]
- Silva, F.M.; Silva, C.L.M. Colour changes in thermally processed cupuaçu (Theobroma grandiflorum) puree: Critical times and kinetics modelling. Int. J. Food Sci. Technol. 1999, 34, 87–94. [Google Scholar] [CrossRef]

| Sample | Neurosporene | α-Carotene | Lutein | Zeaxanthin | Fucoxanthin | β-Carotene | Astaxanthin |
|---|---|---|---|---|---|---|---|
| PLF | 1309.8 ± 73.8 b | 1168.5 ± 73.3 b | 1081.3 ± 64.4 c | 1170.0 ± 69.6 a | 1525.8 ± 92.3 a | 974.9 ± 59.0 a | 1186.1 ± 67.5 a |
| PLD | 978.3 ± 71.8 ab | 844.9 ± 149.8 ab | 682.7 ± 42.3 abc | 847.4 ± 153.7 a | 1317.8 ± 485.6 a | 746.2 ± 174.8 a | 838.5 ± 156.7 a |
| PLR | 559.5 ± 119.4 ab | 493.9 ± 97.4 ab | 456.7 ± 88.8 ab | 494.2 ± 96.1 a | 645.6 ± 126.5 a | 412.5 ± 80.8 a | 490.8 ± 94.5 a |
| PLRT | 700.2 ± 283.5 ab | 696.8 ± 250.3 ab | 677.2 ± 18.9 abc | 732.8 ± 257.0 a | 979.3 ± 581.3 a | 625.7 ± 255.6 a | 756.3 ± 260.7 a |
| PUF | 1224.7 ± 54.4 ab | 1097.5 ± 56.6 ab | 1020.8 ± 58.8 bc | 1104.6 ± 63.4 a | 1441.2 ± 82.1 a | 920.8 ± 52.4 a | 1116.3 ± 66.2 a |
| PUR | 695.4 ± 373.8 ab | 608.0 ± 336.2 ab | 564.2 ± 316.3 abc | 610.5 ± 342.2 a | 807.6 ± 448.0 a | 516.0 ± 286.2 a | 609.3 ± 345.7 a |
| PUD | 742.8 ± 283.5 ab | 633.1 ± 250.3 ab | 419.0 ± 81.2 a | 635.1 ± 257.0 a | 1008.3 ± 581.3 a | 562.4 ± 255.6 a | 627.4 ± 260.7 a |
| PURT | 439.4 ± 256.3 a | 427.3 ± 250.3 a | 409.6 ± 241.9 a | 443.2 ± 261.8 a | 590.0 ± 345.4 a | 377.0 ± 220.7 a | 454.6 ± 267.4 a |
| Sample | Phycoerythrin | Phycoerythrocyanin | Phycocyanin | Allophycocyanin | Allophycocyanin-β |
|---|---|---|---|---|---|
| PLF | 1.16 ± 0.01 b | 0.70 ± 0.01 ab | 0.71 ± 0.00 c | 0.31 ± 0.01 abc | 0.10 ± 0.02 a |
| PLD | 0.97 ± 0.22 ab | 0.99 ± 0.31 b | 0.79 ± 0.01 c | 0.48 ± 0.03 c | 0.57 ± 0.16 a |
| PLR | 1.04 ± 0.41 b | 0.61 ± 0.28 ab | 0.62 ± 0.29 bc | 0.25 ± 0.13 abc | 0.11 ± 0.10 a |
| PLRT | 0.23 ± 0.04 a | 0.21 ± 0.04 a | 0.19 ± 0.04 ab | 0.18 ± 0.05 ab | 0.20 ± 0.05 a |
| PUF | 1.33 ± 0.08 b | 0.82 ± 0.07 b | 0.82 ± 0.09 c | 0.41 ± 0.09 bc | 0.38 ± 0.25 a |
| PUD | 0.90 ± 0.31 ab | 0.88 ± 0.18 b | 0.73 ± 0.06 c | 0.43 ± 0.00 c | 0.36 ± 0.30 a |
| PUR | 0.78 ± 0.11 ab | 0.46 ± 0.07 ab | 0.50 ± 0.05 abc | 0.18 ± 0.05 ab | 0.06 ± 0.03 a |
| PURT | 0.21 ± 0.03 a | 0.19 ± 0.02 a | 0.16 ± 0.02 a | 0.14 ± 0.02 a | 0.18 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, C.; Sapatinha, M.; Mendes, R.; Bandarra, N.M.; Gonçalves, A. Dehydration, Rehydration and Thermal Treatment: Effect on Bioactive Compounds of Red Seaweeds Porphyra umbilicalis and Porphyra linearis. Mar. Drugs 2024, 22, 166. https://doi.org/10.3390/md22040166
Pires C, Sapatinha M, Mendes R, Bandarra NM, Gonçalves A. Dehydration, Rehydration and Thermal Treatment: Effect on Bioactive Compounds of Red Seaweeds Porphyra umbilicalis and Porphyra linearis. Marine Drugs. 2024; 22(4):166. https://doi.org/10.3390/md22040166
Chicago/Turabian StylePires, Carla, Maria Sapatinha, Rogério Mendes, Narcisa M. Bandarra, and Amparo Gonçalves. 2024. "Dehydration, Rehydration and Thermal Treatment: Effect on Bioactive Compounds of Red Seaweeds Porphyra umbilicalis and Porphyra linearis" Marine Drugs 22, no. 4: 166. https://doi.org/10.3390/md22040166
APA StylePires, C., Sapatinha, M., Mendes, R., Bandarra, N. M., & Gonçalves, A. (2024). Dehydration, Rehydration and Thermal Treatment: Effect on Bioactive Compounds of Red Seaweeds Porphyra umbilicalis and Porphyra linearis. Marine Drugs, 22(4), 166. https://doi.org/10.3390/md22040166








