Abstract
It is important to improve the production of bioactive secondary products for drug development. The Escherichia coli—Streptomyces shuttle vector pSET152 and its derived vector pIB139 containing a strong constitutive promoter ermEp* are commonly used as integrative vectors in actinomycetes. Four new integrative vectors carrying the strong constitutive promoter kasOp*, hrdBp, SCO5768p, and SP44, respectively, were constructed and proven to be functional in different mangrove-derived Streptomyces host strains by using kanamycin resistance gene neo as a reporter. Some biosynthetic genes of elaiophylins, azalomycin Fs, and armeniaspirols were selected and inserted into these vectors to overexpress in their producers including Streptomyces sp. 219807, Streptomyces sp. 211726, and S. armeniacus DSM 43125, resulting in an approximately 1.1–1.4-fold enhancement of the antibiotic yields.
1. Introduction
Natural products are critical sources of drug resources. Streptomyces strains, harboring complex secondary metabolic gene clusters, are the most important producers of antibiotics and other bioactive secondary metabolites. The Escherichia coli—Streptomyces shuttle vector pSET152 [1] and its derived vector pIB139 [2,3] containing strong constitutive promoter ermEp* are commonly employed in high-yield strain breeding. They are non-replicative in streptomycetes but integrate into the chromosome to yield stable recombinant strains, thus avoiding the possible problems associated with autonomously replicating plasmids. pSET152 and pIB139 were widely used in gene function analysis, secondary metabolite biosynthetic gene cluster mining, and silent gene cluster activation [4,5,6]. They also introduced some homologous or heterologous genes into Streptomyces to increase antibiotic production [7,8,9,10].
ermEp* is a mutated promoter of the erythromycin resistance gene of Saccharopolyspora erythrea [2]. During our research, we found that the ermEp* did not express a high level in some Streptomyces spp. It is necessary to enrich molecular tools for gene manipulation by constructing new pSET152-derived vectors with other strong constitutive promoters. kasOp* and hrdBp, an engineered promoter of the SARP family regulator gene and a native promoter of the principal sigma factor gene in S. coelicolor, constitutively transcribe gene expression more strongly than the ermEp* [11,12]. SCO5768p is also a strong constitutive promoter scanned from the Streptomyces species, which was twice as strong as ermEp* in S. venezuelae [13]. SP44 is a synthesized constitutive promoter, which was twice as strong as kasOp* in S. avermitilis [14]. As we all know, the promoter strength comparisons were performed using particular genes in special strains under specific conditions, so these findings should not be extrapolated to draw general conclusions.
In this work, we constructed four pSET152-derived vectors with the strong constitutive promoters reported [15], such as kasOp*, hrdBp, SCO5768p, and SP44. Kanamycin resistance gene neo was used as a reporter to test their transcriptional levels in Streptomyces strains. These vectors were applied in gene overexpression to increase the antibiotic production of the mangrove-derived Streptomyces strains, including elaiophylin producer Streptomyces sp. 219807 [16], azalomycin F producer Streptomyces sp. 211726 [17,18,19], and armeniaspirol producter S. armeniacus DSM 43125 [4,20].
2. Results
2.1. Construction of Recombinant Plasmids Harboring Strong Constitutive Promoters and the Corresponding Reporter Plasmids
The native promoters hrdBp, SCO5768p, and kasOp* were amplified by PCR, and the synthetic promoter SP44 was synthetized to develop four new integrating vectors pWHU1288-pWHU1291 (Figure 1). The construction of the following plasmids is listed in Table S1. Agarose gel electrophoresis analysis of PCR products or recombinant plasmids digested with restriction enzymes is shown in Figures S1 and S2.
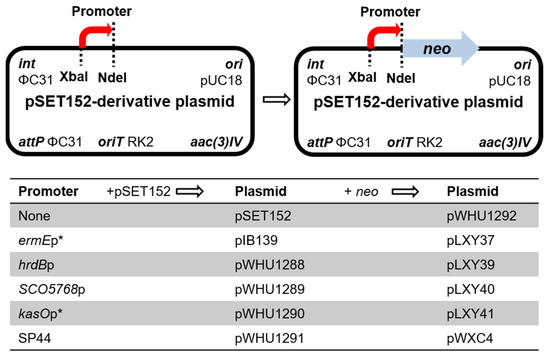
Figure 1.
Genetic map of the base vectors.
Next, we need a reporter gene to test whether the four promoters express normally in Streptomyces strains. The kanamycin resistance gene neo was amplified from plasmid pHZ1358 [21] and inserted into pIB139 to generate plasmid pLXY37; the neo gene is controlled by the promoter ermEp*. pLXY37 was conjugated into Streptomyces strains, including S. coelicolor M145, S. lividans TK24, S. albus J1074, and S. venezuelae ISP5230. The recombinant strains and their corresponding wild-type strains were inoculated in TSBY liquid medium containing 0–50 μg/mL kanamycin and cultured at 30 °C for 48 h. The recombinant strains harboring pLXY37 showed obvious kanamycin resistance, as compared to the wild-type strains (Figure S3). The results proved the neo gene is functional, and can be used to reveal the strength of promoters.
The gene neo was also inserted into pSET152 to generate a promoter-less reporter plasmid pWHU1292 as the negative control. pWHU1292 was conjugated into S. ceolicolor M145 and the recombinant strain S. ceolicolor M145::pWHU1292 was inoculated in TSBY liquid medium containing 0–25 μg/mL kanamycin and cultured at 30 °C for 48 h. Compared with S. ceolicolor M145::pLXY37 as the positive control and the wild-type strain S. ceolicolor M145 as the negative control, S. ceolicolor M145::pWHU1292 was inhibited completely at the concentration of 18–25 μg/mL kanamycin (Figure S4). The results revealed that the neo gene can be used to test the promoter when the concentration of kanamycin is above 20 μg/mL. Then the neo gene was inserted into pWHU1288-pWHU1291, respectively, to generate the corresponding reporter plasmids pLXY39-pLXY41 and pWXC4 to test the strength of promoters in host strains (Figure 1).
2.2. Comparison of Promoter Strength in Different Streptomyces Strains
The reporter plasmids were conjugated into six different Streptomyces strains, including S. coelicolor M145, S. lividans TK24, S. olivaceus CGMCC 4.1369, Streptomyces sp. 219807, Streptomyces sp. 211726, and S. armeniacus DSM 43125, respectively. The kanamycin resistance level of the recombinant strains (Table 1 and Figures S5–S10) is a direct reflection of promoter activity.

Table 1.
Kanamycin resistance levels conferred by different promoters in different Streptomyces strains.
In S. coelicolor M145, the strength of promoters is placed in the order of SP44 > hrdBp > kasOp*, ermEp* > SCO5768p. S. lividans and S. coelicolor are closely related species belonging to the S. violaceouruber sub-clade, but the promoter strengths in S. lividans TK24 and S. coelicolor M145 are different. The strength of promoters in S. lividans TK24 is placed in the order of SP44 > SCO5768p > kasOp* > hrdBp, ermEp*. Firstly, SCO5768p, the weakest promoter in S. ceolicolor M145, exhibited higher activity than the other natural promoters and ranked second only to the artificial promoter SP44 in S. lividans TK24. Secondly, contrary to the result in S. ceolicolor M145, the activity of kasOp* was higher than ermEp* and the expression level of hrdBp was similar to ermEp* in S. lividans TK24.
S. olivaceus is a member of glucose isomerase producers as food enzymes approved by the National Health Commission of the P. R. China (GB 2760-2014). The artificial promoter SP44 is also the most potent in S. olivaceus CGMCC 4.1369. The other promoters have similar expression levels.
In elaiophylin producer Streptomyces sp. 219807, the strength of promoters is placed in the order of SP44 > hrdBp, kasOp* > ermEp* > SCO5768p. The strength of promoters in azalomycin F producer Streptomyces sp. 211726 is placed in the order of SP44 > hrdBp > kasOp*, ermEp*, SCO5768p.
S. armeniacus DSM 43125 has been found to biosynthesize armeniaspirols, which are potent antibiotics against Gram-positive bacteria [4,22,23]. In S. armeniacus DSM 43125, SCO5768p and kasOp* show the best performances with kanamycin resistance up to 2200 μg/mL. The activities of SP44 (1800 μg/mL) are also superior to those of ermEp*. The strength of hrdBp is similar to ermEp*. The strength of promoters in S. armeniacus DSM 43125 is placed in the order of kasOp*, SCO5768p > SP44 > hrdBp, ermEp*.
In summary, the promoters SP44, kasOp*, and hrdBp showed better or similar activities compared with ermEp* in the Streptomyces strains tested. The strength of SCO5768p varies significantly among diverse strains. Next, we selected pWHU1288 containing hrdBp or pWHU1290 containing kasOp* to construct plasmids for overexpressing genes in Streptomyces sp. 219807, Streptomyces sp. 211726, and S. armeniacus DSM 43125.
2.3. Enhancement of Elaiophylin Production
Elaiophylin (ELA) is a glycosylated macrodiolide antibiotic with various biological activities (Figure 2a). Streptomyces sp. 219807 is an elaiophylin producer from mangrove soil collected in Sanya of China [16] (Figure 2b). In order to obtain the biosynthetic gene cluster of elaiophylin, the whole genome of Streptomyces sp. 219807 was sequenced. A DNA region about 64-kb carrying 28 ORFs (Figure 2c, Table S3, accession no. PP236859) was believed to be involved in elaiophylin biosynthesis based on the proposed functions of the genes. Based on the reported elaiophylin biosynthetic gene cluster and biosynthetic pathway in other Streptomyces strains, the roles of biosynthetic genes in the cluster can be suggested (Figure S11).
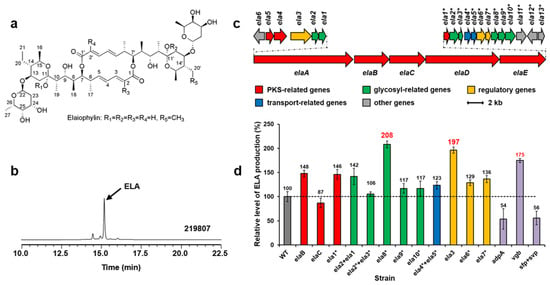
Figure 2.
Structures of elaiophylins (a). HPLC analysis (252 nm) of extracts from Streptomyces sp. 219807 culture (b). ELA, elaiophylin. The elaiophylin biosynthetic gene cluster in Streptomyces sp. 219807 (c). Relative levels of elaiophylin production by Streptomyces sp. 219807 derivative strains detected and quantified by HPLC (d): WT, 219807::pWHU1288; elaB, 219807::pNN1; elaC, 219807::pDQ139; ela1*, 219807::pDQ137; ela2 + ela1, 219807::pLXY45; ela2* + ela3*, 219807::pLXY44; ela8*, 219807::pLXY48; ela9*, 219807::pLXY50; ela10*, 219807::pLXY49; ela4* + ela5*, 219807::pLXY47; ela3, 219807::pLXY51; ela6*, 219807::pLXY52; ela7*, 219807::pLXY46; adpA, 219807::pLXY55; vgb, 219807::pLXY56; sfp + svp, 219807::pWHU2449. Error bars indicate the standard deviation (n = 3).
Three polyketide synthases (PKS) genes (Figure 2c,d, red), seven sugar biosynthesis-related genes (green), two transfer-related genes (blue), and three regulator genes (yellow) in the cluster were amplified from the chromosome of Streptomyces sp. 219807 by PCR, inserted into pWHU1288 (the vector carrying hrdBp) to generate recombinant plasmids, which were conjugated in Streptomyces sp. 219807, respectively. The resulting recombinant strains were confirmed by PCR. The cultures of the derivative strains were extracted and tested by using HPLC and LC-MS. The results showed that the elaiophylin production of the Streptomyces sp. 219807 wild-type strain was about 0.98 g/L in this work and that the best two genes at increasing elaiophylin production were the NAD(P)-dependent oxidoreductase gene ela8* (about 2.04 g/L, increase of 108%) and the LuxR family transcriptional regulator gene ela3 (about 1.93 g/L, increase of 97%). Then ela8* and ela3 were selected and inserted into pWHU1291 (the vector carrying the promoter SP44) to generate recombinant plasmids, which were conjugated in Streptomyces sp. 219807 to increase the elaiophylin production further, respectively. However, the results showed that the production decreased about 30% unexpectedly (Figure S12a).
Three heterologous genes were also conjugated in strain Streptomyces sp. 219807, respectively, including AraC family transcriptional global regulator gene adpA from S. coelicolor, synthetic Vitreoscilla hemoglobin gene vgb, phosphopantetheinyl transferase (PPtase) gene sfp from Bacillus subtilis, and svp from S. verticillus. These genes have been proven to increase antibiotic production or activate silent gene clusters [7,8]. HPLC analysis showed that the overexpression of vgb increased the elaiophylin production about 75% (Figure 2d purple). The overexpression of adpA or sfp + svp decreased the elaiophylin production about 50%.
2.4. Enhancement of Azalomycin F production
Azalomycin F (Azl F) is a complex of ployhydroxy macrocyclic lactones with broad-spectrum antimicrobial activities (Figure 3a). Streptomyces sp. 211726 isolated from mangrove soil is a remarkable producer of azalomycin F (Figure 3b) [17,18]. A circa 130-kb DNA region carrying 23 ORFs (Figure 3c) was proved to be involved in azalomycin F biosynthesis [18,19]. Several genes, as shown in Figure 3c and d: four precursor biosynthesis-related genes (green), two transfer-related genes (blue), and three regulator genes (yellow) in the cluster, were amplified from the chromosome of Streptomyces sp. 211726 by PCR, and inserted into pWHU1288 to generate recombinant plasmids, which were conjugated in the strain Streptomyces sp. 211726 for overexpression, respectively. The derivative strains were fermented and the cultures were extracted and tested by using HPLC and LC-MS. Compared to the wild-type strain Streptomyces sp. 211726 as control (about 2.80 g/L), the best two genes increasing azalomycin F production were the 4-guanidinobutyryl-CoA ligase gene azl4 (about 6.61 g/L, increase of 136%) and the TetR family transcriptional regulator gene azl6 (about 5.43 g/L, increase of 94%), as shown in Figure 3d. pWHU1291 was also used to overexpress azl4 and azl6 in Streptomyces sp. 211726, respectively, in order to further improve the azalomycin F production. The analysis showed a decrease in production, similar to the results observed in Streptomyces sp. 219807 (Figure S12b).
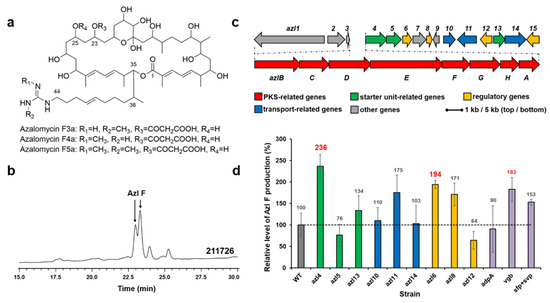
Figure 3.
Structures of azalomycin Fs (a). HPLC analysis (241 nm) of extracts from Streptomyces sp. 211726 culture (b). Azl F, azalomycin F mixtures. The azalomycin biosynthetic gene cluster in Streptomyces sp. 211726 (c). Relative levels of azalomycin F production by Streptomyces sp. 211726 derivative strains detected and quantified by HPLC (d): WT, 211726::pWHU1288; azl4, 211726::pMX301; azl5, 211726::pMX302; azl13, 211726::pMX308; azl10, 211726::pMX305; azl11, 211726::pMX306; azl14, 211726::pMZ309; azl6, 211726::pMX303; azl8, 211726::pMX304; azl12, 211726::pMX307; adpA, 211726::pLXY55; vgb, 211726::pLXY56; sfp + svp, 211726::pWHU2449. Error bars indicate the standard deviation (n = 3).
The genes adpA, vgb, sfp, and svp were also conjugated in Streptomyces sp. 211726, respectively. HPLC analysis showed that the best gene increasing azalomycin F production was vgb (increase of 83%), as shown in Figure 3d (purple).
2.5. Enhancement of Armeniaspirol Production
Armeniaspirols (Arm A, B, and C as shown in Figure 4a,b), with a unique chlorinated spiro[4.4]non-8-ene scaffold, are potent antibiotics against Helicobacter pylori and Gram-positive pathogens [20,22]. We cloned the armeniaspirol biosynthetic gene cluster from S. armeniacus DSM 43125 in previous work (Figure 4c) [4]. Two PKS genes (arm6 and arm7) and three regulator genes (arm1, arm24 and arm25) in the cluster were amplified from the chromosome of S. armeniacus DSM 43125 by PCR, and inserted into pWHU1290 (the vector carrying kasOp*) to generate recombinant plasmids, which were conjugated in the strain S. armeniacus DSM 43125 for overexpression, respectively. The cultures of S. armeniacus DSM 43125 and its derivative strains were extracted and tested by using HPLC and LC-MS. The results are shown in Figure 4d. The armeniaspirol production of the wild-type strain S. armeniacus DSM 43125 was 0.95 mg/L. The best two genes increasing armeniaspirol production were the PKS gene arm6 (about 2.03 mg/L, increase of 114%) and the regulator gene arm24 (about 1.69 mg/L, increase of 78%).
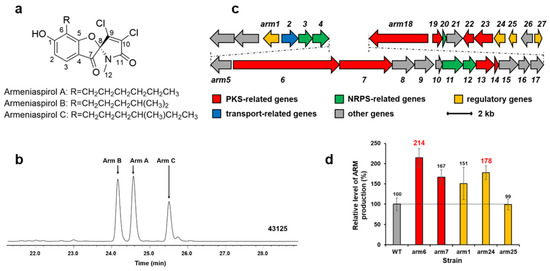
Figure 4.
Structures of armeniaspirol (a). HPLC analysis (300 nm) of extracts from S. armeniacus DSM 43125 culture (b). Arm, armeniaspirol. The armeniaspirol biosynthetic gene cluster in S. armeniacus DSM 43125 (c). Relative levels of armeniaspirol production by S. armeniacus DSM 43125 derivative strains detected and quantified by HPLC (d): WT, 43125::pWHU1290; arm6, 43125::pZQ11; arm7, 43125::pZQ12; arm1, 43125::pYQ1; arm24, 43125::pYQ2; arm25, 43125::pYQ3. Error bars indicate the standard deviation (n = 3).
3. Discussion
Based on the comparison of promoter strength using kanamycin resistance gene neo as a reporter, SP44 is the strongest promoter in most of the tested strains with S. armeniacus being an exception. However, for unknown reasons, SP44 was ineffective in enhancing the yield of elaiophylin and azalomycin F. kasOp* and hrdBp showed similar or higher activity than the ermEp* in all the tested strains and they also had good performance in the improvement of antibiotic production. The activity of SCO5768p was not detected in some strains and the reason remains unexplained. In summary, pWHU1288 harboring hrdBp and pWHU1290 harboring kasOp* are favorable choices for the genetic manipulation of Streptomyces species.
pWHU1288 and pWHU1290 were used in gene overexpression to increase the antibiotic production of three Streptomyces strains, and we obtained high-yielding strains compared with the wild-type strains. Gene ela8*, azl4, and arm6 overexpression doubled elaiophylin, azalomycin F, and armeniaspirol production, respectively. They are all biosynthetic genes of their corresponding gene clusters, which suggests that the products of these genes may be rate-limiting enzymes in the biosynthetic pathway. The gene cluster for a secondary metabolite harbors variable amounts of biosynthetic genes. Identifying the gene responsible for the rate-limiting step is challenging, especially when dealing with a gene cluster containing numerous biosynthetic genes. Trying each one individually becomes unrealistic. Therefore, pathway-specific activator genes of a one-component regulatory system are viable choices. Gene ela3 is the only one-component regulatory system gene of the elaiophylin biosynthetic gene cluster, and overexpression of ela3 increased elaiophylin production about 97%. The same goes for azalomycin F biosynthesis and armeniaspirol biosynthesis. Overexpression of the histidine kinase gene or the response regulator gene of a two-component regulatory system individually did not explicitly improve antibiotic production in the elaiophylin producer and armeniaspirol producer. We also tried co-overexpression of two genes of a two-component regulatory system, but no apparent effects were observed in this work (not shown).
Vitreoscilla hemoglobin is an oxygen-binding protein that promotes oxygen delivery under low oxygen conditions to increase the efficiency of cell metabolism. Normally, oxygen supply is insufficient during the shake flask fermentation, so overexpression of vgb increased antibiotic production in Streptomyces sp. 219807 and Streptomyces sp. 211726, as expected. Gene adpA did not improve the biosynthesis of azalomycin Fs in Streptomyces sp. 211726 and even repressed the biosynthesis of elaiophylins in Streptomyces sp. 219807. AdpA is a global transcriptional activator triggering morphological differentiation and secondary metabolism in Streptomyces. However, genes repressed by AdpA were also reported [24]. It is reasonable that the expressions of secondary biosynthetic gene clusters are either unaffected or repressed by AdpA. Phosphopantetheinyl transferase catalyzes the conversion of the carrier proteins of polyketide synthases and nonribosomal peptide synthases from the apo form to the active form (holo form). Overexpression of the corresponding genes into actinomycete strains achieved a significantly high activation ratio at which strains produced new metabolites [8,25,26]. At the same time, the metabolites produced in some wild-type strains were either eliminated or diminished in their PPtase-overexpressing strains [8]. Thus, it was not inevitable that azalomycin F production went up and elaiophylin production went down when PPtase genes were overexpressed in the producers. In summary, gene vgb is a promising candidate for enhancing antibiotic production.
4. Materials and Methods
4.1. General Materials and Experimental Procedures
The bacterial strains and plasmids used in this work are listed in Table S1. Primer sequences are listed in Table S2. Reagents and solvents purchased from Sigma-Aldrich were of the highest quality available and were used without further purification. Restriction enzymes, T4 DNA ligase, and DNA polymerase were purchased from New England Biolabs and used according to the manufacturer’s specifications. DNA primers were synthesized by TsingKe Co. Ltd. (Wuhan, China). Growth media and conditions used for E. coli and Streptomyces strains and standard methods for handling E. coli and Streptomyces in vivo and in vitro were as described previously, unless otherwise noted. All DNA manipulations were performed following standard procedures. DNA sequencing was carried out at TsingKe Co. Ltd. (Wuhan, China). Genome sequencing of Streptomyces sp. 219807 was performed by BGI Co. Ltd. (Wuhan, China) using the Illumina HiSeq 2000 System. ORFs of the secondary metabolite biosynthetic gene clusters were identified using antiSMASH (http://antismash.secondarymetabolites.org, accessed on 22 July 2018) [27], FramePlot (http://nocardia.nih.go.jp/fp4/, accessed on 22 August 2018) [28], and secondFind (http://biosyn.nih.go.jp/2ndfind/, accessed on 22 August 2018). The NRPS-PKS online tools (http://www.nii.ac.in/~pksdb/sbspks/master.html, accessed on 11 September 2018) and (http://nrps.igs.umaryland.edu/, accessed on 11 September 2018) were used to analyze PKSs [29].
The construction of plasmids is listed in Table S1. The plasmids were transferred into E. coli ET12567/pUZ8002, and the unmethylated plasmid was conjugated into Streptomyces strains, using apramycin to select the respective exconjugants. The exconjugants were confirmed by PCR.
4.2. Determination of Promoter Strength in Streptomyces Strains
Spores of the Streptomyces strains were harvested and resuspended in sterile water, and optical density at 450 nm was measured and normalized to the same level. Kanamycin-resistant analyses were carried out using either method 1: the spore suspension was inoculated in the liquid TSBY medium (0.5% yeast extract, 3% tryptone soya broth, 10.3% sucrose, pH 7.2) with different concentrations of kanamycin and cultured at 28 °C and 200 rpm for 3 days before the photograph was taken; or method 2: the spore suspension was series diluted, spotted on SFM medium (2% soy flour, 2% mannitol, 2% agar) or fermentation medium (FM medium, formula as shown below) with 2% agar supplemented with different concentrations of kanamycin, and the plates were incubated at 30 °C for 6 days before the photograph was taken.
4.3. Fermentation, Extraction, and Quantitative Analysis of Elaiophylins
The cultivation of Streptomyces sp. 219807-derived strains and the analysis of the resulting products were as previously described [16]. The strains were cultured in liquid TSBY medium at 28 °C and 200 rpm for 3 days, respectively. Then 10% (v/v) of the culture was transferred in 50 mL of fresh fermentation medium (0.2% yeast extract, 1% glucose, 2.5% dextrin, 2% oatmeal, 1% cotton seed flour, 0.5% Fish meal, 0.3% CaCO3, pH 7.2) and fermented at 30 °C and 200 rpm for 8 days.
The mycelium and the culture supernatant were separated by centrifugation. The clarified supernatant after centrifugation was extracted with an equal volume of ethyl acetate three times. The ethyl acetate extracts were dried with anhydrous Na2SO4, filtered, and evaporated to dryness under reduced pressure. The mycelium was extracted with 30 mL of 80% (v/v) acetone water solution, which was combined with the culture supernatant extracts. The products were filtered through a 0.22 μm Nylon membrane before subjection to HPLC or LC-ESI-HRMS.
HPLC (Shimadzu, SPD-M20A/LC-20AT, Kyoto, Japan) and LC-ESI-HRMS (Thermo Scientific LTQ Orbitrap XL, positive ion mode, Waltham, MA, USA) were used to analyze samples. The HPLC conditions (Thermo Scientific C18 reversed-phase HPLC column, 250 × 4.6 mm, 5 μm; mobile phase A: water; mobile phase B: acetonitrile; UV detection λ: 252 nm) were as follows: elution gradient I (for HPLC): 10% B for 2 min, 10–100% B for 13 min, 100% B for 10 min, 100–10% B for 10 min, 10% B for 10 min at a flow rate of 1 mL/min; elution gradient II (for LC-ESI- HRMS): 10–100% B for 40 min, 100% B for 20 min, 100–10% B for 10 min, 10% B for 5 min at a flow rate of 0.5 mL/min.
The identity of resultant elaiophylin metabolites was confirmed by direct comparison of retention time and mass spectra with pure authentic standards. The production of elaiophylins was measured with the external standard law. The identities of azalomycin Fs and armeniaspirols were the same unless otherwise noted.
4.4. Fermentation, Extraction, and Quantitative Analysis of Azalomycin Fs
Streptomyces sp. 211726 and all derived strains were cultured in liquid TSBY medium at 30 °C with shaking at 200 rpm for 2 days, respectively. Then 10% (v/v) of the culture was transferred in 25 mL of fresh fermentation medium (0.2% yeast extract, 1% glucose, 3.5% soluble starch, 0.4% casein, pH 7.2–7.4) and fermented at 30 °C and 200 rpm for 10 days.
The mycelium and the culture supernatant were separated by centrifugation. The mycelium was extracted with 25 mL of methanol, which was combined with the clarified culture supernatant after centrifugation. The products were filtered through a microporous membrane (0.22 μm, nylon) before HPLC analysis.
Each sample was analyzed by HPLC (Shimadzu, SPD-M20A/LC 20AT, Kyoto, Japan) with a SHIMADZU Shim-pack VP-ODS C18 column (250 × 4.6 mm, 5 μm) at a flow rate of 1 mL/min using a mobile phase of (A) water and (B) methanol. The separation gradient was as follows: 70% B for 2 min, 70–90% B for 23 min, 90–70% B for 3 min, 70% B for 2 min. Azalomycin F was analyzed by LC-ESI-HRMS (Thermo Electron LTQ-ESI-HRMS, positive ion mode, Waltham, MA, USA) with a SHIMADZU Shim-pack VP-ODS C18 column (250 × 4.6 mm, 5 μm) at a flow rate of 0.2 mL/min using a mobile phase of (A) H2O with 0.1% formic acid and (B) methanol. The separation gradient was as follows: 0–2 min, 80% B; 2–25 min, 80–100% B; 25–28 min, 100–80% B; 28–43 min, 80% B. The mass spectrometer was set to full scan (from 200 to 2000 m/z).
4.5. Fermentation, Extraction, and Quantitative Analysis of Armeniaspirols
The cultivation of S. armeniacus strains and the analysis of the resulting products were as previously described [4]. The S. armeniacus strains were cultured in liquid ISP-2 medium (0.4% yeast extract, 1% malt extract, 0.4% glucose, pH 7.0) containing 2 g/L CaCO3 at 30 °C and 200 rpm for 3 days, respectively. Then 10% (v/v) of the culture was transferred in 50 mL of fresh ISP-2 medium and fermented at 30 °C and 200 rpm for 6 days.
The culture was separated from the biomass fraction and the supernatant fraction by centrifugation. The supernatant fraction was extracted with an equal volume of ethyl acetate twice. The biomass fraction was extracted with methanol, disrupted by sonication, and centrifuged to remove the cell debris. The retained supernatant was evaporated under reduced pressure, resuspended in water, and twice extracted with an equal volume of ethyl acetate. The combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated on a rotovap. The residue was re-dissolved in methanol and filtered with a microporous membrane (0.22 μm, nylon).
HPLC (Shimadzu, SPD-M20A/LC-20AT, Kyoto, Japan) and LC-ESI-HRMS (Thermo Scientific LTQ Orbitrap XL, negative ion mode, Waltham, MA, USA) were used to analyze each sample. The HPLC conditions (mobile phase A: water; mobile phase B: acetonitrile; UV detection λ: 300 nm) were as follows: elution gradient I (for Thermo Scientific C18 reversed-phase HPLC column, 250 × 4.6 mm, 5 μm, used in HPLC and LC-ESI-HRMS): 5–95% B for 25 min, 95% B for 10 min, 95–5% B for 5 min, 5% B for 5 min at a flow rate of 1 mL/min.
5. Conclusions
Four strong constitutive promoters, kasOp*, hrdBp, SCO5768p, and SP44, were inserted in the integrative vector pSET152 to generate four new vectors, and three of them, pWHU1288 with hrdBp, pWHU1290 with kasOp*, and pWHU1291 with SP44, were proven to be functional in different host strains of six Streptomyces species, namely S. coelicolor M145, S. lividans TK24, S. olivaceus CGMCC 4.1369, Streptomyces sp. 219807, Streptomyces sp. 211726, and S. armeniacus DSM 43125. Furthermore, pWHU1288 was selected to overexpress NAD(P)-dependent oxidoreductase gene ela8* in marine Streptomyces sp. 219807 and the elaiophylin production increased about 108%. Similarly, pWHU1288 harboring the 4-guanidinobutyryl-CoA ligase gene azl4 enhanced the elaiophylin production of marine Streptomyces sp. 211726 about 136%. In addition, pWHU1290 was used to overexpress the PKS gene arm6 in S. armeniacus DSM 43125 and armeniaspirol production improved about 114%. In brief, pWHU1288 and pWHU1290 exhibit efficient gene activation and expression, and flexible host compatibility, which are useful in synthetic biology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md22020094/s1, Table S1: Bacterial strains and plasmids used in this study; Table S2: Primers used in this study; Figures S1–S2: Agarose gel electrophoresis analysis of PCR products or recombinant plasmids digested with restriction enzymes; Figures S3–S10: Determination of promoter activity by using kanamycin resistance gene neo as a reporter gene; Table S3: Deduced functions and sequence comparison of elaiophylin biosynthetic genes; Figure S11: Proposed biosynthetic pathway of elaiophylin; Figure S12: Relative levels of elaiophylin (and azalomycin F) production by Streptomyces sp. 219807 (and 211726) derivative strains detected and quantified by HPLC.
Author Contributions
D.Z., K.H., and Z.D. conceived and designed the research. M.Z. (Mingxia Zhao), Z.Y., X.L., Y.L. (Yaqi Liu), Y.Z., M.Z. (Mengqian Zhang), Y.L. (Yangli Li), and X.W. performed the experiments. M.Z. (Mingxia Zhao), Z.Y., X.L., and Y.L. (Yaqi Liu) analyzed data. D.Z., K.H., M.Z. (Mingxia Zhao), and Z.Y. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Fundamental Resources Investigation Program of China (2021FY100900) and the National Key R&D Program of China (2018YFC0311004).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The authors declare that all data of this study are available within the article and its Supplementary Materials file or from the corresponding authors upon request.
Acknowledgments
We thank Xudong Qu (Shanghai Jiao Tong University, Shanghai, China) for providing the template DNA carrying kasOp* and the plasmid carrying sfp and svp. We thank Delin You (Shanghai Jiao Tong University, Shanghai, China) for providing the plasmid carrying adpA and vgb. We thank Huarong Tan’s Group (State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) for providing the template DNA carrying hrdBp. We also thank Yuhui Sun (Wuhan University, Wuhan, China) for the template DNA carrying neo.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bierman, M.; Logan, R.; O’Brien, K.; Seno, E.T.; Rao, R.N.; Schoner, B.E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 1992, 116, 43–49. [Google Scholar] [CrossRef]
- Bibb, M.J.; Janssen, G.R.; Ward, J.M. Cloning and analysis of the promoter region of the erythromycin resistance gene (ermE) of Streptomyces erythraeus. Gene 1985, 38, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.J.; Hughes-Thomas, Z.A.; Martin, C.J.; Bohm, I.; Mironenko, T.; Deacon, M.; Wheatcroft, M.; Wirtz, G.; Staunton, J.; Leadlay, P.F. Increasing the efficiency of heterologous promoters in actinomycetes. J. Mol. Microbiol. Biotechnol. 2002, 4, 417–426. [Google Scholar] [PubMed]
- Qiao, Y.; Yan, J.; Jia, J.; Xue, J.; Qu, X.; Hu, Y.; Deng, Z.; Bi, H.; Zhu, D. Characterization of the Biosynthetic Gene Cluster for the Antibiotic Armeniaspirols in Streptomyces armeniacus. J. Nat. Prod. 2019, 82, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, S.; Zhu, L.; Zhang, C.; Xiang, W.; Deng, Z.; Ikeda, H.; Cane, D.E.; Zhu, D. Substitution of a Single Amino Acid Reverses the Regiospecificity of the Baeyer-Villiger Monooxygenase PntE in the Biosynthesis of the Antibiotic Pentalenolactone. Biochemistry 2016, 55, 6696–6704. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Burgo, Y.; Santos-Aberturas, J.; Rodriguez-Garcia, A.; Barreales, E.G.; Tormo, J.R.; Truman, A.W.; Reyes, F.; Aparicio, J.F.; Liras, P. Activation of Secondary Metabolite Gene Clusters in Streptomyces clavuligerus by the PimM Regulator of Streptomyces natalensis. Front. Microbiol. 2019, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bai, L.; Zhu, D.; Lei, X.; Liu, G.; Deng, Z.; You, D. Enhancing macrolide production in Streptomyces by coexpressing three heterologous genes. Enzym. Microb. Technol. 2012, 50, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, W.; Wang, S.; Yan, X.; Jia, X.; Pierens, G.K.; Chen, W.; Ma, H.; Deng, Z.; Qu, X. Activation of Natural Products Biosynthetic Pathways via a Protein Modification Level Regulation. ACS Chem. Biol. 2017, 12, 1732–1736. [Google Scholar] [CrossRef]
- Liu, K.; Hu, X.R.; Zhao, L.X.; Wang, Y.; Deng, Z.; Tao, M. Enhancing Ristomycin A Production by Overexpression of ParB-Like StrR Family Regulators Controlling the Biosynthesis Genes. Appl. Environ. Microbiol. 2021, 87, e0106621. [Google Scholar] [CrossRef]
- Yun, K.; Zhang, Y.; Li, S.; Wang, Y.; Tu, R.; Liu, H.; Wang, M. Droplet-Microfluidic-Based Promoter Engineering and Expression Fine-Tuning for Improved Erythromycin Production in Saccharopolyspora erythraea NRRL 23338. Front. Bioeng. Biotechnol. 2022, 10, 864977. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Wang, J.; Xiang, S.; Feng, X.; Yang, K. An engineered strong promoter for streptomycetes. Appl. Environ. Microbiol. 2013, 79, 4484–4492. [Google Scholar] [CrossRef]
- Du, D.; Zhu, Y.; Wei, J.; Tian, Y.; Niu, G.; Tan, H. Improvement of gougerotin and nikkomycin production by engineering their biosynthetic gene clusters. Appl. Microbiol. Biotechnol. 2013, 97, 6383–6396. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Li, X.; Yin, S.; Wang, W.; Yang, K. Genome-wide identification and evaluation of constitutive promoters in streptomycetes. Microb. Cell Factories 2015, 14, 172. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, Y.; Zhao, X.; Hu, Y.; Xiang, S.; Miao, J.; Lou, C.; Zhang, L. Exploiting a precise design of universal synthetic modular regulatory elements to unlock the microbial natural products in Streptomyces. Proc. Natl. Acad. Sci. USA 2015, 112, 12181–12186. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hwang, S.; Lee, Y.; Cho, S.; Palsson, B.; Cho, B.K. Synthetic Biology Tools for Novel Secondary Metabolite Discovery in Streptomyces. J. Microbiol. Biotechnol. 2019, 29, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tian, E.; Xu, D.; Ma, M.; Deng, Z.; Hong, K. Halichoblelide D a New Elaiophylin Derivative with Potent Cytotoxic Activity from Mangrove-Derived Streptomyces sp. 219807. Molecules 2016, 21, 970. [Google Scholar] [CrossRef]
- Yuan, G.; Lin, H.; Wang, C.; Hong, K.; Liu, Y.; Li, J. 1H and 13C assignments of two new macrocyclic lactones isolated from Streptomyces sp. 211726 and revised assignments of azalomycins F3a, F4a and F5a. Magn. Reson. Chem. MRC 2011, 49, 30–37. [Google Scholar] [CrossRef]
- Yuan, G.; Hong, K.; Lin, H.; She, Z.; Li, J. New azalomycin F analogs from mangrove Streptomyces sp. 211726 with activity against microbes and cancer cells. Mar. Drugs 2013, 11, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhai, G.; Liu, Y.; Li, Y.; Shi, Y.; Hong, K.; Hong, H.; Leadlay, P.F.; Deng, Z.; Sun, Y. An Iterative Module in the Azalomycin F Polyketide Synthase Contains a Switchable Enoylreductase Domain. Angew. Chem. 2017, 56, 5503–5506. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, C.; Liu, Y.; Huang, Y.; Bai, Y.; Hang, X.; Zeng, L.; Zhu, D.; Bi, H. Armeniaspirol A: A novel anti-Helicobacter pylori agent. Microb. Biotechnol. 2022, 15, 442–454. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, X.; Liu, J.; Bao, K.; Zhang, G.; Tu, G.; Kieser, T.; Deng, Z. ‘Streptomyces nanchangensis’, a producer of the insecticidal polyether antibiotic nanchangmycin and the antiparasitic macrolide meilingmycin, contains multiple polyketide gene clusters. Microbiology 2002, 148 (Pt 2), 361–371. [Google Scholar] [CrossRef]
- Dufour, C.; Wink, J.; Kurz, M.; Kogler, H.; Olivan, H.; Sable, S.; Heyse, W.; Gerlitz, M.; Toti, L.; Nusser, A.; et al. Isolation and structural elucidation of armeniaspirols A-C: Potent antibiotics against gram-positive pathogens. Chemistry 2012, 18, 16123–16128. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xie, F.; Hoffmann, J.; Wang, Q.; Bauer, A.; Bronstrup, M.; Mahmud, T.; Muller, R. Armeniaspirol Antibiotic Biosynthesis: Chlorination and Oxidative Dechlorination Steps Affording Spiro[4.4]non-8-ene. Chembiochem A Eur. J. Chem. Biol. 2019, 20, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Higo, A.; Hara, H.; Horinouchi, S.; Ohnishi, Y. Genome-wide distribution of AdpA, a global regulator for secondary metabolism and morphological differentiation in Streptomyces, revealed the extent and complexity of the AdpA regulatory network. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2012, 19, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, Q.; Zhu, Y.; Zhang, L.; Zhang, W.; Ma, L.; Zhang, H.; Zhang, C. Proximicins F and G and Diproximicin A: Aminofurans from the Marine-Derived Verrucosispora sp. SCSIO 40062 by Overexpression of PPtase Genes. J. Nat. Prod. 2020, 83, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Yan, X.; Deng, Z.; Lei, C.; Qu, X. Expanding the Chemical Diversity of Fasamycin Via Genome Mining and Biocatalysis. J. Nat. Prod. 2022, 85, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Ishikawa, J.; Hotta, K. FramePlot: A new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol. Lett. 1999, 174, 251–253. [Google Scholar] [CrossRef]
- Anand, S.; Prasad, M.V.; Yadav, G.; Kumar, N.; Shehara, J.; Ansari, M.Z.; Mohanty, D. SBSPKS: Structure based sequence analysis of polyketide synthases. Nucleic Acids Res. 2010, 38, W487–W496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).