Tuning the Bioactive Properties of Dunaliella salina Water Extracts by Ultrasound-Assisted Extraction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impact of Ultrasound-Assisted Extraction Parameters
2.1.1. On the Yield
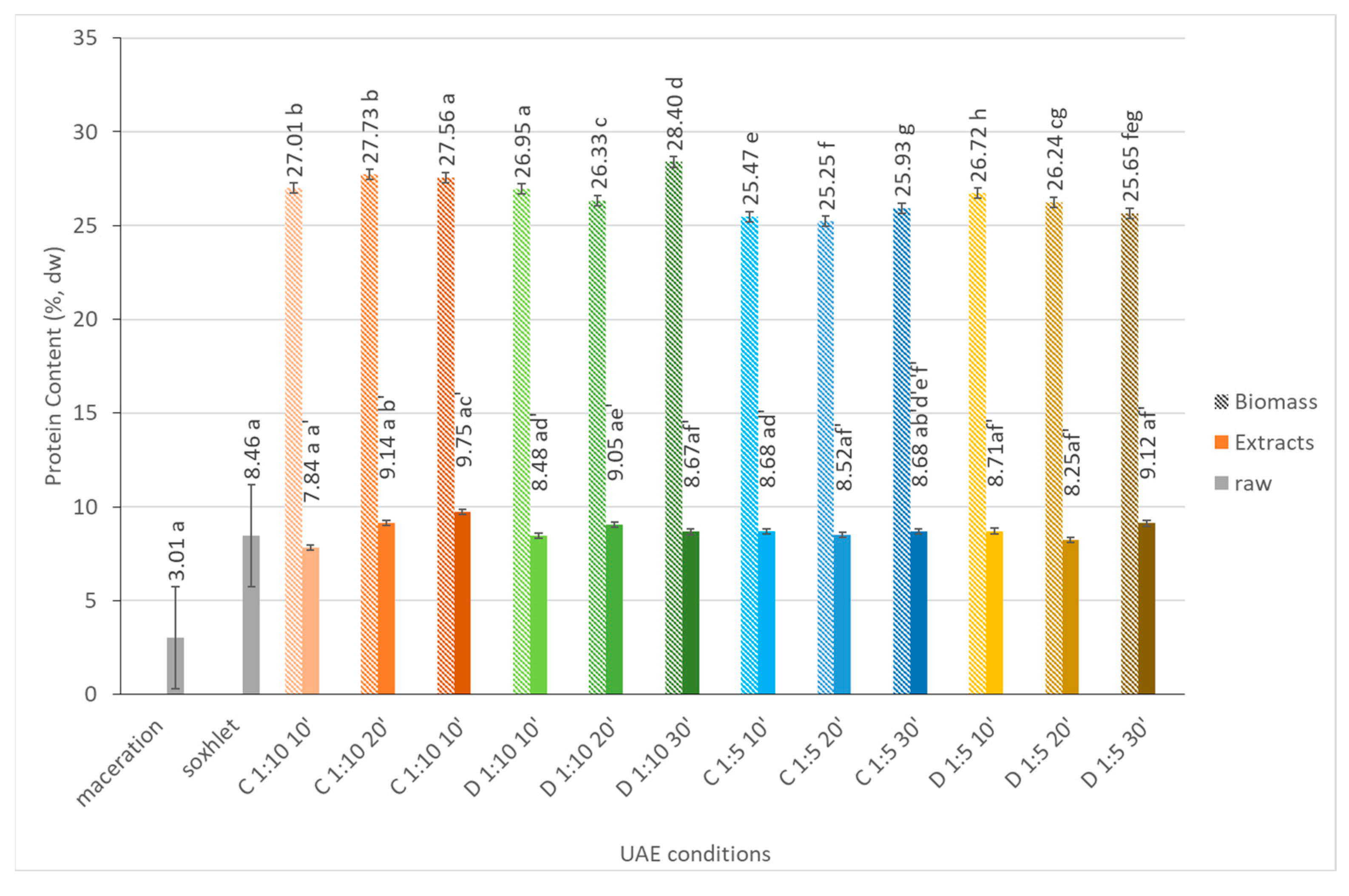
2.1.2. Protein Content
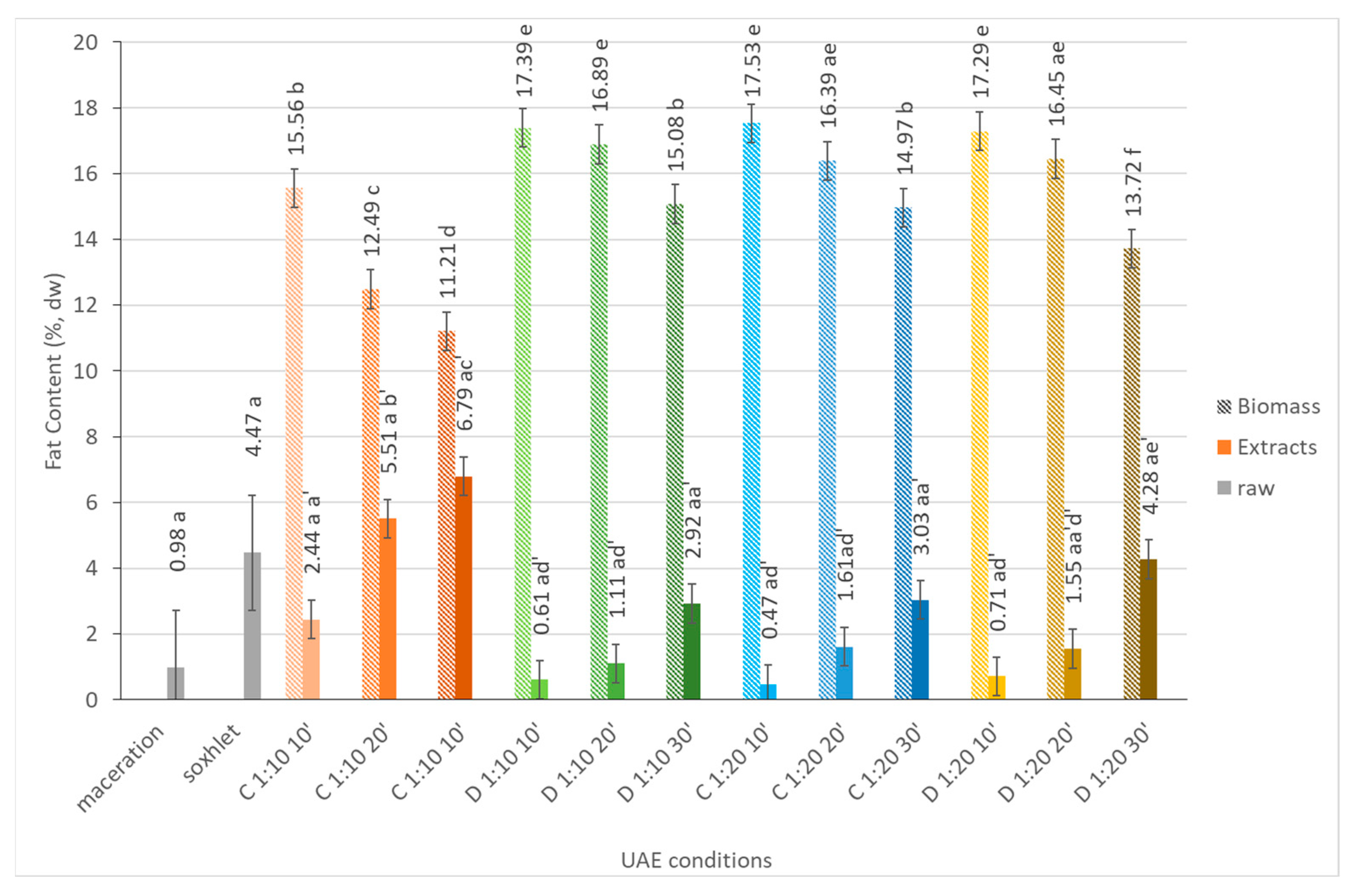
2.1.3. Fat Content
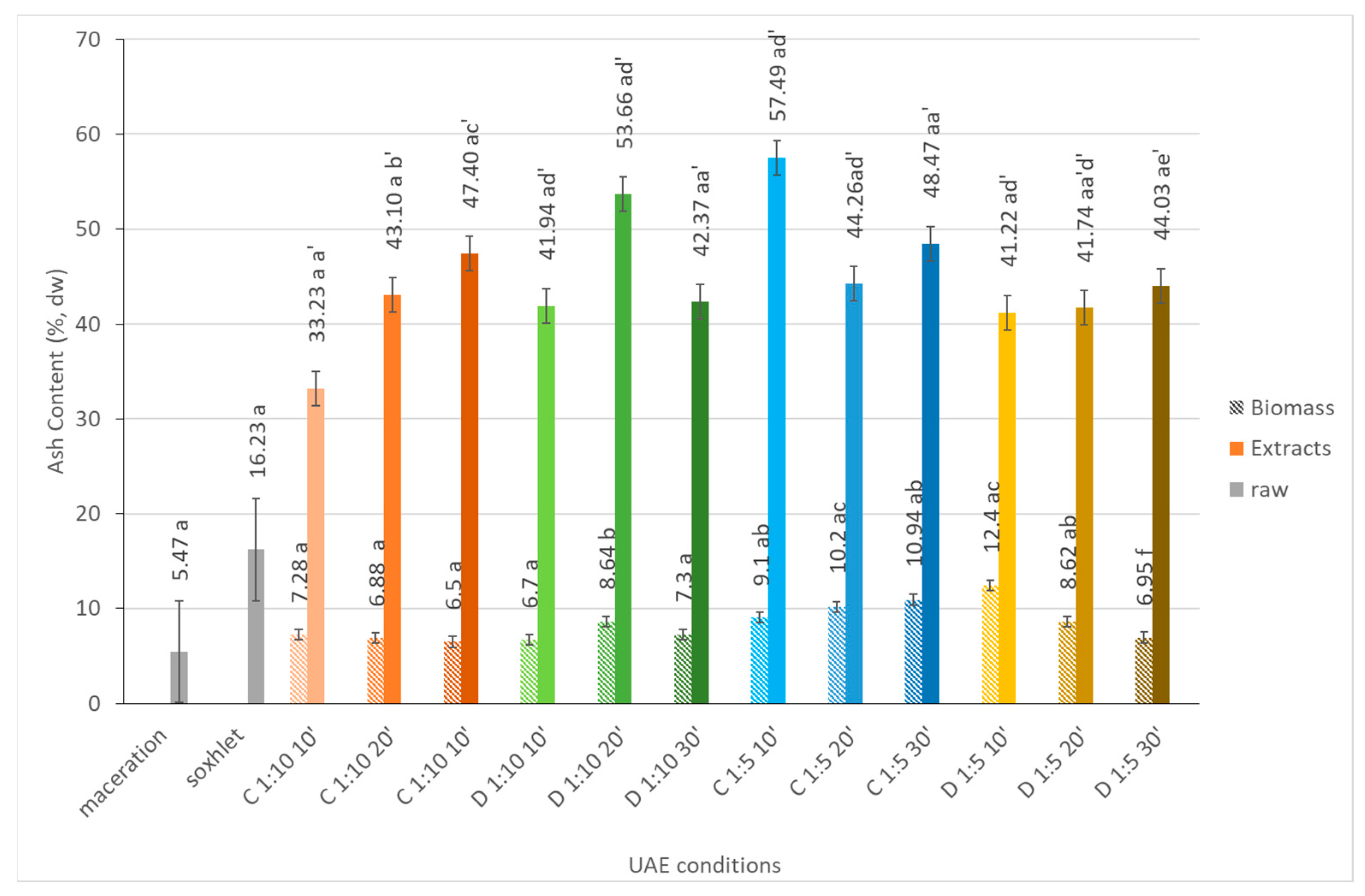
2.1.4. Ash
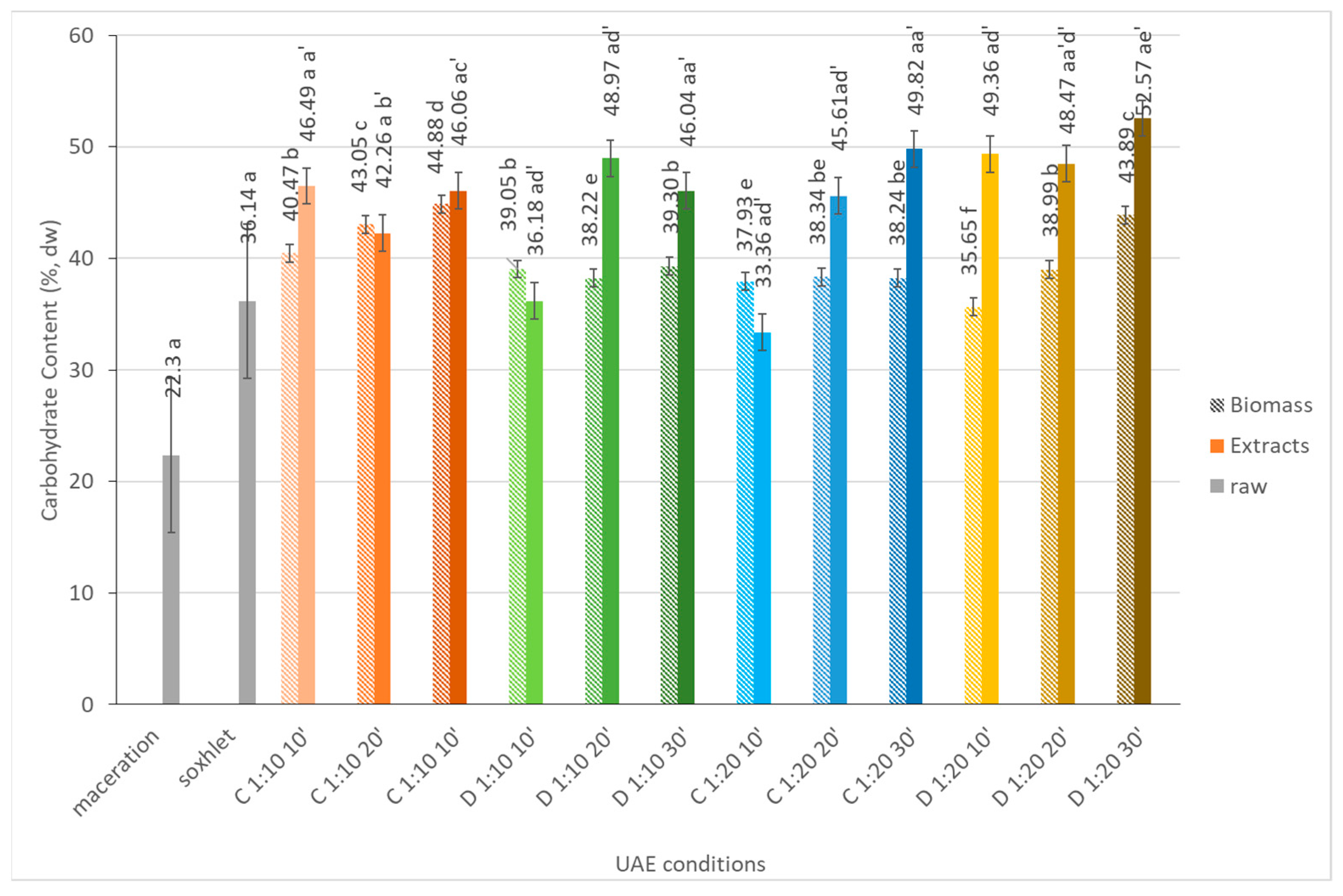
2.1.5. Carbohydrates
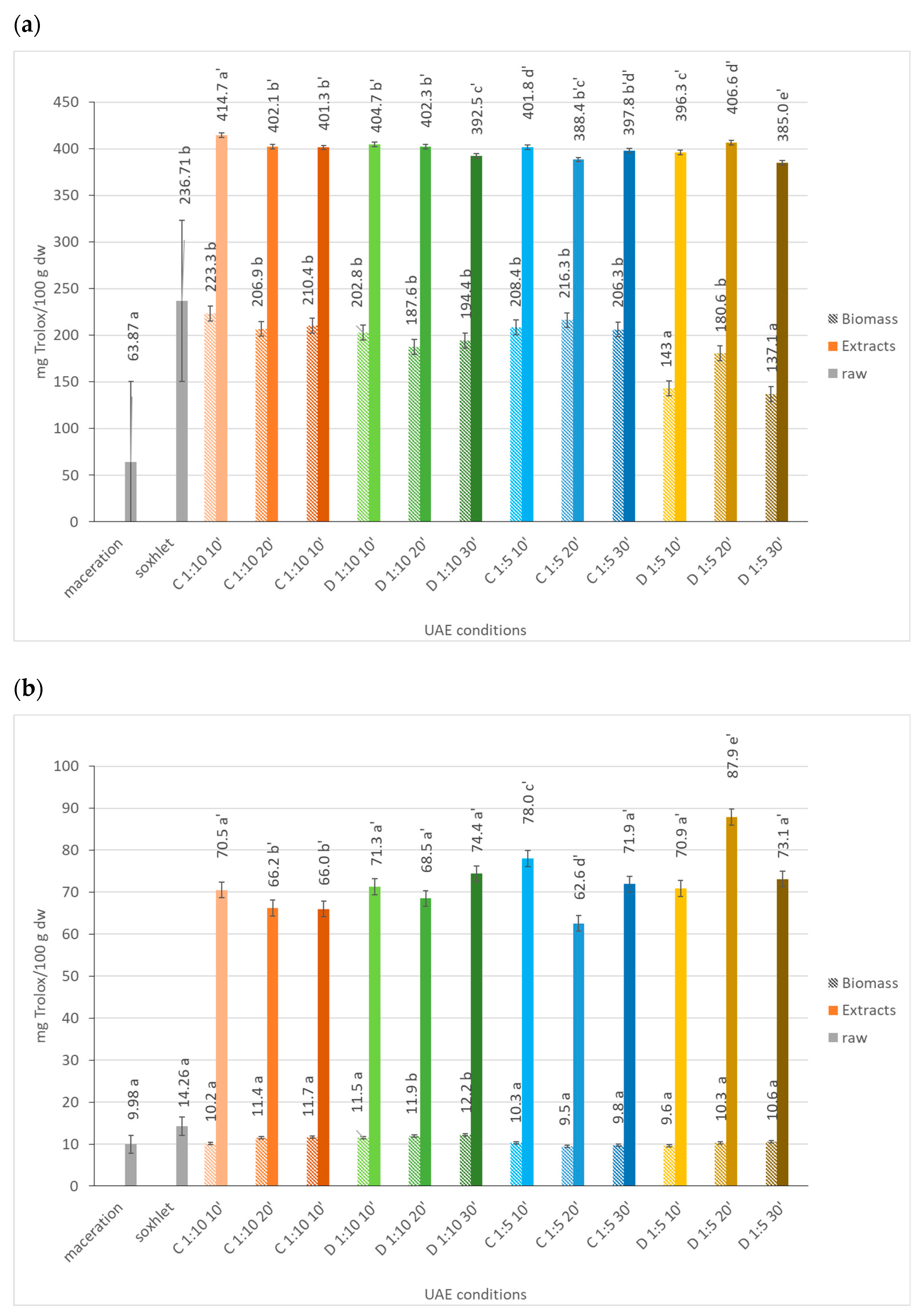
2.1.6. Antioxidant Potential
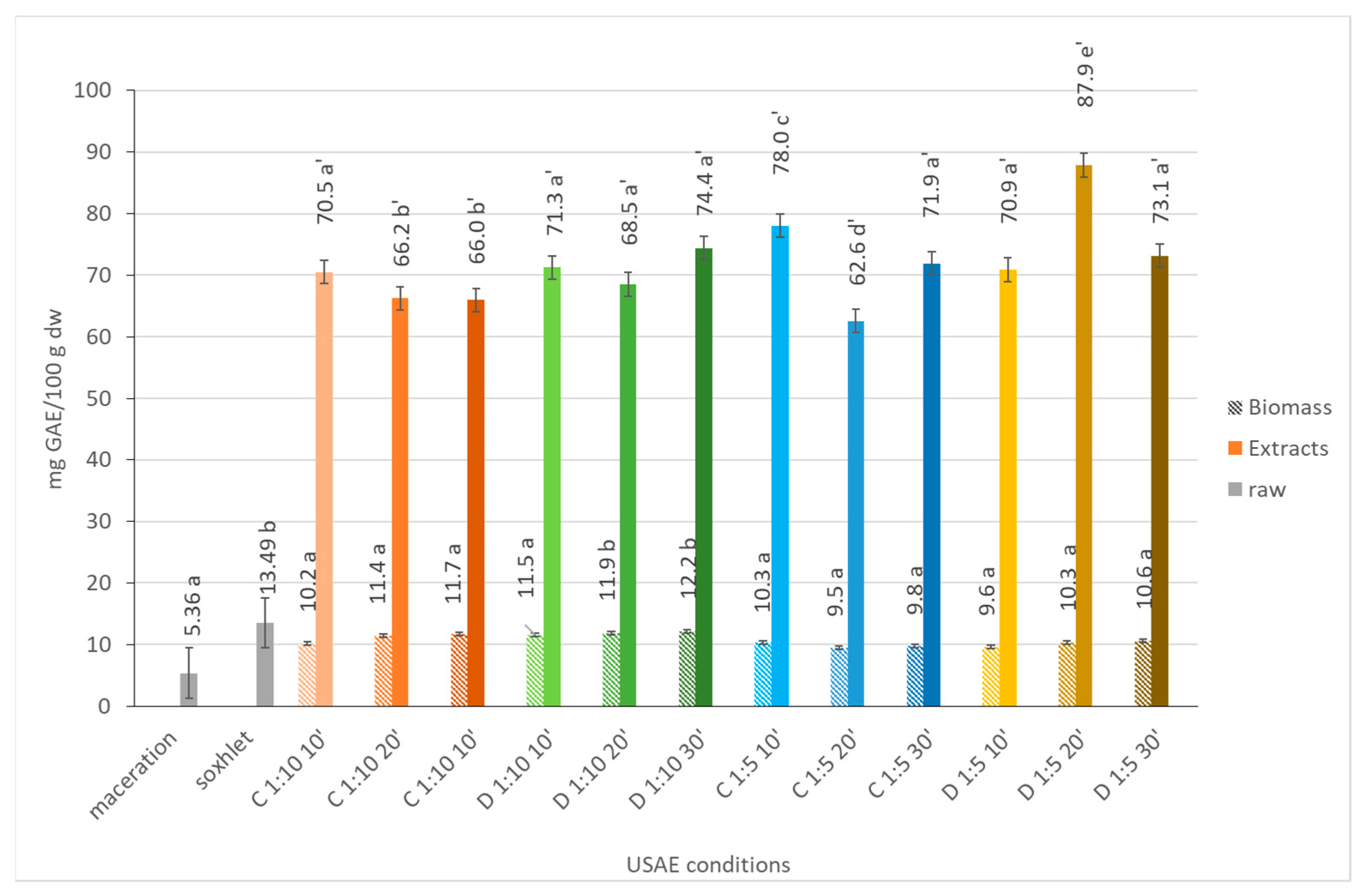
2.1.7. Total Phenolic Content
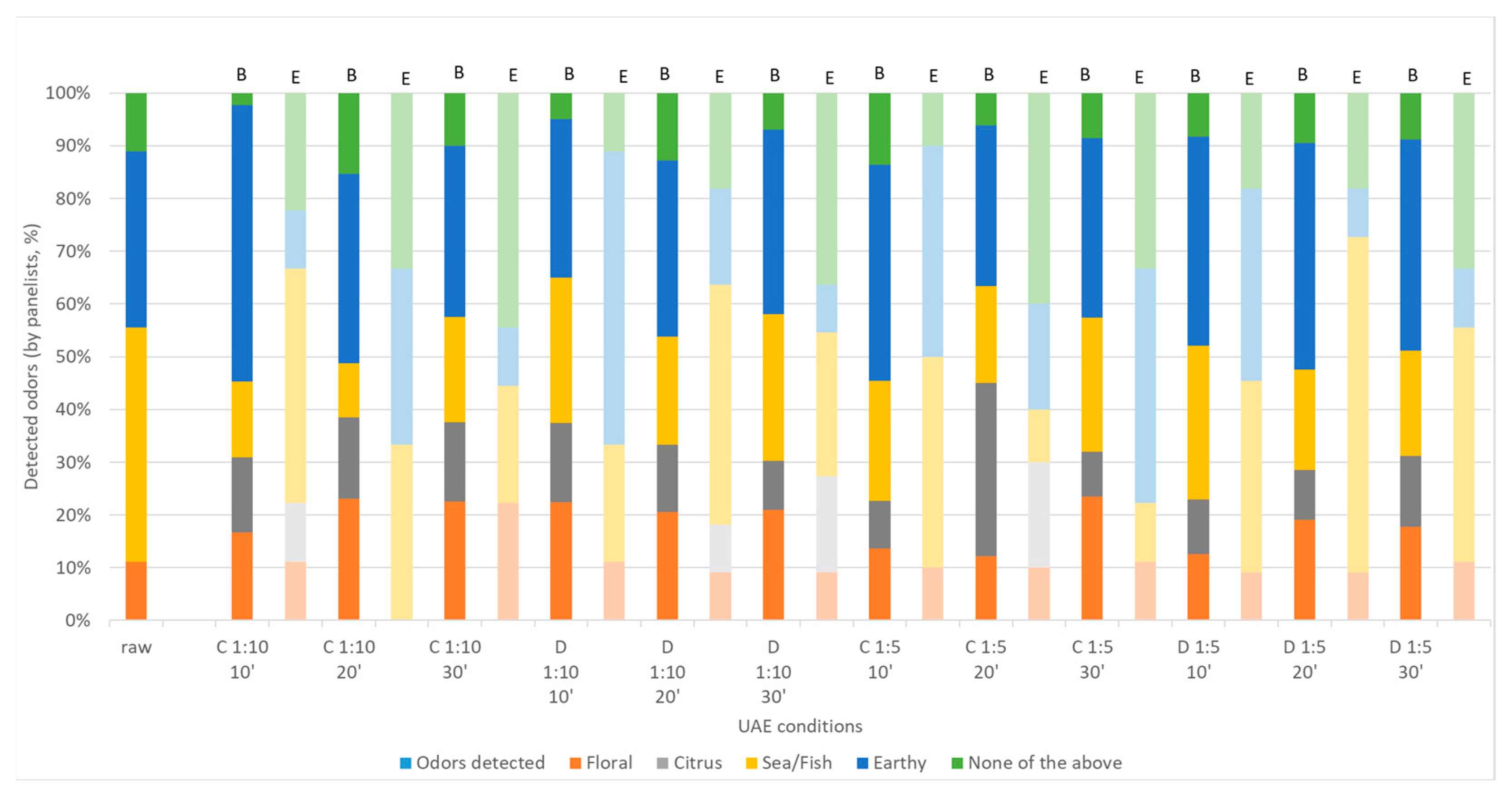
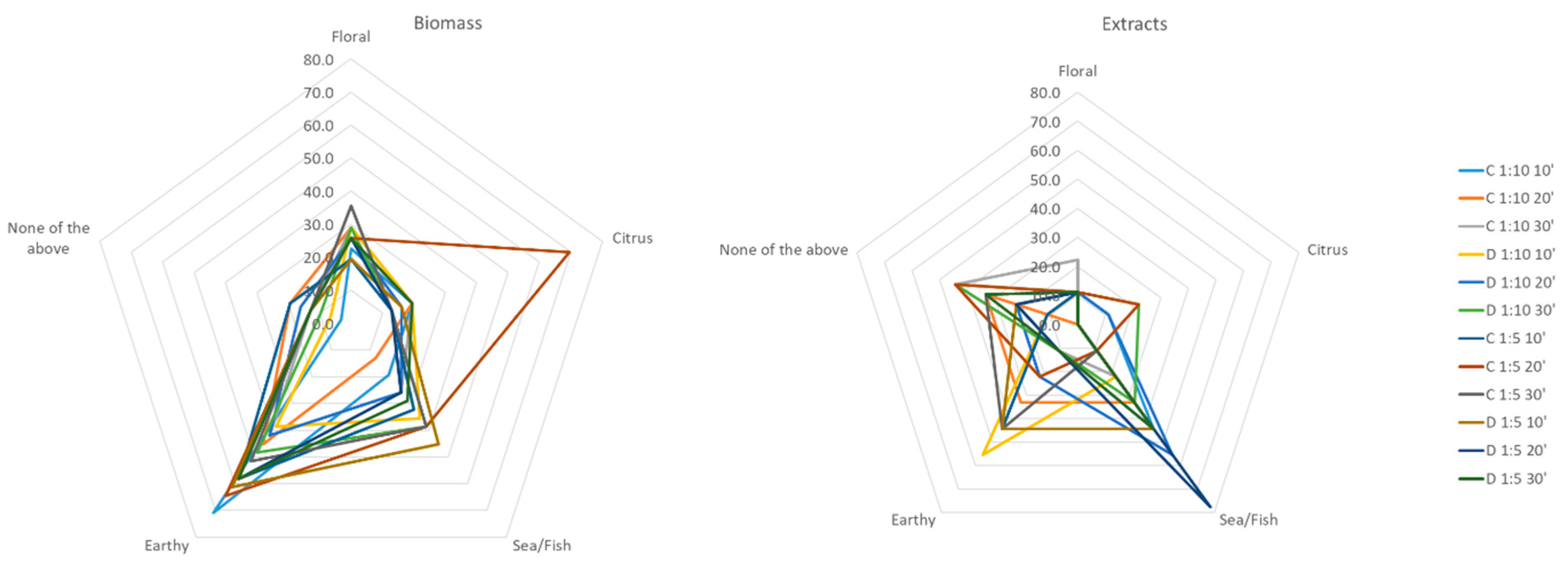
2.2. Sensory Analysis
3. Methods and Materials
3.1. Samples and Chemicals
3.2. Conventional Extraction
3.2.1. Soxhlet Extraction
3.2.2. Maceration
3.3. Ultrasound-Assisted Extraction
3.4. Nutritional Composition
3.5. Antioxidant Capacity Evaluation
3.5.1. FRAP
3.5.2. DPPH
3.6. Total Phenolic Content
3.7. Sensory Evaluation
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical Polyphenols: New Analytical Challenges and Opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J.; Peschel, A.O. Consumer perception of plant-based proteins: The value of source transparency for alternative protein ingredients. Food Hydrocoll. 2019, 96, 20–28. [Google Scholar] [CrossRef]
- Dey, S.; Rathod, V.K. Ultrasound Assisted Extraction of β-Carotene from Spirulina Platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ozcan Cetin, E.H.; Cetin, M.S.; Özbay, M.B.; Yaman, N.M.; Könte, H.C.; Ekizler, F.A.; Tak, B.T.; Kara, M.; Temizhan, A.; Özcan, F.; et al. The Other Side of the Medallion in Heart Failure: Reverse Metabolic Syndrome. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2041–2050. [Google Scholar] [CrossRef]
- Nunes, M.C.; Ferreira, J.; Raymundo, A. Volatile Fingerprint Impact on the Sensory Properties of Microalgae and Development of Mitigation Strategies. Curr. Opin. Food Sci. 2023, 51, 101040. [Google Scholar] [CrossRef]
- Hyrslova, I.; Krausova, G.; Mrvikova, I.; Stankova, B.; Branyik, T.; Malinska, H.; Huttl, M.; Kana, A.; Doskocil, I. Functional Properties of Dunaliella salina and Its Positive Effect on Probiotics. Mar. Drugs 2022, 20, 781. [Google Scholar] [CrossRef]
- Chavan, Y.; Singhal, R.S. Ultrasound-Assisted Extraction (UAE) of Bioactives from Arecanut (Areca Catechu L.) and Optimization Study Using Response Surface Methodology. Innov. Food Sci. Emerg. Technol. 2013, 17, 106–113. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat. Molecules 2017, 22, 638. [Google Scholar] [CrossRef]
- Purohit, A.J.; Gogate, P.R. Ultrasound-Assisted Extraction of β-Carotene from Waste Carrot Residue: Effect of Operating Parameters and Type of Ultrasonic Irradiation. Sep. Sci. Technol. 2015, 50, 1507–1517. [Google Scholar] [CrossRef]
- Custódio, L.; Fernandes, E.; Escapa, A.L.; Fajardo, A.; Aligué, R.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. Antioxidant and Cytotoxic Activities of Carob Tree Fruit Pulps Are Strongly Influenced by Gender and Cultivar. J. Agric. Food Chem. 2011, 59, 7005–7012. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic Content and In Vitro Antioxidant Characteristics of Wine Industry and Other Agri-Food Solid Waste Extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic Assisted Aqueous Extraction of Catechin and Gallic Acid from Syzygium Cumini Seed Kernel and Evaluation of Total Phenolic, Flavonoid Contents and Antioxidant Activity. Chem. Eng. Process. Process Intensif. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Ultrasound Waves Increase the Yield and Carotenoid Content of Lipid Extracted From Cephalothorax of Pacific White Shrimp (Litopenaeus Vannamei). Eur. J. Lipid Sci. Technol. 2018, 120, 1700495. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Cui, Y.; Yuan, W. Ultrasound for Microalgal Cell Disruption and Product Extraction: A Review. Ultrason. Sonochem. 2022, 87, 106054. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Patil, C.; Moholkar, V.S. Mechanistic Assessment of Microalgal Lipid Extraction. Ind. Eng. Chem. Res. 2010, 49, 2979–2985. [Google Scholar] [CrossRef]
- Mecozzi, M.; Amici, M.; Romanelli, G.; Pietrantonio, E.; Deluca, A. Ultrasound Extraction and Thin Layer Chromatography–Flame Ionization Detection Analysis of the Lipid Fraction in Marine Mucilage Samples. J. Chromatogr. A 2002, 963, 363–373. [Google Scholar] [CrossRef]
- Wiyarno, B.; Yunus, R.M.; Mel, M. Extraction of Algae Oil from Nannocloropsis Sp.: A Study of Soxhlet and Ultrasonic-Assisted Extractions. J. Appl. Sci. 2011, 11, 3607–3612. [Google Scholar] [CrossRef]
- Gutte, K.B.; Sahoo, A.K.; Ranveer, R.C. Effect of Ultrasonic Treatment on Extraction and Fatty Acid Profile of Flaxseed Oil. OCL 2015, 22, D606. [Google Scholar] [CrossRef]
- Bigelow, T.A.; Xu, J.; Stessman, D.J.; Yao, L.; Spalding, M.H.; Wang, T. Lysis of Chlamydomonas Reinhardtii by High-Intensity Focused Ultrasound as a Function of Exposure Time. Ultrason. Sonochem. 2014, 21, 1258–1264. [Google Scholar] [CrossRef]
- Martínez, N.; Callejas, N.; Morais, E.G.; Vieira Costa, J.A.; Jachmanián, I.; Vieitez, I. Obtaining Biodiesel from Microalgae Oil Using Ultrasound-Assisted in-Situ Alkaline Transesterification. Fuel 2017, 202, 512–519. [Google Scholar] [CrossRef]
- Hromádková, Z.; Kováčiková, J.; Ebringerová, A. Study of the Classical and Ultrasound-Assisted Extraction of the Corn Cob Xylan. Ind. Crops Prod. 1999, 9, 101–109. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, X.; Wang, L.; Zhou, S.; Feng, H.; Chen, W.N.; Lau, R. Ultrasound Assisted Extraction of Carbohydrates from Microalgae as Feedstock for Yeast Fermentation. Bioresour. Technol. 2013, 128, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Kocberber Kilic, N.; Erdem, K.; Donmez, G. Bioactive Compounds Produced by Dunaliella Species, Antimicrobial Effects and Optimization of the Efficiency. Turk. J. Fish. Aquat. Sci. 2019, 19, 923–933. [Google Scholar] [CrossRef]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal Derivatives as Potential Nutraceutical and Food Supplements for Human Health: A Focus on Cancer Prevention and Interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef]
- Ahmed, F.; Zhou, W.; Schenk, P.M. Pavlova Lutheri Is a High-Level Producer of Phytosterols. Algal Res. 2015, 10, 210–217. [Google Scholar] [CrossRef]
- D-FACTORY (THE MICRO ALGAE BIOREFINERY). Available online: https://cordis.europa.eu/project/id/613870/reporting (accessed on 30 July 2023).
- Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Continuous and Pulsed Ultrasound-Assisted Extraction of Carob’s Antioxidants: Processing Parameters Optimization and Identification of Polyphenolic Composition. Ultrason. Sonochem. 2021, 76, 105630. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Grape (Vitis vinifera) Seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef]
- Terpinc, P.; Čeh, B.; Ulrih, N.P.; Abramovič, H. Studies of the Correlation between Antioxidant Properties and the Total Phenolic Content of Different Oil Cake Extracts. Ind. Crops Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Kilicgun, H.; Altiner, D. Correlation between Antioxidant Effect Mechanisms and Polyphenol Content of Rosa Canina. Pharmacogn. Mag. 2010, 6, 238. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 10th ed.; Method 08-01, 46-10, 10-10B, and 54-21; The Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Norma Portuguesa. Cereais e Derivados-Determinação Do Teor de Matéria Gorda Total. 1991. Available online: http://www.Esa.Ipsantarem.Pt/Norma_IPQ_homepage_206.Pdf (accessed on 30 July 2023).
- Kirsten, W.J. Automatic Methods for the Simultaneous Determination of Carbon, Hydrogen, Nitrogen, and Sulfur, and for Sulfur Alone in Organic and Inorganic Materials. Anal. Chem. 1979, 51, 1173–1179. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae Biomass as an Additional Ingredient of Gluten-Free Bread: Dough Rheology, Texture Quality and Nutritional Properties. Algal Res. 2020, 50, 101998. [Google Scholar] [CrossRef]
- Nunes, M.C.; Fernandes, I.; Vasco, I.; Sousa, I.; Raymundo, A. Tetraselmis Chuii as a Sustainable and Healthy Ingredient to Produce Gluten-Free Bread: Impact on Structure, Colour and Bioactivity. Foods 2020, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Declaration of Helsinki 1975, Revised in 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 4 August 2023).
| Yield of Extraction (%) | |||
|---|---|---|---|
| Solid to Solvent Ratio | Time (min) | Continuous Mode | Pulse Mode |
| 1:10 | 10 | 35.78 | 22.82 |
| 20 | 35.91 | 33.36 | |
| 30 | 41.38 | 35.30 | |
| 1:5 | 10 | 23.52 | 14.85 |
| 20 | 15.78 | 14.73 | |
| 30 | 17.47 | 14.67 | |
| Conventional extraction | Time (h) | Yield of extraction (%) | |
| Soxhlet | 18 | 10.36 | |
| Maceration | 24 | 3.14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.P.A.; Grácio, M.; Sousa, I.; Pagarete, A.; Nunes, M.C.; Raymundo, A. Tuning the Bioactive Properties of Dunaliella salina Water Extracts by Ultrasound-Assisted Extraction. Mar. Drugs 2023, 21, 472. https://doi.org/10.3390/md21090472
Ferreira JPA, Grácio M, Sousa I, Pagarete A, Nunes MC, Raymundo A. Tuning the Bioactive Properties of Dunaliella salina Water Extracts by Ultrasound-Assisted Extraction. Marine Drugs. 2023; 21(9):472. https://doi.org/10.3390/md21090472
Chicago/Turabian StyleFerreira, Joana P. A., Madalena Grácio, Isabel Sousa, António Pagarete, M. Cristiana Nunes, and Anabela Raymundo. 2023. "Tuning the Bioactive Properties of Dunaliella salina Water Extracts by Ultrasound-Assisted Extraction" Marine Drugs 21, no. 9: 472. https://doi.org/10.3390/md21090472
APA StyleFerreira, J. P. A., Grácio, M., Sousa, I., Pagarete, A., Nunes, M. C., & Raymundo, A. (2023). Tuning the Bioactive Properties of Dunaliella salina Water Extracts by Ultrasound-Assisted Extraction. Marine Drugs, 21(9), 472. https://doi.org/10.3390/md21090472