Abstract
The combination of liquid chromatography coupled to high resolution mass spectrometry (LC-HRESMS)-based dereplication and antiproliferative activity-guided fractionation was applied on the Red Sea-derived soft coral Sarcophyton sp. This approach facilitated the isolation of five new cembrane-type diterpenoids (1–5), along with two known analogs (6 and 7), as well as the identification of 19 further, known compounds. The chemical structures of the new compounds were elucidated while using comprehensive spectroscopic analyses, including one-dimensional (1D) and two-dimensional (2D) NMR and HRMS. All of the isolated cembranoids (1–7) showed moderate in vitro antiproliferative activity against a human breast cancer cell line (MCF-7), with IC50 ranging from 22.39–27.12 µg/mL. This class of compounds could thus serve as scaffold for the future design of anticancer leads.
1. Introduction
Marine natural products are characterized by their diversity in chemical structures, along with biological activities [1]. The Red Sea is considered to be one of the most important marine spots comprising high biodiversity. About 40% of the soft corals that are identified worldwide are native to the Red Sea, however only a few species that have been chemically examined in the last decades [1,2,3]. The genus Sarcophyton (Phylum Cnidaria; Order Alcyonacea; Family Alcyoniidae) contains 46 species with typically mushroom- or toadstool-shaped appearance and it is considered one of the most abundant Red Sea soft corals [1]. Sarcophyton sp. are well recognized as a rich source for a wide range of terpenoid metabolites. Macrocyclic cembrane-type diterpenoids and their derivatives represent the main chemical defense for Sarcophyton species against natural predators [3,4,5]. Previous studies concerning the biological activities of cembranoid analogues revealed that they exhibited a wide range of biological activities, including ichthyotoxic [6], anti-inflammatory [7], anti-bacterial [8], neuroprotective [9], and antitumor [10] properties. In addition, a number of Sarcophyton-derived cembranoid analogues (e.g., sarcophine and its hydroxylated derivatives) were investigated as potential competitive cholinesterase inhibitors [11], selective noncompetitive phosphofructokinase inhibitors [12], and a Na+, K+-ATPase inhibitors [13].
Cancer chemoprevention is based on chemical compounds that inhibit or reverse the development of cancer in normal or pre-neoplastic tissue [14]. A lot of novel marine metabolites were identified as anticancer leads during the past 20 years [15]. Our previous work on sarcophine (7) and its analogs has illustrated the ability of this class of compounds to inhibit growth, proliferation, and migration of the prostate and breast metastatic cancer cell lines PC-3 and MDA-MB-231 [6].
Looking for new natural products with cytotoxic activity that is applicable in cancer therapy is of utmost importance. The discovery process has improved with the evolution of new spectroscopic techniques. Liquid chromatography coupled to high resolution mass spectrometry (LC-HRESMS) can generate information-rich data sets that can assist in the dereplication of previously reported natural products that are present in the crude extracts prior to a tedious isolation attempt. New biological material was collected and extracted in order to extend our previous investigation of the Red Sea Sarcophyton species [6]. Subsequently, bioactivity-guided isolation assisted by LC-HRESMS metabolic profiling led to the isolation of five new cembrane-type diterpenoids (1–5, Figure 1) that are structurally related to sarcophine (7) which was also isolated along with another known cembranoid, sinumaximol G (6). Testing of all isolated compounds against the MCF-7 breast cancer cell line showed a moderate in vitro growth inhibitory activity.
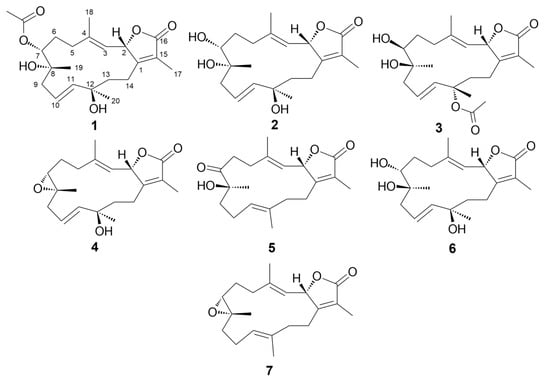
Figure 1.
Structures of isolated compounds (1–7).
2. Results and Discussion
Extraction and fractionation on normal phase (NP) silica gel while using medium pressure liquid chromatography (MPLC) afforded seven major fractions. LC-HRESMS dereplication of these fractions using commercial and public databases led to the putative identification of 19 compounds, which were previously reported from Sarcophyton sp., in addition to 17 molecules with mass data showing no hits in MS databases, most of them in fractions 6 and 7 (Table S1). These LC-HRESMS findings, together with the in vitro cytotoxicity results against MCF-7 cell line using the acquired MPLC fractions, led to prioritization of fractions 6 and 7 for further chromatographic purification on silica gel and Sephadex LH-20 to isolate the active components. Preliminary 1H NMR spectroscopic analysis revealed that all the major compounds in fractions 6 and 7 had a cembranoid backbone [16,17], differing either in the degree of oxidation or the configuration of one or more chiral centers.
2.1. Identification of the Isolated Compounds
Compound 1 was obtained as colorless oil. Its molecular formula was established as C22H32O6 that is based on HRESIMS data (Figure S1) that showed an [M + H]+ ion at m/z 393.2264 (calcd m/z 393.2272). The 1H NMR spectrum (Table 1 and Table 2), together with the multiplicity-edited HSQC (Figures S2–S5), were in harmony with a sarchophine (7) skeleton that was previously isolated from other Sarcophyton species [6,16,18]. 1H NMR and 13C NMR (Table 2), together with multiplicity-edited HMQC, showed the following characteristic resonances: (i) the presence of an α,β-unsaturated-γ-lactone functionality: δC 122.9 (C-15), δc 161.4 (C-1), and δc 175.4 (C-16). (ii) Two olefinic moieties at δc 142.0 (C-4) and δc/H 122.9/4.81 (C-3), as well as at δc/H 124.5/ 5.48 (C-10) and δC/H 140.0/5.47 (C-11). HMBC correlations confirmed the positions of the olefinic moieties (Figure 2 and Figures S6 and S7) H-5/C-4, H-18/C-4, H-18/C-3, H-9/C-10, H-9/C-11, H-10/C-8, and H-10/C-12. (iii) Three oxygen functionalities; an oxomethine at δC/H 79.2/5.48 (C-2) and two oxygenated quaternary carbons at δC 74.3 (C-8) and δC 74.4 (C-12). (iv) Four methyl singlets at [δH 1.86 (H-17), δH 1.85 (H-18), δH 1.20 (H-19), and δH 1.36 (H-20)], and five methylene groups [δH 2.14, 1.90 (H-5); δH 1.90, 1.75 (H-6); δH 2.28, 2.20 (H-9); δH 1.70, 1.68 (H-13); and, δH 2.18, 2.48 (H-14)]. HMBC correlations between H2-14 and δC 161.4 (C-1), H-3 and δC 35.5 (C-5), H2-5 and δC 142.0 (C-4) and H2-6 and δC 35.5 (C-5) and H2-6 /H2-5 and δC 74.4 (C-7) established the carbon linkages from C-14 to C-7. Furthermore, correlations between H2-9 and δC 74.3 (C-8)/124.5 (C-10)/140.0 (C-11); H-10 and δC 74.3 (C-8)/74.4 (C-12); H2-13 and δC 74.4 (C-12)/140.0 (C-11)/23.0 (C-14); and finally, H2-14 and δC 161.4 (C-1)/79.2 (C-2) established the connectivity of the 14-membered ring. The positions of the methyl groups were recognized through HMBC correlations between δH 1.85 (H3-18, s) and C-3 and C-4; δH 1.20 (H3-19, s) and C-9; δH 1.36 (H3-20, s) and C-11 and C-12; and, δH 1.86 (H3-17, s) and C-1, C-15, and C-16. These NMR spectroscopic features were very similar to the reported spectroscopic data of the known compound sinumaximol G, which was previously isolated from the Soft Coral Sinularia maxima [16] and also isolated in the present work (Compound 6). The 1H NMR and 13C NMR spectroscopic data of compound 1 showed differences in the chemical shifts of C-7/δC 74.4 and H-7/δH 4.82, which indicated changes of the substitution at C-7. In addition, the presence of additional resonances in the NMR spectral data for acetate moiety [δC 170.3 (C-1’), δC/H 21.1/2.08 (C-2’)], along with the HMBC correlations of H3-2’/C-1’, H-7/C-1’, and H-7/C-19, suggested that the OH group in sinumaximol G at C-7 has been acetylated in compound 1. The relative configuration of 1 was determined on the basis of coupling constants and NOESY experiments (Figures S8 and S9). The vicinal coupling constant of 14.0 Hz between H-2 (δH 5.48) and H-3 (δH 4.81), together with the 1H-1H NOESY correlations (Figure 3) between the methyl protons H3-18 (δH 1.85), and both H-2 (δH 5.48) and H-7 (δH 4.82), suggested a β-orientation of both H-2 and H-7. Moreover, the 1H-1H NOESY correlations between H-3 (δH 4.81) and H-20 (δH 1.36) suggested that H3-20 were in the α-orientation. Alterations in the 1H NMR chemical shift of H3-19 (δH 1.2), together with the 1H-1H NOESY correlations between H3-19 (δH 1.2) and H3-18 (δH 1.85), indicated changes in the orientation of the methyl group at C-8 to be in the β-orientation. Therefore, compound 1 was assigned as a new natural product and given the name 7-acetyl-8-epi- sinumaximol G (1).

Table 1.
1H NMR spectral data of 1–5 (600 MHz, CDCl3).

Table 2.
NMR spectral data of 1-5 (150 MHz, CDCl3).
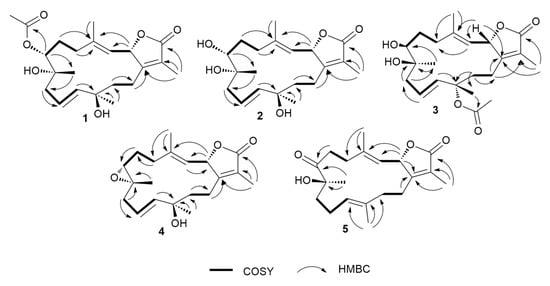
Figure 2.
1H-1H COSY and key HMBC correlations of compounds 1–5.
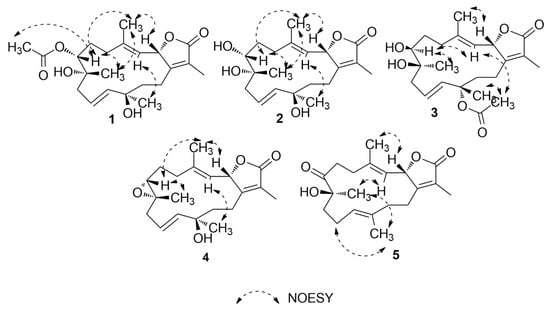
Figure 3.
1H-1H NOESY correlations of compounds 1–5.
The molecular formula of compound 2 was determined to be the same as that of sinumaximol G (6) (C20H30O5); m/z 351.2160 [M + H]+ ion (calcd m/z 351.2166) (Figure S10). The one-dimensional (1D) and two-dimensional (2D) NMR spectroscopic features (Table 1 and Table 2; Figures S11–S15) also indicated that 2 had an almost identical structure as 1 and sinumaximol G (6) [16]. The difference between those two compounds was the deshielding of the H3-19 methyl protons (δH 1.34), which indicated that the configuration of the methyl group at C-8 is altered to the β-configuration. The 1H-1H NOESY correlation (Figure 3) between H3-19 (δH 1.34) and H3-18 (δH 1.83) further confirmed this change, and hence compound 2 was identified as a new natural product, which we named 8-epi- sinumaximol G (2).
Compound 3 was isolated as colorless oil. HRESIMS analysis (Figure S16) resulted in m/z 393.2264 [M + H]+ ion (calcd m/z 393.2272), giving a molecular formula of C22H32O6. The 1D NMR spectral analyses (Table 2) together with the multiplicity-edited HSQC spectrum of compound 3 showed remarkable similarity to that of compound 1 (Figures S17–S20). The difference between the two compounds was the change in the location of the acetyl moiety, which HMBC correlations confirmed (Figure 2 and Figure S21) of H3-20/C-1’ and H3-2’/C-12, and NOESY correlation (Figure 3 and Figure S22) between H3-20 (δH 1.56) and H3-2’ (δH 2.0). Changes in the 1H NMR chemical shifts of H-7 (δH 3.39) and 13C NMR of C-12 (δC 82.3), along with the NOESY (Figure 3) correlations between H-3 (δH 4.85) and both H-7 (δH 3.39) and H3-2′ (δH 2.00), indicated that H-7 and the acetyl moiety were both in α-orientation. In addition, agreement of the 1H NMR and 13C NMR chemical shifts at C-2 (δH 5.43, δC 79.3) and C-8 (δC 74.9) with that of 6 and the previously reported sinumaximol G (1), together with the NOESY correlations between H3-19 (δH 1.54) and H-7 (δH 3.39), and between H3-18 (δH 1.82) and H-2 (δH 5.43), suggested an β-orientation of H-2 and α-orientation of H-19. Therefore, compound 3 was identified as a new natural product which we named 12-acetyl-7, 12-epi- sinumaximol G (3).
Compound 4 was obtained as a colorless solid. Its molecular formula was deduced as C20H28O4 based on the HRESIMS [M + H]+ ion at m/z 333.2035 (calcd m/z 333.2066) (Figure S23). The 1H and 13C NMR spectral data (Table 1 and Table 2), together with the 2D spectral data of compound 4 (Figures S24–S28), was closely related to that of sarcophine (7) [18]. HMBC correlations (Figure 2) between H-10 (δH 5.76) and C-9 (δC 42.8)/C-11(δC 140.4)/C-12 (δC 37.1), H-13 (δH 1.78, 1.80), and C-1 (δC 161.7)/C-11 (δC 140.4)/C-12 (δC 37.1)/C-14 (δC 23.8), and H-20 (δH 1.38) and C-11 (δC 140.4)/C-12 (δC 37.1)/C-13 (δC 40.7) revealed the presence of a double bond at C-10/C-11 and it also indicated the presence of an oxygenated quaternary carbon at C-12. The NOESY correlations (Figure 3 and Figure S29) between the methyl protons H3-18 (δH 1.78) and both H-2 (δH 5.43) and H-7 (δH 3.53), suggested a β-orientation of both H-2 and H-7. Similarly, NOESY correlations between H-7 (δH 3.53) and H3-19 (δH 1.31) indicated a β-orientation of the methyl group at C-8. Additionally, the NOESY correlations between H-3 (δH 4.96) and H3-20 (δH 1.38) suggested the methyl group at C-12 to be in α-orientation. Therefore, compound 4 was identified as a new natural product, to which we gave the name 12-hydroxysarcoph-10-ene (4).
Compound 5 was obtained as colorless oil. The molecular formula was deduced as C20H28O4 based on HRESIMS [M + H]+ ion at m/z 333.2035 (calcd m/z 333.2066) (Figure S30), which indicated seven degrees of unsaturation. The 1H and 13C NMR spectral data (Table 1 and Table 2; Figures S31–S33) were in harmony with the skeleton of 8-epi-sarcophinone that was previously isolated from the Soft Coral Sorcophytum glaucum [19]. Considering 13C NMR chemical shifts, the following functional groups were identified; (i) an α,β-unsaturated-γ-lactone functionality: δC 124.0 (C-15), δC 163.4 (C-1) δC 175.1 (C-16). (ii) olefinic carbons at δC 120.2/144.0 (C-3/C-4) and δC 123.9/135.1 (C-11/C-12), and (iii) a ketone functional group at δC 213.7 (C-7) and alcohol functionalities at δC 79.3 (C-2) and δC 78.2 (C-8). The COSY spectrum (Figure S34) revealed the coupling of four hydrocarbon regions common to sarchophine skeletons: δH 5.44 (d, J = 12.0 Hz, H-2) and 4.95 (d, J = 12.0 Hz, H-3); δH 2.22 /2.47 (m, H2-5) and 2.76 /2.93 (m, H2-6); δH 1.98 (m, H2-9), 2.20/2.44 (m, H2-10) and 4.70 (br. t, J = 6 Hz, H-11) and δH 1.81/1.95 (m, H2-13) and 2.21/2.44 (m, H2-14). HMBC correlations (Figure 2 and Figure S35) between H-2 and δC 163.4 (C-1), H-2 and δC 120.2 (C-3), H-2 and δC 144.0 (C-4), H-3 and δC 17.8 (C-18), H3-18 and δC 120.2 (C-3), H3-18 and δC 31.5 (C-5), H2-5 and δC 144.0 (C-4), H2-5 and δC 34.2 (C-6) and H2-6/H2-5 and δC 213.7 (C-7); these correlations confirmed the linkage from C-1 to C-7. Additional correlations between H3-19 and δC 213.7 (C-7), H2-9, and δC 78.2 (C-8), H2-10 and δC 35.8 (C-9), H-11 and δC 25.7 (C-10), H3-20 and δC 123.9 (C-11), H2-10 and δC 135.1 (C-12), H3-20 and δC 39.4 (C-13), H2-13 and δC 163.4 (C-1), and H2-14 and δC 163.4 (C-1) confirmed the 14-membered ring skeleton. 1H-1H NOESY correlations (Figure 3 and Figure S36) between H3-18 (δH 1.89) and H-2 (δH 5.44), and between H3-19 (δH 1.26) and both H-3 (δH 4.95) and H-20 (δH 1.53) suggested a β-orientation of H-2 and a α-orientation of H-19. The chemical shifts of H-3 at δH 4.95 and H-11 at δH 4.70 are very characteristic for E configuration at the C-11/C-12 and C-3/C-4 double bonds [6,16,20], which was further confirmed by the 1H-1H NOESY correlations of the two olefinic methyls at C-4 and C-12 with H-2 and H2-10, respectively. Based on these data, compound 5 was considered to be a new natural product, which was named 8-hydroxy-epi-sarcophinone (5).
Compounds 6 and 7 were previously isolated from the Soft Corals Sinularia maxima and Sorcophytum glaucum, and identified as sinumaximol G (6) [16] and sarcophine (7) [18], respectively. Their structures were confirmed based on a comparison of their HRMS and NMR data with literate.
2.2. Antiproliferative Activity
Our previous in vitro screening results showed that, among several cancer cell lines, sarchophine (7), along with other analogs, were only active against breast (MDA-MB-231) and prostate (PC-3) cancer cell lines [6]. We consequently chose a breast carcinoma cell line (MCF-7) to assess the in vitro anticancer effect of the isolated compounds (1–7). All of the tested compounds induced dose-dependent cell death with IC50 values (Table 3) of (22.39–27.12 µg/mL). Although all of the tested compounds have significant cytotoxic potentials against MCF-7, they have slightly reduced (approx. two-fold) anticancer effects in comparison to the standard anticancer drug doxorubicin (IC50: 12.78 µg/mL). In the light of these results, along with the previous reports [6,16,19,20,21] on other cembrane-type diterpenoids, we can conclude that the presence of an α, β-unsaturated-γ-lactone moiety is an essential feature for the antiproliferative properties of these class of compounds. Additionally, changing in the orientation or acetylation of hydroxyl groups at C-7, C-8, and C-12 almost have no effect on their cytotoxicity.

Table 3.
In-vitro antiproliferative activity of isolated metabolites (1–7) against MCF-7 cells.
3. Materials and Methods
3.1. General Experimental Procedures
Silica gel 60 (Natland, 63–200 µm) and solvent systems consisting of n-hexane-EtOAc (9.5:0.5 to 70:30) were used for column chromatography. Pre-coated silica gel plates (Merck, Darmstadt, Germany, Kieselgel 60 F254, 0.25 mm) were used for TLC analyses. 1% vanillin in concentrated H2SO4 was used as the visualizing reagent. LC/MS was conducted on a Thermo MS system (LTQ XL/LTQ Orbitrap Discovery) coupled to a Thermo Instruments HPLC system with Accela PDA detector: The data were processed using Xcalibur 2.0.7. 1H and 13C NMR spectra were recorded in CDCl3 on a JEOL ECA-600 spectrometer (600 MHz for 1H and 150 MHz for 13C, respectively). All of the chemical shifts (δ) are given in ppm units with reference to TMS as an internal standard, and coupling constants (J) are reported in Hz.
3.2. Extraction and Fractionation
The soft coral Sarcophyton sp. was collected from the Egyptian Red Sea off the coast of Hurghada (GPS coordinates N 27°15048”, E 33°4903”) at depths of 5–7 m in March 2016 and then frozen for storage. A voucher specimen (NIOF404/2016) was reserved at the National Institute of Oceanography and Fisheries, Red Sea Branch, Invertebrates Department. The frozen soft coral (700 g) was extracted four times with isopropanol. The extract was concentrated under vacuum to give 70 g raw material and then chromatographed on silica gel while using n-hexane/EtOAc to afford seven fractions. Only fractions 6 and 7 exhibited in vitro cytotoxicity against MCF-7 cell lines. In addition, LC-HRESMS dereplication results were used to prioritize those fractions, as they showed several unknown molecular formulas. Further chromatographic separation of fractions 6 and 7 on silica gel while using a gradient of n-hexane/EtOAc yielded compounds 1–7, which were purified on Sephadex LH-20 using 10% aqueous MeCN.
7-Acetyl-8-epi-sinumaximol G (1): colorless oil; +4.8 (c 0.54, CH2Cl2); 1H NMR and 13C NMR data, see Table 1 and Table 2; HR-EI-MS [M + H]+ m/z 393.2262 (calc. 393.2272, C22H32O6).
8-epi-Sinumaximol G (2): colorless oil; +5.4 (c 0.50, CH2Cl2); 1H NMR and 13C NMR data, see Table 1 and Table 2; HR-FAB-MS [M + H]+ m/z 351.2160 (calc. 351.2166, C20H30O5).
12-Acetyl-7, 12-epi- sinumaximol G (3): colorless oil; +1.7 (c 0.58, CH2Cl2); 1H NMR and 13C NMR data, see Table 1 and Table 2; HR-EI-MS [M + H]+ m/z 393.2264 (calc. 393.2272, C22H32O6).
12-Hydroxysarcoph-10-ene (4): colorless oil; +4.6 (c 0.46, CH2Cl2); 1H NMR and 13C NMR data, see Table 1 and Table 2; HR-EI-MS [M + H]+ m/z 343.2840 (calc. 343.2843, C20H38O4).
8-Hydroxy-epi-sarcophinone (5): colorless oil; +5.1 (c 0.40, CH2Cl2); 1H NMR and 13C NMR data, see Table 1 and Table 2; HR-EI-MS [M + H]+ m/z 343.2849 (calc. 343.2843, C20H38O4).
Sinumaximol G (6): = +4.1 (c=0.48, CH2Cl2); lit. +3.4 (c 0.52, CH2Cl2) [16].
Sarcophine (7): +79.6 (c 0.9, CH2Cl2); lit. +92 (c 1.0, CHCl3) [6].
3.3. LC-HRESIMS Analysis and Dereplication
The following conditions were used for LC-HRESIMS analysis: capillary voltage 45 V, capillary temperature 260 °C, auxiliary gas flow rate 10–20 arbitrary units, sheath gas flow rate 40-50 arbitrary units, spray voltage 4.5 kV, and mass range 100–2000 amu (maximum resolution 30000). Gradient separation was achieved while using a SunFire C18 RP analytical HPLC column (5µm, 4.6 × 150 mm, Waters) with a mobile phase of 0–100% MeOH over 30 min. at a flow rate of 1 mL/min. Multiple available databases (SciFinder: https://sso.cas.org/as/E9dPQ/resume/as/authorization.ping, MarinLit: http://pubs.rsc.org/marinlit/, Dictionary of Natural Products: http://dnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml, PubChem: https://pubchem.ncbi.nlm.nih.gov/, Chemspider: http://www.chemspider.com/) were used for the dereplication and annotation of known compounds.
3.4. In Vitro Antiproliferative Activity
The human breast cancer cell line (MCF-7) was obtained from the American Type Culture Collection (ATCC, Manassas, USA). They were seeded in 96 well microtiter plates at a concentration of 1000–2000 cells/well, 100 µL/well. After 24 h, the cells were incubated for 72 h with the compounds to be tested. Dulbecco’s Modified Eagle Medium (DMEM) with 10% foetal calf serum, sodium pyruvate, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37 °C and 5% CO2 was used as culture medium. The medium was discarded at the end of the incubation. The cells were fixed with 150 μL cold trichloroacetic acid with 10% final concentration for 1 h at 4 °C. The plates were washed with distilled water (automatic washer Tecan, Germany) and then stained with 50 μL 0.4% Sulforhodamine B dissolved in 1% acetic acid for 30 min at room temperature in the dark. The plates were washed with 1% acetic acid to remove unbound dye and air-dried (24 h). The dye was solubilized with 150 µL/well of 10 mM tris base (pH 7.4) for 5 min. on a shaker at 1600 rpm. The optical density (OD) of each well was spectrophotometrically measured at 490 nm with an ELISA microplate reader. The mean background absorbance was automatically subtracted and the mean values of each tested compound and doxorubicin concentration was calculated. The experiment was repeated three times for each tested compound. The percentage of cell survival was calculated by using the following formula: surviving percent = [O.D. (treated cells)/O.D. (control cells)] x100. The IC50 values (the concentrations of compound required to produce 50% inhibition of cell growth) were also calculated.
4. Conclusions
The present study provides an additional chemical characterization of the Red Sea soft coral Sarcophyton. LC-HRESMS-based dereplication, in combination with biological activity-guided fractionation, allowed for the accelerated characterization of further new bioactive cembrane-type diterpenoids. All of the isolated compounds demonstrated moderate in vitro antiproliferative activity against breast cancer cell line MCF-7. This finding adds to our existing knowledge and understanding regarding the cytotoxic activity of cembranoid diterpenes, indicating that this class of compounds can be utilized as a potential scaffold for the future design of potent anticancer agents.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/7/411/s1. Figures S1–S36: HRESIMS, 1D and 2D NMR spectra of isolated compounds.
Author Contributions
H.M.H. designed the experiments; H.M.H. and M.E.R. performed the experiments and isolated the compounds; H.M.H., A.M.S., M.H.H., M.R. and M.E.R. performed data acquisition and structure elucidation; O.M.A. performed the biological assays; H.M.H., A.B.M., M.E.R., S.S., M.R., E.A., F.A.B., T.A.M.G., U.R.A. drafted and revised the manuscript.
Funding
This research received no external funding, and the APC was funded by Technical University of Dresden (TU Dresden).
Acknowledgments
We thank the College of Physical Sciences, University of Aberdeen, for provision of infrastructure and facilities in the Marine Biodiscovery Centre, and Marcel Jaspars, Marine Biodiscovery Centre for NMR and LC-HRMS analysis of our samples.This work was didicated to the soul of our collaborator, Tarek Abd-Elaziz Mohammed, who passed away last year.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-Ezz, R.F.A.; Ahmed, S.A.; Radwan, M.M.; Ayoub, N.A.; Afifi, M.S.; Ross, S.A.; Szymanski, P.T.; Fahmy, H.; Khalifa, S.I. Bioactive cembranoids from the Red Sea soft coral Sarcophyton glaucum. Tetrahed. Lett. 2013, 54, 989–992. [Google Scholar] [CrossRef]
- Al-Lihaibi, S.S.; Alarif, W.M.; Abdel-Lateff, A.; Ayyad, S.-E.N.; Abdel-Naim, A.B.; El-Senduny, F.F.; Badria, F.A. Three new cembranoid-type diterpenes from red sea soft coral Sarcophyton glaucum: Isolation and antiproliferative activity against HepG2 cells. Eur. J. Med. Chem. 2014, 81, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera Sarcophyton, Sinularia, and Lobophytum since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-F.; Chen, W.-T.; Li, X.-W.; Wang, H.-Y.; Guo, Y.-W. New bicyclic cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum. Sci. Rep. 2017, 7, 46584. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-F.; Kurtán, T.; Mándi, A.; Yao, L.-G.; Li, J.; Lan, L.-F.; Guo, Y.-W. Structural, stereochemical, and bioactive studies of cembranoids from Chinese soft coral Sarcophyton trocheliophorum. Tetrahedron 2018, 74, 1933–1941. [Google Scholar] [CrossRef]
- Hassan, H.M.; Sallam, A.A.; Mohammed, R.; Hifnawy, M.S.; Youssef, D.T.; El Sayed, K.A. Semisynthetic analogues of the marine cembranoid sarcophine as prostate and breast cancer migration inhibitors. Bioorg. Medicin. Chem. 2011, 19, 4928–4934. [Google Scholar] [CrossRef] [PubMed]
- Polastro, F.; Golin, S.; Chianese, G.; Putra, M.Y.; Moriello, A.S.; de Petrocellis, L.; García, V.; Munoz, E.; Taglialatela-Scafeiti, O.; Appendino, G. Neuroactive and anti-inflammatory frankincense cembranes: A structure-activity study. J. Nat. Prod. 2016, 79, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.; Guirguis, A.; El-Naggar, W. Antibacterial and antifungal agents from Egyptian marine organisms. Int. J. Pharmacog. 1997, 35, 284–287. [Google Scholar] [CrossRef]
- Badria, F.A.; Guirguis, A.N.; Perovic, S.; Steffen, R.; Müller, W.E.; Schröder, H.C. Sarcophytolide: A new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology 1998, 131, 133–143. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Elshamy, A.I.; Mohamed, T.A.; Hamed, A.R.; Ibrahim, M.A.A.; Ohta, S.; Paré, P.W. Cembrene diterpenoids with ether linkages from Sarcophyton ehrenbergi: An anti-proliferation and molecular-docking assessment. Mar. Drugs 2017, 15, 192. [Google Scholar] [CrossRef]
- Neeman, I.; Fishelson, I.; Kashman, Y. Sarcophine—A new toxin from the soft coral Sarcophyton glaucum (Alcyonaria). Toxicon 1974, 12, 593–598. [Google Scholar] [CrossRef]
- Erman, A.; Neeman, I. Inhibition of phosphofructokinase by the toxic cembranolide sarcophine isolated from the soft-bodied coral Sarcophyton glaucum. Toxicon 1976, 15, 207–215. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Orabi, K.Y.; Dunbar, D.C.; Hammann, M.T.; Avery, M.A.; Sabnis, Y.A.; Mossa, J.S.; El Feraly, F.S. Transformation of lactone to lactam in sarcophine and antimalarial activity of resulting N-substituted azasarcophines. Tetrahedron 2002, 58, 3699–3708. [Google Scholar] [CrossRef]
- Hong, W.K.; Sporn, M.B. Recent advances in chemoprevention of cancer. Science 1997, 278, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Arif, J.M.; Al-Hazzani, A.A.; Kunhi, M.; Al-Khodairy, F. Novel marine compounds: Anticancer or genotoxic? J. Biomed. Biotechnol. 2004, 2, 93–98. [Google Scholar] [CrossRef]
- Thao, N.P.; Nam, N.H.; Cuong, N.X.; Quang, T.H.; Tai, B.H.; Luyen, B.T.T.; Chae, D.; Kim, S.; Koh, Y.-S.; van Kiem, P.; et al. Diterpenoids from the soft coral sinularia maxima and their inhibitory effects on lipopolysaccharide-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. Chem. Pharm. Bull. 2012, 60, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.G.; Liu, H.L.; Guo, Y.W.; Mollo, E. New cembranoids from the hainan soft coral Sarcophyton glaucum. Helv. Chim. Acta 2009, 92, 1085–1091. [Google Scholar] [CrossRef]
- Bernstein, J.; Shmeuli, U.; Zadock, E.; Kashman, Y.; Neeman, I. Sarcophine, a new epoxy cembranolide from marine origin. Tetrahedron 1974, 30, 2817–2824. [Google Scholar] [CrossRef]
- Hegazy, M.E.; Gamal Eldeen, A.M.; Shahat, A.A.; Abdel-Latif, F.F.; Mohamed, T.A.; Whittlesey, B.R.; Paré, P.W. Bioactive hydroperoxyl cembranoids from the red sea soft coral Sarcophyton glaucum. Mar. Drugs. 2012, 10, 209–222. [Google Scholar] [CrossRef]
- Su, J.H.; Lin, Y.F.; Lu, Y.; Yeh, H.C.; Wang, W.H.; Fan, T.Y.; Sheu, J.H. Oxygenated cembranoids from the cultured and wild-type soft corals Sinularia flexibilis. Chem. Pharm. Bull. 2009, 57, 1189–1192. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Chiang, M.Y.; Duh, C.Y. Cembranoids from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2010, 73, 197–203. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).