Abstract
New four eunicellin-based diterpenoids, krempfielins J–M (1–4) were isolated from the organic extract of a Taiwanese soft coral Cladiella krempfi. The structures of the new metabolites were elucidated on the basis of extensive spectroscopic analysis. The structure of compound 2 is rare due to the presence of the highly oxygenated pattern. Anti-inflammatory activity of 1–6 to inhibit the superoxide anion generation and elastase release in FMLP/CB-induced human neutrophils was also evaluated, and 2 and 4 were shown to possess the ability to inhibit the elastase release.
1. Introduction
Many recent studies about the discovery of versatile structures and bioactivities of eunicellin-type compounds isolated from soft corals have been reported [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. The soft coral Cladiella krempfi has been found to generate several types of metabolites including eunicellin-type diterpenoids [16,17] and pregnane-type steroids [18,19,20]. Our previous study on the bioactive secondary metabolites of a Taiwanese soft coral Cladiella krempfi also resulted in the isolation of a series of new eunicellin-based diterpenoids, krempfielins A–I [21,22]. In this paper, we further report the discovery of four new eunicellin-based diterpenoids, krempfielins J–M (1–4), along with two known diterpenoids sclerophytin F (5) [23] and litophynol B (6) [24] (Chart 1). The ability of these compounds to inhibit the superoxide anion generation and elastase release in FMLP/CB-induced human neutrophils was also evaluated. The results showed that at 10 μM compounds 2 and 4 effectively inhibited the elastase release in FMLP/CB-induced human neutrophils.
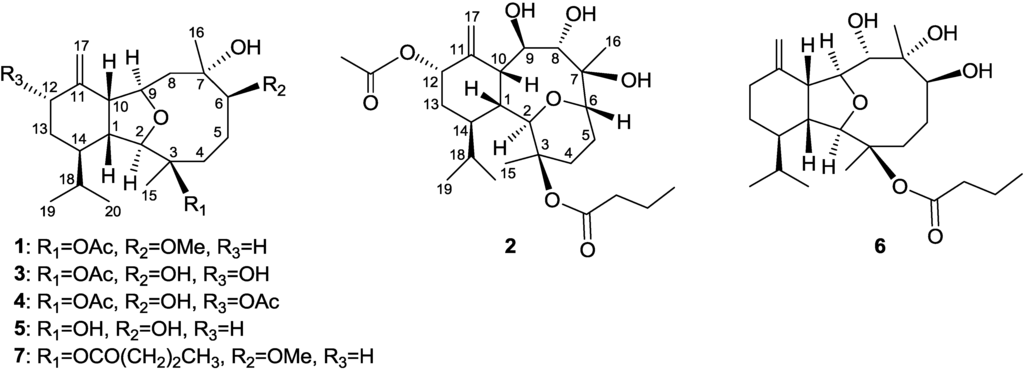
Chart 1.
Structures of metabolites 1–6.
2. Results and Discussion
Krempfielin J (1) showed the pseudomolecular ion peak [M + Na]+ at m/z 417.2616 in the HRESIMS and the molecular formula was determined as C23H38O5. NMR spectroscopic data of 1 (Table 1) showed the presence of one acetoxy group (δC 169.6 and 22.4; δH 2.12, s, 3H) and one methoxyl group (δC 57.0 and δH 3.34, 3H, s). The NMR data of 1 was found to be similar to the known compound, 7 [22,25] (Chart 1). Comparison of the NMR data of them revealed that the only difference between both compounds arises from the replacement of the n-butyryloxy group at C-3 in 7 by one acetoxy group in 1. The stereochemistry of 1 was confirmed by comparison of the NMR data and NOE correlations of both 7 and 1.

Table 1.
13C and 1H NMR data for compounds 1–4.
| 1 a | 2 b | 3 a | 4 a | |||||
|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | |
| 1 | 45.6, CH c | 2.20 dd | 37.8, CH | 3.24 m | 44.2, CH | 2.31 t (8.0) | 44.2, CH | 2.31 ddd |
| (10.8, 8.0) d | (8.0, 6.0, 2.0) | |||||||
| 2 | 92.1, CH | 3.59 br s | 80.9, CH | 3.58 m | 90.4, CH | 3.69 br s | 90.2, CH | 3.69 d (2.0) |
| 3 | 86.6, C | 83.8, C | 86.6, C | 86.6, C | ||||
| 4 | 36.8, CH2 | 1.75 m | 28.8, CH2 | 1.92 m | 34.6, CH2 | 1.94 m | 34.9, CH2 | 1.92 m |
| 2.61 dd | 2.90 dd | 2.48 m | 2.52 dd | |||||
| (14.4, 9.2) | (14.8, 7.0) | (14.8, 8.8) | ||||||
| 5 | 26.8, CH2 | 1.33 m | 19.9, CH2 | 1.50 m | 30.4, CH2 | 1.42 m | 30.5, CH2 | 1.45 m |
| 1.59 m | 1.71 m | 1.71 m | 1.68 m | |||||
| 6 | 90.6, CH | 4.12 d (6.0) | 70.1, CH | 3.68 m | 79.0, CH | 4.57 d (8.4) | 79.1, CH | 4.58 d (7.2) |
| 7 | 75.9, C | 75.5, C | 76.6, C | 76.5, C | ||||
| 8 | 45.0, CH2 | 1.82 m | 79.0, CH | 3.62 m | 46.0, CH2 | 1.85 m | 45.9, CH2 | 1.83 m |
| 9 | 78.5, CH | 4.18 dd | 69.9, CH | 4.39 t (8.0) | 79.4, CH | 4.53 m | 78.8, CH | 4.40 m |
| (14.8, 7.2) | ||||||||
| 10 | 53.9, CH | 2.95 t (7.2) | 50.0, CH | 2.51 br s | 51.0, CH | 2.86 m | 51.4, CH | 2.93 dd |
| (7.2, 6.0) | ||||||||
| 11 | 147.6, C | 145.3, C | 148.0, C | 143.0, C | ||||
| 12 | 31.5, CH2 | 2.04 m | 71.6, CH | 5.29 m | 70.7, CH | 4.37 s | 72.5, CH | 5.44 dd |
| 2.25 m | (5.6, 2.8) | |||||||
| 13 | 24.6, CH2 | 1.00 m | 31.9, CH2 | 1.58 m | 31.2, CH2 | 1.46 m | 29.0, CH2 | 1.42 m |
| 1.70 m | 2.17 m | 1.84 m | 1.90 m | |||||
| 14 | 44.0, CH | 1.26 m | 40.1, CH | 1.48 m | 36.5, CH | 1.82 m | 37.2, CH | 1.70 m |
| 15 | 23.1, CH3 | 1.41 s | 26.3, CH3 | 1.56 s | 23.2, CH3 | 1.48 s | 23.2, CH3 | 1.47 s |
| 16 | 23.6, CH3 | 1.13 s | 13.7, CH3 | 1.19 s | 22.6, CH3 | 1.17 s | 22.8, CH3 | 1.18 s |
| 17 | 109.4, CH2 | 4.64 s | 105.0, CH2 e | 5.04 s | 111.8, CH2 | 4.87 s | 114.9, CH2 | 4.97 s |
| 4.68 s | 5.23 s | 5.06 s | 5.14 s | |||||
| 18 | 29.1, CH | 1.72 m | 28.3, CH | 2.03 m | 29.0, CH | 1.80 m | 28.9, CH | 1.80 m |
| 19 | 15.6, CH3 | 0.79 d (6.8) | 21.5, CH3 | 1.06 d (6.5) | 16.4, CH3 | 0.85 d (6.4) | 16.2, CH3 | 0.83 d (6.8) |
| 20 | 21.9, CH3 | 0.97 d (6.8) | 21.5, CH3 | 1.09 d (6.5) | 21.9, CH3 | 1.00 d (6.4) | 21.8, CH3 | 0.96 d (6.8) |
| 3-n-butyrate | 172.6, C | |||||||
| 37.5, CH2 | 2.22 m | |||||||
| 2.30 m | ||||||||
| 18.6, CH2 | 1.64 m | |||||||
| 13.8, CH3 | 0.95 t (7.5) | |||||||
| 3-OAc | 169.6, C | 169.5, C | 169.4, C | |||||
| 22.4, CH3 | 2.12 s | 22.4, CH3 | 2.07 s | 22.4, CH3 | 2.07 s | |||
| 6-OMe | 57.0, CH3 | 3.34 s | ||||||
| 6-OAc | ||||||||
| 8-OAc | ||||||||
| 12-OAc | 170.0, C | 170.3, C | ||||||
| 21.2, CH3 | 2.14 s | 21.5, CH3 | 2.06 s | |||||
a 13C and 1H spectra recorded at 100 and 400 MHz in CDCl3; b 13C and 1H spectra recorded at 125 and 500 MHz in CDCl3; c Deduced from DEPT; d J values (Hz) in parentheses; e Broad signal.
The new metabolite krempfielin K (2) was found to have the molecular formula C26H42O8 and six degrees of unsaturation, as indicated from the HRESIMS. The IR absorptions at νmax 3444 and 1732 cm−1 revealed the presence of hydroxy and ester carbonyl functionalities. The 13C carbons spectral data (Table 1) were assigned by the assistance of DEPT spectrum to six methyls (including one acetate methyl δC 21.2), five sp3 methylenes, one sp2 methylene, nine sp3 methines (including five oxymethines), two sp3 and three sp2 quaternary carbons (including two ester carbonyls). The NMR spectroscopic data of 2 (Table 1) showed the presence of one 1,1-disubstituted double bond (δC 105.0, CH2 and 145.3, C; δH 5.23, s and 5.04, s, each 1H). Two ester carbonyls (δC 172.6 and 170.0) were assigned from the 13C NMR spectrum and were HMBC correlated with the methylene (δH 2.30 m and 2.22 m, each 1H) of an n-butyrate and those of an acetate methyl (δH 2.14, s, 3H), respectively. Therefore, the remaining three degrees of unsaturation identified compound 2 as a tricyclic molecule. 1H-1H COSY and HMBC correlations (Figure 1) were further used to establish the molecular skeleton of 2. The COSY experiment assigned five isolated consecutive proton spin systems. Above evidences and HMBC correlations from H-2 and H-6 (δH 3.58 and 3.68) to C-6 and C-2 (δC 70.1 and 80.9), respectively, implied the presence of an ether linkage between C-2 and C-6, and suggested that 2 is an 2,6-ether linked eunicellin-based diterpenoid [1,26]. Furthermore, the acetoxy group attaching at C-12 was confirmed from the HMBC correlations from H-12 (δH 5.29) and acetate methyl protons (δH 2.14) to the carbonyl carbon appearing at 170.0 (C). Thus, the remaining one n-butyryloxy group had to be positioned at C-3, an oxygen-bearing quaternary carbon resonating at δ 83.8 ppm. On the basis of above analysis, the planar structure of 2 was established. The relative structure of 2 was elucidated by the analysis of NOESY correlations, as shown in Figure 2. The observation of the NOE interactions between H-1 and H-6, H-8 and H-10; H-10 and both H-8 and H-12, revealed that they are all β-oriented. Also, the correlations between H-2 and H-9, H-14 and H3-15; H-9 and H3-16 suggested that of all of H-2, H-9, H-14, H3-15 and H3-16 are α-oriented. The relative configuration of 2 was thus established. The highly oxygenated pattern of 2 at C-2, C-3, C-6, C-7, C-8, C-9 and C-12 is rare in known eunicellins.
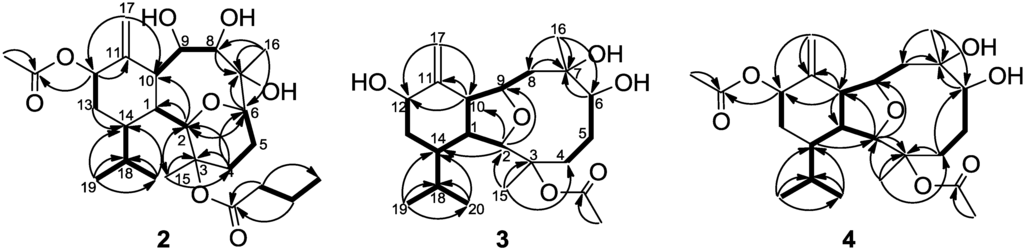
Figure 1.
Selected 1H-1H COSY (▬) and HMBC (→) correlations of 2–4.
Krempfielin L (3) showed the molecular ion peak [M + Na]+ at m/z 419.2407 in the HRESIMS and established a molecular formula of C22H36O6, implying five degrees of unsaturation. The IR absorptions at νmax 3419 and 1734 cm−1 revealed the presence of hydroxy and ester carbonyl functionalities. The 13C NMR spectrum of 3 showed signals of 22 carbons (Table 1), which were characterized by the DEPT spectrum as five methyls (including one acetate methyl), five methylenes (including one sp2 methylene), eight methines (including four oxygenated carbons), and four quaternary carbons (including one ester carbonyl and one sp2 quaternary carbon of an olefin). The 1H and 13C NMR spectral data of 3 (Table 1) also showed the presence of one acetoxy group (δH 2.07, s, 3H; δC 22.4, CH3 and 169.5, C). The remaining three degrees of unsaturation again identified 3 as a tricyclic diterpenoid. The molecular framework was established by 1H-1H COSY and HMBC correlations (Figure 1). Comparison of the NMR data of 3 with those of the known compound sclerophytin E [27] revealed that 3 is the C-12 hydroxylated derivative of sclerophytin E. The stereochemistry of compound 3 was determined by the NOESY spectrum as shown in Figure 2.
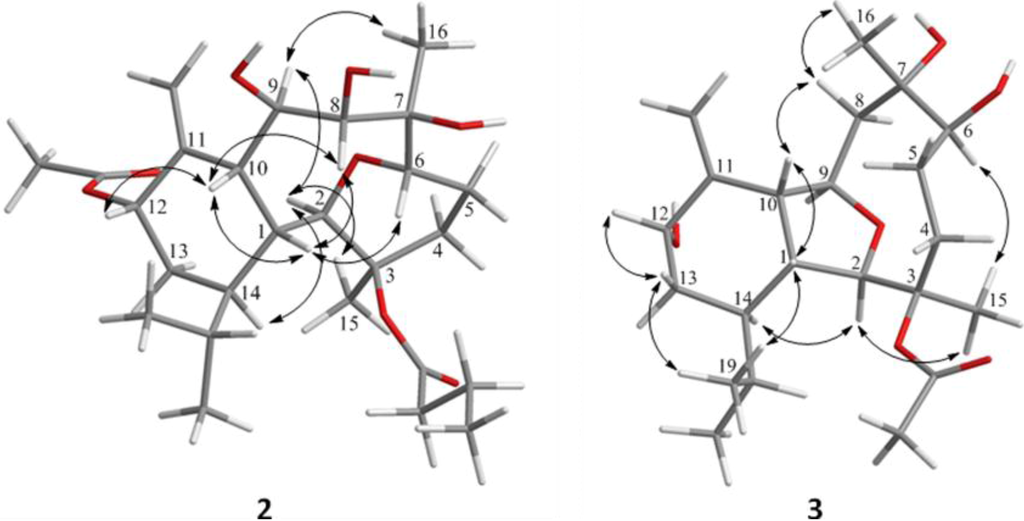
Figure 2.
Key NOESY correlations for 2 and 3.
The HRESIMS (m/z 461.2518 [M + Na]+) of krempfielin M (4) established the molecular formula of C24H38O7. The 1H and 13C NMR spectral data of 4 (Table 1) revealed that the structure of metabolite 4 should be similar to that of 3, as the 1H NMR spectral data of 4 are almost identical with those of 3 except for the presence of one additional acetyl group (δH 2.06, s, 3H) in 4. Furthermore, the placement of an acetoxy group at C-12 was established by the HMBC experiment which showed correlations from an oxymethine (δH 5.44, H-12) and acetate methyl (δH 2.06) to the ester carbonyl carbon appearing at δC 170.3 (C) (Figure 1). The NOE correlations of 4 also showed that the stereochemistry of this metabolite is identical with that of 3. Thus the structure of metabolite 4 was determined.
The in vitro anti-inflammatory effects of the diterpenoids 1–6 were tested by examining the inhibitory activity of these compounds toward the release of elastase in N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (FMLP/CB)-induced human neutrophils cells (Table 2). At a concentration of 10 μM, all of these compounds could not significantly reduce the expression of superoxide anion, relative to the control cells stimulated with FMLP/CB only. At the same concentration, compounds 2 and 4 were found to effectively inhibit the elastase release (45.51 ± 2.69% and 27.30 ± 5.42% inhibition, respectively) in the same FMLP/CB-stimulated cells.

Table 2.
Effect of pure compounds on elastase release in FMLP/CB-induced human neutrophils.
| Compound | Elastase | ||
|---|---|---|---|
| Inh% | IC50 (μM) | ||
| 1 | 19.42 ± 7.89 | * | >10 |
| 2 | 45.51 ± 2.69 | *** | >10 |
| 3 | 18.67 ± 5.75 | * | >10 |
| 4 | 27.30 ± 5.42 | ** | >10 |
| 5 | −0.32 ± 0.81 | >10 | |
| 6 | 6.15 ± 3.42 | >10 | |
Percentage of inhibition (Inh%) was measured at 10 μM. Results are presented as mean ± S.E.M. (n = 3 or 4); * p < 0.05, ** p < 0.01 and *** p < 0.001 compared with the control value.
3. Experimental Section
3.1. General Experimental Procedures
Melting point was determined using a Fisher-Johns melting point apparatus. Optical rotations were measured on a JASCO P-1020 polarimeter. IR spectra were recorded on a JASCO FT/IR-4100 infrared spectrophotometer. ESIMS were obtained with a Bruker APEX II mass spectrometer. The NMR spectra were recorded either on a Varian UNITY INOVA-500 FT-NMR and a Varian 400MR FT-NMR. Silica gel (Merck, 230–400 mesh) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F-254, 0.2 mm) were used for analytical thin layer chromatography (TLC). High performance liquid chromatography was performed on a Hitachi L-7100 HPLC apparatus with an octadecylsilane (ODS) column (250 × 21.2 mm, 5 μm).
3.2. Animal Material
C. krempfi was collected by hand using scuba off the coast of Penghu islands of Taiwan in June 2008, at a depth of 5–10 m, and stored in a freezer until extraction. A voucher sample (specimen No. 200806CK) was deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Separation
The octocoral (1.1 kg fresh wt) was collected and freeze-dried. The freeze-dried material was minced and extracted exhaustively with EtOH (3 × 10 L). The EtOH extract of the frozen organism was partitioned between CH2Cl2 and H2O. The CH2Cl2-soluble portion (14.4 g) was subjected to column chromatography on silica gel and eluted with EtOAc in n-hexane (0%–100% of EtOAc, stepwise) and then further with MeOH in EtOAc with increasing polarity to yield 41 fractions. Fraction 28, eluted with n-hexane–EtOAc (1:2), was rechromatoraphed over a reversed-phase column (RP-18) using acetone–H2O (10:1) as the mobile phase to afford six subfractions (A1–A6). Subfraction A3 was repeatedly separated by reverse phase HPLC (CH3CN–H2O, 1.4:1.1) to afford compound 1 (2.6 mg). Fraction 31, eluted with n-hexane–EtOAc (1:10), was rechromatoraphed over a silica gel open column using n-hexane–acetone (3:1) as the mobile phase to afford eight subfractions (B1–B8). Subfraction B5 separated by reverse phase HPLC (CH3CN–H2O, 1:1 to 1:1.6) to afford compounds 2 (5.1 mg), 3 (2.8 mg), 5 (52.3 mg) and 6 (13.4 mg). Subfraction B6 by reverse phase HPLC (CH3CN–H2O, 1:1.5) to afford compounds 4 (8.4 mg).
3.3.1. Krempfielin J (1)
Colorless oil; [α]23D = +52 (c 0.85, CHCl3); IR (neat) νmax 3479, 2958, 2930, 2872, 1737, 1644, 1463, 1369, 1244, and 1100 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 417 [M + Na]+; HRESIMS m/z 417.2616 [M + Na]+ (calcd. for C23H38O5Na, 417.2617).
3.3.2. Krempfielin K (2)
White powder; mp 162–163 °C; [α]25D = −58 (c 1.5, CHCl3); IR (neat) νmax 3444, 2963, 1731, 1651, 1464, 1380, 1240, 1177, 1087, and 1045 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 505 [M + Na]+; HRESIMS m/z 505.2780 [M + Na]+ (calcd. for C26H42O8Na, 505.2777).
3.3.3. Krempfielin L (3)
Colorless oil; [α]25D = +26 (c 0.8, CHCl3); IR (neat) νmax 3419, 2958, 1734, 1457, 1369, 1246, 1063 and 1026 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 419 [M + Na]+; HRESIMS m/z 419.2407 [M + Na]+ (calcd. for C22H36O6Na, 419.2409).
3.3.4. Krempfielin M (4)
Colorless oil; [α]25D = +24 (c 2.4, CHCl3); IR (neat) νmax 3452, 2960, 1736, 1435, 1370, 1243, 1199, 1075, and 1024 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 461 [M + Na]+; HRESIMS m/z 461.2518 [M + Na]+ (calcd. for C24H38O7Na, 461.2515).
3.4. In Vitro Anti-Inflammatory Assay—Superoxide Anion Generation and Elastase Release by Human Neutrophils
Human neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Measurements of superoxide anion generation and elastase release were carried out according to previously described procedures [28,29]. LY294002, a phosphatidylinositol-3-kinase inhibitor, was used as a positive control for inhibition of superoxide anion generation and elastase release with IC50 values of 1.88 ± 0.45 and 4.12 ± 0.92 μM, respectively. Briefly, superoxide anion production was assayed by monitoring the superoxide dismutase-inhibitable reduction of ferricytochrome c. Elastase release experiments were performed using MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide as the elastase substrate [30].
4. Conclusions
New eunicellin-based diterpenoids were isolated together with known ones from the soft coral Cladiella krempfi. Compounds 2 and 4 could significantly inhibit the release of elastase in FMLP/CB-induced human neutrophils. Thus, compounds 2 and 4, in particular 2, could be promising anti-inflammatory agents and may warrant further biomedical investigation.
Acknowledgements
This research was supported by grants from the National Science Council of Taiwan (NSC 100-2320-B-110-001-MY2) and National Sun Yat-sen University–Kaohsiung Medical University Joint Project (NSYSUKMU 02C030117), Taiwan, awarded to Jyh-Horng Sheu.
References
- Ahmed, A.F.; Wu, M.-H.; Wang, G.-H.; Wu, Y.-C.; Sheu, J.-H. Eunicellin-based diterpenoids, australins A–D, isolated from the soft coral Cladiella australis. J. Nat. Prod. 2005, 68, 1051–1055. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Chen, B.-W.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Simplexins A–I, eunicellin-based diterpenoids from the soft coral Klyxum simplex. J. Nat. Prod. 2009, 72, 994–1000. [Google Scholar] [CrossRef]
- Chen, B.-W.; Wu, Y.-C.; Chiang, M.Y.; Su, J.-H.; Wang, W.-H.; Fan, T.-Y.; Sheu, J.-H. Eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Tetrahedron 2009, 65, 7016–7022. [Google Scholar]
- Chen, Y.-H.; Tai, C.-Y.; Hwang, T.-L.; Weng, C.-F.; Li, J.-J.; Fang, L.-S.; Wang, W.-H.; Wu, Y.-C.; Sung, P.-J. Cladielloides A and B: New eunicellin-type diterpenoids from an Indonesian octocoral Cladiella sp. Mar. Drugs 2010, 8, 2936–2945. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chang, S.-M.; Huang, C.-Y.; Chao, C.-H.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. Hirsutalins A–H, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. J. Nat. Prod. 2010, 73, 1785–1791. [Google Scholar] [CrossRef]
- Hassan, H.M.; Khanfar, M.A.; Elnagar, A.Y.; Mohammed, R.; Shaala, L.A.; Youssef, D.T.A.; Hifnawy, M.S.; El Sayed, K.A. Pachycladins A–E, prostate cancer invasion and migration inhibitory eunicellin-based diterpenoids from the Red Sea soft coral Cladiella pachyclados. J. Nat. Prod. 2010, 73, 848–853. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Wen, Z.-H.; Sung, P.-J.; Sheu, J.-H. Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010, 8, 2363–2366. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Lu, Y.; Chen, B.-W.; Huang, C.-Y.; Wen, Z.-H.; Kuo, Y.-H.; Sheu, J.-H. Simplexins J–O, eunicellin-based diterpenoids from a Dongsha Atoll soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011, 84, 626–632. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, C.-Y.; Su, Y.-D.; Chang, Y.-C.; Lu, M.-C.; Weng, C.-F.; Su, J.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Discovery of new eunicellins from an Indonesian octocoral Cladiella sp. Mar. Drugs 2011, 9, 934–943. [Google Scholar] [CrossRef]
- Hsu, F.-J.; Chen, B.-W.; Wen, Z.-H.; Huang, C.-Y.; Dai, C.-F.; Su, J.-H.; Wu, Y.-C.; Sheu, J.-H. Klymollins A–H, bioactive eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle. J. Nat. Prod. 2011, 74, 2467–2471. [Google Scholar] [CrossRef]
- Chen, B.-W.; Huang, C.-Y.; Wen, Z.-H.; Su, J.-H.; Wang, W.-H.; Sung, P.-J.; Wu, Y.-C.; Sheu, J.-H. Klysimplexins U–X, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011, 84, 1237–1242. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Tsai, C.-W.; Wang, W.-H.; Wen, Z.-H.; Huang, C.-Y.; Ung, P.-J.; Wu, Y.-C.; Sheu, J.-H. Klysimplexins I–T, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2011, 9, 834–844. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Huang, C.-Y.; Tai, C.-J.; Sung, P.-J.; Liaw, C.-C.; Sheu, J.-H. Simplexins P–S, eunicellin-based diterpenes from the soft coral Klyxum simplex. Mar. Drugs 2012, 10, 1203–1211. [Google Scholar] [CrossRef]
- Williams, D.E.; Amlani, A.; Dewi, A.S.; Patrict, B.O.; van Ofwegen, L.; Mui, A.L.-F.; Andersen, R.J. Australin E isolated from the soft coral Cladiella sp. collected in pohnpei activates the inositol 5-phosphatase SHIP1. Aust. J. Chem. 2010, 63, 895–900. [Google Scholar] [CrossRef]
- Ospina, C.A.; Rodríguez, A.D. Bioactive compounds from the gorgonian Briareum polyanthes. Correction of the structures of four asbestinane-type diterpenes. J. Nat. Prod. 2006, 69, 1721–1727. [Google Scholar] [CrossRef]
- Sarma, N.S.; Chavakula, R.; Rao, I.N.; Kadirvelraj, R.; Row, T.N.G.; Saito, I. Crystal and molecular structure of sclerophytin F methyl ether from the soft coral Cladiella krempfi. J. Nat. Prod. 1993, 56, 1977–1980. [Google Scholar] [CrossRef]
- Cai, Y.-S.; Yao, L.-G.; Pascale, A.D.; Irace, C.; Mollo, E.; Taglialatela-Scafati, O.; Guo, Y.-W. Polyoxygenated diterpenoids of the eunicellin-type from the Chinese soft coral Cladiella krempfi. Tetrahedron 2013, 69, 2214–2219. [Google Scholar] [CrossRef]
- Lan, W.-J.; Lin, C.-W.; Su, J.-Y.; Zeng, L.-M. Two steroidal glycosides from the soft coral Cladiella krempfi. Chem. J. Chin. Univ. 2003, 24, 2019–2021. [Google Scholar]
- Huang, X.-P.; Deng, Z.-W.; Ofwegen, L.V.; Li, J.; Fu, H.-Z.; Zhu, X.-B.; Lin, W.-H. Two new pregnane glycosides from soft coral Cladiella krempfi. J. Asian Nat. Prod. Res. 2006, 8, 287–291. [Google Scholar] [CrossRef]
- Huang, X.-P.; Deng, Z.-W.; Zhu, X.-B.; Ofwegen, L.V.; Proksch, P.; Lin, W.-H. Krempenes A–D: A series of unprecedented pregnane-type steroids from the marine soft coral Cladiella krempfi. Helv. Chim. Acta 2006, 89, 2020–2026. [Google Scholar] [CrossRef]
- Tai, C.-J.; Su, J.-H.; Huang, M.-S.; Wen, Z.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2011, 9, 2036–2045. [Google Scholar] [CrossRef]
- Tai, C.-J.; Su, J.-H.; Huang, C.-Y.; Huang, M.-S.; Wen, Z.-H.; Dai, C.-F.; Sheu, J.-H. Cytotoxic and anti-inflammatory eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2013, 11, 788–799. [Google Scholar] [CrossRef]
- Alam, M.; Sharma, P.; Zektzer, A.S.; Martin, G.E.; Ji, X.; Helm, D. Sclerophytin C–F: Isolation and structures of four new diterpenes from the soft coral Sclerophytum capitalis. J. Org. Chem. 1989, 54, 1896–1900. [Google Scholar] [CrossRef]
- Miyamoto, T.; Yamada, K.; Ikeda, N.; Komori, T.; Higuchi, R. Bioactive terpenoids from octocorallia, I. Bioactive diterpenoids: Litophynols A and B from the mucus of the soft coral Litophyton sp. J. Nat. Prod. 1994, 57, 1212–1219. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kusatsu, T.; Tsuha, K.; Hamada, T.; Okamura, H.; Furukawa, T.; Akiyama, S.; Doe, M.; Morimoto, Y.; Iwase, F.; Takemura, K. Cytotoxic eunicellin-type diterpenes from the soft coral Litophyton viscudium. Heterocycles 2011, 83, 2149–2155. [Google Scholar] [CrossRef]
- Roussis, V.; Fenical, W.; Vagias, C.; Kornprobst, J.-M.; Miralles, J. Labiatamides A, B, and other eunicellan diterpenoids from the Senegalese gorgonian Eunicella labiata. Tetrahedron 1996, 52, 2735–2742. [Google Scholar] [CrossRef]
- Rao, C.B.; Rao, D.S.; Satyanarayana, C.; Rao, D.V.; Kassühlke, K.E.; Faulkner, D.J. New cladiellane diterpenes from the soft coral Cladiella australis of the Andaman and Nicobar Islands. J. Nat. Prod. 1994, 57, 574–580. [Google Scholar] [CrossRef]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido) benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Wang, C.-C.; Kuo, Y.-H.; Huang, H.-C.; Wu, Y.-C.; Kuo, L.-M.; Wu, Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Leu, Y.-L.; Kao, S.-H.; Tang, M.-C.; Chang, H.-L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic. Biol. Med. 2006, 41, 1433–1441. [Google Scholar] [CrossRef]
- Samples Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).