Abstract
Background and Objectives: The tumor-node-metastasis (TNM) classification system is the standard for staging gastric cancer and predicting survival. However, its accuracy can be compromised by insufficient lymph node (LN) dissection during surgery or inadequate pathologic examination. Alternative staging systems, such as the lymph node ratio (LNR) and log odds of positive lymph nodes (LODDS), may provide better prognostic value when LN examination is suboptimal. Because the current N staging system was not able to accurately stratify patients relative to their survival outcomes in our series, this study assessed the prognostic impact of LNR and LODDS on overall survival (OS) of patients who underwent radical gastrectomy for cancer. Materials and Methods: Between March 2005 and June 2025, the authors performed gastrectomy for gastric carcinoma in 114 patients. Out of these patients, 39 were excluded (19 had stage IV, while 20 underwent palliative gastrectomy with D1 lymphadenectomy). Thus, the study cohort included 75 patients who underwent curative gastrectomy, with 4 (5.3%) of them dying postoperatively. Potential prognostic factors associated with OS (including age, sex, tumor location, T stage, N stage, TNM stage, LNR, and LODDS) were evaluated by univariate and multivariate analysis. Because the recurrence data were missing in 41 patients, the disease-free survival (DFS) analysis would not be meaningful. Results: The OS analysis was based on the 71 patients surviving postoperatively. Because successive N stage groups could not accurately stratify patients according to their OS, we used X-tile software version 3.6.1 to identify two cut-offs (both for LNR and LODDS) that were able to stratify patients in three subgroups with significantly distinct survival outcomes. Multivariate analysis found that both LODDS and LNR systems were independent prognostic factors for OS. Conclusions: LNR and LODDS provide more detailed insights into lymph node status and have demonstrated potential for enhancing prognostic accuracy compared to N staging, even in patients who underwent curative gastrectomy with D2 lymphadenectomy. Although LNR and LODDS are usually useful in patients who underwent suboptimal lymphadenectomy, the current study demonstrated that these systems could improve prognostic stratification even in patients with more than 15 retrieved LNs. However, due to the small sample size, the current observations and proposed cut-offs of LNR and LODDS have to be validated in larger studies including such patients.
1. Introduction
Gastric cancer is a major global health issue, being the fifth-most prevalent cancer worldwide and the fifth leading cause of cancer-related deaths [1,2]. Its incidence varies significantly, being the highest in East Asian countries and in many regions of Central and Eastern Europe. Although cancer rates have steadily declined over the last half-century, mainly for noncardia cancers due to H. Pylori eradication and better food preservation, recent studies have shown rising trends in younger populations, with autoimmune gastritis and gastric microbiome dysbiosis potentially driving tumors near the esophagogastric junction [3,4,5,6,7].
Surgery is the primary treatmesoftnt for early-stage cancers, whereas other therapies, such as chemotherapy, may be administered before and/or after surgery to reduce tumor size or prevent recurrence. Surgical removal of all or part of the stomach remains the cornerstone treatment for gastric cancer. The type of gastrectomy (total, proximal, or distal) depends on the tumor’s location and extent, stage of cancer, and patient factors, with the aim of removing the tumor with clear margins (R0 resection) and preserving healthy tissue and function [8]. Lymph node dissection plays a crucial role in the staging and surgical management of patients with gastric cancer. Therefore, R0 radical gastrectomy with D2 lymphadenectomy is mandatory to achieve radicality in patients with gastric cancer. Although few authors suggested the performance of D2-plus or D3 lymphadenectomy in selected patients, [9,10] none of the current guidelines recommend such an extensive lymph node dissection [11,12,13,14].
The accuracy of staging and prognosis in gastric cancer can be significantly compromised when an insufficient number of lymph nodes (LNs) is examined. Numerous studies have suggested that a minimum of 15 LNs should be retrieved to ensure optimal staging, with over 25 nodes recommended for accurate classification of N stage [11,15,16]. Failure to meet these requirements may result in a phenomenon known as “stage migration” or the Will Rogers phenomenon [17]. However, few authors suggested that, even in patients who underwent radical gastrectomy with D2 lymphadenectomy, lymph node ratio (LNR) and log odds of positive lymph nodes (LODDS) could further refine the prognosis of such patients [15,18,19].
These methods have been proposed in addition to the commonly used staging systems for gastric adenocarcinoma, such as those by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) [20]. These approaches aim to provide more reliable prognostic information even in cases where the minimum recommended number of LNs was retrieved.
This study aimed to identify the key prognostic factors based on the number of retrieved and positive LNs affecting overall survival (OS) in a cohort of patients who underwent radical surgery for gastric cancer. Thus, we analyzed various clinical and pathological variables using univariate and multivariate statistical methods to assess the prognostic accuracy of pN stage, LNR, and LODDS.
2. Materials and Methods
2.1. Patients and Data Collection
Patients who underwent surgical intervention for gastric adenocarcinoma between March 2005 and July 2025, with either curative or palliative gastric resection, were retrospectively assessed. It is important to note that a single surgical team conducted operations on these patients from March 2005 to November 2023, at the Fundeni Clinical Institute, and from November 2023 to the conclusion of the study at the Emergency University Hospital Bucharest.
From this cohort, we excluded patients who underwent palliative surgery in the context of metastatic disease and those who underwent only D1 lymphadenectomy because of comorbid conditions. Inclusion criteria were as follows: histologically confirmed gastric adenocarcinoma, non-metastatic gastric carcinoma, and D2 lymphadenectomy. Patients with stage IV gastric adenocarcinoma and those who died during hospitalization for gastric resection or within the first 30 days postoperatively were also excluded from OS analysis.
This retrospective study was approved by the Ethics Committee of Fundeni Clinical Institute, Bucharest, under the number 15384/14.04.2025, and by the Ethics Committee of Emergency University Hospital Bucharest under number 12872/18.02.2025.
2.2. Surgical and Oncologic Management
All patients with gastric cancer operated on by our team were evaluated by a multidisciplinary team. The diagnosis was established preoperatively by upper endoscopy and biopsy in all patients. Contrast-enhanced CT of the thorax, abdomen, and pelvis was performed for staging purposes. For TNM staging, we used the UICC 8th edition and performed appropriate conversion in patients whose tumor stage presented with older TNM editions [20]. Especially since 2020, all patients with locally advanced gastric cancer and without primary tumor complications (e.g., digestive stenosis or severe anemia due to hemorrhagic gastric cancer), have benefited from neoadjuvant chemotherapy according to current guidelines [11,13,14,21,22,23]. The most commonly used neoadjuvant chemotherapy regimen was FLOT (docetaxel, oxaliplatin, leucovorin, and 5-fluorouracil).
Staging has been performed according to the 8th Edition of the AJCC staging system.
Follow-up recommendations consist of the evaluation of CEA and CA 19-9 levels, as well as CT scan of thorax, abdomen, and pelvis every 3 months during the first 2 years after operation and every 6 months for the next 3 years. Upper GI endoscopy is recommended at 1 year postoperatively and every 2 years during the next 4 years.
Unfortunately, 41 (57.7%) patients were lost to follow-up, and the only available data regarding their survival outcome were the date of their death or their alive status at 1 July 2025. By this reason, a meaningful analysis of the disease-free survival (DFS) of the patients included in this study cannot be performed.
2.3. Statistical Analysis
Upon completion of the database search, the analysis was conducted using IBM SPSS Statistics (Version 27). Continuous variables were reported as mean (±standard deviation) or median [IQR 25–QR 75]. Categorical variables have been presented as number and percentage. The survival outcome of the study was overall survival (OS), defined as the interval between radical gastric resection and either the date of the patient’s death or the study’s conclusion (1 July 2025) for the patients who were alive at that moment. X-tile software version 3.6.1 was used to establish the cut-off values for both LNR and LODDS which could categorize the patients into three subgroups with significantly distinct prognoses. The cut-off values of LNR and LODDS were identified as the values associated with the maximum chi square (and the minimum p value).
OS curves were generated using the Kaplan–Meier method. In univariate analysis, the log-rank test was used to compare the OS rates between the groups. All variables with a p value < 0.05 at univariate analysis were introduced in multivariate analysis. A backward stepwise Cox proportional hazards regression model was used to identify the independent prognostic factors associated with OS. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. Statistical significance was set at p < 0.05.
3. Results
We retrospectively evaluated all the 114 patients who underwent surgery for gastric cancer between March 2005 and July 2025.
Out of 114 patients, 39 (34.2%) underwent palliative gastrectomy (19 metastatic patients, 20 underwent only D1 lymphadenectomy), while 75 (65.8%) had radical gastric resection (including D2 lymph node dissection) (Figure 1).
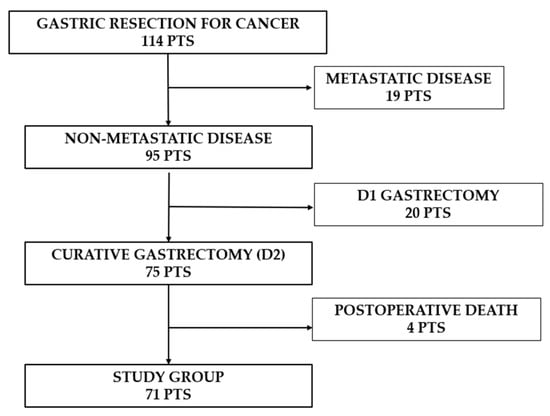
Figure 1.
Flowchart showing the number of patients enrolled in the study group.
Because the survival outcomes after gastrectomy are significantly poorer in the context of metastatic disease or D1 lymphadenectomy, we further evaluated the short-term postoperative outcomes only for non-metastatic patients undergoing D2 lymphadenectomy (75 patients).
3.1. Clinico-Pathologic Characteristics of the Entire Group
The average age of the patients was 62.6 years, with a standard deviation of 9.5. The patient group was categorized into those aged 65 or younger (69.2%) and those older than 65 (30.8%), highlighting the predominance of younger patients in this cohort (Table 1).

Table 1.
Clinico-pathological characteristics of all patients.
The study population comprised a higher proportion of males (62.7%) compared to females (37.3%). The antrum was identified as the most common site for tumor occurrence (53.3%), followed by the body of the stomach (41.3%) and a smaller proportion of patients had the primary tumor located at the gastroesophageal junction (GEJ) (5.4%). The cohort was nearly equally divided between those undergoing total gastrectomy (50.6%) and those undergoing subtotal gastrectomy (49.4%).
The majority of patients (76%) did not receive neoadjuvant chemotherapy, whereas 24% (18 patients) did, suggesting that most patients proceeded directly to surgery without prior chemotherapy.
The median number of harvested LNs per patient was 26 [IQR 20–39]. The distribution of evaluated LNs is presented in Figure 2.
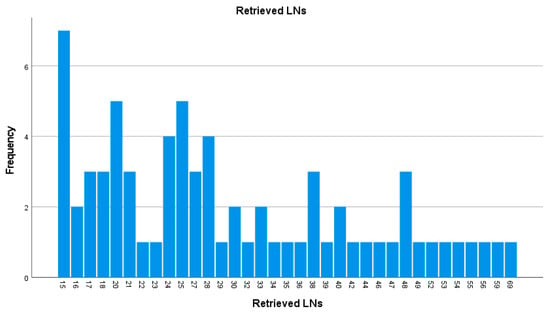
Figure 2.
Histogram displaying the distribution of total nodes examined per patient (LNs—lymph nodes).
A significant proportion of patients had no lymph node involvement N0 (38.6%), yet 61.4% had positive nodes—N1 (18.6%), N2 (22.7%), and N3 (20.1%).
Patients were distributed across various stages, with Stage IIA being the most common (28%), followed by Stage IIIA (24%), indicating that the study population largely consisted of patients with advanced-stage, non-metastatic cancer (Table 1).
3.2. Short-Term Outcomes
Postoperative complications that occurred during hospitalization or 30 days after the surgical procedure were assessed using the Dindo Clavien grading system (Table 1) [24,25,26]. While 47 (62.6%) patients had an uneventful postoperative course, 21 (28%) patients developed minor postoperative complications and 7 (9.3%) had major complications eventually leading to the death of 4 (5.3%) patients.
3.3. Setting of LNR and LODDS Cut-Off Values
Because 4 patients died postoperatively due to other causes than malignancy’s progression, the survival analysis was based on 71 patients who survived after operation.
The median number of positive LNs per patients was 2 [IQR 0–6], and the median number of negative LNs was 24 [IQR 15–35] (Figure 3).

Figure 3.
(a) Number of positive LNs per patient. (b) Number of negative LNs per patient.
LNR represents the proportion of the positive LNs from the entire number of examined LNs. LODDS was calculated using the following formula: LODDS = log ([NPLN + 0.5]/[NDLN-NPLN + 0.5]), where NPLN is the number of positive LNs and NDLN is the number of dissected LNs. X-Tile software (version 3.6.1; Yale University School of Medicine, New Haven, CT, USA) was used to identify the optimal LODDS and LNR cut-off values able to stratify patients into three groups with significantly different OS rates. By using X-tile software, we determined the two cut-offs associated with the maximal survival difference (or the highest log-rank χ2 value) among the three groups [27].
For LODDS, the two cut-off points were established at −2.2 and −0.6 and the patients were divided into three groups according to the LODDS value: LODDS 0 ≤ −2.2, LODDS 1 > −2.2, ≤ −0.6, and LODDS 2 > −0.6 (Figure 4).

Figure 4.
X-tile plots of the LODDS*100 (log odds of positive lymph nodes multiplied by 100) and corresponding OS curves, in patients with radical gastrectomy for gastric adenocarcinoma. (a) Histogram showing LODDS: the values of −2.2 and −0.6 divided the patients into three subgroups. (b) Comparative survival curves of the three subgroups. (c) OS rates were associated with the highest chi square and the lowest p value. Blue bars/curve—LODDS0 group, gray bars/curve—LODDS1 group, purple bars/curve— LODDS2 group.
The same method was used to find cut-off values for LNR, also establishing two values at 0.08 and 0.33; patients were divided according to the LNR value into three groups: LNR0 ≤ 0.08, LNR1 > 0.08, ≤ 0.33 and LNR2 > 0.33 (Figure 5).

Figure 5.
X-tile plots of the LNR*100 (lymph node ratio multiplied by 100) and corresponding OS curves, in patients with radical gastrectomy for gastric adenocarcinoma. (a) Histogram showing LNR: the value of 0.08 and 0.33 divided the patients into three subgroups. (b) Comparative survival curves of the three subgroups. (c) OS rates were associated with the highest chi square and the lowest p value. Blue bars/curve—LNR0 group, gray bars/curve—LNR1 group, purple bars/curve—LNR2.
3.4. Long-Term Outcomes (OS)
For the study group of 71 patients, the median OS was 117 months, with 1-, 3-, 5- and 10-year OS rates of 92.3%, 71.2%, 65.1%, and 43.6%, respectively.
To evaluate the factors significantly associated with OS, we initially performed a univariate analysis and, subsequently, multivariate analysis.
The variables included in univariate analysis were demographic data (age and sex), treatment factors (neoadjuvant chemotherapy, type of gastrectomy), tumor characteristics (location, grading, T-, N-, and TNM stage), postoperative complications, LNR, and LODDS.
Several factors did not have a statistically significant association with OS in univariate analysis (Table 2), while poor differentiation, higher N stage, higher LNR and LODDS were all significantly associated with poorer OS (Table 3).

Table 2.
Univariate analysis of variables that did not show statistical significance (p > 0.05).

Table 3.
Multivariate analysis of variables with statistical significance in univariate analysis.
However, when consecutive N stages have been compared one by one, the difference in OS was not statistically significant. Thus, the median OS for N0 vs. N1 was 120 months vs. NR (p = 0.443). Between the N1 and N2 stages, the difference in median OS was also not statistically significant (NR vs. 77 mo.; p = 0.372). Furthermore, the difference in OS was not significant between the N2 and N3 stages (77 vs. 24 mo.; p = 0.103). Thus, the N stage could not adequately stratify patients relative to their OS (Figure 6).

Figure 6.
Comparative OS rates according to the N stage.
By this reason, we used other staging systems based on the number of evaluated LNs and positive LNs, such as LNR and LODDS, to adequately stratify the patients relative to their overall survival outcome.
In multivariate analysis, tumor grading was not an independent factor associated with prognosis (p = 0.474). Regarding the N stage, only the N3 stage was an independent factor associated with significantly poorer OS (HR = 54.502, 95% CI: 2.188–1357.612, p = 0.015), while N1 and N2 were not independent risk factors (for N1-stage: HR = 1.361, 95% CI: 0.163–11.394; for N2-stage: HR = 11.465, 95% CI: 0.964–136.329; p > 0.05 for both). Both LNR and LODDS were independent factors associated with prognosis, and the two cut-offs determined by X-tile software were able to stratify patients into three categories, each of them being independently associated with a significantly distinct prognostic (LNR/LODDS1: HR = 52.450, 95% CI: 4.354–631.774, p = 0.002; LNR/LODDS2: HR = 260.715, 95% CI: 9.834–6912.215, p < 0.001) (Table 3).
4. Discussion
Gastric cancer remains one of the leading causes of cancer worldwide, ranking among the top five leading causes of cancer-related mortality [1,28]. Without surgery, the overall survival rate at 5 years is dismal (less than 5%), while in patients, radically resected 5 year OS rates increase, according to stage of the disease, from 36–50% in stage III to 70% in stage II patients and over 90% in early gastric cancer [29,30,31].
In our study group, the majority of patients had locally advanced disease with 5-year OS rates of 77.1%, 72%, and 51.8% in stages I, II, and III, respectively, similar to survival rates reported in previously mentioned studies [29,30,31,32].
Traditional staging systems, such as the UICC/AJCC systems, do not always accurately estimate OS. Numerous studies have suggested that the use of alternative staging systems, either alongside or instead of the classical TNM staging system, can improve the prognostic estimation of survival. The traditional TNM staging system shows limited prognostic efficacy when data collection is inadequate, adherence to guidelines is lacking, in instances of early gastric cancer in Asian countries [33,34], as well as in situations of stage migration following neoadjuvant chemotherapy, or when fewer than 15 LNs are retrieved or examined after radical D2 gastrectomy.
Lymph node dissection is crucial in radical gastrectomy, especially in advanced stages, because it allows for more accurate staging and potentially curative removal of the disease. However, studies conducted in the USA from 1998 to 2005 indicated that only 29% of patients had more than 15 nodes assessed [15,35,36,37,38]. For such instances, when postoperative LN assessment was not adequate, alternative staging systems such as LNR and/or LODDS were released.
LNR was initially utilized in breast cancer studies, whereas the LODDS was first introduced for breast cancer and subsequently validated in other cancers. Both are relatively new prognostic systems that have been applied to various cancers, including gastric, pancreatic, and colorectal cancer. Consequently, the LNR and LODDS staging systems have emerged as more significant prognostic factors than the pN (pathological node status) or TNM staging systems, especially in cases with inadequate lymph node dissection [39,40].
In our study, although all patients had at least 15 LNs evaluated, the N stage was not able to adequately stratify patients relative to the OS. This observation is similar to those reported by other authors who recommended the assessment of a minimum of 25 LNs in curative-intent operations performed for gastric cancer, to achieve an adequate prognostic stratification based on TNM staging [11,15,16]. Because 40.8% (29/71) of the patients included in the current study had between 15 and 24 evaluated LNs, and the N stage cannot adequately stratify the patients, we decided to explore the prognostic value of LNR and LODDS in our group of patients who underwent radical gastrectomy with D2 lymphadenectomy.
Our work showed that LNR and LODDS were independent prognostic factors of survival according to multivariate analysis, similar to the findings of Jian-Hui et al. [18].
Other studies have also shown an important relationship between the LNR and LODDS [15,19,41]. They demonstrated, as in our study, that both LNR and LODDS are superior to the pN classification in discriminating the different prognoses of patients, but not in a linear manner, although they are strongly correlated [42]. In these studies, the LODDS system had the potential to discriminate between patients with the same LNR and remained the most appropriate staging system [15,18,41].
In our study, possibly due to the limited sample size, the LODDS did not demonstrate superiority over the LNR, with both staging systems exhibiting equivalent prognostic values. With a larger, more diverse dataset, the two markers might have shown distinct, independent prognostic value, as reported in other studies [15,19,41]. In the context of the small sample size, the modality to determine the cut-off points with X-tile might explain the fact that software found the cut-off values for both LNR and LODDS at levels that divided the entire group into three identical subgroups for corresponding LODDS and LNR (the same patients were included in LNR0 and LODDS0, LNR1 and LODDS1, LNR2 and LODDS2). This is the explanation for the perfect collinearity of LNR and LODDS in this series, and it represents a significant limitation of the current study.
However, there are numerous modalities for calculating cut-off points for stratifying patients according to lymph node status using the percentile method, ROC (Receiver Operating Characteristic) curve analysis, AIC (Akaike Information Criterion), log-rank test and X-tile software [19,42,43,44,45,46]. Each of these methods has strengths and limitations, and the choice of method can significantly affect the resulting stratification. While other methods like ROC curve analysis are useful for assessing the trade-off between sensitivity and specificity, and AIC is good for model quality assessment, X-tile’s direct focus on identifying prognostically significant survival differences made it a suitable tool for achieving this study’s primary objective of improving prognostic stratification. The X-tile method was used in our study alongside the one conducted by Zeng et al. [46] who categorized patients into four subgroups (whereas our study identified three subgroups owing to the smaller sample size).
Regardless of the chosen method, the ultimate objective remains consistent: to create patient subgroups that demonstrate distinct prognostic outcomes, thereby enhancing their ability to predict survival and inform treatment decisions and follow-up regimen.
The frequently used cut-off of LNR set at 0 does not bring any prognostic benefit for N0 patients, and this is the explanation of the fact that the lower cut-off of LNR in our study was identified by X-tile software at 0.08. Similarly, Çapkinoğlu et al. used a single cut-off of 0.09 which divided 193 patients (median of 24 harvested LNs) into two groups with distinct survival outcomes [47]. Our higher LNR cut-off of 0.33 identified with X-tile software was similar to those reported by other studies [48,49,50]. For example, Wang et al. divided 18,043 patients included in the SEER database into four subgroups with significantly different prognosis using the cut-offs of 0.07, 0.3, and 0.7 [51]. In our study, the cut-offs were similar to their lower cut-offs, but because our series has a low sample size, the introduction of a third cut-off would generate four subgroups with a very small number of patients. Regarding the usefulness of LODDS in stratifying patients with gastric cancer, most studies revealed that, in patients with more than 15–20 LNs retrieved, LODDS lost its prognostic significance [15,44]. Thus, Cao et al. found in 877 patients who underwent D2 gastrectomy that the C-index of LODDS was significantly lower than those of LNR when more than 15 LNs have been harvested [44], while Sun et al. observed in 1593 patients with more than 15 evaluated LNs that LODDS lost its significance and was substituted by the N stage [15]. Their results may be explained by the cut-offs used, which cannot adequately stratify the patients with more than 15–20 retrieved LNs (Cao used the cut-offs of −0.5, 0, 0.5, while Sun used the cut-offs of −1.5, −1, −0.5 and 0). Due to the mathematical formula used to calculate LODDS, when more than 15 LNs are evaluated, most patients will be included in the group with very low LODDS value (e.g., less than −1.5 or less than −0.5), and a very small number of patients will be included in the other groups. For example, in our series, 50 (70.4%) patients had LODDS lower than −1.5, 6 (8.5%) had LODDS between −1.5 and −1, 4 (5.6%) patients were between −1 and −0.5, 3 (4.2%) patients had LODDs ranging between −0.5 and 0, 5 (7%) patients had LODDS between 0 and 0.5, and 3 (4.2%) patients had LODDS higher than 0.5. It is obvious that a more balanced number of patients in each group should be achieved by moving the cut-offs toward lower levels, explaining why, in our series, X-tile software found lower levels that were able to divide patients in homogeneous prognostic subgroups. Different from our method to determine cut-offs by using X-tile software, which discriminates patients according to their survival expectancy, most of the above-mentioned authors used rather empirical cut-offs, with LODDS classification intervals being determined by comparing OS rates according to LODDS with an initial interval of 0.5 and combining patients with similar prognosis [15,44]. Our cut-offs for LODDS were much more similar to those of Lee et al. who divided 3929 patients (97% of whom had more than 15 evaluated LNs) into five groups using the cut-offs of −4, −2.5, −2, and −0.5 [42]. The cut-offs that we used (−2.2 and −0.6) roughly included in our first subgroup (less than −2.2) their LODDS 1 and LODDS 2, in the second subgroup (−2.2 to −0.6) their LODDS 3 and LODDS 4, and our last subgroup was similar to their LODDS5 group. The similarity of our cut-offs with those of Lee may be explained by the fact that, in their series, almost 97% of patients had more than 15 retrieved LNs, similar to our cohort. Moreover, although they found that LODDS staging facilitates more accurate prognostic stratification, their results revealed that the receiver operating characteristic (ROC)-AUC curves of N-, LNR-, and LODDS staging systems did not differ significantly in terms of predicting survival [42]. This observation corroborated the previously discussed modality of achieving our cut-offs, may explain the collinearity between LNR and LODDS in our series, and might be a characteristic of the cohorts including patients with sufficient harvested LNs.
Thus, the current study revealed that cut-offs of LNR, and especially LODDS, that are able to discriminate patients into distinct prognostic groups with significantly different OS outcomes should be different in patients with more than 15 evaluated LNs than those frequently used for patients with insufficient retrieved LNs.
4.1. Limitations
One limitation of our study is the relatively small cohort size which precluded the possibility to separately evaluate the patients who underwent upfront surgery vs. those who received neoadjuvant chemotherapy. The cohort size of 18 patients receiving neoadjuvant therapy before surgery is inadequate for further subdivision. Furthermore, the follow-up would be too short, since all these patients were operated during the last 5 years. In this context, our results rather reflect the discriminative ability of LNR and LODDS in patients with gastric carcinoma who underwent upfront D2 gastrectomy. Stage migration in patients receiving neoadjuvant chemotherapy for gastric cancer presents a significant challenge for accurate prognostication using the current staging systems. The effectiveness of neoadjuvant treatment can lead to tumor downstaging and a reduction in lymph node involvement, potentially altering the patient’s staging classification. This phenomenon underscores the need for a new and more adaptive staging system that accounts for the effects of preoperative therapy. Distinct studies including only patients who underwent neoadjuvant therapy are mandatory to determine whether LNR and LODDS staging systems could be useful in the prognostic stratification of such patients and eventually determine the adequate cut-offs. The usefulness of TNM staging, LNR, and LODDS is even more debatable in patients receiving preoperative immunotherapy and chemotherapy, since almost 20% of them had complete pathologic response [52]. Also, due to the small sample size, the cut-off values of LODDS and LNR found in the current study cannot be validated in an independent cohort of patients.
Another limitation is the retrospective nature of the study. As a consequence, follow-up data regarding the time and type of recurrence were available only for 30 patients (42.3%), thus precluding the possibility to evaluate disease-free survival (DFS) in our cohort.
Due to these important limitations, this study should be considered as a pilot study, with future studies being necessary to validate its results.
4.2. Future Directions
Starting from these results, a future larger study involving hundreds of patients with non-metastatic gastric cancer undergoing radical gastrectomy should be performed. Because neoadjuvant therapy is the standard of care in present, the patients should undergo preoperative oncologic therapy. The study protocol should include preoperative TNM staging, surgery should include adequate D2 lymphadenectomy performed by surgeons experienced in gastric cancer operations, pathologic evaluations should follow a dedicated protocol to adequately identify all yielded LNs, and a strict follow-up protocol to determine the time and location of recurrence should be implemented. After a median follow-up of more than 36 months, the OS and DFS results should be evaluated according to the N stage, LNR, and LODDS. The cut-off values of LNR and LODDS should also be determined with X-tile software, and their prognostic accuracy must be evaluated with the ROC curve. Further, these cut-offs should be applied to a distinct series of similar patients, to validate the results achieved in the control group. However, because a randomized controlled trial which demonstrated that preoperative association of immunotherapy to the FLOT regimen significantly improved survival outcomes was published this year [52], it would be better to use this combination therapy preoperatively instead of neoadjuvant chemotherapy alone.
5. Conclusions
In patients who underwent radical D2 gastrectomy, LNR and LODDS were able to better stratify patients relative to their OS than the current TNM staging system. Although previous studies suggested that LNR and LODDS are useful, mainly in case of insufficiently evaluated LNs; the current results emphasize that these staging systems could also be applied in patients with an adequate number of retrieved LNs. However, this should be considered as a pilot study due to the small sample size, requiring further validation in larger studies with more datapoints, especially in terms of the accuracy of these staging systems in patients who underwent preoperative oncologic therapy.
Author Contributions
T.R.G.: Conceptualization, Investigation, Writing—original draft. C.V.: Formal analysis, Data curation. T.D.C.: Formal analysis, Data curation. I.M.G.: Formal analysis, Data curation. A.I.: Formal analysis, Data curation. G.A.P.: Formal analysis, Writing—original draft. M.P.: Formal analysis, Data curation. S.T.A.: Conceptualization, Formal Analysis, Investigation, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Fundeni Clinical Institute (number 15384 from 14.04.2025) and by the Ethics Committee of Emergency University Hospital Bucharest (number 12872/18.02.2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
Upon completion of the database search, the analysis was conducted using IBM SPSS Statistics (Version 27). X-tile software was used to establish the cut-off values for LNR and LODDS. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TNM | Tumor-Node-Metastasis |
| LN | Lymph Node |
| LNR | Lymph Node Ratio |
| LODDS | Log Odds of Positive Lymph Nodes |
| AJCC | American Joint Committee on Cancer |
| UICC | Union for International Cancer Control |
| OS | Overall Survival |
| FLOT | Fluorouracil, Leucovorin, Oxaliplatin, and T docetaxel |
| HR | Hazard Ratio |
| CI | Confidence Intervals |
| EGJ | Esophagogastric junction |
| NPLN | Number of Positive Lymph Nodes |
| NDLN | Number of Dissected Lymph Nodes |
| pN | Pathological Node Status |
| ROC | Receiver Operating Characteristic |
| AIC | Akaike Information Criterion |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 28 September 2025).
- Howson, C.P.; Hiyama, T.; Wynder, E.L. The Decline in Gastric Cancer: Epidemiology of an Unplanned Triumph. Epidemiol. Rev. 1986, 8, 1–27. [Google Scholar] [CrossRef]
- Anderson, W.F.; Rabkin, C.S.; Turner, N.; Fraumeni, J.F., Jr.; Rosenberg, P.S.; Camargo, M.C. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J. Natl. Cancer Inst. 2018, 110, 608–615. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: A population-based modelling study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Dikken, J.L.; Lemmens, V.E.; Wouters, M.W.; Wijnhoven, B.P.; Siersema, P.D.; Nieuwenhuijzen, G.A.; van Sandick, J.W.; Cats, A.; Verheij, M.; Coebergh, J.W.; et al. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur. J. Cancer 2012, 48, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.M.; Buicko Lopez, J.L. Gastric Resection for Malignancy (Gastrectomy). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK560760/ (accessed on 18 August 2025).
- Marrelli, D.; Piccioni, S.A.; Carbone, L.; Petrioli, R.; Costantini, M.; Malagnino, V.; Bagnacci, G.; Rizzoli, G.; Calomino, N.; Piagnerelli, R.; et al. Posterior and Para-Aortic (D2plus) Lymphadenectomy after Neoadjuvant/Conversion Therapy for Locally Advanced/Oligometastatic Gastric Cancer. Cancers 2024, 16, 1376. [Google Scholar] [CrossRef]
- Roviello, F.; Pedrazzani, C.; Marrelli, D.; Di Leo, A.; Caruso, S.; Giacopuzzi, S.; Corso, G.; de Manzoni, G. Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur. J. Surg. Oncol. (EJSO) 2010, 36, 439–446. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- NCC Network. Gastric Cancer. Version 1. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 2 October 2025).
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-H.; Kang, S.J.; Choi, W.; Na Seo, A.; Eom, B.W.; Kang, B.; Kim, B.J.; Min, B.-H.; Tae, C.H.; Choi, C.I.; et al. Korean Practice Guidelines for Gastric Cancer 2024: An Evidence-based, Multidisciplinary Approach (Update of 2022 Guideline). J. Gastric Cancer 2025, 25, 5–114. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, Y.; Li, D.M.; Wang, Z.N.; Zhu, G.L.; Huang, B.J.; Li, K.; Xu, H.M. Log odds of positive lymph nodes. Cancer 2010, 116, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Ichikura, T.; Ogawa, T.; Chochi, K.; Kawabata, T.; Sugasawa, H.; Mochizuki, H. Minimum number of lymph nodes that should be examined for the International Union Against Cancer/American Joint Committee on Cancer TNM classification of gastric carcinoma. World J. Surg. 2003, 27, 330–333. [Google Scholar] [CrossRef]
- Feinstein, A.R.; Sosin, D.M.; Wells, C.K. The Will Rogers Phenomenon: Stage Migration and New Diagnostic Techniques as a Source of Misleading Statistics for Survival in Cancer. N. Engl. J. Med. 1985, 312, 1604–1608. [Google Scholar] [CrossRef]
- Chen, J.-H.; Cai, S.-R.; Wu, H.; Chen, S.-L.; Xu, J.-B.; Zhai, E.-T.; Chen, C.-Q.; He, Y.-L. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumor Biol. 2016, 37, 11105–11113. [Google Scholar] [CrossRef]
- Natalizi, N.; Graziosi, L.; Di Schiena, F.; Donini, A. Is ypN Sufficient to Stage Gastric Cancer Patients After Neoadjuvant Chemotherapy? Indian J. Surg. 2025, 87, 22–30. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Clavien, P.A.; Sanabria, J.R.; Strasberg, S.M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992, 111, 518–526. [Google Scholar] [PubMed]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-Tile: A New Bio-Informatics Tool for Biomarker Assessment and Outcome-Based Cut-Point Optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- 7-stomach-fact-sheet.pdf. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/7-stomach-fact-sheet.pdf (accessed on 28 September 2025).
- Cenitagoya, G.F.; Bergh, C.K.; Klinger-Roitman, J. A prospective study of gastric cancer. “Real” 5-year survival rates and mortality rates in a country with high incidence. Dig. Surg. 1998, 15, 317–322. [Google Scholar] [CrossRef]
- Suzuki, H.; Oda, I.; Abe, S.; Sekiguchi, M.; Mori, G.; Nonaka, S.; Yoshinaga, S.; Saito, Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016, 19, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.G.; Seong, B.O.; Ko, C.S.; Yook, J.H.; Yoo, M.-W.; Kim, B.S.; Lee, I.-S.; Gong, C.S.; Min, S.H.; Kim, S. Life expectancy of patients with early gastric cancer who underwent curative gastrectomy: Comparison with the general population. Sci. Rep. 2025, 15, 5229. [Google Scholar] [CrossRef]
- Mueller, J.L.; Kim, D.H.; Stapleton, S.; Cauley, C.E.; Chang, D.C.; Park, C.H.; Song, K.Y.; Mullen, J.T. Nature versus nurture: The impact of nativity and site of treatment on survival for gastric cancer. Gastric Cancer 2019, 22, 446–455. [Google Scholar] [CrossRef]
- Forman, D.; Pisani, P. Gastric cancer in Japan--honing treatment, seeking causes. N. Engl. J. Med. 2008, 359, 448–451. [Google Scholar] [CrossRef]
- Kooby, D.A.; Suriawinata, A.; Klimstra, D.S.; Brennan, M.F.; Karpeh, M.S. Biologic Predictors of Survival in Node-Negative Gastric Cancer. Ann. Surg. 2003, 237, 828–837. [Google Scholar] [CrossRef]
- Estes, N.C.; MacDonald, J.S.; Touijer, K.; Benedetti, J.; Jacobson, J. Inadequate documentation and resection for gastric cancer in the United States: A preliminary report. Am. Surg. 1998, 64, 680–685. [Google Scholar] [PubMed]
- Schwarz, R.E.; Smith, D.D. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann. Surg. Oncol. 2007, 14, 317–328. [Google Scholar] [CrossRef]
- Coburn, N.G.; Swallow, C.J.; Kiss, A.; Law, C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer 2006, 107, 2143–2151. [Google Scholar] [CrossRef]
- Smith, D.D.; Schwarz, R.R.; Schwarz, R.E. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US-population database. J. Clin. Oncol. 2005, 23, 7114–7124. [Google Scholar] [CrossRef] [PubMed]
- Occhionorelli, S.; Andreotti, D.; Vallese, P.; Morganti, L.; Lacavalla, D.; Forini, E.; Pascale, G. Evaluation on prognostic efficacy of lymph nodes ratio (LNR) and log odds of positive lymph nodes (LODDS) in complicated colon cancer: The first study in emergency surgery. World J. Surg. Oncol. 2018, 16, 186. [Google Scholar] [CrossRef]
- Alexandrescu, S.T.; Selaru, F.M.; Diaconescu, A.S.; Zlate, C.A.; Blanita, D.; Grigorie, R.T.; Zarnescu, N.O.; Herlea, V.; Popescu, I. Prognostic Value of Lymph Node Ratio in Patients with Resected Synchronous Colorectal Liver Metastases and Less Than 12 Examined Lymph Nodes. J. Gastrointest. Surg. 2022, 26, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Deng, J.; Sun, Z.; Wang, Z.; Wang, W.; Liang, H.; Xu, H.; Zhou, Z. Superiority of log odds of positive lymph nodes (LODDS) for prognostic prediction after gastric cancer surgery: A multi-institutional analysis of 7620 patients in China. Surg. Today 2021, 51, 101–110. [Google Scholar] [CrossRef]
- Lee, J.W.; Ali, B.; Park, C.H.; Song, K.Y. Different lymph node staging systems in patients with gastric cancer from Korean: What is the best prognostic assessment tool? Medicine 2016, 95, e3860. [Google Scholar] [CrossRef] [PubMed]
- Marchet, A.; Mocellin, S.; Ambrosi, A.; de Manzoni, G.; Di Leo, A.; Marrelli, D.; Roviello, F.; Morgagni, P.; Saragoni, L.; Natalini, G.; et al. The prognostic value of N-ratio in patients with gastric cancer: Validation in a large, multicenter series. Eur. J. Surg. Oncol. 2008, 34, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Tang, Z.; Yu, Z.; Wang, Q.; Li, Z.; Lu, Q.; Wu, Y. Comparison of the 8th union for international cancer control lymph node staging system for gastric cancer with two other lymph node staging systems. Oncol. Lett. 2019, 17, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Tóth, D.; Bíró, A.; Varga, Z.; Török, M.; Árkosy, P. Comparison of different lymph node staging systems in prognosis of gastric cancer: A bi-institutional study from Hungary. Chin. J. Cancer Res. 2017, 29, 323–332. [Google Scholar] [CrossRef]
- Zeng, W.-J.; Hu, W.-Q.; Wang, L.-W.; Yan, S.-G.; Li, J.-D.; Zhao, H.-L.; Peng, C.-W.; Yang, G.-F.; Li, Y. Lymph node ratio is a better prognosticator than lymph node status for gastric cancer: A retrospective study of 138 cases. Oncol. Lett. 2013, 6, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Çapkinoğlu, E.; Tufan, A.E.; Ömeroğlu, S.; Tanal, M.; Güven, O.; Demir, U. Positive lymph node ratio as a prognostic factor for gastric cancer patients: Is it going to supersede positive lymph node number in guidelines? Medicine 2023, 102, e33757. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Bao, M.; Zhang, C. Prognostic value of different lymph node staging methods for node-positive cardia gastric cancer: A register-based retrospective cohort study. BMJ Open 2021, 11, e050378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Sun, X.; Zeng, L.; Zeng, X.; Xie, G.; Liu, X.; Tao, K.; Zhang, P. Lymph node ratio is a prognostic indicator for locally advanced gastric cancer after neoadjuvant immunochemotherapy. BMC Gastroenterol. 2024, 24, 371. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Hwang, I.; Park, Y.-S.; Gardner, J.; Ro, J.Y. Metastatic lymph node ratio in advanced gastric carcinoma: A better prognostic factor than number of metastatic lymph nodes? Int. J. Oncol. 2010, 36, 1461–1467. [Google Scholar] [CrossRef]
- Wang, J.; Dang, P.; Raut, C.P.; Pandalai, P.K.; Maduekwe, U.N.; Rattner, D.W.; Lauwers, G.Y.; Yoon, S.S. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: Analysis of 18,043 patients from the SEER database. Ann. Surg. 2012, 255, 478–485. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Al-Batran, S.-E.; Wainberg, Z.A.; Muro, K.; Molena, D.; Van Cutsem, E.; Hyung, W.J.; Wyrwicz, L.; Oh, D.-Y.; Omori, T.; et al. Perioperative Durvalumab in Gastric and Gastroesophageal Junction Cancer. N. Engl. J. Med. 2025, 393, 217–230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.