Abstract
Background and Objectives: Japan’s aging population faces growing challenges related to oral frailty, a condition characterized by the decline of oral function associated with physical and nutritional deterioration. Impaired oral function contributes to reduced chewing, swallowing, and saliva secretion, leading to poor appetite and frailty progression. Ninjin’yoeito (NYT), a traditional Kampo formula, has been clinically used to improve systemic weakness and oral symptoms. This study aimed to evaluate the efficacy and safety of NYT in improving oral health among elderly individuals with impaired oral function. Materials and Methods: In this open-label prospective study, patients received NYT daily for 12 weeks. Assessments included oral symptom scores, mucosal moisture, repetitive saliva swallowing tests (RSST), gustatory function by visual analogue scale (VAS), an 11-item oral questionnaire, and immune profiling by flow cytometry. Safety was assessed through hematological and biochemical tests. Results: Symptom scores decreased from 8.27 at baseline to 3.64 at 12 weeks (p = 0.006), while oral condition scores improved from 5.09 to 1.36 (p = 0.006). Mucosal moisture increased (25.1 to 28.1, p = 0.03), and RSST frequency improved (2.18 to 4.55, p = 0.046). Questionnaire scores declined from 5.1 to 2.0 (p < 0.001). VAS-taste was unchanged overall (p = 0.21) but improved in low baseline patients. Laboratory findings showed no adverse changes, with favorable lipid trends. Immune analysis revealed a decline in NKG2D expression (p = 0.02), whereas other activating and inhibitory markers remained stable. Conclusions: NYT was well tolerated and associated with gradual improvements in oral and physical symptoms among elderly individuals with impaired oral function. These findings provide preliminary evidence supporting the feasibility of Kampo-based approaches for maintaining oral health in aging populations and warrant further validation in larger controlled trials.
1. Introduction
Japan is currently experiencing a rapid demographic shift toward a super-aged society, in which maintaining oral function has become an essential component of elderly healthcare [1]. Oral frailty—defined as a decrease in oral function accompanied by a decrease in mental and physical function —has recently emerged as a critical public health issue [2,3]. This condition not only impairs chewing, swallowing, and saliva secretion but also contributes to nutritional decline, weight loss, and frailty progression among older adults [4,5,6,7,8]. Previous epidemiological studies have reported that more than 20–30% of Japanese individuals aged ≥ 75 years experience some degree of oral frailty, highlighting its high prevalence and impact on quality of life [9].
Oral function is closely related to overall health, nutritional status, and longevity. Decreased salivary flow, dry mouth, and swallowing difficulty can reduce food intake and digestion efficiency, leading to malnutrition and increased mortality risk [7,8,10]. Furthermore, poor oral function affects communication, social interaction, and psychological well-being, which are key components of healthy aging [3,11,12]. Aging is also accompanied by immunosenescence and metabolic decline, which increase vulnerability to diseases and further exacerbate frailty [13,14]. Therefore, maintaining oral function and nutritional balance is considered a cornerstone of frailty prevention and comprehensive geriatric care.
Ninjin’yoeito (NYT), also known as Ren Shen Yang Rong Tang in Traditional Chinese Medicine, is a classical Kampo prescription approved by the Japanese Ministry of Health, Labour and Welfare. NYT consists of extracts from 12 medicinal herbs: Ginseng radix, Atractylodis macrocephalae rhizoma, Poria, Glycyrrhizae radix, Angelicae radix, Rehmanniae radix, Astragali radix, Cinnamomi cortex, Paeoniae radix, Citrus unshiu peel, Schisandrae fructus, and Polygalae radix. NYT has been prescribed since the 16th century in Japan and other Asian countries for a wide range of conditions, including weakness and neuropathic pain [15], as well as asthenia and general debility [16]. It has also been reported to improve the nutritional status of frail older adults [17]. Regarding oral function, Miyazaki et al. demonstrated that NYT alleviated oxybutynin hydrochloride-induced xerostomia in patients with psychogenic urinary frequency or unstable bladder [18], and additional case reports have described improvements in dry mouth and appetite following two months of treatment [19]. Moreover, experimental studies have shown that NYT may modulate immune responses, including effects on regulatory T-cell populations [20]. These findings lead us to believe that NYT may help enhance physical strength, oral health, nutritional status, and immune balance in elderly individuals, supporting its potential as a multifaceted therapeutic option.
However, evidence regarding its impact on oral function and nutrition in older adults remains limited, and few studies have comprehensively examined its safety profile through hematological and biochemical parameters. Therefore, the present study was designed as an exploratory, open-label pilot trial to evaluate the potential of NYT in improving oral health among elderly individuals. In addition to assessing preliminary efficacy and safety, this study also aimed to examine the feasibility of long-term follow-up in this population, providing groundwork for future large-scale clinical studies focusing on geriatric oral health promotion.
2. Materials and Methods
2.1. Participants
This exploratory, open-label pilot study evaluated the feasibility and preliminary effects of NYT in older adults with impaired oral function. Participants aged ≥ 65 years were recruited at Kanazawa University Hospital between July 2019 and September 2022 using convenience sampling, which is appropriate for pilot feasibility research. In accordance with the CONSORT 2010 Extension for Pilot and Feasibility Trials, a formal sample size calculation was not performed, as the primary objective was to assess feasibility rather than to test predefined hypotheses. Impaired oral function was defined according to standardized Japanese oral frailty criteria. Participants were required to meet at least one of the following conditions: (1) two or more positive responses on a three-item screening questionnaire (“difficulty chewing hard foods,” “coughing or choking when drinking,” and “oral dryness”); (2) abnormal clinical oral findings, including poor oral hygiene, oral malodor, reduced facial or oral expressiveness, articulation difficulties, or residual food after swallowing; or (3) a RSST score < 3. Suitability for NYT administration was further determined based on Kampo diagnostic principles [21]. Participants were considered appropriate if they exhibited classical qi-and-blood deficiency patterns, such as post-illness fatigue, reduced vitality, appetite loss, cold extremities, night sweats, or anemia [22,23]. Oral status was clinically evaluated to confirm that all participants had adequate natural dentition or prosthetic support to perform chewing, swallowing, and salivary assessments, although detailed prosthodontic classifications were not systematically recorded.
Eligibility assessments were independently conducted by two experienced physicians using the Severity of Symptoms Questionnaire and the Oral Condition Questionnaire, with discrepancies resolved by consensus. Exclusion criteria included hypersensitivity to NYT, severe acute illness, significant hepatic or renal dysfunction, active cancer treatment, use of conflicting medications, and inability to comply with study procedures. All participants provided written informed consent. The study adhered to the Declaration of Helsinki and was approved by the Medical Ethics Committee of Kanazawa University (approval no. 000039882; approval date: 28 March 2019; UMIN ID: UMIN000039882).
A total of 11 participants aged > 65 years (9 females and 2 males; mean age, 76.4 ± 5.7 years; range, 70–88 years) were enrolled. The mean height was 156.2 ± 8.6 cm (range, 143–173 cm), and participants had an average of 3.5 comorbidities. The most common comorbid conditions were hypertension (36.4%), dyslipidemia (36.4%), type 2 diabetes mellitus (18.2%), glaucoma (18.2%), allergic rhinitis (18.2%), and insomnia (18.2%). Baseline demographic and clinical characteristics of the participants are summarized in Table 1.

Table 1.
Baseline characteristics of study participants.
2.2. Clinical and Oral Assessment Methods
Clinical and oral conditions were assessed using a combination of objective measurements and patient-reported questionnaires. Symptom severity was evaluated using the Severity of Symptoms Questionnaire, while oral conditions were assessed using the Oral Condition Questionnaire. Oral mucosal moisture was objectively measured using a MOUKAS oral hygrometer (Life Co., Ltd., Saitama, Japan). Swallowing function was assessed using the RSST. The complete Severity of Symptoms Questionnaire and Oral Condition Questionnaire are provided in Supplementary Tables S1 and S2.
The Severity of Symptoms Questionnaire and the Oral Condition Questionnaire were developed by the clinical team based on Japanese oral frailty guidelines. Although these instruments have not undergone formal psychometric validation, conceptually similar validated tools have been reported in previous studies, including the 8-item Oral Frailty Index developed by Nomura et al. [24], and 11-item oral frailty screening instruments evaluated in geriatric cohorts [25]. These referenced instruments are cited for contextual comparison only and were not directly used in the present study. Therefore, assessments based on the questionnaires developed for this study are interpreted as exploratory.
Additional patient-reported assessments included gustatory function, evaluated using VAS, and an 11-item oral function questionnaire. This 11-item oral function questionnaire is a self-reported instrument distinct from the 11-item oral frailty screening tools described in previous studies [22]. It is widely used in clinical practice in Japan but has not been formally validated for oral frailty assessment, and this limitation is acknowledged. The full questionnaire is provided as Supplementary Table S3. The latter is widely used in clinical practice in Japan; however, its lack of formal validation for oral frailty assessment is acknowledged.
Immunological assessments were conducted on an exploratory basis using flow cytometry, including analysis of lymphocyte subsets and the expression of activating and inhibitory receptors, such as NKG2D, NKp46, PD-1, and TIM-3.
2.3. Evaluation of NYT Treatment on Symptom Severity
The severity of six symptoms (post-illness fatigue, irritability, appetite loss, somnolence, cold extremities, and anemia) was assessed at baseline and weeks 4, 8, and 12 using the Six-Symptom Severity Questionnaire developed by the study clinicians. Symptoms were scored as 3 (severe), 2 (moderate), 1 (mild), or 0 (none), and total scores were calculated at each time point. This questionnaire has not been psychometrically validated, nor has its reliability been established in prior studies. To improve assessment consistency, two senior physicians independently rated all items, resolving disagreements by consensus. The absence of formal validation remains a methodological limitation of this pilot study. A sample questionnaire is shown in Supplementary Table S1.
2.4. Evaluation of NYT Treatment Oral Function Symptoms by Physician
Oral function was assessed using an Oral Condition Questionnaire at baseline and after 4, 8, and 12 weeks of treatment. The questionnaire evaluates oral conditions and observable findings, including visible oral hygiene-related findings (such as deposits or discoloration on teeth, dentures, or the tongue), oral malodor, poor facial expressions, speech problems (e.g., unclear pronunciation, dysarthria, and reduced speech), and food residue after swallowing. According to Kampo diagnostic principles, these observable oral findings are considered clinically relevant because they may reflect impaired digestive function, reduced vital energy (qi), and decreased self-care ability, which are associated with age-related physiological characteristics [23].
Each symptom was scored by a physician as follows: 3 points for severe, 2 points for moderate, 1 point for mild, and 0 points for no signs. The total score for the five symptoms was recorded at each time point and compared across assessments. A sample of the questionnaire is provided in Supplementary Table S2.
2.5. Evaluation of NYT Treatment on Oral Mucosal Moisture
Oral mucosal moisture was evaluated using a portable oral hygrometer (MOUKAS Oral Hygrometer; Life Co., Ltd., Saitama, Japan). Measurements were performed at the oral mucosa under standardized conditions, with the probe gently placed in contact with the mucosal surface for a few seconds until the moisture value was automatically recorded according to the manufacturer’s instructions. Dry mouth was defined as a hygrometer value below 28.0. Assessments were conducted at baseline and after 4, 8, and 12 weeks of treatment.
2.6. Evaluation of NYT Treatment on Swallowing Frequency Using the Repetitive Saliva Swallowing Test
The RSST was conducted by gently placing the examiner’s second and third fingers on the patient’s laryngeal prominence and hyoid bone. The patient was instructed to swallow saliva as many times as possible within 30 s. The examiner recorded the number of times the laryngeal protuberance passed under the second finger during this period, which was noted as the RSST swallowing frequency. An RSST score of <3 swallows indicated impaired swallowing function [26]. RSST measurements were conducted at baseline and after 4, 8, and 12 weeks of NYT treatment.
2.7. Visual Analogue Scale (VAS) for Taste
Gustatory function was assessed using a 100 mm VAS. Participants were instructed to rate their current subjective taste perception on a straight horizontal line anchored at 0 mm (“no taste perception”) and 100 mm (“usual taste perception”), with the latter reflecting their own typical or desirable taste ability rather than an objective normative standard. Each participant marked the point on the line that best represented their perceived taste function at the time of assessment, and the distance in millimeters from the 0 mm anchor was recorded as the VAS score. Evaluations were conducted at baseline and at 4, 8, and 12 weeks of NYT treatment.
2.8. Patient-Reported Oral Function Questionnaire
Oral function was additionally evaluated using an 11-item patient-reported questionnaire assessing multiple domains relevant to oral frailty, including difficulties in chewing hard foods, swallowing, speaking, oral dryness, halitosis, taste recognition, and food residue. Each item was scored as 1 point for “yes” (impaired) and 0 points for “no” (normal), yielding a total score ranging from 0 (no impairment) to 11 (severe impairment). Assessments were performed at baseline and at 4, 8, and 12 weeks. A sample of the questionnaire is provided in Supplementary Table S3.
2.9. Hematological and Biochemical Safety Assessment
Safety was assessed using routine hematological and biochemical examinations conducted at baseline and after 12 weeks of NYT treatment. Hematological parameters included white blood cell count, red blood cell count, hemoglobin (Hb), hematocrit (Ht), platelet count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), mean platelet volume (MPV), platelet distribution width (PDW), and differential leukocyte counts (neutrophils, eosinophils, basophils, lymphocytes, and monocytes). Biochemical parameters included C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), lactate dehydrogenase (LDH), total bilirubin (T-Bil), serum electrolytes (Na, K, Cl), creatinine (CRE), blood urea nitrogen (BUN), lipid profile [low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG)], and blood pressure. Changes from baseline were analyzed to monitor potential adverse effects of NYT treatment.
2.10. Immunological Assessment Through Blood Tests
To evaluate immune system changes, flow cytometry was used to quantify immune cells and their biomarkers before and after 12 weeks of NYT treatment. Blood samples were collected in 3.5 mL ethylenediaminetetraacetic acid tubes and centrifuged at 3000× g for 8 min to separate the serum. Peripheral blood mononuclear cells (PBMCs) were isolated from the remaining blood through gradient centrifugation. A portion of the PBMCs from each sample was cryopreserved for later use. PBMCs were stained with antibodies specific to cell surface markers for natural killer (NK) lymphocytes and T cells, including the following: anti-CD3, CD4, CD8, CD56, NKG2D, NKp46, NKp30, TLR4, DNAM-1, NKG2A, 4-1BB, ICOS, OX40, GITR, PD-1, CTLA4, LAG3, TIGIT, and TIM3 (BioLegend, San Diego, CA, USA). The stained cells were analyzed using a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA), and the data were analyzed using FlowJo software (version 10; Tree Star, Ashland, OR, USA).
2.11. Dietary NYT Supplementation
The Kampo formula NYT used in this study was Kracie Ninjinyoeito Extract Fine Granules (KB-108, Kracie Pharmaceutical Co., Tokyo, Japan), a Japanese-standard Kampo product classified under product number 875200, approval number: 16100AMZ03510000, and YJ code: 5200117C1043. Each 7.5 g daily dose (two packets) contains 3.75 g of Ninjinyoeito extract powder (Kracie Pharmaceutical Co., Ltd., 3-20-20 Kaigan, Minato-ku, Tokyo, Japan [108-8080]). The product is a dry extract mixture, light brown to brown in color, with component ratios as listed in Table 2.

Table 2.
Component ratios of Ninjinyoeito supplementation.
2.12. Statistical Analysis
Given the exploratory pilot design and the small sample size, all statistical analyses were conducted with the primary objective of identifying preliminary trends rather than performing confirmatory hypothesis testing. Data obtained from the severity and oral function questionnaires, oral mucosal moisture measurements, RSST tests, VAS scores, and the 11-item oral questionnaire were first examined for normality using the Shapiro–Wilk test. Variables that were not normally distributed were analyzed using the non-parametric Friedman test with Bonferroni-adjusted post hoc pairwise comparisons. For normally distributed variables, repeated-measures ANOVA was used; however, these results were interpreted cautiously within an exploratory framework. Linear mixed-effects models (LMMs) were included only to illustrate time-dependent changes and to estimate within-subject and between-subject variability. These models were not used for confirmatory inference, thereby reducing the risk of type I and type II errors associated with high-dimensional modeling in small samples. For immune-cell subset markers and laboratory parameters (hematological and biochemical tests), paired t-tests or Wilcoxon signed-rank tests were applied according to data distribution. Because multiple immune markers were evaluated, the Benjamini–Hochberg false discovery rate (FDR) correction was applied. Global FDR-adjusted p-values are reported in Supplementary Table S4, and, for biologically related immunological pathways (T-cell subsets, activating NK receptors, inhibitory checkpoints, co-stimulatory receptors, and TLR4), subgroup-specific FDR-adjusted p-values are presented in Supplementary Table S4 to avoid over-correction across unrelated markers in this exploratory pilot analysis. All statistical tests were two-sided, and p < 0.05 was considered statistically significant within the context of exploratory analysis. Statistical analyses were performed using SPSS Statistics version 28 (IBM, Armonk, NY, USA).
Because of the small sample size and the imbalance in sex distribution (only two male participants), formal adjustment for covariates such as age and gender was not statistically feasible. Multivariable modeling was avoided to prevent unstable coefficient estimates and overfitting. Accordingly, analyses focused on within-subject comparisons without covariate adjustment, and the inability to control for age and sex effects is acknowledged as a methodological limitation of this exploratory pilot study.
3. Results
3.1. Study Design
Figure 1 illustrates the study design and timeline for patients with impaired oral function who received NYT (7.5 g/day) for 12 weeks. Eligible participants (n = 13) were assessed at baseline and re-examined at 4, 8, and 12 weeks. Assessments included symptom questionnaires (severity, oral function, 11-item questionnaire, and VAS), oral mucosal moisture (Moukas hygrometer), and RSST. Blood and biochemical parameters were measured at baseline and week 12. During follow-up visits, 2 patients did not attend re-examinations due to personal reasons, resulting in n = 12 at weeks 4 and 8, and n = 11 at week 12.
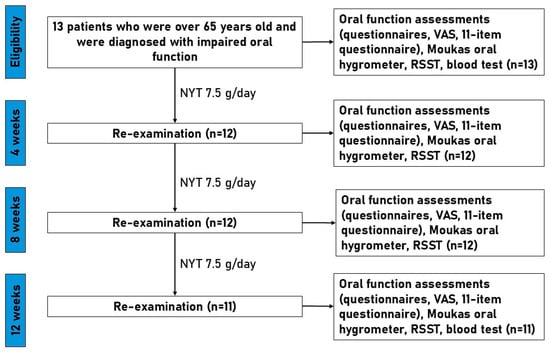
Figure 1.
Flowchart of study design. NYT, Ninjinyoeito; RSST, repetitive saliva swallowing test.
3.2. Changes in Primary Outcomes After NYT Treatment
As shown in Figure 2, all four predefined primary outcomes demonstrated consistent improvement during 12 weeks of NYT treatment. The mean symptom severity score decreased from 8.27 at baseline to 7.45 at 4 weeks, 6.00 at 8 weeks, and 3.64 at 12 weeks, with repeated-measures ANOVA indicating significant reductions at 8 weeks (p = 0.029) and 12 weeks (p = 0.006). Oral mucosal moisture increased progressively from 25.09 at baseline to 28.07 at week 12, exceeding the clinical threshold of 28.0, and reached statistical significance (p = 0.03). Oral condition scores improved markedly from 5.09 at baseline to 1.36 at 12 weeks (p = 0.006, Friedman test). RSST swallowing frequency rose from 2.18 at baseline to 4.55 at 12 weeks, surpassing the diagnostic cut-off of three swallows/30 s and showing a significant increase (p = 0.046). These improvements were observed consistently across multiple domains, including systemic symptom burden, oral moisture status, clinician-assessed oral condition, and swallowing function, indicating a broad effect of NYT on both general and oral health-related outcomes.
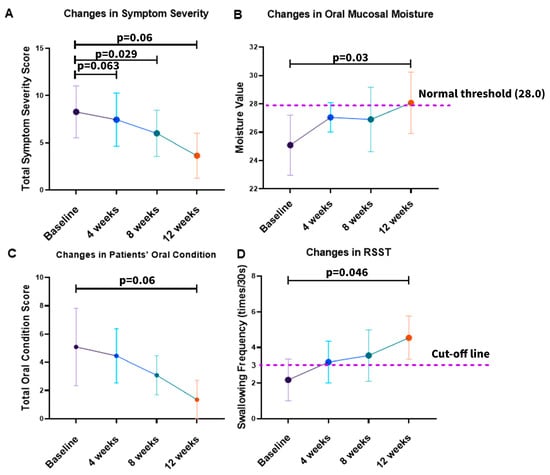
Figure 2.
Changes in primary outcomes during NYT treatment: (A) total symptom severity score; (B) oral mucosal moisture measured by the MOUKAS Oral Hygrometer; (C) total oral condition score; (D) swallowing function assessed by the RSST. Data are presented as group means with 95% confidence intervals.
To further quantify the longitudinal changes observed in the primary outcomes, linear mixed-effects (LME) regression analysis was performed, and the detailed results are summarized in Table 3. The analysis demonstrated significant time-dependent reductions in symptom severity (Coef. = −0.384, 95% CI −0.493 to −0.275, p < 0.001) and oral condition scores (Coef. = −0.314, 95% CI −0.432 to −0.195, p < 0.001). In contrast, oral mucosal moisture (Coef. = +0.220, 95% CI +0.093 to +0.347, p < 0.001) and RSST swallowing frequency (Coef. = +0.186, 95% CI +0.084 to +0.289, p < 0.001) increased significantly over time. Random-effect parameters, including the standard deviations of the intercept and slope, indicated inter-individual variability in baseline status and rates of change; however, the overall pattern consistently supported clinically meaningful and temporally consistent improvements in primary outcomes during NYT treatment.

Table 3.
Linear mixed-effects regression results for primary outcomes and secondary outcomes.
3.3. Changes in Taste Function and Oral Function Questionnaire
As shown in Figure 3, the VAS score for taste function exhibited only a modest upward trend during the 12-week treatment. The mean score increased from 84.1 ± 25.2 at baseline to 85.9 ± 21.7 at 4 weeks, 86.7 ± 19.1 at 8 weeks, and 90.5 ± 14.8 at 12 weeks. These changes did not reach statistical significance (Friedman test, p = 0.21). A substantial proportion of participants reported high baseline taste function scores (≥80/100), suggesting a ceiling effect that may have limited the ability to detect further improvements at the group level. Notably, one participant (male patient No. 11) with a markedly low baseline score (16/100) demonstrated a progressive and clinically meaningful increase to 53/100 at week 12. Linear mixed-effects regression analysis (Table 3) confirmed this finding, showing a non-significant overall trend (Coef. = +0.498, 95% CI −0.071 to +1.066, p = 0.086).
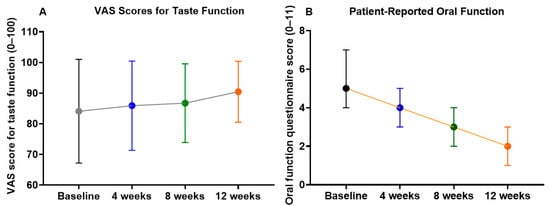
Figure 3.
Changes in patient-reported taste and oral function during 12 weeks of NYT treatment. (A) taste function assessed using a VAS; (B) patient-reported oral function assessed using the 11-item questionnaire. Values represent group means with 95% confidence intervals.
In contrast, the oral function questionnaire scores (0–11) demonstrated significant improvement during NYT treatment. The mean score declined from 5.1 ± 1.3 at baseline to 4.0 ± 1.2 at 4 weeks, 3.2 ± 1.2 at 8 weeks, and 2.0 ± 0.6 at 12 weeks, with the Friedman test revealing a significant overall difference across time points (p < 0.001). Post hoc pairwise comparisons showed that scores at 8 and 12 weeks were significantly lower than baseline (p < 0.01 and p < 0.001, respectively), indicating progressive improvement in subjective oral function symptoms. LME regression (Table 3) supported this result, with a significant time-dependent reduction (Coef. = −0.252, 95% CI −0.317 to −0.187, p < 0.001).
Together, these results suggest that while the effect of NYT on taste function was limited at the group level, likely due to a ceiling effect, patient-reported oral function showed robust and progressive improvement during the intervention period.
3.4. Safety Outcomes
No serious adverse events were observed during the study period. In addition, no significant differences were observed in hematological and biochemical parameters after 12 weeks of NYT administration (Table 4). Most values, including WBC, hemoglobin, hematocrit, platelet counts, and renal function markers (CRE, BUN), remained stable throughout the study period.

Table 4.
Hematological and biochemical parameters before and after NYT treatment.
Inflammatory and hepatic markers showed minor fluctuations: CRP levels increased slightly but remained within the normal range. Liver enzymes (AST, ALT) exhibited an upward trend; however, their values stayed within the normal reference range, and the changes did not reach statistical significance.
Notably, the lipid profile demonstrated a favorable tendency. LDL-C levels decreased, while HDL-C levels increased, with HDL-C approaching statistical significance (p = 0.057). Triglycerides also showed a slight decrease. Together, these findings suggest that the NYT administration was not associated with adverse hematological or biochemical effects and may contribute to modest improvements in lipid metabolism.
3.5. Immunological Outcomes
The overall proportion of lymphocytes remained stable during the intervention (75.8% ± 7.1 at baseline vs. 76.4% ± 4.9 after treatment, p = 0.807). CD3+CD56− T cells decreased modestly (23.1% ± 4.9 vs. 21.7% ± 5.3, p = 0.398), with a slight downward trend in the CD4+ subset (5.44% ± 3.3 vs. 4.81% ± 2.7, p = 0.366), whereas CD8+ T cells remained unchanged (16.4% ± 4.6 vs. 17.0% ± 5.7, p = 0.699). CD3−CD56+ NK cells also showed no significant differences (Figure 4). Among activating receptors, NKG2D expression decreased significantly from 26.8% ± 6.2 to 22.9% ± 5.8 (Δ = −3.94, 95% CI: −7.91 to −0.03, p = 0.026). NKp46+ and NKp30+ cells demonstrated downward but non-significant trends, while inhibitory receptors, including NKG2A, CTLA-4, PD-1, and TIGIT, remained stable. GITR showed a non-significant tendency to increase (Δ = +8.11 ± 12.83, p = 0.077). Detailed numerical values for all immune markers, including additional co-stimulatory and inhibitory receptors (e.g., TLR4, ICOS, LAG-3, TIM-3), are presented in Supplementary Table S4. Correlation matrix analysis of Δ values revealed weak-to-moderate positive associations among most markers, suggesting preservation of overall immune network balance following NYT treatment. Notably, reductions in NKG2D correlated negatively with changes in CD3+CD56−CD4+ T cells, whereas other co-stimulatory and inhibitory receptors showed minimal inter-relationships (Figure 5).
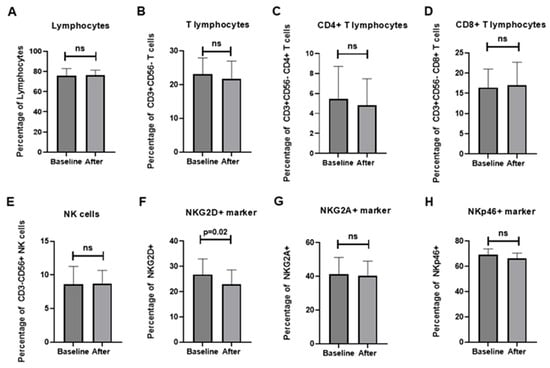
Figure 4.
Effects of NYT treatment on lymphocyte and NK/T-cell subsets: (A) total lymphocytes; (B) CD3+CD56− T cells; (C) CD4+ T cells; (D) CD8+ T cells; (E) CD3−CD56+ NK cells; (F) NKG2D+ cells; (G) NKG2A+ cells; (H) NKp46+ cells. Data are presented as mean ± SD before and after 12 weeks of treatment. ns, not significant; p < 0.05 was considered statistically significant.
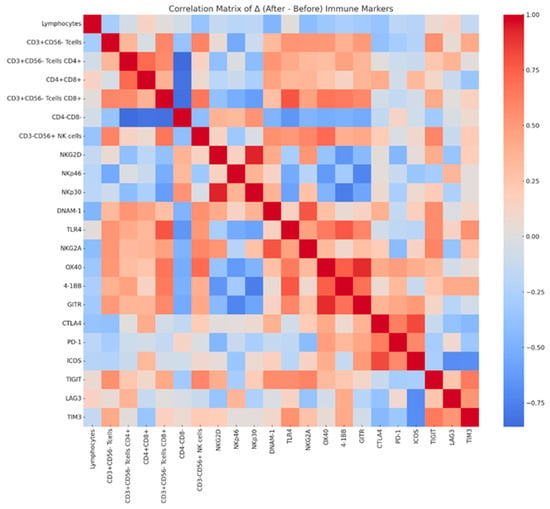
Figure 5.
Correlation matrix of changes (Δ) in immune cell subsets and receptor expression before and after NYT administration. The heatmap shows Pearson correlation coefficients between changes in immune cell populations and regulatory markers, with red indicating positive correlations, blue indicating negative correlations, and lighter colors indicating weaker correlations.
4. Discussion
Our findings suggest that NYT significantly alleviates physical symptoms such as fatigue, anorexia, irritability, and drowsiness, as previously reported [27]. Among its components, Panax ginseng is a well-known herbal treatment for chronic fatigue syndrome, with established efficacy and safety [28]. Ogawa-Ochiai et al. also demonstrated that Panax ginseng reduces fatigue in patients with frailty and significantly enhances NK cell activity [29,30].
Atractylodes macrocephala, another major NYT component, is traditionally used to address digestive and gastrointestinal issues. It promotes gastric emptying by stimulating stomach and intestinal activity, while its macrocephalic lactone I content enhances salivary amylase activity, intestinal absorption, and overall gastrointestinal regulation [31]. These properties may explain the capacity of NYT in reducing appetite loss. Furthermore, Schisandra chinensis has demonstrated its ability to improve endurance and energy metabolism in exercised rats. This effect is linked to the upregulation of peroxisome proliferator-activated receptor-gamma coactivator-1α, a key regulator of skeletal muscle energy metabolism [32]. Additionally, polysaccharides and α-cubebenoate derived from Schisandra fruit exhibit anti-fatigue effects through mechanisms such anti-inflammatory activity [33].
This study also revealed a substantial reduction in oral symptom severity, including improved oral mucosal moisture following NYT treatment. Enhanced mucosal moisture reduces the risk of xerostomia (dry mouth), a condition associated with complications such as bad breath, poor oral hygiene, dental decay, and difficulties in speaking and swallowing. Consistent with prior research, NYT has shown potential in alleviating dry mouth symptoms [34]. It can be observed that after treatment with NYT, there have been physical changes in the oral symptoms of elderly individuals. Recent studies have demonstrated that chewing, swallowing, digestion, and nutrient absorption are interconnected processes [35]. Oropharyngeal dysphagia, characterized by difficulties in swallowing and food residue post-swallowing, increases with age and poses serious health issues, including malnutrition [36]. In this study, NYT not only mitigated dry mouth but also improved speech clarity and swallowing function. These results suggest that NYT may contribute to the improvement of oral health and overall quality of life in elderly individuals. Moreover, considering NYT as a supplementary intervention to improve nutritional intake merits further investigation.
The cost-effective, innocuous, and simple nature of the RSST makes it an optimal screening tool for detecting dysphagia in various patient populations. In this study, swallowing frequency on the RSST significantly increased following NYT treatment, suggesting a beneficial effect for patients with swallowing dysfunction. This improvement holds particular significance for elderly or stroke patients, as aging is the strongest risk factor for stroke [37,38]. Dysphagia affects more than 50% of stroke survivors [39]. These results indicate that NYT could enhance swallowing ability in elderly patients and individuals recovering from a stroke. Given the interrelationship between age, stroke, and swallowing dysfunction, NYT shows potential for improving functional symptoms and nutritional status in this population. Additionally, swallowing is critical to maintaining the body’s nutritional functions. The observed improvement in swallowing frequency following NYT treatment offers hope that this traditional herbal medicine could become an effective intervention for alleviating functional symptoms and enhancing nutritional intake in elderly individuals, particularly those with stroke-related dysphagia.
In addition to objective findings, subjective symptom burden was also evaluated using the VAS and the 11-item questionnaire. Both instruments showed consistent trends toward improvement in domains such as fatigue, loss of appetite, and irritability, supporting the clinical relevance of NYT in reducing patient-perceived discomfort. These subjective assessments complement the objective measures, highlighting the holistic benefits of NYT for elderly patients with multiple complaints.
In this study, we observed a modest reduction in total T lymphocytes, primarily driven by a decline in the CD4+ T-cell population, while CD8+ T cells remained stable. NKG2D expression also decreased following NYT administration. Because NKG2D is expressed across multiple lymphocyte subsets—including NK cells, CD8+ T cells, CD4+CD8+ double-positive cells, and CD4+ T cells—it is important to consider which population underlies this change. Our correlation analysis did not reveal a strong or definitive association between NKG2D expression and any specific T-cell subset. Nevertheless, previous studies have highlighted that aberrant NKG2D expression on CD4+ T cells is a hallmark of immune aging, whereas CD4+CD8+ double-positive cells are rare in peripheral blood and unlikely to account for the observed reduction [40,41]. Thus, while our findings suggest a parallel decline in CD4+ T cells and NKG2D, further investigation is required to clarify whether these phenomena are mechanistically linked.
The immune system plays a central role in defending against pathogens and malignancies. CD8+ T cells and NK cells are well-established mediators of tumor rejection through interferon-γ production and cytotoxicity [42]. CD4+ T cells are equally essential for orchestrating immune responses. Prior studies have reported the presence of NKG2D+CD4+ cells in patients with cancer, autoimmune disease, or chronic infection [40,43,44,45]. In the elderly, naïve T-cell compartments decline while memory populations increase, with aberrant CD4+NKG2D+ cells emerging as a feature of immunosenescence [40,46,47]. Unlike NKG2D+ NK or CD8+ cells, CD4+NKG2D+ cells exert immunosuppressive effects by inhibiting T-cell activity through Fas ligand pathways [48]. Accordingly, a reduction in such cells could theoretically mitigate immune aging and restore immune competence. Prior animal studies have shown that NYT decreases CD4+ T-cell levels in tumor models [49], and miR-16 has been implicated in regulating CD4+NKG2D+ activity by directly targeting NKG2D [50]. While our data cannot conclusively attribute NKG2D reduction to CD4+ subsets, the possibility that NYT modulates this pathway remains of interest.
Taken together, these results indicate that NYT produced only limited shifts in immune markers, maintaining global immune balance while modestly reducing CD4+ T cells and NKG2D expression. This dual effect may reflect a subtle attenuation of senescent or suppressive CD4+NKG2D+ cells, though confirmatory mechanistic studies are needed. Importantly, most other immune markers—including activating receptors such as NKp46 and NKp30, as well as inhibitory checkpoints such as PD-1, TIM-3, and TIGIT—remained stable over the 12-week treatment period, indicating that NYT did not induce broad immunosuppression but rather exerted a selective regulatory effect on specific immune subsets associated with aging. Such selective modulation may represent a fine-tuning mechanism that helps maintain immunological balance. Although a modest reduction in total T lymphocytes—mainly CD4+ T cells—was observed, this change remained within age-appropriate physiological ranges and did not coincide with any clinical signs of immunosuppression. In frail older adults, such fluctuations often reflect immunoregulatory adjustments rather than immune deterioration, particularly when other key immune markers remained stable. Nonetheless, given the small sample size and exploratory design, this finding should be interpreted with caution. Larger studies with detailed immunophenotyping are required to determine whether this reduction represents a beneficial modulation of senescent CD4+NKG2D+ cells or simply natural biological variability.
No clinically relevant adverse changes were observed in hematological or biochemical parameters, supporting the overall safety of NYT in elderly patients. Moreover, favorable trends in lipid metabolism were noted, suggesting that NYT may also contribute to maintaining cardiovascular health, although these findings warrant further confirmation in larger studies.
The participants in this study presented with multiple comorbidities and extensive polypharmacy, both of which may have influenced baseline oral function, immune status, and responsiveness to NYT. Common comorbidities included hypertension, dyslipidemia, diabetes mellitus, osteoporosis, glaucoma, rheumatoid arthritis, spinal canal stenosis, coronary arteriosclerosis, post-stroke sequelae, pulmonary MAC disease, and various inflammatory or autoimmune conditions. Many patients were also receiving multiple concomitant medications such as antihypertensives, statins, antidiabetic agents, psychotropic drugs, proton-pump inhibitors, bisphosphonates, analgesics, magnesium preparations, and antirheumatic therapies. Several of these medications—particularly psychotropics, antihypertensives, and PPIs—are known to cause xerostomia, impair taste perception, alter swallowing function, or modulate immune activity [51,52,53]. Therefore, both comorbidity burden and polypharmacy may act as important confounders that contribute to variability in oral and immunological outcomes. As this pilot study was not powered to adjust for these factors, larger studies will be necessary to determine their independent and combined influence on treatment effects.
This study has several limitations. First, the sample size was very small (n = 11), which limits the statistical power and generalizability of the findings. Because of this limited cohort size and the imbalance in sex distribution (only two male participants), adjustments for covariates such as age and gender were not feasible, and all statistical comparisons should be interpreted as exploratory. Second, the questionnaires used to assess symptom severity and oral condition were developed by the clinical team and have not undergone formal psychometric validation; although similar oral frailty screening tools have shown validity in previous studies, the absence of formal validation for the instruments used here remains a limitation. Third, detailed oral prosthodontic information (e.g., presence of fixed partial dentures, removable partial dentures, or complete dentures) was not systematically recorded, which may affect the interpretation of oral functional outcomes. Fourth, immune assessments were limited to peripheral blood and examined only selected immune-cell markers; more extensive immunophenotyping—including analyses of naïve/memory subsets, senescent markers, and tissue-level immune responses—was not performed. Fifth, although a modest reduction in CD4+ T cells was observed, this pilot study was not designed to evaluate immunological safety endpoints, and the clinical significance of this finding remains uncertain. Finally, although an FDR correction was applied for immune-cell markers, the analyses remain exploratory due to multiple comparisons and small sample size. Larger, controlled studies with comprehensive clinical and immunological assessments are required to confirm and extend these preliminary observations.
5. Conclusions
This pilot study suggests that NYT administration may be associated with gradual improvements in oral and nutritional symptoms among elderly individuals with impaired oral function. Participants reported reduced fatigue and swallowing difficulties, indicating potential benefits of NYT for supporting oral health and nutritional maintenance in older adults. Additionally, exploratory immune analyses hinted at mild modulatory effects without global suppression, which could be relevant for future mechanistic investigations. Overall, these findings provide preliminary insight into the possible role of NYT in promoting oral health and general well-being among the elderly, highlighting the need for larger controlled studies to confirm its efficacy and clarify underlying mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina62010048/s1, Table S1: Severity of Symptoms Questionnaire (example for one patient); Table S2: Oral Condition Assessment Questionnaire (example for one patient); Table S3: 11-item Questionnaire for Oral Function (example for one patient); Table S4: Immune-related markers before and after 12 weeks of NYT administration.
Author Contributions
Conceptualization, T.Y. and K.O.-O.; methodology, T.Y. and K.O.-O.; data curation, A.S., H.L. and A.T.; formal analysis, Q.T.N., A.S., H.L. and L.L.; writing—original draft preparation, Q.T.N., A.S. and H.L.; writing—review and editing, T.Y. and K.O.-O.; supervision, A.K.; validation, A.K.; project administration, T.Y. and K.O.-O.; funding acquisition, T.Y. and K.O.-O. All authors critically reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the internal research fund of Kanazawa University. No grant number is applicable. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Kanazawa University (UMIN ID: UMIN000039882; date of approval 28 March 2019).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The materials described in this study’s findings, including all relevant raw data, will be freely available to any scientist for non-commercial use upon request to the corresponding author, provided that patient confidentiality is not compromised.
Acknowledgments
The authors would like to thank Ayako Kimura from the Kampo Clinical Center at Hiroshima University. Additionally, the authors would like to thank Isao Horikawa from Horikawa Clinic for his assistance throughout the research process.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BUN | Blood urea nitrogen |
| Cl | Chloride |
| CRE | Creatinine |
| CRP | C-reactive protein |
| DBP | Diastolic blood pressure |
| FDR | False Discovery Rate |
| Hb | Hemoglobin |
| HDL-C | High-density lipoprotein cholesterol |
| Ht | Hematocrit |
| K | Potassium |
| LME | Linear mixed-effects (regression) |
| LDL-C | Low-density lipoprotein cholesterol |
| LDH | Lactate dehydrogenase |
| LMR | Lymphocyte-to-monocyte ratio |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| Na | Sodium |
| NK | Natural killer (cells) |
| NLR | Neutrophil-to-lymphocyte ratio |
| NYT | Ninjin’yoeito |
| PBMCs | Peripheral blood mononuclear cells |
| PDW | Platelet distribution width |
| PLR | Platelet-to-lymphocyte ratio |
| PLT | Platelet count |
| RDW | Red cell distribution width |
| RSST | Repetitive saliva swallowing test |
| SBP | Systolic blood pressure |
| T-Bil | Total bilirubin |
| TG | Triglycerides |
| VAS | Visual analogue scale |
| WBC | White blood cell |
| γ-GTP | Gamma-glutamyl transpeptidase |
References
- Muto, G.; Yokoyama, K.; Endo, M. Solutions against declining birthrates confronting Japan’s aging society by supporting female workers in harmonizing work with their health and social issues: Fertility, chronic illness, and raising children. Nihon Eiseigaku Zasshi 2018, 73, 200–209. [Google Scholar] [CrossRef]
- Radwan-Oczko, M.; Bandosz, K.; Rojek, Z.; Owczarek-Drabińska, J.E. Clinical study of oral mucosal lesions in the elderly—Prevalence and distribution. Int. J. Environ. Res. Public Health 2022, 19, 2853. [Google Scholar] [CrossRef]
- Watanabe, Y.; Okada, K.; Kondo, M.; Matsushita, T.; Nakazawa, S.; Yamazaki, Y. Oral health for achieving longevity. Geriatr. Gerontol. Int. 2020, 20, 526–538. [Google Scholar] [CrossRef]
- Jensen, G.L.; McGee, M.; Binkley, J. Nutrition in the elderly. Gastroenterol. Clin. N. Am. 2001, 30, 313–334. [Google Scholar] [CrossRef]
- de Souto Barreto, P.; Cesari, M.; Morley, J.; Roberts, S.; Landi, F.; Cederholm, T.; Rolland, Y.; Vellas, B.; Fielding, R. Appetite loss and anorexia of aging in clinical care: An ICFSR task force report. J. Frailty Aging 2022, 11, 129–134. [Google Scholar] [CrossRef]
- Walls, A.; Steele, J. The relationship between oral health and nutrition in older people. Mech. Ageing Dev. 2004, 125, 853–857. [Google Scholar] [CrossRef]
- Xu, F.; Laguna, L.; Sarkar, A. Aging-related changes in quantity and quality of saliva: Where do we stand in our understanding? J. Texture Stud. 2019, 50, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Mojon, P.; Budtz-Jørgensen, E.; Rapin, C.-H. Relationship between oral health and nutrition in very old people. Age Ageing 1999, 28, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, E.; Toniolo, L. Nutrition, diet and healthy aging. Nutrients 2021, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Turnheim, K. Drug therapy in the elderly. Exp. Gerontol. 2004, 39, 1731–1738. [Google Scholar] [CrossRef]
- Buranarom, N.; Komin, O.; Matangkasombut, O. Hyposalivation, oral health, and Candida colonization in independent dentate elders. PLoS ONE 2020, 15, e0242832. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam-MacDonald, A.M.; Barbon, C.E.; Steele, C.M. A review of swallow timing in the elderly. Physiol. Behav. 2018, 184, 12–26. [Google Scholar] [CrossRef]
- Montecino-Rodriguez, E.; Berent-Maoz, B.; Dorshkind, K. Causes, consequences, and reversal of immune system aging. J. Clin. Investig. 2013, 123, 958–965. [Google Scholar] [CrossRef]
- Haynes, L. Aging of the immune system: Research challenges to enhance the health span of older adults. Front. Aging 2020, 1, 602108. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Nonaka, M.; Uzu, M.; Ohshima, K.; Uezono, Y. Multifunctional actions of ninjinyoeito, a Japanese Kampo medicine: Accumulated scientific evidence based on experiments with cells and animal models, and clinical studies. Front. Nutr. 2018, 5, 93. [Google Scholar] [CrossRef]
- Nakae, H.; Hiroshima, Y.; Hebiguchi, M. Kampo medicines for frailty in locomotor disease. Front. Nutr. 2018, 5, 31. [Google Scholar] [CrossRef]
- Lee, H.-G.; Arai, I.; Kwon, S. A Herbal Prescription of Insamyangyeongtang as a Therapeutic Agent for Frailty in Elderly: A Narrative Review. Nutrients 2024, 16, 721. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Yamada, A.; Saitou, M. Effect of Ninjin-Youei-tou on xerostomia induced by oxybutynin hydrochloride. Shinyaku Rinsho 1994, 43, 2613–2617. [Google Scholar]
- Yamaguchi, K.; Mukai, H.; Hamada, T.; Sugihara, K. Three cases report of intraoral discomforts with after cancer treatment successfully treated with Ninjinyoeito (TJ108). Pain Kampo Med. 2012, 22, 56–62. [Google Scholar]
- Kiyomi, A.; Matsuda, A.; Nara, M.; Yamazaki, K.; Imai, S.; Sugiura, M. Immunological differences in human peripheral blood mononuclear cells treated with traditional Japanese herbal medicines hochuekkito, juzentaihoto, and ninjin’yoeito from different pharmaceutical companies. Evid. Based Complement. Alternat. Med. 2021, 2021, 7605057. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Matsuura, K.; Gao, P.; Hottenbacher, L.; Tokunaga, H.; Nishimura, K.; Imazu, Y.; Reissenweber, H.; Witt, C.M. Traditional Japanese Kampo medicine: Clinical research between modernity and traditional medicine—The state of research and methodological suggestions for the future. Evid. Based Complement. Alternat. Med. 2011, 2011, 513842. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Michihara, S.; Kimura, Y.; Morinaga, A.; Miyakawa, K.; Tsushima, N.; Otani, K.; Jinnai, A.; Aso, Y.; Okabayashi, A.J.T.; et al. Review of frequently used Kampo prescriptions: Part 4, Ninjin’yoeito. Tradit. Kampo Med. 2023, 10, 224–252. [Google Scholar] [CrossRef]
- Japan Society for Oriental Medicine (JSOM). Evidence-Based Clinical Practice Guidelines for Kampo Medicine 2021. Available online: https://www.jsom.or.jp/medical/ebm/cpg/index.html (accessed on 13 December 2025).
- Nomura, Y.; Ishii, Y.; Chiba, Y.; Suzuki, S.; Suzuki, A.; Suzuki, S.; Morita, K.; Tanabe, J.; Yamakawa, K.; Ishiwata, Y. Structure and validity of questionnaire for oral frail screening. Healthcare 2021, 9, 45. [Google Scholar] [CrossRef]
- Lyu, W.; Tanaka, T.; Son, B.-K.; Yoshizawa, Y.; Akishita, M.; Iijima, K. Validity of a simple self-reported questionnaire “Eleven-Check” for screening of frailty in Japanese community-dwelling older adults: Kashiwa cohort study. Arch. Gerontol. Geriatr. 2024, 117, 105257. [Google Scholar] [CrossRef]
- Oguchi, K.; Saitoh, E.; Baba, M.; Kusudo, S.; Tanaka, T.; Onogi, K. The repetitive saliva swallowing test (RSST) as a screening test of functional dysphagia (2) validity of RSST. Jpn. J. Rehabil. Med. 2000, 37, 383–388. [Google Scholar] [CrossRef]
- Okada, K.-I.; Kawai, M.; Hirono, S.; Miyazawa, M.; Kitahata, Y.; Kobayashi, R.; Ueno, M.; Hayami, S.; Shimokawa, T.; Yamaue, H. Impact of Ninjin’Yoeito on fatigue in patients receiving nab-paclitaxel plus gemcitabine therapy: A prospective, single-arm, phase II open label, nonrandomized, historically-controlled study. Curr. Ther. Res. 2020, 93, 100605. [Google Scholar] [CrossRef]
- Yang, J.; Shin, K.-M.; Abu Dabrh, A.M.; Bierle, D.M.; Zhou, X.; Bauer, B.A.; Mohabbat, A.B. Ginseng for the treatment of chronic fatigue syndrome: A systematic review of clinical studies. Glob. Adv. Health Med. 2022, 11, 2164957X221079790. [Google Scholar] [CrossRef] [PubMed]
- Uehara, S.; Ogawa, K.; Arimitsu, J.; Okuyama, H. “Ninjinto”(ginseng decoction), a traditional Japanese herbal medicine, improves gastrointestinal symptoms and immune competence in patients with chronic intestinal failure. Evid. Based Complement. Alternat. Med. 2015, 2015, 462586. [Google Scholar] [CrossRef]
- Ogawa-Ochiai, K.; Kawasaki, K. Panax ginseng for frailty-related disorders: A review. Front. Nutr. 2018, 5, 140. [Google Scholar] [CrossRef]
- Ruqiao, L.; Yueli, C.; Xuelan, Z.; Huifen, L.; Xin, Z.; Danjie, Z.; Le, S.; Yanxue, Z. Rhizoma Atractylodis macrocephalae: A review of photochemistry, pharmacokinetics and pharmacology. Die Pharm. 2020, 75, 42–55. [Google Scholar]
- Kim, Y.-J.; Yoo, S.-R.; Chae, C.-K.; Jung, U.J.; Choi, M.-S. Omija fruit extract improves endurance and energy metabolism by upregulating PGC-1 α expression in the skeletal muscle of exercised rats. J. Med. Food 2014, 17, 28–35. [Google Scholar] [CrossRef]
- Chi, A.; Zhang, Y.; Kang, Y.; Shen, Z. Metabolic mechanism of a polysaccharide from Schisandra chinensis to relieve chronic fatigue syndrome. Int. J. Biol. Macromol. 2016, 93, 322–332. [Google Scholar] [CrossRef]
- Yamaguchi, K. Traditional Japanese herbal medicines for treatment of odontopathy. Front. Pharmacol. 2015, 6, 176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Almotairy, N.; Merzo, J.J.; Wendin, K.; Rothenberg, E.; Grigoriadis, A.; Sandborgh-Englund, G.; Trulsson, M. Chewing and its influence on swallowing, gastrointestinal and nutrition-related factors: A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 63, 11987–12017. [Google Scholar] [CrossRef]
- Ney, D.M.; Weiss, J.M.; Kind, A.J.; Robbins, J. Senescent swallowing: Impact, strategies, and interventions. Nutr. Clin. Pract. 2009, 24, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Yousufuddin, M.; Young, N. Aging and ischemic stroke. Aging 2019, 11, 2542. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Hayes, M. Influence of age and health behaviors on stroke risk: Lessons from longitudinal studies. J. Am. Geriatr. Soc. 2010, 58, S325–S328. [Google Scholar] [CrossRef]
- González-Fernández, M.; Ottenstein, L.; Atanelov, L.; Christian, A.B. Dysphagia after stroke: An overview. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 187–196. [Google Scholar] [CrossRef]
- Allez, M.; Tieng, V.; Nakazawa, A.; Treton, X.; Pacault, V.; Dulphy, N.; Caillat–Zucman, S.; Paul, P.; Gornet, J.M.; Douay, C. CD4+ NKG2D+ T cells in Crohn’s disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology 2007, 132, 2346–2358. [Google Scholar] [CrossRef]
- Waschbisch, A.; Sammet, L.; Schröder, S.; Lee, D.; Barrantes-Freer, A.; Stadelmann, C.; Linker, R.A. Analysis of CD4+ CD8+ double-positive T cells in blood, cerebrospinal fluid and multiple sclerosis lesions. Clin. Exp. Immunol. 2014, 177, 404–411. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Alonso-Arias, R.; Moro-García, M.A.; López-Vázquez, A.; Rodrigo, L.; Baltar, J.; García, F.M.S.; Jaurrieta, J.J.S.; López-Larrea, C. NKG2D expression in CD4+ T lymphocytes as a marker of senescence in the aged immune system. Age 2011, 33, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Azimi, N.; Jacobson, S.; Tanaka, Y.; Corey, L.; Groh, V.; Spies, T. Immunostimulation by induced expression of NKG2D and its MIC ligands in HTLV-1-associated neurologic disease. Immunogenetics 2006, 58, 252–258. [Google Scholar] [CrossRef]
- Duftner, C.; Goldberger, C.; Falkenbach, A.; Würzner, R.; Falkensammer, B.; Pfeiffer, K.P.; Maerker-Hermann, E.; Schirmer, M. Prevalence, clinical relevance and characterization of circulating cytotoxic CD4+ CD28-T cells in ankylosing spondylitis. Arthritis Res. Ther. 2003, 5, R292–R300. [Google Scholar] [CrossRef]
- Appay, V.; Sauce, D.; Prelog, M. The role of the thymus in immunosenescence: Lessons from the study of thymectomized individuals. Aging 2010, 2, 78. [Google Scholar] [CrossRef]
- Koch, S.; Larbi, A.; Derhovanessian, E.; Özcelik, D.; Naumova, E.; Pawelec, G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Smythe, K.; Dai, Z.; Spies, T. Fas ligand–mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nat. Immunol. 2006, 7, 755–762. [Google Scholar] [CrossRef]
- Takaku, S.; Shimizu, M.; Takahashi, H. Japanese Kampo medicine ninjin’yoeito synergistically enhances tumor vaccine effects mediated by CD8+ T cells. Oncol. Lett. 2017, 13, 3471–3478. [Google Scholar] [CrossRef]
- Zhu, T.; Lin, Z.; Han, S.; Wei, Y.; Lu, G.; Zhang, Y.; Xiao, W.; Wang, Z.; Jia, X.; Gong, W. Low miR-16 expression induces regulatory CD4+ NKG2D+ T cells involved in colorectal cancer progression. Am. J. Cancer Res. 2021, 11, 1540. [Google Scholar] [PubMed]
- Teoh, C.X.W.; Thng, M.; Lau, S.; Taing, M.-W.; Chaw, S.Y.; Siskind, D.; Kisely, S. Dry mouth effects from drugs used for depression, anxiety, schizophrenia and bipolar mood disorder in adults: Systematic review. BJPsych Open 2023, 9, e53. [Google Scholar] [CrossRef]
- Martinez-Acitores, L.R.; de Azcarate, F.H.R.; Casañas, E.; Serrano, J.; Hernandez, G.; López-Pintor, R.M. Xerostomia and salivary flow in patients taking antihypertensive drugs. Int. J. Environ. Res. Public Health 2020, 17, 2478. [Google Scholar] [CrossRef] [PubMed]
- Teare, J.; Spedding, C.; Whitehead, M.; Greenfield, S.; Challacombe, S.; Thompson, R. Omeprazole and dry mouth. Scand. J. Gastroenterol. 1995, 30, 216–218. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.