Abstract
Background and Objectives: Dichrostachys glomerata and Cissus quadrangularis, two species traditionally used in Cameroon, are recognized for their weight-reducing potential. This study examined the effects of standardized extracts of these botanicals on glucagon-like peptide-1 (GLP-1), dipeptidyl peptidase-4 (DPP-4), and key metabolic outcomes in individuals with excess body weight. Materials and Methods: In this 16-week, randomized, double-blind, placebo-controlled trial, 248 adults (126 women and 122 men; mean age 41.3 ± 0.3 years; BMI 25–34.9 kg/m2) were assigned to receive 400 mg D. glomerata extract (DGE), 300 mg C. quadrangularis extract (CQE), semaglutide (dose-escalated from 3 mg to 14 mg), or placebo, administered once daily. These are all standard clinical regimens. Primary assessments included changes in GLP-1 levels and DPP-4 activity. Secondary evaluations included body composition, caloric intake, satiety response, fasting glucose levels, and lipid profiles. Results: Participants receiving DGE or CQE displayed notable elevations in circulating GLP-1 (+38.6 pg/mL and +42.2 pg/mL, respectively; p < 0.01) and significant reductions in DPP-4 activity (−15.3% and −17.8%; p < 0.01) compared with placebo. Both extracts produced substantial improvements in body weight (−5.2% and −5.8%), body fat (−10.3% and −10.9%), energy intake (−16.2% and −17.5%), and satiety (+25.6% and +27.4%) (p < 0.01). Significant changes in fasting glucose and serum lipid levels were also observed (p < 0.05). These responses are comparable to those of semaglutide. Moreover, GLP-1 increments showed strong negative correlations with body fat percentage (r = −0.91 to −0.92; p < 0.001) and DPP-4 activity (r = −0.97 to −0.98; p < 0.001). Conclusions: Supplementation with D. glomerata and C. quadrangularis extracts enhanced GLP-1 secretion and reduced DPP-4 activity, yielding significant benefits for body composition and metabolic parameters. These findings indicate that both botanicals are promising natural agents for managing obesity through incretin-based mechanisms.
1. Introduction
Obesity and related disorders affect more than 2.3 billion individuals globally and contribute substantially to cardiometabolic morbidity [1]. Glucagon-like peptide-1 (GLP-1) is an important incretin hormone implicated in appetite regulation and weight loss [2]. Its physiological activity is limited by the enzyme dipeptidyl peptidase-4 (DPP-4), which rapidly degrades GLP-1 and thereby contributes to the development and progression of obesity and other metabolic diseases. Pharmacological agents such as DPP-4 inhibitors (gliptins, such as sitagliptin, saxagliptin, and vildagliptin) can enhance incretin signaling, but their use may be constrained by adverse effects and cost barriers [3]. Therefore, natural alternatives with fewer adverse effects are needed.
Dichrostachys glomerata and Cissus quadrangularis, traditionally used in Africa and Asia, promote weight loss; however, their underlying mechanisms remain unclear [4]. Clinical [5] and preclinical studies [6,7,8] suggest potential roles in DPP-4 inhibition, appetite suppression, and lipid metabolism. Despite these findings, evidence on GLP-1 and DPP-4 modulation in humans is lacking and constitutes an important gap in the field. This clinical trial evaluated the effects of these regimens on GLP-1, DPP-4, and other metabolic parameters in overweight and obese adults.
2. Materials and Methods
2.1. Test Materials
Dichrostachys glomerata extract (DGE) and Cissus quadrangularis extract (CQE), which are known for their anti-obesity effect, were supplied by Gateway Health Alliances Inc. (Fairfield, CA, USA). Each extract was standardized and accompanied by a certificate of analysis (COA) confirming identity, purity, and batch quality.
- DGE: Standardized to contain ≥25% total polyphenols, expressed as gallic acid equivalents (GAE).
- CQE: Standardized to contain ≥2.5% ketosteroids and ≥15% total flavonoids, expressed as quercetin equivalents (QE).
Both extracts were manufactured under Good Manufacturing Practice (GMP) conditions. Product characteristics are reported in Table 1.

Table 1.
Test Product Details.
Quality control and phytochemical verification were conducted at the Laboratory of Nutritional Biochemistry, University of Yaoundé I, using spectrophotometric assays for total phenolics and flavonoids. Chromatographic fingerprints were generated by HPLC-DAD to ensure batch uniformity and chemical consistency.
For comparison, oral semaglutide (Rybelsus®) was purchased commercially (Novo Nordisk A/S, Bagsværd, Gladsaxe Municipality, Denmark) and repackaged into visually matched capsules. Placebo capsules contained dextrin powder (400 mg) and were indistinguishable from the active capsules in size, color, and shape to preserve blinding.
2.2. Participants and Study Design
This randomized, double-blind, placebo-controlled, parallel-group clinical trial was registered at ClinicalTrials.gov (Identifier: NCT06827002) and conducted at the National Obesity Center of the Yaounde Central Hospital and the Laboratory of Nutrition and Nutritional Biochemistry, University of Yaoundé I. Ethical approval was granted by the Institutional Review Board (IRB) of the University of Yaoundé I (Approval code: BTC-JIRB2023-084) on 26 March 2023. Prior to Ethical Approval and to being within the required funding start date, study activities were limited to non-interventional, minimal-risk preparatory procedures, including site preparation and staff training. No participant enrollment, informed consent, randomization, intervention administration, or collection of outcome data occurred during this period. This study adhered to CONSORT 2010 and SPIRIT guidelines. Written informed consent was obtained from all the participants prior to enrolment, and the study complied with the principles of the Declaration of Helsinki (revised in 2013). Participant confidentiality and data privacy were maintained throughout the study.
This randomized, double-blind, placebo-controlled, parallel-group clinical trial was conducted at the National Obesity Center of the Yaounde Central Hospital and the Laboratory of Nutrition and Nutritional Biochemistry, University of Yaoundé I, and was subsequently registered at ClinicalTrials.gov (Identifier: NCT06827002).
The sample size (n) was calculated for the primary endpoint (change in postprandial GLP-1) using a two-sided, two-sample test for differences in means. Bonferroni correction was applied for the four planned pairwise comparisons (CQE vs. placebo, CQE vs. semaglutide, DGE vs. placebo, DGE vs. semaglutide) (α′ = 0.05/4 = 0.0125). The following formula was used:
- where
n = required sample size per group;
α = nominal Type-I error (two-sided) with Z1 − α2 the corresponding normal quantile;
1 − β = desired power (80%), and Z1 − α2 was that quantile;
σ2 = variance; and
Δ = the minimum clinically meaningful difference between groups.
Allowing for a 15% dropout rate, the number of participants required per group increased to 62, yielding a total planned sample size of (n = 62 × 4) = 248.
A total of 248 overweight or obese adults (126 females and 122 males; age, 25–59 years) were recruited between March and September, 2023 through public advertisements and community health centers in Yaoundé, Cameroon.
2.3. Inclusion and Exclusion Criteria
- ➢
- Inclusion criteria
- BMI between 25–34.9 kg/m2;
- Stable body weight (±2 kg) over the previous 3 months;
- Willingness to maintain usual dietary and physical activity patterns
- ➢
- Exclusion criteria
- Diagnosed and uncontrolled diabetes mellitus;
- Recently suffered from a stroke or heart attack;
- Impaired kidney or liver function;
- Pregnancy or lactation;
- Use of weight-loss medications or herbal supplements within the previous 3 months;
- Known allergies to study materials.
2.4. Randomization
After screening, eligible participants were randomized (1:1:1:1) into four groups:
- DGE 400 mg/day (dosage based on prior literature [9]);
- CQE 300 mg/day (dosage based on prior literature [10]);
- Oral semaglutide (dose-escalation: 3 → 7 → 14 mg/day);
- Placebo (400 mg dextrin/day).
Randomization was performed by an IT technician not involved in the study using block randomization with a computer-generated random sequence (SPSS Version 26 random number generator). Allocation concealment was maintained using sequentially numbered, opaque, and sealed envelopes. Investigators and participants remained blinded to the group assignments.
2.5. Intervention Protocol
Participants consumed one capsule daily of one of the following: DGE, CQE, semaglutide or placebo for 16 consecutive weeks with water, 30 min before breakfast. Compliance was monitored through capsule counts at weeks 4, 8, 12, and 16, and through self-reported adherence logs. Missed doses were documented.
To help mitigate adverse gastrointestinal effects, semaglutide was administered following the dose-ascending protocol described by [11]. The starting dose of once-daily oral semaglutide was 3 mg (weeks 0–4), escalating to 7 mg (weeks 4–8), and then 14 mg (weeks 8–16).
2.6. Dietary and Exercise Restrictions
No specific dietary or exercise regimen was prescribed. Participants maintained their usual lifestyle patterns and refrained from initiating new nutritional supplements or medications that could affect their metabolism. Compliance with lifestyle maintenance was assessed through weekly self-reports and a review of 7-day food diaries.
2.7. Blood Sample Collection
Venous blood samples were collected at baseline (week 0) and at weeks 4, 8, 12 and 16 after an overnight fast and again 2 and 3 h postprandially. Samples were transferred into pre-chilled EDTA tubes with (PW) or without (PWO) sitagliptin. Plasma was separated by centrifugation (3000 rpm, 10 min, 4 °C) and stored at −80 °C until analysis.
2.8. Outcome Measures
2.8.1. Primary Outcomes
- GLP-1 levels: PW was used to measure active GLP-1 in fasting as well as 2 and 3 h postprandial plasma. Concentrations were measured using the RayBio® Human GLP-1 (Active) ELISA Kit (Catalog #ELH-GLP1-1, RayBiotech, Peachtree Corners, GA, USA).
- DPP-4 activity: PWO was assayed using a Human DPP-4/CD26 Immunoassay Kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions.
2.8.2. Secondary Outcomes
- Anthropometric parameters: height (m) was measured using a stadiometer, weight (kg) using a digital scale (Omron HBF-511), BMI calculated as kg/m2, and body fat (%) estimated through bioelectric impedance (HD Touch Body composition scale).
- Metabolic parameters: Fasting glucose was measured with an Accu-Chek® glucometer; total cholesterol, triglycerides, and HDL-c were measured using Roche Diagnostics kits (Mannheim, Germany); and LDL cholesterol levels were calculated using the Friedewald formula [12].
- Energy intake: Estimated from 7-day food diaries using the FAO Food Composition Tables for Cameroon. The energy intake (kcal/day) was calculated as follows:
- Satiety: Evaluated using the Visual Analogue Scale (VAS) questionnaire validated by Cazzo et al. [13].
2.9. Statistical Analysis
Data were analyzed using SPSS (version 24.0; IBM Corp., Chicago, IL, USA) and cross-validated using R version 4.3.1. Continuous variables are presented as mean ± standard error of the mean (SEM). The normality of data distributions was assessed using the Kolmogorov–Smirnov test as well as the visual inspection of Q–Q plots, and the homogeneity of variances was verified using Levene’s test. Outliers were identified as standardized residuals exceeding ±3 SD and evaluated for biological plausibility.
Between-group differences at baseline and at each time point were assessed using one-way analysis of variance (ANOVA) for normally distributed variables and the Kruskal–Wallis test for non-normal variables. Longitudinal changes were analyzed using repeated-measures ANOVA, with time (weeks 0, 4, 8, 12, and 16) as the within-subject factor and treatment group (placebo, DGE, and CQE) as the between-subject factor. Mauchly’s test assessed sphericity, and Greenhouse–Geisser corrections were applied when this assumption was violated. Post hoc pairwise comparisons were adjusted using the Benjamini–Hochberg false discovery rate (FDR) procedure.
Effect sizes were expressed as partial η2 (0.01 = small, 0.06 = medium, 0.14 = large) with corresponding 95% confidence intervals. Correlations among GLP-1, DPP-4 activity, body fat percentage, and energy intake were assessed using Pearson’s or Spearman’s correlation coefficients, as appropriate.
Predictors of the GLP-1 response and weight change were identified using multivariate linear regression analyses adjusted for baseline BMI, age, and sex. The model assumptions (linearity, homoscedasticity, multicollinearity, and normality of the residuals) were verified prior to interpretation. Standardized beta coefficients and adjusted R2 values were quantified for predictor contributions.
An a priori power analysis conducted in G*Power v3.1 indicated that a total sample size of 75 participants (25 per group) would provide 80% power to detect a medium effect size (f = 0.25) at α = 0.05 in a repeated-measures ANOVA with five time points. This sample size also provided adequate power to detect a minimum correlation coefficient of at least r = 0.30 with 95% confidence. All tests were two-tailed, and statistical significance was set at p ≤ 0.05.
3. Results
3.1. Participant Baseline Characteristics
Among the 284 individuals screened, 248 participants (126 females and 122 males; mean age = 41.3 ± 0.55 years; mean BMI = 30.7 ± 2.4 kg/m2) met the inclusion criteria and were randomized equally into four groups: DGE (400 mg/day), CQE (300 mg/day), semaglutide (oral, 3–14 mg/day), or placebo (400 mg dextrin). A total of 228 participants (91.9%) completed the 16-week intervention (Figure 1). Withdrawals were as a result of loss to follow-up (n = 5), stomach and general discomfort (n = 6), and non-compliance with capsule intake (n = 9). The baseline demographic and metabolic characteristics did not differ significantly across groups (Table 2).
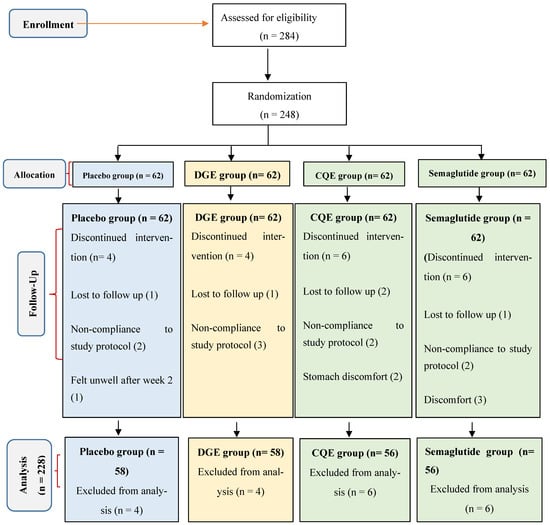
Figure 1.
CONSORT flow diagram for clinical trial.

Table 2.
Demographic and baseline characteristics of participants.
3.2. Effects on Circulating GLP-1 and DPP-4 Activity
Postprandial GLP-1 concentrations increased significantly from baseline in all active treatment groups compared with placebo (time × treatment interaction, p < 0.001). At week 16, mean GLP-1 levels at three hours postprandial rose by +38.6 pg/mL in the DGE group, +42.2 pg/mL in the CQE group, and +46.8 pg/mL in the semaglutide group, compared with +4.7 pg/mL in the placebo group (Table 3). Similar patterns were observed at two hours postprandial. Post hoc Tukey tests confirmed significant differences between the DGE/CQEs and placebo groups (p < 0.01), while no significant differences were detected between the botanical groups and semaglutide (p > 0.05).

Table 3.
The effect of DGE and CQE on GLP-1 level and DPP4 Activity.
DPP-4 enzymatic activity decreased significantly in the DGE (−15.3%) and CQE (−17.8%) groups relative to placebo (−2.9%, p < 0.001), with the semaglutide group exhibiting the highest reduction (−23.5%) (Table 3).
3.3. Anthropometric and Metabolic Parameters
3.3.1. Anthropometric Parameters
Body weight decreased significantly after 16 weeks in the DGE (−4.3 kg) and CQE (−4.7 kg) and semaglutide (−4.8 kg) groups compared with placebo (−0.7 kg, p < 0.05). Correspondingly, BMI and body fat percentage declined significantly in all active treatment groups (p > 0.05) (Table 4).

Table 4.
The effect of DGE and CQE on anthropometric parameters.
3.3.2. Metabolic Parameters
Fasting glucose and triglyceride levels decreased significantly in all active treatment groups relative to placebo group (Table 5). LDL-C levels exhibited modest reductions and HDL-C levels modest increases in the DGE and CQE groups; however, these changes were not statistically significant after adjustment for multiple comparisons (adjusted p > 0.05) (Table 5).

Table 5.
The effect of DGE and CQE on metabolic parameters.
3.4. Energy Intake and Satiety Scores
Daily energy intake declined significantly from baseline values in all groups, but with larger reductions observed in the DGE (−470.2 kcal/day) and CQE (−513.8 kcal/day) groups compared with placebo (−92 kcal/day, p < 0.05). VAS-based satiety scores increased by +25.6% in the DGE group and +27.4% in the CQE groups, compared with +5.3% in the placebo group (p < 0.01) (Table 6). Exploratory analysis indicated positive correlations between changes in satiety and GLP-1 levels. GLP-1 levels were negatively correlated with DPP-4 activity (Table 7).

Table 6.
The effect of DGE and CQE on energy intake and Satiety.

Table 7.
Correlation matrix between GLP-1, DPP-4, body fat, and energy intake in treated groups at week 16.
3.5. Safety and Tolerability
No serious adverse events were observed. Mild gastrointestinal discomfort occurred primarily at the beginning of the intervention in the semaglutide group and in less than 3% of participants in the DGE and CQE groups. All events resolved without requiring discontinuation.
4. Discussion
This randomized controlled trial demonstrated that supplementation with DGEs and CQEs significantly enhanced the incretin axis in overweight and obese adults, as evidenced by increased circulating GLP-1 concentrations and reduced DPP-4 activity. These endocrine effects translated into clinically meaningful improvements, including reductions in body weight, body fat, energy intake, as well as enhanced satiety and favorable changes in lipid profile and fasting blood glucose. Collectively, these findings extend prior in vitro and preclinical evidence indicating that polyphenol-rich extracts modulate incretin signaling [14].
The dual mechanism of stimulating GLP-1 secretion and inhibiting its enzymatic degradation through DPP-4 suppression is characteristic of polyphenol-rich plant extracts. The flavonoid glycosides and related phytochemicals in DGE and CQE likely act as allosteric or competitive inhibitors of DPP-4, thereby prolonging the half-life of endogenous GLP-1 [15]. These actions operate in parallel, although at a nutraceutical scale, to the pharmacodynamics of synthetic GLP-1 receptor agonists and DPP-4 inhibitors such as semaglutide and sitagliptin. The degree of GLP-1 elevation observed in this trial suggests partial engagement of comparable signaling pathways. Moreover, accumulating evidence indicates that CQE polyphenols may directly stimulate GLP-1 secretion from intestinal L-cells through AMPK activation and intracellular calcium mobilization [16,17].
The reductions in body weight and adiposity likely reflected both incretin-mediated appetite regulation and the direct metabolic effects of these extracts. GLP-1 enhances satiety through hypothalamic signaling, which aligns with the increased VAS satiety scores and decreased energy intake observed in this study. CQE constituents also exert peripheral antiadipogenic and lipolytic effects. For instance, a lupenone-rich CQE fraction suppressed lipid accumulation in 3T3-L1 adipocytes [5], while a human trial demonstrated CQE-induced upregulation of uncoupling protein (UCP) mRNA, indicating increased white adipose tissue browning [18]. Browning enhances energy expenditure and fat oxidation, thereby contributing to reductions in central adiposity.
These metabolic benefits may be further potentiated by the anti-inflammatory properties of these extracts. Cissus quadrangularis inhibits key pro-inflammatory pathways through specific pharmacophores [19]. By attenuating chronic low-grade inflammation mediated by cytokines such as TNF-α and IL-6—drivers of insulin resistance and adipose dysfunction—CQE fosters a metabolically favorable environment. This anti-inflammatory shift enhances insulin signaling and promotes the transition of the adipose tissue toward oxidative rather than storage functions, thereby reinforcing improvements in both body composition and glucose homeostasis.
The improvements in lipid profile and reductions in total cholesterol, triglycerides, and LDL-C align with known roles of GLP-1 in lipid metabolism, including the inhibition of hepatic lipogenesis and stimulation of lipoprotein lipase activity. Similar lipid-lowering effects have been reported in other clinical trials evaluating weight-loss supplementation [20]. The moderate but significant reduction in fasting glucose can be attributed to the combined effects of enhanced insulin secretion through GLP-1 signaling, improved insulin sensitivity associated with weight loss, and the β-cell protective properties of CQE [17].
Additionally, reductions in fasting glucose may result from the direct inhibition of carbohydrate-digesting enzymes. CQE was recently identified as a potent inhibitor of α-amylase and α-glucosidase [17]. Inhibiting these enzymes delays carbohydrate digestion and glucose absorption, reduces postprandial glycemic excursions, and lowers overall glycemic load. This mechanism complements incretin-mediated insulin enhancement, providing a multifaceted phytotherapeutic approach to glycemic control.
These findings are consistent with prior clinical trials demonstrating the anti-obesity and metabolic effects of Cissus quadrangularis [10,20] and Dichrostachys glomerata [4,15]. The magnitude of weight loss and metabolic improvement observed is consistent with prior reports, reinforcing the reproducibility of these effects. However, variations in extract standardization, duration, and dosage underscore the need for uniform formulations to optimize translational outcomes.
Despite these promising findings, this study has several limitations. The most significant of these is the asymmetric dosing protocols. We compared fixed doses of herbal extracts to a titrated, maximally effective dose of semaglutide. This design, while pragmatic, prevents a direct pharmacological comparison of the intrinsic efficacy or potency of the compounds. It cannot determine whether the tested doses of DGE or CQE represent their optimal efficacy (D_max), nor can it establish milligram-for-milligram equivalence. Comparability claims pertain specifically to the tested regimens, not to the agents in principle. Also, the 16-week duration restricts inferences regarding long-term adaptations to sustained GLP-1 elevation. Additionally, the study included overweight but nondiabetic adults, limiting generalizability to individuals with diabetes or normal BMI. Future studies should incorporate mechanistic endpoints, such as adipocyte gene expression, insulin sensitivity indices, and AMPK activation assays, to delineate the molecular pathways linking incretin modulation to metabolic outcomes.
5. Conclusions
In this 16-week trial, a fixed daily dose of DGE (400 mg) or CQE (300 mg) enhanced GLP-1 bioavailability and signaling, leading to significant improvements in body composition, appetite regulation, and metabolic health comparable to the standard clinical titration regimen of oral semaglutide (3 → 7 → 14 mg) as measured at the study endpoints. Beyond incretin modulation, these extracts may directly influence adipose tissue browning, lipid oxidation, and anti-inflammatory activity, offering a comprehensive nutraceutical approach to obesity management. Their multi-targeted biochemical effects warrant long-term, mechanistic studies to establish standardized and clinically validated formulations.
Author Contributions
Conceptualization, J.O. and G.T.; methodology, J.Y. and C.A.; software, J.Y.; validation, R.M., M.A. and J.O.; formal analysis, M.A.; investigation, C.A. and F.N.; data curation, I.M.; writing—original draft preparation, J.O.; writing—review and editing, J.Y. and M.A.; visualization, G.T.; supervision, J.O.; project administration, J.O.; funding acquisition, R.M. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Cameroon Nutrition and Dietetic Research Center, of the J&A Oben Foundation with the Grant ID-JAOF 122025).
Institutional Review Board Statements
Ethical approval was granted by the Institutional Review Board (IRB) of the University of Yaoundé I (Approval code: BTC-JIRB2023-084), date of approval: 26 March 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data utilized in this study is available upon reasonable request from the corresponding author.
Acknowledgments
The authors would like to thank all the participants of the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body Mass Index |
| DPP4 | Dipeptidyl Peptidase 4 |
| GLP-1 | Glucagon-Like Peptide-1 |
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 February 2024).
- Van Bloemendaal, L.; Ten Kulve, J.S.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1–T16. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, M.; Kumar, S.; Sood, A.K. Acute pancreatitis with gliptins: Is it a clinical reality? Indian J. Endocrinol. Metab. 2013, 17, S323–S325. [Google Scholar] [CrossRef] [PubMed]
- Youovop, J.; Takuissu, G.; Mbopda, C.; Nwang, F.; Ntentié, R.; Mbong, M.; Azantsa, B.; Singh, H.; Oben, J. The effects of DGE (Dichrostachys glomerata extract) on body fat percentage and body weight: A randomized, double-blind, placebo-controlled clinical trial. Funct. Foods Health Dis. 2023, 13, 334–346. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Cissus quadrangularis enhances UCP1 mRNA, indicative of white adipocyte browning and decreases central obesity in humans in a randomized trial. Sci. Rep. 2021, 11, 2008. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Lee, S.K.; Min, D.E.; Choi, B.K.; Lee, D.R. Anti-obesity effect of Dyglomera® is associated with activation of the AMPK signaling pathway in 3T3-L1 adipocytes and mice with high-fat diet-induced obesity. Molecules 2022, 27, 3288. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Le, B.; Lee, D.R.; Choi, B.K.; Yang, S.H. Cissus quadrangularis extract (CQR-300) inhibits lipid accumulation by downregulating adipogenesis and lipogenesis in 3T3-L1 cells. Toxicol. Rep. 2018, 5, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Conde, K.; Fang, S.; Xu, Y. Unraveling the serotonin saga: From discovery to weight regulation and beyond—A comprehensive scientific review. Cell Biosci. 2023, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Hausenblas, H.A.; Lynch, T.A.; Befus, S.M.; Braverman, T.L.; Hooper, S.L. Efficacy of Dichrostachys glomerata supplementation on overweight and mildly obese adults’ weight, mood, and health-related quality of life: A randomized double-blind placebo-controlled trial. J. Diet. Suppl. 2024, 21, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Kuate, D.; Nash, R.J.; Bartholomew, B.; Penkova, Y. The use of Cissus quadrangularis (CQR-300) in the management of components of metabolic syndrome in overweight and obese participants. Nat. Prod. Commun. 2015, 10, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Blundell, J.; Tetens Hoff, S.; Dahl, K.; Bauer, R.; Baekdal, T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Cazzo, E.; Pareja, J.; Chaim, E.; Coy, C.; Magro, D. Glucagon-like peptides 1 and 2 are involved in satiety modulation after modified biliopancreatic diversion: Results of a pilot study. Obes. Surg. 2017, 27, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Alkhalidy, H.; Liu, D. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef] [PubMed]
- Kuate, D.; Etoundi, B.; Ngondi, J.; Wan Muda, W.; Oben, J. Anti-inflammatory, anthropometric, and lipomodulatory effects of Dyglomera® (aqueous extract of Dichrostachys glomerata) in obese participants with metabolic syndrome. Funct. Foods Health Dis. 2013, 3, 416–427. [Google Scholar] [CrossRef]
- Paul, S.; Das, P.K.; Saha, S.; Dhiwar, P.S.; Khanra, R.; Chatterjee, A.; Zanchi, F.B. Exploration of antidiabetic and anti-apoptotic activity of Cissus quadrangularis stem through mechanistic pathway: An in vitro, in silico and in vivo approach. Appl. Biochem. Biotechnol. 2025, 197, 6507–6529. [Google Scholar] [CrossRef] [PubMed]
- Lakthan, T.; Limpachayaporn, P.; Rayanil, K.O.; Charoenpanich, P.; Phuangbubpha, P.; Charoenpanich, A. Lupenone-rich fraction derived from Cissus quadrangularis L. suppresses lipid accumulation in 3T3-L1 adipocytes. Life 2023, 13, 1724. [Google Scholar] [CrossRef] [PubMed]
- Mondal, I.; Zilani, M.N.H.; Lisany, N.F.; Yasmin, F.; Bibi, S.; Biswas, P.; Tauhida, S.J.; Rahman, M.S.; Albadrani, G.M.; Al-Ghadi, M.Q.; et al. Cissus quadrangularis revealed as a potential source of anti-inflammatory and antidiabetic pharmacophore in experimental and computational studies. Chem. Biodivers. 2025, 22, e00903. [Google Scholar] [CrossRef] [PubMed]
- Sowinski, R.J.; Grubic, T.J.; Dalton, R.L.; Schlaffer, J.; Reyes-Elrod, A.G.; Jenkins, V.M.; Williamson, S.; Rasmussen, C.; Murano, P.S.; Earnest, C.P.; et al. An examination of a novel weight loss supplement on anthropometry and indices of cardiovascular disease risk. J. Diet. Suppl. 2021, 18, 478–506. [Google Scholar] [CrossRef] [PubMed]
- Sawangjit, R.; Puttarak, P.; Saokaew, S.; Chaiyakunapruk, N. Efficacy and safety of Cissus quadrangularis L. in clinical use: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2017, 31, 555–567. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.