The Prophylactic and Therapeutic Use of the Heli-FX EndoAnchor System in Patients Undergoing Endovascular Aortic Aneurysm Repair—A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Definitions
2.5. Risk of Bias Assessment
2.6. Data Analysis
2.7. Ethics
3. Results
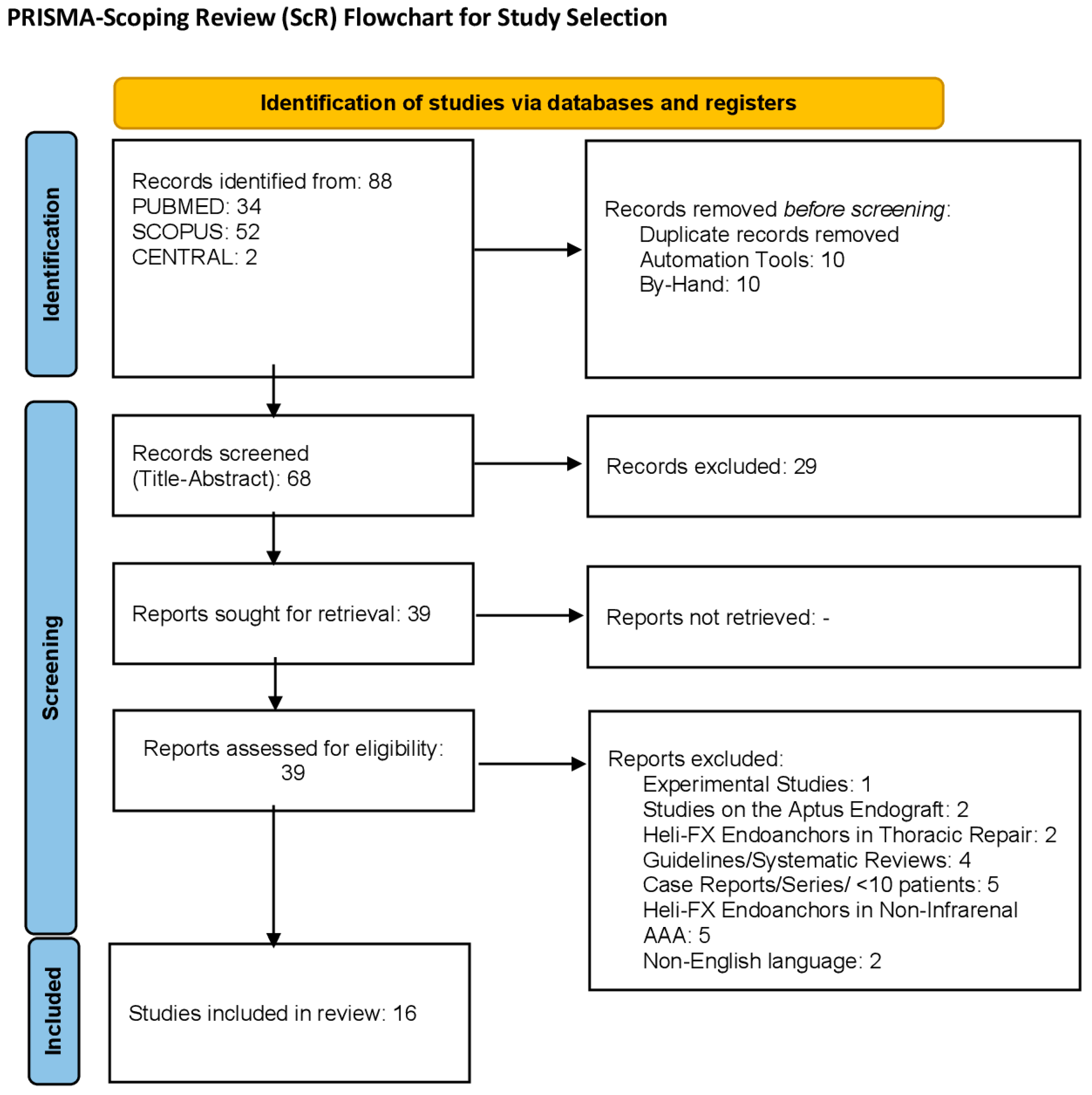
3.1. Study Selection
3.2. Study Population
3.3. Morphological Features
3.4. Technical Outcomes
3.5. Quality Assessment
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paravastu, S.C.; Jayarajasingam, R.; Cottam, R.; Palfreyman, S.J.; Michaels, J.A.; Thomas, S.M. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014, CD004178. [Google Scholar] [CrossRef] [PubMed]
- Kontopodis, N.; Gavalaki, A.; Galanakis, N.; Kantzas, M.; Ioannou, C.; Geroulakos, G.; Kakisis, J.; Antoniou, G.A. Systematic Review with Meta-Analysis of Endovascular Versus Open Repair of Abdominal Aortic Aneurysm Repair in the Young. J. Endovasc. Ther. 2023, 32, 276–289. [Google Scholar] [CrossRef]
- Pitoulias, G.A.; Valdivia, A.R.; Hahtapornsawan, S.; Torsello, G.; Pitoulias, A.G.; Austermann, M.; Gandarias, C.; Donas, K.P. Conical neck is strongly associated with proximal failure in standard endovascular aneurysm repair. J. Vasc. Surg. 2017, 66, 1686–1695. [Google Scholar] [CrossRef]
- Nana, P.; Spanos, K.; Kouvelos, G.; Arnaoutoglou, E.; Giannoukas, A.; Matsagkas, M. Conical Aortic Neck as a Predictor of Outcome after Endovascular Aneurysm Exclusion: Midterm Results. Ann. Vasc. Surg. 2023, 90, 77–84. [Google Scholar] [CrossRef]
- Karaolanis, G.; Antonopoulos, C.N.; Koutsias, S.; Antoniou, G.A.; Beropoulis, E.; Torsello, G.; Taneva, G.T.; Donas, K.P. A Systematic Review of the Use of Endoanchors in Endovascular Aortic Aneurysm Repair. Vascular 2020, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Qamhawi, Z.; Barge, T.F.; Makris, G.C.; Patel, R.; Wigham, A.; Anthony, S.; Uberoi, R. Systematic Review of the Use of Endoanchors in Endovascular Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 748–756. [Google Scholar] [CrossRef]
- de Vries, J.P.; Ouriel, K.; Mehta, M.; Varnagy, D.; Moore, W.M., Jr.; Arko, F.R.; Joye, J.; Jordan, W.D., Jr. Global Registry ANCHOR Trial. Analysis of EndoAnchors for endovascular aneurysm repair by indications for use. J. Vasc. Surg. 2014, 60, 1460–1467.e1. [Google Scholar] [CrossRef]
- Jordan, W.D., Jr.; Mehta, M.; Ouriel, K.; Arko, F.R.; Varnagy, D.; Joye, J.; Moore, W.M., Jr.; de Vries, J.P. One-year results of the ANCHOR trial of EndoAnchors for the prevention and treatment of aortic neck complications after endovascular aneurysm repair. Vascular 2016, 24, 177–186. [Google Scholar] [CrossRef]
- Goudeketting, S.R.; van Noort, K.; Ouriel, K.; Jordan, W.D., Jr.; Panneton, J.M.; Slump, C.H.; de Vries, J.P.M. Influence of aortic neck characteristics on successful aortic wall penetration of EndoAnchors in therapeutic use during endovascular aneurysm repair. J. Vasc. Surg. 2018, 68, 1007–1016. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Badawy, A. Endograft platform does not influence aortic neck dilatation after infrarenal endovascular aneurysm repair with primary endostapling. Vascular 2021, 29, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Deaton, D.H.; Mehta, M.; Kasirajan, K.; Chaikof, E.; Farber, M.; Glickman, M.H.; Neville, R.F.; Fairman, R.M. The phase I multicenter trial (STAPLE-1) of the Aptus endovascular repair system: Results at 6 months and 1 year. J. Vasc. Surg. 2009, 49, 851–857; discussion 857–858. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 6.4 (updated August 2023); Cochrane: London, UK, 2023. [Google Scholar]
- Jordan, W.D., Jr.; Mehta, M.; Varnagy, D.; Moore, W.M., Jr.; Arko, F.R.; Joye, J.; Ouriel, K.; de Vries, J.-P. Results of the ANCHOR prospective, multicenter registry of EndoAnchors for type Ia endoleaks and endograft migration in patients with challenging anatomy. J. Vasc. Surg. 2014, 60, 885–892. [Google Scholar] [CrossRef]
- Oderich, G.S.; Forbes, T.L.; Chaer, R.; Davies, M.G.; Lindsay, T.F.; Mastracci, T.; Singh, M.J.; Timaran, C.; Woo, E.Y. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J. Vasc. Surg. 2021, 73, 4S52S. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Blankensteijn, J.D.; Harris, P.L.; White, G.H.; Zarins, C.K.; Bernhard, V.M.; Matsumura, J.S.; May, J.; Veith, F.J.; Fillinger, M.F.; et al. Reporting standards for endovascular aortic aneurysm repair. J. Vasc. Surg. 2002, 35, 1048–1060. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Avci, M.; Vos, J.A.; Kolvenbach, R.R.; Verhoeven, E.L.; Perdikides, T.; Resch, T.A.; Espinosa, G.; Böckler, D.; De Vries, J.P. The use of endoanchors in repair EVAR cases to improve proximal endograft fixation. J. Cardiovasc. Surg. 2012, 53, 419–426. [Google Scholar]
- Perdikides, T.; Melas, N.; Lagios, K.; Saratzis, A.; Siafakas, A.; Bountouris, I.; Kouris, N.; Avci, M.; Van den Heuvel, D.A.; de Vries, J.P. Primary endoanchoring in the endovascular repair of abdominal aortic aneurysms with an unfavorable neck. J. Endovasc. Ther. 2012, 19, 707–715. [Google Scholar] [CrossRef]
- Goudeketting, S.R.; Wille, J.; van den Heuvel, D.A.F.; Vos, J.A.; de Vries, J.P.M. Midterm Single-Center Results of Endovascular Aneurysm Repair with Additional EndoAnchors. J. Endovasc. Ther. 2019, 26, 90–100. [Google Scholar] [CrossRef]
- Giudice, R.; Borghese, O.; Sbenaglia, G.; Coscarella, C.; De Gregorio, C.; Leopardi, M.; Pogany, G. The use of EndoAnchors in endovascular repair of abdominal aortic aneurysms with challenging proximal neck: Single-centre experience. JRSM Cardiovasc Dis. 2019, 8, 2048004019845508. [Google Scholar] [CrossRef]
- Reyes Valdivia, A.; Beropoulis, E.; Pitoulias, G.; Pratesi, G.; Alvarez Marcos, F.; Barbante, M.; Gandarias, C.; Torsello, G.; Bisdas, T.; Donas, K. Multicenter Registry about the Use of EndoAnchors in the Endovascular Repair of Abdominal Aortic Aneurysms with Hostile Neck Showed Successful but Delayed Endograft Sealing within Intraoperative Type Ia Endoleak Cases. Ann. Vasc. Surg. 2019, 60, 61–69. [Google Scholar] [CrossRef]
- Ho, V.T.; George, E.L.; Dua, A.; Lavingia, K.S.; Sgroi, M.D.; Dake, M.D.; Lee, J.T. Early Real-World Experience with EndoAnchors by Indication. Ann. Vasc. Surg. 2020, 62, 30–34. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Kim, H.K.; Valdivia, A.R. Improved Midterm Outcomes Using Standard Devices and EndoAnchors for Endovascular Repair of Abdominal Aortic Aneurysms with Hyperangulated Necks. Cardiovasc. Interv. Radiol. 2020, 43, 971–980. [Google Scholar] [CrossRef]
- Reyes Valdivia, A.; Chaudhuri, A.; Milner, R.; Pratesi, G.; Reijnen, M.M.; Tinelli, G.; Schuurmann, R.; Barbante, M.; Babrowski, T.A.; Pitoulias, G.; et al. Endovascular aortic repair with EndoAnchors demonstrate good mid-term outcomes in physician-initiated multicenter analysis-The PERU registry. Vascular 2022, 30, 27–37. [Google Scholar] [CrossRef]
- Bordet, M.; Oliny, A.; Miasumu, T.; Tresson, P.; Lermusiaux, P.; Della Schiava, N.; Millon, A. EndoSuture aneurysm repair versus fenestrated aneurysm repair in patients with short neck abdominal aortic aneurysm. J. Vasc. Surg. 2023, 77, 28–36.e3. [Google Scholar] [CrossRef] [PubMed]
- Arko, F.R., 3rd; Pearce, B.J.; Henretta, J.P.; Fugate, M.W.; Torsello, G.; Panneton, J.M.; Peng, Y.; Edward Garrett, H., Jr. Five-year outcomes of endosuture aneurysm repair in patients with short neck abdominal aortic aneurysm from the ANCHOR registry. J. Vasc. Surg. 2023, 78, 1418–1425.e1. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hadi, O.; Zhong, J.; Tingerides, C.; Shaw, D.; McPherson, S.; Puppala, S.; Walker, P. Midterm Outcomes of Primary and Secondary Use of an Endoanchor System for Thoracic and Abdominal Aortic Endovascular Aortic Repair. J. Vasc. Interv. Radiol. 2023, 34, 1938–1945. [Google Scholar] [CrossRef]
- Reyes Valdivia, A.; Oikonomou, K.; Milner, R.; Pitoulias, A.; Reijnen, M.M.P.J.; Pfister, K.; Tinelli, G.; Csobay-Novák, C.; Pratesi, G.; Ferreira, L.M.; et al. PERU registry collaborators. Endosutured Aneurysm Repair of Abdominal Aortic Aneurysms with Short Necks Achieves Acceptable Midterm Outcomes-Results from the Peru Registry. Ann. Vasc. Surg. 2024, 106, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Sivak, J.; Suchac, M.; Daxner, M.; Kmetkova, K.; Sykora, J.; Zapletalova, J.; Zelenak, K.; Simkova, I. Use of EndoAnchors during index endovascular aortic aneurysm repair in patients with hostile proximal aortic neck anatomy. Bratisl. Lek. Listy. 2024, 125, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Melas, N.; Perdikides, T.; Saratzis, A.; Saratzis, N.; Kiskinis, D.; Deaton, D.H. Helical EndoStaples enhance endograft fixation in an experimental model using human cadaveric aortas. J. Vasc. Surg. 2012, 55, 1726–1733. [Google Scholar] [CrossRef]
- Nana, P.; Kölbel, T.; Behrendt, C.A.; Kouvelos, G.; Giannoukas, A.; Haulon, S.; Spanos, K. Systematic review of reintervention with fenestrated or branched devices after failed previous endovascular aortic aneurysm repair. J. Vasc. Surg. 2023, 77, 1806–1814.e2. [Google Scholar] [CrossRef]
- Tang, E.W.C.; Lau, A.C.K.; Cheng, J.C.H.; Wong, J.C.-Y.; Chan, Y.C. Effectiveness and Safety of Endoanchors in Abdominal and Thoracic Endovascular Aneurysm Repair: A Systematic Review and Meta-analysis. J. Endovasc. Ther. 2024; in press. [Google Scholar] [CrossRef]
- Wanhainen, A.; Van Herzeele, I.; Bastos Gonçalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’oRia, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. European Society for Vascular Surgery 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef]
- de Guerre, L.; O’Donnell, T.; Varkevisser, R.; Swerdlow, N.; Li, C.; van Herwaarden, J.; Schermerhorn, M.; Patel, V. Hostile neck anatomy is associated with higher perioperative type IA endoleaks and lower long-term survival after elective endovascular aneurysm repair. J. Vasc. Surg. 2019, 70, e45. [Google Scholar] [CrossRef]
- Kawka, M.; Caradu, C.; Scicluna, R.; Bicknell, C.; Bown, M.; Gohel, M.; Pouncey, A.L. Sex Specific Differences in Abdominal Aortic Aneurysm Morphology Based on Fully Automated Volume Segmented Imaging: A Multicentre Cohort Study and Propensity Score Matched Analysis. Eur. J. Vasc. Endovasc. Surg. 2025; in press. [Google Scholar] [CrossRef]
- Trogolo-Franco, C.; Dossabhoy, S.S.; Sorondo, S.M.; Tran, K.; Stern, J.R.; Lee, J.T. Sex Related Differences in Perioperative Outcomes after Complex Endovascular Aortic Aneurysm Repair. Ann. Vasc. Surg. 2025, 110, 236–245. [Google Scholar] [CrossRef]
- Spanos, K.; Karathanos, C.; Saleptsis, V.; Giannoukas, A.D. Systematic review and meta-analysis of migration after endovascular abdominal aortic aneurysm repair. Vascular 2016, 24, 323–326. [Google Scholar] [CrossRef]
- Chatzelas, D.A.; Loutradis, C.N.; Pitoulias, A.G.; Kalogirou, T.E.; Pitoulias, G.A. A systematic review and meta-analysis of proximal aortic neck dilatation after endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2023, 77, 941–956.e1. [Google Scholar] [CrossRef]
| Author | Journal | Publication Year | Study Period | Type of Analysis | Number of Participating Centers |
|---|---|---|---|---|---|
| Avci et al. [19] | J Cardiovasc Surg | 2012 | 2010–2011 | Prospective | 1 |
| Perdikides et al. [20] | J Endovasc Ther | 2012 | 2010–2012 | Prospective | 2 |
| Jordan Jr WD et al. [15] | J Vasc Surg | 2014 | 2012–2014 | Prospective | 43 |
| Jordan Jr WD et al. [8] | Vascular | 2015 | 2012–2014 | Prospective | 43 |
| Goudeketting et al. [21] | J Endovasc Ther | 2019 | 2010–2017 | Retrospective | 1 |
| Giudice et al. [22] | JRSM Cardiovasc Dis | 2019 | 2015–2018 | Retrospective | 1 |
| Valdivia et al. [23] | Ann Vasc Surg | 2019 | 2011–2017 | Prospectively Collected, Retrospective Analysis | 4 |
| Ho et al. [24] | Ann Vasc Surg | 2020 | 2016–2018 | Prospectively Collected, Retrospective Analysis | 1 |
| Chaudhuri et al. [10] | Cardiovasc Intervent Radiol | 2020 | 2013–2019 | Prospectively Collected, Retrospective Analysis | 2 |
| Chaudhuri et al. [25] | Vascular | 2021 | 2013–2020 | Prospectively Collected, Retrospective Analysis | 1 |
| Valdivia et al. [26] | Vascular | 2022 | 2010–2019 | Prospectively Collected, Retrospective Analysis | 7 |
| Bordet et al. [27] | J Vasc Surg | 2023 | 2012–2020 | Retrospective | 1 |
| Arko et al. [28] | J Vasc Surg | 2023 | 2012–2015 | Prospective | 43 |
| Abdel-Hadi et al. [29] | J Vasc Interv Radiol | 2023 | 2017–2021 | Retrospective | 1 |
| Valdivia et al. [30] | Ann Vasc Surg | 2024 | 2010–2019 | Prospectively Collected Retrospective Analysis | 9 |
| Sivak et al. [31] | Bratisl Med J | 2024 | 2018–2021 | Retrospective | 1 |
| Number of Patients | Sex (Male/Female) | Age | Index EVAR | Revision (EL Ia/Device Migration) | Intact/Rupture AAA | Endograft Type | |
|---|---|---|---|---|---|---|---|
| Avci et al. [19] | 11 | 8/3 | 77 (59–88) | 0 | 11 | 11/0 | Talent, Excluder, AneuRx |
| Perdikides et al. [20] | 13 | 13/0 | 73 (62–82) | 13 | 0 | 13/0 | Endurant, Zenith |
| Jordan Jr WD et al. [15] | 319 | 238/81 | 74.1 ± 8.2 | 242 | 77 | 315/4 | Excluder, Zenith, Endurant, AneuRx, Talent, Other |
| Jordan Jr WD et al. [8] | 100 | 80/20 | 73 ± 8 | 73 | 27 | NA | Excluder, Zenith, Endurant, AneuRx, Talent, Other |
| Goudeketting et al. [21] | 51 | 38/13 | 75 (53–78) | 31 | 20 | 50/1 | Endurant, Talent, Valiant, Zenith, Excluder |
| Giudice et al. [22] | 17 | NA | NA | 9 | 8 | 17/0 | Endurant |
| Valdivia et al. [23] | 46 | 43/3 | 74.4 ± 8.1 | 46 [Therapeutic for Intraoperative EL Ia (22), Prophylactic (24)] | 0 | 45/1 | Endurant, Incraft |
| Ho et al. [24] | 31 | 24/7 | 78.4–80.2 | 20 [Therapeutic for Intraoperativie EL Ia (10), Prophylactic (10)] | 11 | 31/0 | Endurant, Excluder |
| Chaudhuri et al. [10] | 41 | 33/8 | 76.8 ± 8.9 | 35 | 6 | 36/5 | Zenith Flex, Zenith Alpha, Endurant, Excluder C3 |
| Chaudhuri et al. [25] | 84 | 76/8 | 73.7 ± 7.8 | 84 | 0 | 84/0 | Zenith Flex, Zenith Alpha, Zenith Aorto-Uni-Iliac, Endurant, Exluder, Incraft |
| Valdivia et al. [26] | 221 | 184/37 | 75.6 (8.3) | 175 | 46 | 213/8 | Endurant, Zenith, Excluder, Incraft, E-tegra |
| Bordet et al. [27] | 18 | 18/0 | 74 ± 7 | 18 | 0 | 18/0 | Endurant |
| Arko et al. [28] | 70 | 51/19 | 71.3 ± 8.1 | 70 | 0 | 70/0 | Endurant |
| Abdel-Hadi et al. [29] | 42 | NA | NA | 29 | 13 | NA | NA |
| Valdivia et al. [30] | 76 | 62/14 | 79.5 ± 7.5 | 76 | 0 | NA | Endurant, Zenith, Excluder, Incraft, E-tegra |
| Sivak et al. [31] | 24 | 22/2 | 73 ± 6.8 | 24 | 0 | 24/0 | Endurant |
| Author | Heli-FX Endoanchor Deployment Criteria | Cohort Unfavorable Neck Criteria (Number of Patients) |
|---|---|---|
| Avci et al. [19] | EL Ia, Device Migration, EL Ia + Device Migration | El Ia (4), Device Migration (1), EL Ia + Device Migration (6) |
| Perdikides et al. [20] | Neck Length < 15 mm, Neck Diameter > 29 mm, Angulation >45°, Conical Configuration, Irregural Shape (tapered, hourglass, barrel, bulged) | Angulation (8), Conical (7), Wide (7), Short (5), Bulged (2), Barrel (1), Irregular (1) |
| Jordan Jr WD et al. [15] | Index EVAR [Hostile Neck (Neck Length <10 mm, Neck Diameter > 28 mm, Angulation >60°, Conical Configuration, Mural Thrombus or Calcium > 2 mm, Intraoperative EL Ia or Misdeployment)], Revision [EL Ia, Device Migration] | Index EVAR [Hostile Neck (186), Residual Intraoperative EL Ia (52), Misdeployed Endograft (4)], Revision [EL Ia (45), Device Migration + EL Ia (21), Device Migration (11)] |
| Jordan Jr WD et al. [8] | Index EVAR [Hostile Neck, Intraoperative EL Ia or Misdeployment), Revision [EL Ia, Device Migration] | Index EVAR [Hostile Neck (63)], Revision [Hostile Neck (20)] |
| Goudeketting et al. [21] | Neck Length < 10 mm, Neck Diameter > 28 mm, Angulation >60°, Mural Thrombus or Calcium >2 mm thickness or <50% circumference, Conical Configuration) | Hostile Neck (48), Wide Neck (12), |
| Giudice et al. [22] | Hostile Neck Characteristics (Angulation >60o, Conical Configuration, Significant Thrombus or Calcium) | Angulation (3), Significant Thrombus (8), Conical Configuration (4) |
| Valdivia et al. [23] | Hostile Neck Characteristics (Angulation >60°, Conical Configuration, Significant Thrombus or Calcium, Neck Length < 10 mm, Neck Diameter > 32 mm) | Index EVAR [Hostile Neck (186), Residual Intraoperative EL Ia (52), Misdeployed Endograft (4)], Revision [EL Ia (45), Device Migration + EL Ia (21), Device Migration (11)] |
| Ho et al. [24] | Hostile Neck Characteristics, Residual Intraoperative EL Ia, EL Ia | Hostile Neck (10), Intraoperative EL Ia (11), Therapeutic EL Ia (10) |
| Chaudhuri et al. [10] | Angulation > 60°, EL Ia | Angulation >60° (35), Therapeutic EL Ia (6) |
| Chaudhuri et al. [25] | NR | Conical Configuration (22) |
| Valdivia et al. [26] | Hostile Neck [Neck Length < 10 mm + Angulation > 60° or >2 mm or >50% circumference thrombus or >50% circumference calcification or Conical Configuration or Neck Diameter > 28 mm or Asymmetric Neck Bulges] | Conical Configuration (82), Bulge Shape (12) |
| Bordet et al. [27] | Neck Length < 15 mm | Neck Length < 15 mm (18) |
| Arko et al. [28] | Index EVAR [Hostile Neck, Intraoperative EL Ia or Misdeployment), Revision [EL Ia, Device Migration] | NR |
| Abdel-Hadi et al. [29] | Hostile Neck (Neck Length < 10 mm, Angulation > 60°, Neck Diameter > 28 mm) | Hostile Neck (28) |
| Valdivia et al. [30] | Hostile Neck [Neck Length < 10 mm + Angulation >60° or >2 mm or >50% circumference thrombus or >50% circumference calcification or Conical Configuration or Neck Diameter > 28 mm or Asymmetric Neck Bulges] | Neck Length ≥ 4 and <7 mm (17), Neck Length ≥ 7 and <10 mm (59) |
| Sivak et al. [31] | Neck Length < 15 mm, Neck Diameter > 28 mm, Angulation >60°, Reverse Taper Configuration, >50% Circumference Calcification/Thrombus | Neck Length < 15 mm (13), Angulation >60° (7), Wide Neck (2) |
| Author | Number of Heli-FX Endoanchors | Aneurysm Diameter (mm) | Neck Diameter (mm) | Neck Leght (mm) |
|---|---|---|---|---|
| Avci et al. [19] | 6 (4–9) | NR | NR | NR |
| Perdikides et al. [20] | 4 (3–10) | 56 (50–98) | 32 (26–34) | 16 (8–35) |
| Jordan Jr WD et al. [15] | Index EVAR: 5 (4–6), Revision: 7 (5–8) | 58 ± 13 (51–63) | 27 ± 4 (25–30) | 16 ± 13 (7–23) |
| Jordan Jr WD et al. [8] | 5.3 ± 1.8 | 58 ± 16 | 27 ± 5 | 16 ± 13.7 |
| Goudeketting et al. [21] | Index EVAR: 6 (5–7), Revision: 6 (5–9) | 63.7 (57.3–71) | 27.7 (23, 30.1) | 9 (5,18) |
| Giudice et al. [22] | 5 (4–10) | 60 (43–88) | 29.5 | 10.7 |
| Valdivia et al. [23] | 6 ± 1.9 | 58.2 ± 8 | 25.5 | 8 (3–38) |
| Ho et al. [24] | 8.3–10 | 68.7 | NR | NR |
| Chaudhuri et al. [10] | 6 ± 2 | 71.6 ± 16 | 24.5 ± 4.3 | 19.18 ± 12 |
| Chaudhuri et al. [25] | 7 ± 2 | 65.1 (12.2) | 25 (22–31) | 19 (13–28) |
| Valdivia et al. [26] | 6 ± 3 | 63 ± 15.1 | 26 (7) | 13 (10) |
| Bordet et al. [27] | 4–8 | 54 (52–61) | 25 (22–26) | 8 (6–12) |
| Arko et al. [28] | NR | NR | NR | NR |
| Abdel-Hadi et al. [29] | 7 (3–10) | NR | NR | NR |
| Valdivia et al. [30] | 6 (3) | 57 (12.8) | 24 (6) | 8 (3) |
| Sivak et al. [31] | 7 (4–12) | NR | 22.9 (20–28.2) | 15 (7–46) |
| Author | Technical Success | Procedural Success | Residual Intraoperative EL Ia | Freedom from EL Ia (Maximum FU) | Follow-Up (months) |
|---|---|---|---|---|---|
| Avci et al. [19] | 100% (Revision only) | 82% (Revision only) | 2 | 100% | 10 (3–18) |
| Perdikides et al. [20] | 85% (Index EVAR only) | 85% (Index EVAR only) | 2 | 100% | 7 (2–17) |
| Jordan Jr WD et al. [15] | 95% (Index EVAR: 96.3%, Revision: 90.9%) | 87.5% (Index EVAR: 89.7%, Revision: 80.5%) | 29 (Index EVAR: 19, Revision: 10) | 100% | 9.3 ± 4.7 |
| Jordan Jr WD et al. [8] | 93% (Index EVAR: 95%, Revision: 89%) | 89% (Index EVAR: 92%, Revision: 81%) | 9 (Index EVAR: 3, Revision: 3) | Index EVAR 95% (95% CI 89–100%), Revision 77% (95% CI 60–95%) | 18 ± 4 |
| Goudeketting et al. [21] | 99% (Index EVAR: NR, Revision: NR) | 80% (Index EVAR: 87%, Revision: 70%) | 10 (Index EVAR: 4, Revision: 6) | Index EVAR 88.9% (95% CI 76.9–100%), Revision 84% (95% CI 62.4–100%) | 23.9 (13.4, 35.6) |
| Giudice et al. [22] | 100% (Index EVAR: 100%, Revision: 100%) | 100% (Index EVAR:100%, Revision: 100%) | 0 | 100% | 13 (4–39) |
| Valdivia et al. [23] | 97.8% (Index EVAR only) | NR | 9 (Index EVAR only) | 100% | 15 ± 12.9 |
| Ho et al. [24] | 100% (Index EVAR: 100%, Revision: 100%) | Revision (45.4%), Therapeutic for Intraoperative EL Ia during index EVAR (100%), Prophylactic (100%) | 5 (Index EVAR: 0, Revision:5) | NR | 9.4–13.6 |
| Chaudhuri et al. [10] | 100% (Index EVAR:100%, Revision:100%) | 97.6% (Index EVAR:100%, Revision: 84%) | 0 | 97.5% | 18.5 (13.3–23.9) |
| Chaudhuri et al. [25] | NR | NR | 1 (Index EVAR only) | 100% | 28.5 (12–43) |
| Valdivia et al. [26] | 94.3% (Index EVAR: NR, Revision: NR) | 89.1% (Index EVAR: NR, Revision: NR) | 23 (Index EVAR:NR, Revision: NR) | Index EVAR 96%, Revision 86% | 27 (12–48) |
| Bordet et al. [27] | 100% (Index EVAR only) | 94% (Index EVAR only) | 1 (Index EVAR only) | 100% | 23 (19–33) |
| Arko et al. [28] | NR | NR | NR | NR | 60 |
| Abdel-Hadi et al. [29] | 98% (Index EVAR:100%, Revision: 92.4%) | 96% (Index EVAR: 100%, Revision: 94.7%) | 2 (Index EVAR: 0, Revision: 2) | 93% | 38 (2–71) |
| Valdivia et al. [30] | 86.8% (Index EVAR only) | 86.8% (Index EVAR only) | 10 (Index EVAR only) | 84% | 40.5 (12–61) |
| Sivak et al. [31] | 100% (Index EVAR only) | 100% (Index EVAR Only) | 0 | 95.8% | 22.5 (2–31.5) |
| Author | Secondary Interventions for Proximal Neck Complications (EL Ia, Migration, AND) | New Postoperative EL Ia (Maximum FU) | Postoperative Neck Diameter (mm) |
|---|---|---|---|
| Avci et al. [19] | 0 | 0 | >5 Decrease |
| Perdikides et al. [20] | 0 | 0 | 32 (26–34) |
| Jordan Jr WD et al. [15] | 8 (Index EVAR: 1, Revision: 7) (Reintervention for EL Ia: 7, Reintervention for Device Migration: 1) | 0 | NR |
| Jordan Jr WD et al. [8] | 4 (Index EVAR: 1, Revision 3) (Reintervention for EL Ia: 4, Reintervention for Device MigrationJ | 1 | NR |
| Goudeketting et al. [21] | 5 (Index EVAR: 1, Revision: 4) (Reintervention for EL Ia: 5, Reintervention for Device Migration: 0) | 6 | 2.5 (0.6, 3.8) Increase |
| Giudice et al. [22] | 0 | 0 | NR |
| Valdivia et al. [23] | 0 | 0 | 26.5 |
| Ho et al. [24] | 1 (Index EVAR: 0, Revision: 1) (Reintervention for EL Ia: 1, Reintervention for Device Migration: 0) | 0 | NR |
| Chaudhuri et al. [10] | 0 | 0 | 3.2 ± 3.7 Increase |
| Chaudhuri et al. [25] | 0 | 0 | 4.6 (5.7) Increase |
| Valdivia et al. [26] | 10 (Index EVAR: 6, Revision: 4) (Reintervention for EL Ia: NR, Reintervention for Device Migration: NR) | 3 | NR |
| Bordet et al. [27] | 0 | 0 | No Change |
| Arko et al. [28] | 3 (Index EVAR only) (Reintervention for EL Ia: 3) | 9 | NR |
| Abdel-Hadi et al. [29] | 5 (Index EVAR: NR, Revision: NR) (Reintervention for EL Ia: 5, Reintervention for Device Migration: 0) | NR | NR |
| Valdivia et al. [30] | 5 (Index EVAR only) (Reintervention for EL Ia: 5, Reintervention for Device Migration: 0) | 1 | NR |
| Sivak et al. [31] | 1 (Index EVAR Only) (Reintervention for EL Ia: 1, Reintervention for Device Migration: 0) | 1 | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dakis, K.; Apostolidis, G.; Nana, P.; Kouvelos, G.; Arnaoutoglou, E.; Giannoukas, A.; Matsagkas, M.; Spanos, K. The Prophylactic and Therapeutic Use of the Heli-FX EndoAnchor System in Patients Undergoing Endovascular Aortic Aneurysm Repair—A Scoping Review. Medicina 2026, 62, 40. https://doi.org/10.3390/medicina62010040
Dakis K, Apostolidis G, Nana P, Kouvelos G, Arnaoutoglou E, Giannoukas A, Matsagkas M, Spanos K. The Prophylactic and Therapeutic Use of the Heli-FX EndoAnchor System in Patients Undergoing Endovascular Aortic Aneurysm Repair—A Scoping Review. Medicina. 2026; 62(1):40. https://doi.org/10.3390/medicina62010040
Chicago/Turabian StyleDakis, Konstantinos, George Apostolidis, Petroula Nana, George Kouvelos, Eleni Arnaoutoglou, Athanasios Giannoukas, Miltiadis Matsagkas, and Konstantinos Spanos. 2026. "The Prophylactic and Therapeutic Use of the Heli-FX EndoAnchor System in Patients Undergoing Endovascular Aortic Aneurysm Repair—A Scoping Review" Medicina 62, no. 1: 40. https://doi.org/10.3390/medicina62010040
APA StyleDakis, K., Apostolidis, G., Nana, P., Kouvelos, G., Arnaoutoglou, E., Giannoukas, A., Matsagkas, M., & Spanos, K. (2026). The Prophylactic and Therapeutic Use of the Heli-FX EndoAnchor System in Patients Undergoing Endovascular Aortic Aneurysm Repair—A Scoping Review. Medicina, 62(1), 40. https://doi.org/10.3390/medicina62010040








