Association Between Genotoxic Effects of Ageing Dental Restorations and Micronuclei in Oral Mucosal Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MN PI | Micronuclei Plaque Index |
| GI | Silness–Löe Gingival Index |
| PBI | Mühlemann Papillary Bleeding Index |
| HE | hematoxylin–eosin |
References
- Shahi, S.; Özcan, M.; Dizaj, S.M.; Sharifi, S.; Husain, N.A.-H.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Mary, S.J.; Girish, K.L.; Sathyan, P. Genotoxic Effects of Silver Amalgam and Composite Restorations: Micronuclei-Based Cohort and Case–Control Study in Oral Exfoliated Cells. Contemp. Clin. Dent. 2018, 9, 249–254. [Google Scholar]
- Schmaltz, G.; Widbiller, M. Biocompatibility of amalgam vs composite-a review. Oral Health Prev. Dent. 2022, 20, 149–156. [Google Scholar]
- Gavran, M.T.; Željezić, D.; Vranić, L.; Vrani, D.N.; Grabarević, L.; Jurić-Kaćunarić, D.; Tadin, A.; Šegović, S.; Galić, N. Assessment of cytotoxic and genotoxic effect of modern dental materials in vivo. Acta Stomatol. Croat. 2023, 57, 216–228. [Google Scholar] [CrossRef]
- Ratner, B.D. The biocompatibility manifesto: Biocompatibility for the twenty-first century. J. Cardiovasc. Transl. Res. 2011, 4, 523–527. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- De Marini, D.M. The Role of Genotoxicity in Carcinogenesis; National Center for Biotechnology Information (NCBI): Bethesda, Maryland, USA (NCBI is part of the U.S. National Library of Medicine, based in Bethesda), 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570347/ (accessed on 14 June 2025).
- World Health Organization. 10 Chemicals of Public Health Concern. 2025. Available online: https://www.who.int/news-room/photo-story/detail/10-chemicals-of-public-health-concern (accessed on 14 June 2025).
- United States Environmental Protection Agency. Basic Information About Mercury. 2025. Available online: https://www.epa.gov/mercury/basic-information-about-mercury (accessed on 14 June 2025).
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef]
- Homme, K.G.; Kern, J.K.; Haley, B.E.; Geier, D.A.; King, P.G.; Sykes, L.K.; Geier, M.R. New science challenges old notion that mercury dental amalgam is safe. Biometals 2014, 27, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Amalgam (Part 2): Safe Use and Phase Down of Dental Amalgam. Adopted by the FDI General Assembly: 27–29 September 2021, Sydney, Australia. Available online: https://www.fdiworlddental.org/amalgam-part-2-safe-use-and-phase-down-dental-amalgam (accessed on 20 January 2025).
- Sánchez-Alarcón, J.; Milić, M.; Bustamante-Montes, L.P.; Isaac-Olivé, K.; Valencia-Quintana, R.; Ramírez-Durán, N. Genotoxicity of mercury and its derivatives demonstrated in vitro and in vivo in human populations studies. Systematic review. Toxics 2021, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Siblerud, R.; Mutter, J. An overview of evidence that mercury from dental fillings may be an etiological factor in many health disorders. J. Biomed. Res. Environ. Sci. 2021, 2, 472–485. [Google Scholar] [CrossRef]
- Bartel-Steinbach, M.; Lermen, D.; Gwinner, F.; Schäfer, M.; Göen, T.; Conrad, A.; Weber, T.; von Briese, H.; Kolossa-Gehring, M. Long-term monitoring of mercury in young German adults: Time trend analyses from the German Environmental Specimen Bank, 1995–2018. Environ. Res. 2022, 207, 112592. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, J.T.; Dahl, J.E.; Karlsson, S.; Morisbak, E.; Becher, R. Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation oft he MAP-kinases p38, JNK and ERK. Dent. Mater. 2007, 23, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.H.; Huang, F.M.; Lee, S.S.; Li, Y.C.; Chang, Y.C. BisGMA stimulates prostaglandin E2 production in macrophages via cyclooxygenase-2, cytosolic phospholipase A2, and mitogen-activated protein kinases family. PLoS ONE 2013, 8, e82942. [Google Scholar] [CrossRef]
- Schubert, A.; Ziegler, C.; Bernhard, A.; Bürgers, R.; Miosge, N. Cytotoxic effects to mouse and humangingival fibroblasts of a nanohybrid ormocer versus dimethacrylate-based composites. Clin. Oral. Investig. 2019, 23, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Saks, M.; Upreti, S.; Rajendra, S.V.; Dang, R. Genotoxicity: Mechanisms, testing guidelines and methods. Glob. J. Pharm. Pharm. Sci. 2017, 1, 133–138. [Google Scholar]
- Carvalho, A.P.; Moura, M.F.; Costa, F.O.; Cota, L.-O.-M. Correlation between different plaque indexes and bleeding on probing: A concurrent validity study. J. Clin. Exp. Dent. 2023, 15, e9–e16. [Google Scholar] [CrossRef]
- Tankova, H.; Lazarova, Z. A comparative study of full mouth bleeding score (FMBS) and gingival index Loe and Silness (GILS) in assessing gingival status of children aged 10–14 years. J. IMAB Annu. Proc. Sci. Pap. 2024, 30, 5387–5391. [Google Scholar] [CrossRef]
- Tolbert, P.E.; Shy, C.M.; Allen, J.W. Micronuclei and other nuclear anomalies in buccal smears: A field test in snuff users. Am. J. Epidemiol. 1991, 134, 840–850. [Google Scholar] [CrossRef]
- Siwach, P.; Thakur, A. Micronuclei count in oral exfoliated cells—A tool for biomonitoring DNA damage in tobacco users. Int. J. Health Sci. Res. 2018, 8, 111–117. [Google Scholar]
- Arul, P.; Shetty, S.; Masilamani, S.; Akshatha, C.; Kumar, B.J.N.N. Evaluation of Micronucleus in Exfoliated Buccal Epithelial Cells Using Liquid-based Cytology Preparation in Petrol Station Workers. Indian J. Med. Paediatr. Oncol. 2017, 38, 273–276. [Google Scholar] [CrossRef]
- Shah, S.; Singaraju, S.; Bertin, E.T.; Medhini, S.; Ashish, S. Quantification of micronuclei in exfoliated cells of human immunodeficiency virus/AIDS-infected female patients. J. Oral. Maxillofac. Pathol. 2019, 23, 301. [Google Scholar] [PubMed]
- Hayashi, M. The micronucleus test is–most widely used in vivo genotoxicity test. Genes Environ. 2016, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Al Yadav, V.; Fuentes, J.L.; Krishnan, A.; Singh, N.; Vahora, D. Guidance for the use and interpretation of assays for monitoring anti-gentotoxicity. Life Sci. 2024, 337, 122341. [Google Scholar] [CrossRef] [PubMed]
- Benvindo-Souza, M.; Assis, R.A.; Oliveira, E.S.; Borges, R.E.; Santos, L.S. The micronucleus test for the oral mucosa: Global trends and new questions. Environ. Sci. Pollut. Res. Int. 2017, 24, 27724–27730. [Google Scholar] [CrossRef]
- Tadin, A.; Gavic, L.; Roguljic, M.; Jerkovic, D.; Zeljezic, D. Nuclear morphological changes in gingival epithelial cells of patients with periodontitis. Clin. Oral Investig. 2019, 10, 3749–3757. [Google Scholar] [CrossRef]
- Nersesyan, A.; Proietti, S.; Knasmueller, S.; Bonassi, S. High correlation between micronuclei in lymphocytes and buccal cells in humans provides further validation of their use as biomarkers of DNA damage and cancer risks in vivo. Mutagenesis 2025, 66, geaf006. [Google Scholar] [CrossRef]
- Visalli, G.; Baluce, B.; La Maestra, S.; Micale, R.T.; Cingano, L.; De Flora, S.; Di Pietro, A. Genotoxic damage in the oral mucosa cells of subjects carrying restorative dental fillings. Arch. Toxicol. 2013, 87, 179–187. [Google Scholar] [CrossRef]
- Dudas, C.; Kardos, E.; Szekely, M.; Ádám, L.; Bardocz-Veres, Z.; Szőllősi, E.; Jánosi, K.M.; Kerekes-Máthé, B. Effect of glass fiber reinforcement on marginal microleakage in class II composite restoration: An in vitro pilot study. Dent. J. 2024, 12, 410. [Google Scholar] [CrossRef]
- Krupina, K.; Goginashvili, A.; Cleveland, D.W. Causes and consequences of micronuclei. Curr. Opin. Cell Biol. 2021, 70, 91–99. [Google Scholar] [CrossRef]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef]
- Crasta, K.; Ganem, N.J.; Dagher, R.; Lantermann, A.B.; Ivanova, E.V.; Pan, Y.; Nezi, L.; Protopopov, A.; Chowdhury, D.; Pellman, D. DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012, 482, 53–58. [Google Scholar] [CrossRef]
- Hatch, E.M.; Fischer, A.H.; Deerinck, T.J.; Hetzer, M.W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 2013, 154, 47–60. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA damage in micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.F.; Lindoso, J.C.; de Oliveira Conde, N.C.; da Silva, L.M.; Loguercio, A.D.; Pereira, J.V. Evaluation of the genotoxic potential of different delivery methods of at-home bleaching gels: A single-blind, randomized clinical trial. Clin. Oral. Investig. 2019, 23, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Alp, G.; Çakmak, G.; Sert, M.; Burgaz, Y. Corrosion potential in artificial saliva and possible genotoxic and cytotoxic damage in buccal epithelial cells of patients who underwent Ni-Cr based porcelain-fused-to-metal fixed dental prostheses. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 827, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Deli, G. Modern detection methods for the effects of ionizing radiation on the human body. Honvédorvos 2019, 71, 31–45. [Google Scholar] [CrossRef]
- Russo, A.; Degrassi, F. Molecular cytogenetics of the micronucleus: Still surprising. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 836, 36–40. [Google Scholar] [CrossRef]
- Umbreit, N.T.; Zhang, C.Z.; Lynch, L.D.; Blaine, L.J.; Cheng, A.M.; Richard, T.; Sun, L.; Almubarak, H.F.; Judge, K.; Mitchell, T.J.; et al. Mechanisms generating cancer genome complexity from a single cell division error. Science 2020, 368, 240. [Google Scholar] [CrossRef]
- Shoshani, O.; Brunner, S.F.; Yaeger, R.; Ly, P.; Nechemia-Arbely, Y.; Kim, D.H.; Fang, R.; Castillon, G.A.; Yu, M.; Li, J.S.Z.; et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature 2020, 591, 137–141. [Google Scholar] [CrossRef]
- Ray, J.G.; Ganguly, M.; Rao, B.S.; Sanjit, M.; Basudev, M.; Keya, C. Clinico-epidemiological profile of oral potentially malignant and malignant conditions among areca nut, tobacco and alcohol users in Eastern India: A hospital based study. J. Oral. Maxillofac. Pathol. 2013, 17, 45–50. [Google Scholar] [CrossRef]
- Liu, S.; Pellman, D. The coordination of nuclear envelope assembly and chromosome segregation in metazoans. Nucleus 2020, 11, 35–52. [Google Scholar] [CrossRef]
- Hatch, E.M. Nuclear envelope rupture: Little holes, big openings. Curr. Opin. Cell Biol. 2018, 52, 66–72. [Google Scholar] [CrossRef]
- Fonseca, C.L.; Malaby, H.L.; Sepaniac, L.A.; Martin, W.; Byers, C.; Czechanski, A.; Messinger, D.; Tang, M.; Ohi, R.; Reinholdt, L.G.; et al. Mitotic chromosome alignment ensures mitotic fidelity by promoting interchromosomal compaction during anaphase. J. Cell Biol. 2019, 218, 1148–1163. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, M.; Chen, Y.; Agustinus, A.S.; Mazzagatti, A.; Duran, M.A.; Deyell, M.; Bronder, D.; Hickling, J.; Hong, C.; Scipioni, L.; et al. Micronuclear collapse from oxidative damage. Science 2024, 385, 8691. [Google Scholar] [CrossRef] [PubMed]
- Reichl, F.X.; Simon, S.; Esters, M.; Seiss, M.; Kehe, K.; Kleinsasser, N.; Hickel, R. Cytotoxicity of dental composite (co) monomers and the amalgam component Hg(2+) in human gingival fibroblasts. Arch. Toxicol. 2006, 80, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Gavic, L.; Gorseta, K.; Glavina, D.; Zeljezic, D.; Galic, N.; Tadin, A. In vivo assessment of genotoxicity in buccal cells of children undergoing tooth restoration. Cent. Eur. J. Public Health 2019, 27, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Tadin, A.; Gavic, L.; Roguljic, M.; Babić, M.; Galić, I.; Želježić, D. Assessment of cytogenetic damage to exfoliated gingival cells in patients with chronic periodontitis. Acta Clin. Croat. 2021, 60, 209–215. [Google Scholar] [CrossRef]
- Ceppi, M.; Biasotti, B.; Fenech, M.; Bonassi, S. Human population studies with the exfoliated buccal micronucleus assay: Statistical and epidemiological issues. Mutat. Res. 2010, 705, 11–19. [Google Scholar] [CrossRef]
- Hopf, N.B.; Danuser, B.; Bolognesi, C.; Wild, P. Age related micronuclei frequency ranges in buccal and nasal cells in a healthy population. Environ. Res. 2020, 180, 108824. [Google Scholar] [CrossRef]
- Bloching, M.; Reich, W.; Schubert, J.; Grummt, T.; Sandner, A. Micronucleus rate of buccal mucosal epithelial cells in relation to oral hygiene and dental factors. Oral Oncol. 2008, 44, 220–226. [Google Scholar] [CrossRef]
- Ahmed, R.H.; Aref, M.I.; Hassan, R.M.; Mohammed, N.R. Cytotoxic effect of composite resin and amalgam filling materials on human labial and buccal epithelium. Nat. Sci. 2010, 8, 48–53. [Google Scholar]
- Caministeanu, F.; Perieanu, V.S.; Popa, A.S.; Manolescu, L.S.C.; Stetiu, A.A.; Costea, R.C.; Burlibasa, M.; Vorovenci, A.; Costea, R.M.; Serbanescu, C.M.; et al. Assessing the genotoxic impact of Ni-Cr alloys in dental prosthodontics: A preliminary comparative analysis with and without beryllium. Oral 2025, 5, 32. [Google Scholar] [CrossRef]
- Dhar, V.; Pilcher, L.; Fontana, M.; González-Cabezas, C.; Keels, M.A.; Mascarenhas, A.K.; Nascimento, M.; Platt, J.A.; Sabino, G.J.; Slayton, R.; et al. Evidence-based clinical practice guideline on restorative treatments for caries lesions: A report from the American Dental Association. J. Am. Dent. Assoc. 2023, 154, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, A.; Baig, M.R.; Baskaradoss, J.K.; Alsanea, S.; AlMousawi, A. Factors influencing the selection of materials and luting agents for crown restorations. Dent. J. 2025, 13, 207. [Google Scholar] [CrossRef]
- Tadin, A.; Galic, N.; Mladinic, M.; Marovic, D.; Kovacic, I.; Zeljezic, D. Genotoxicity in gingival cells of patients undergoing tooth restoration with two different dental composite materials. Clin. Oral Investig. 2014, 18, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, T.; Manaktala, N.; Pralhad, S.; Natarajan, S. Assessment and comparison of nuclear changes seen in gingivitis and periodontitis using fluorescent microscopy. Rev. Esp. Patol. 2019, 52, 208–213. [Google Scholar] [CrossRef]
- Tolbert, P.E.; Shy, C.M.; Allen, J.W. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat. Res. 1992, 271, 69–77. [Google Scholar] [CrossRef]
- Gupta, J.; Gupta, K.; Agarwal, R. Comparison of different stains in exfoliated oral mucosal cell micronucleus of potentially malignant disorders of oral cavity. J. Cancer Res. Ther. 2019, 15, 615–619. [Google Scholar] [CrossRef] [PubMed]
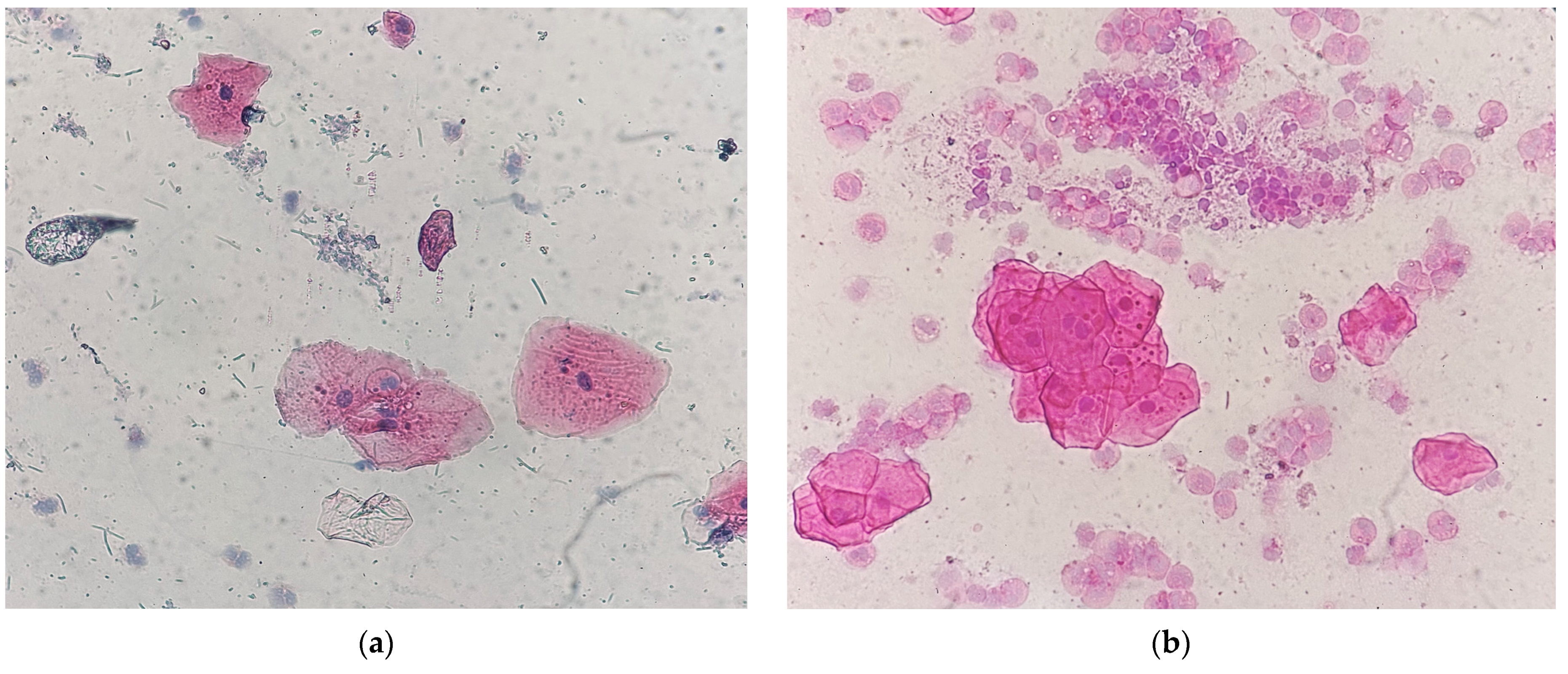
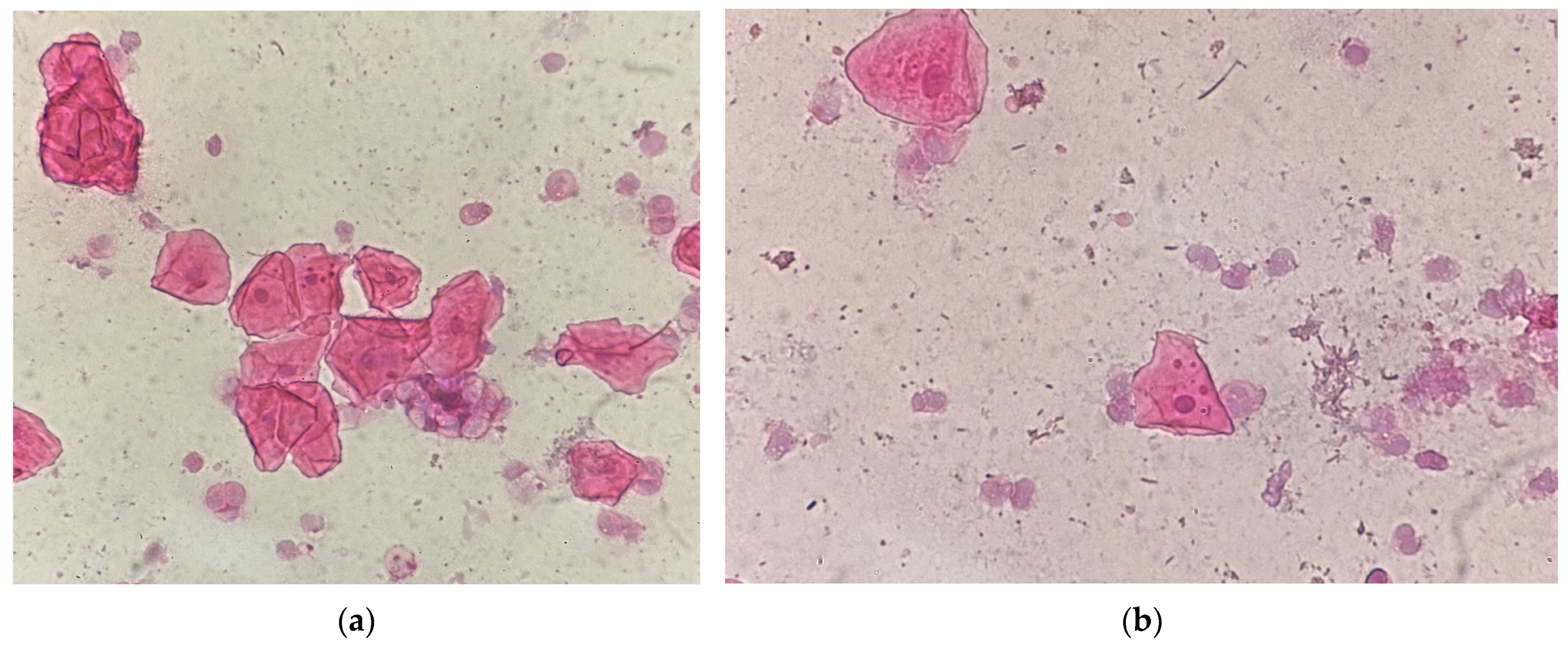

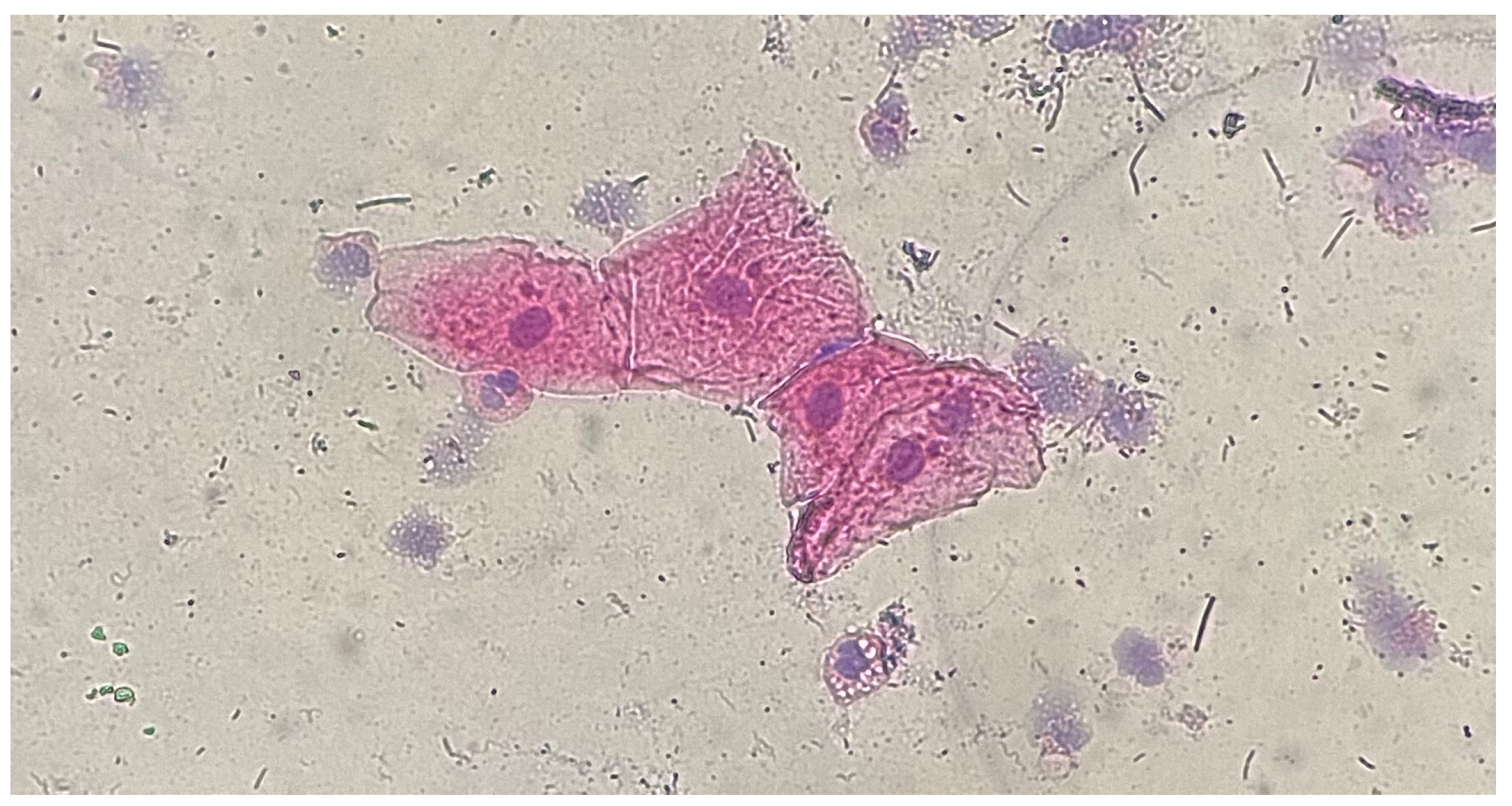
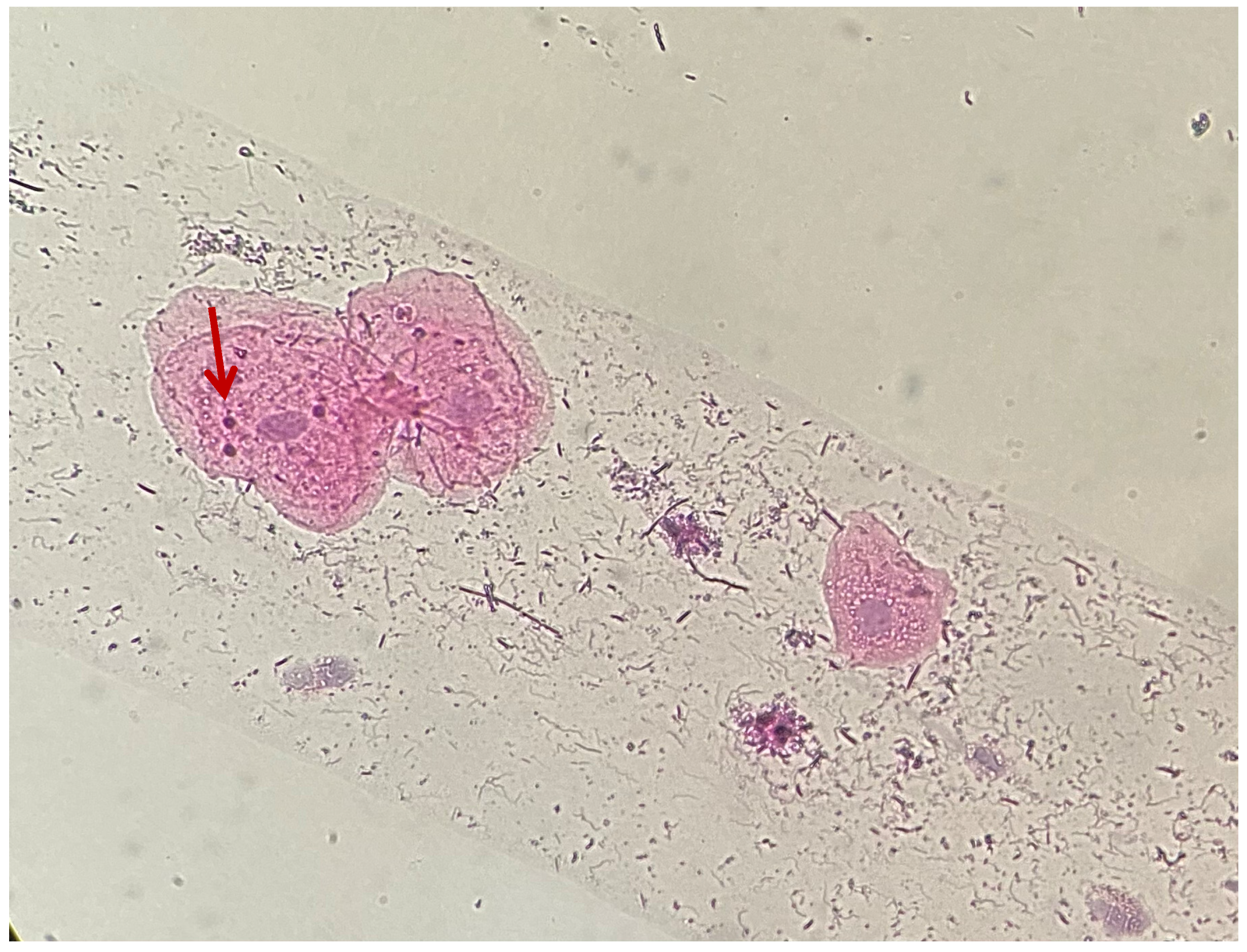
| Type of Restoration | Type of Index | Index Level | Occurrence in Percentage (Absolute Value) |
|---|---|---|---|
| 1–5 years Composite | PI | 0 | 17.95% (n = 7) |
| 1 | 41.03% (n = 16) | ||
| 2 | 41.03% (n = 16) | ||
| GI | 0 | 25.64% (n = 10) | |
| 1 | 48.72% (n = 19) | ||
| 2 | 25.64% (n = 10) | ||
| PBI | 0 | 58.97% (n = 23) | |
| 1 | 23.08% (n = 9) | ||
| 2 | 5.13% (n = 2) | ||
| 3 | 12.82% (n = 5) | ||
| 5–10 years Composite | PI | 0 | 44.00% (n = 11) |
| 1 | 28.00% (n = 7) | ||
| 2 | 28.00% (n = 7) | ||
| GI | 0 | 40.00% (n = 10) | |
| 1 | 36.00% (n = 9) | ||
| 2 | 24.00% (n = 6) | ||
| PBI | 0 | 60.00% (n = 15) | |
| 1 | 12.00% (n = 3) | ||
| 2 | 20.00% (n = 5) | ||
| 3 | 8.00% (n = 2) | ||
| >10 years composite | PI | 0 | 17.65% (n = 6) |
| 1 | 64.71% (n = 22) | ||
| 2 | 17.65% (n = 6) | ||
| GI | 0 | 5.88% (n = 2) | |
| 1 | 82.35% (n = 28) | ||
| 2 | 11.76% (n = 4) | ||
| PBI | 0 | 70.59% (n = 24) | |
| 1 | 11.76% (n = 4) | ||
| 2 | 17.65% (n = 6) | ||
| Amalgam | PI | 0 | 17.65% (n = 3) |
| 1 | 29.41% (n = 5) | ||
| 2 | 52.94% (n = 9) | ||
| GI | 0 | 11.76% (n = 2) | |
| 1 | 52.94% (n = 9) | ||
| 2 | 35.29% (n = 6) | ||
| PBI | 0 | 52.94% (n = 9) | |
| 1 | 5.88% (n = 1) | ||
| 2 | 41.18% (n = 7) |
| Type of Restoration | Number of Micronuclei | Occurrence in Percentage |
|---|---|---|
| 1–5 years composite | <5 | 69% |
| 5–10 | 31% | |
| 5–10 years composite | <5 | 82% |
| 5–10 | 18% | |
| >10 years composite | <5 | 77% |
| 5–10 | 23% | |
| Amalgam >10 years | <5 | 14% |
| 5–10 | 86% |
| Type of Restoration | Size of Micronuclei | Occurrence in Percentage | Location of Micronuclei | Occurrence in Percentage | Staining of Micronuclei | Occurrence in Percentage |
|---|---|---|---|---|---|---|
| 1–5 years Composite | Uniform sizes | 87% | Around the nucleus | 94% | Same as the nucleus | 81% |
| Different sizes | 13% | Far from the nucleus | 6% | Hyperchromatic | 19% | |
| 5–10 years Composite | Uniform sizes | 64% | Around the nucleus | 91% | Same as the nucleus | 82% |
| Different sizes | 36% | Far from the nucleus | 9% | Hyperchromatic | 18% | |
| >10 years Composite | Uniform sizes | 69% | Around the nucleus | 77% | Same as the nucleus | 85% |
| Different sizes | 31% | Far from the nucleus | 23% | Hyperchromatic | 15% | |
| Amalgam | Uniform sizes | 43% | Around the nucleus | 57% | Same as the nucleus | 100% |
| Different sizes | 57% | Far from the nucleus | 43% | Hyperchromatic | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedek, C.; Kerekes-Máthé, B.; Bardocz-Veres, Z.; Szabó, B.; Iacob, A.; Stoica, A.; Dako, T.; Kovács, M.; Dénes, L.; Bereșescu, L. Association Between Genotoxic Effects of Ageing Dental Restorations and Micronuclei in Oral Mucosal Cells. Medicina 2025, 61, 1363. https://doi.org/10.3390/medicina61081363
Benedek C, Kerekes-Máthé B, Bardocz-Veres Z, Szabó B, Iacob A, Stoica A, Dako T, Kovács M, Dénes L, Bereșescu L. Association Between Genotoxic Effects of Ageing Dental Restorations and Micronuclei in Oral Mucosal Cells. Medicina. 2025; 61(8):1363. https://doi.org/10.3390/medicina61081363
Chicago/Turabian StyleBenedek, Csilla, Bernadette Kerekes-Máthé, Zsuzsanna Bardocz-Veres, Boglárka Szabó, Alina Iacob, Alexandra Stoica, Timea Dako, Mónika Kovács, Lóránd Dénes, and Liana Bereșescu. 2025. "Association Between Genotoxic Effects of Ageing Dental Restorations and Micronuclei in Oral Mucosal Cells" Medicina 61, no. 8: 1363. https://doi.org/10.3390/medicina61081363
APA StyleBenedek, C., Kerekes-Máthé, B., Bardocz-Veres, Z., Szabó, B., Iacob, A., Stoica, A., Dako, T., Kovács, M., Dénes, L., & Bereșescu, L. (2025). Association Between Genotoxic Effects of Ageing Dental Restorations and Micronuclei in Oral Mucosal Cells. Medicina, 61(8), 1363. https://doi.org/10.3390/medicina61081363







