The Role of Allografts in Revision ACL Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. The Allograft
2.2. Graft Preparation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IKDC | International Knee Documentation Committee |
| YBT | Y Balance Test |
| ACL | Anterior Cruciate Ligament |
| FDA | Food and Drug Administration |
| AATB | American Association of Tissue Banks |
| VAS | Visual Analog Scale for Pain |
References
- Paschos, N.K.; Howell, S.M. Anterior Cruciate Ligament Reconstruction: Principles of Treatment. EFORT Open Rev. 2016, 1, 398–408. [Google Scholar] [CrossRef]
- Monllau, J.C.; Perelli, S.; Costa, G.G. Anterior Cruciate Ligament Failure and Management. EFORT Open Rev. 2023, 8, 231–244. [Google Scholar] [CrossRef]
- Lansdown, D.A.; Riff, A.J.; Meadows, M.; Yanke, A.B.; Bach, B.R. What Factors Influence the Biomechanical Properties of Allograft Tissue for ACL Reconstruction? A Systematic Review. Clin. Orthop. Relat. Res. 2017, 475, 2412–2426. [Google Scholar] [CrossRef]
- Grassi, A.; Nitri, M.; Moulton, S.G.; Marcheggiani Muccioli, G.M.; Bondi, A.; Romagnoli, M.; Zaffagnini, S. Does the Type of Graft Affect the Outcome of Revision Anterior Cruciate Ligament Reconstruction? Bone Jt. J. 2017, 99-B, 714–723. [Google Scholar] [CrossRef]
- Brown, M.J.; Carter, T. ACL Allograft: Advantages and When to Use. Sports Med. Arthrosc. 2018, 26, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Grassi, A.; Cialdella, S.; Costa, G.; Pizza, N.; Macchiarola, L.; Dal Fabbro, G.; Lo Presti, M.; Zaffagnini, S. Good Stability and Mid-Term Subjective Outcomes after Repeated Anterior Cruciate Ligament (ACL) Revision Surgery Using Allografts. Knee Surg. Sport. Traumatol. Arthrosc. 2023, 31, 3353–3361. [Google Scholar] [CrossRef]
- Baawa-Ameyaw, J.; Plastow, R.; Begum, F.A.; Kayani, B.; Jeddy, H.; Haddad, F. Current Concepts in Graft Selection for Anterior Cruciate Ligament Reconstruction. EFORT Open Rev. 2021, 6, 808–815. [Google Scholar] [CrossRef]
- Sun, K.; Tian, S.; Zhang, J.; Xia, C.; Zhang, C.; Yu, T. Anterior Cruciate Ligament Reconstruction with BPTB Autograft, Irradiated versus Non-Irradiated Allograft: A Prospective Randomized Clinical Study. Knee Surg. Sport. Traumatol. Arthrosc. 2009, 17, 464–474. [Google Scholar] [CrossRef]
- Hanna, A.J.; Johns, W.L.; Perez, A.R.; Kemler, B.; Onor, G.I.; Freedman, K.B.; Dodson, C.C.; Ciccotti, M.G. Patients’ under 25 Subjective Readiness to Return to Sport after ACL Reconstruction with Bone-Patellar-Bone Grafts: Autograft vs. Allograft. J. Orthop. 2024, 55, 149–156. [Google Scholar] [CrossRef]
- Jacobs, C.A.; Burnham, J.M.; Makhni, E.C.; Malempati, C.S.; Swart, E.C.; Johnson, D.L. Allograft Augmentation of Hamstring Autograft for Younger Patients Undergoing Anterior Cruciate Ligament Reconstruction: Clinical and Cost-Effectiveness Analyses. Am. J. Sports Med. 2017, 45, 892–899. [Google Scholar] [CrossRef]
- Hamada, M.; Shino, K.; Mitsuoka, T.; Abe, N.; Horibe, S. Cross-Sectional Area Measurement of the Semitendinosus Tendon for Anterior Cruciate Ligament Reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Mroz, T.E.; Joyce, M.J.; Steinmetz, M.P.; Lieberman, I.H.; Wang, J.C. Musculoskeletal Allograft Risks and Recalls in the United States. J. Am. Acad. Orthop. Surg. 2008, 16, 559–565. [Google Scholar] [CrossRef]
- Sikka, R.S.; Narvy, S.J.; Vangsness, C.T. Anterior Cruciate Ligament Allograft Surgery: Underreporting of Graft Source, Graft Processing, and Donor Age. Am. J. Sports Med. 2011, 39, 649–655. [Google Scholar] [CrossRef]
- Samsell, B.J.; Moore, M.A. Use of Controlled Low Dose Gamma Irradiation to Sterilize Allograft Tendons for ACL Reconstruction: Biomechanical and Clinical Perspective. Cell Tissue Bank. 2012, 13, 217–223. [Google Scholar] [CrossRef]
- Park, S.S.-H.; Dwyer, T.; Congiusta, F.; Whelan, D.B.; Theodoropoulos, J. Analysis of Irradiation on the Clinical Effectiveness of Allogenic Tissue When Used for Primary Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2015, 43, 226–235. [Google Scholar] [CrossRef]
- Roberson, T.A.; Abildgaard, J.T.; Wyland, D.J.; Siffri, P.C.; Geary, S.P.; Hawkins, R.J.; Tokish, J.M. “Proprietary Processed” Allografts: Clinical Outcomes and Biomechanical Properties in Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2017, 45, 3158–3167. [Google Scholar] [CrossRef]
- Carter, T.; Rabago, M. Allograft Anterior Cruciate Ligament Reconstruction in Patients Younger than 25 Years. J. Knee Surg. 2015, 29, 322–328. [Google Scholar] [CrossRef]
- Balsly, C.R.; Cotter, A.T.; Williams, L.A.; Gaskins, B.D.; Moore, M.A.; Wolfinbarger, L. Effect of Low Dose and Moderate Dose Gamma Irradiation on the Mechanical Properties of Bone and Soft Tissue Allografts. Cell Tissue Bank. 2008, 9, 289–298. [Google Scholar] [CrossRef]
- Greaves, L.L.; Hecker, A.T.; Brown, C.H. The Effect of Donor Age and Low-Dose Gamma Irradiation on the Initial Biomechanical Properties of Human Tibialis Tendon Allografts. Am. J. Sports Med. 2008, 36, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Aguila, C.; Delcroix, G.J.-R.; Kaimrajh, D.N.; Milne, E.L.; Temple, H.T.; Latta, L.L. Effects of Gamma Irradiation on the Biomechanical Properties of Peroneus Tendons. Open Access J. Sport. Med. 2016, 7, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Tejwani, S.G.; Chen, J.; Funahashi, T.T.; Love, R.; Maletis, G.B. Revision Risk After Allograft Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2015, 43, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Maestro Fernández, A.; Pipa Muñiz, I.; Rodríguez García, N.; Maestro, A. Two-Stage Anterior Cruciate Ligament Reconstruction Revision Surgery for Severe Bone Defects with Anterolateral Ligament Reconstruction Technique. Arthrosc. Tech. 2020, 9, e327–e337. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Rodríguez, N.; Pipa, I.; Toyos, C.; Lanuza, L.; Machado, F.; Castaño, C.; Maestro, S. Influence of Extra-Articular Augmentation on Clinical Outcomes and Survival in Patients Undergoing Anterior Cruciate Ligament Reconstruction: A Pseudorandomized Study. Medicina 2025, 61, 116. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, R.A.; Stine, R.; Torg, J.S. Lachman Test Evaluated. Quantification of a Clinical Observation. Clin. Orthop. Relat. Res. 1987, 216, 141–150. [Google Scholar] [CrossRef]
- Galway, R.D.; Beaupre, A.; McIntosh, D.L. Pivot Shift: A Clinical Sign of Symptomatic Anterior Cruciate Insufficiency. Bone Jt. Surg. Br. 1972, 54B, 763–764. [Google Scholar]
- Tegner, Y.; Lysholm, J. Rating Systems in the Evaluation of Knee Ligament Injuries. Clin. Orthop. Relat. Res. 1985, 43–49. [Google Scholar] [CrossRef]
- Gustavsson, A.; Neeter, C.; Thomeé, P.; Grävare Silbernagel, K.; Augustsson, J.; Thomeé, R.; Karlsson, J. A Test Battery for Evaluating Hop Performance in Patients with an ACL Injury and Patients Who Have Undergone ACL Reconstruction. Knee Surg. Sport. Traumatol. Arthrosc. 2006, 14, 778–788. [Google Scholar] [CrossRef]
- Hertel, J.; Braham, R.A.; Hale, S.A.; Olmsted-Kramer, L.C. Simplifying the Star Excursion Balance Test: Analyses of Subjects with and without Chronic Ankle Instability. J. Orthop. Sport. Phys. Ther. 2006, 36, 131–137. [Google Scholar] [CrossRef]
- Stiffler, M.R.; Sanfilippo, J.L.; Brooks, M.A.; Heiderscheit, B.C. Star Excursion Balance Test Performance Varies by Sport in Healthy Division I Collegiate Athletes. J. Orthop. Sport. Phys. Ther. 2015, 45, 772–780. [Google Scholar] [CrossRef]
- Hertel, J. Sensorimotor Deficits with Ankle Sprains and Chronic Ankle Instability. Clin. Sports Med. 2008, 27, 353–370. [Google Scholar] [CrossRef]
- Gribble, P.A.; Hertel, J.; Plisky, P. Using the Star Excursion Balance Test to Assess Dynamic Postural-Control Deficits and Outcomes in Lower Extremity Injury: A Literature and Systematic Review. J. Athl. Train. 2012, 47, 339–357. [Google Scholar] [CrossRef]
- Irrgang, J.J.; Anderson, A.F.; Boland, A.L.; Harner, C.D.; Kurosaka, M.; Neyret, P.; Richmond, J.C.; Shelborne, K.D. Development and Validation of the International Knee Documentation Committee Subjective Knee Form. Am. J. Sports Med. 2001, 29, 600–613. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Zein, A.; Ali, M.; Ali, H.; Saleh Elsaid, A.N.; Mahmoud, A.Z.; Osman, M.K.; Mohamed Soliman, A.M. Combined Anatomic Reconstruction of the Anterior Cruciate and Anterolateral Ligaments Using Hamstring Graft Through a Single Femoral Tunnel and with a Single Femoral Fixation. Arthrosc. Tech. 2017, 6, e567–e577. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Thaunat, M.; Freychet, B.; Pupim, B.H.B.; Murphy, C.G.; Claes, S. Outcome of a Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Technique with a Minimum 2-Year Follow-Up. Am. J. Sports Med. 2015, 43, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, A.P.; Iorio, R.; De Carli, A.; Bonifazi, A.; Iorio, C.; Gatti, A.; Rossi, C.; Ferretti, A. An Extra-Articular Procedure Improves the Clinical Outcome in Anterior Cruciate Ligament Reconstruction with Hamstrings in Female Athletes. Int. Orthop. 2013, 37, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Monaco, E.; Maestri, B.; Conteduca, F.; Mazza, D.; Iorio, C.; Ferretti, A. Extra-Articular ACL Reconstruction and Pivot Shift. Am. J. Sports Med. 2014, 42, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- American Association of Tissue Banks (AATB). AATB Standards for Tissue Banking, 15th ed.; AATB: McLean, VA, USA, 2024. [Google Scholar]
- Barker, J.U.; Drakos, M.C.; Maak, T.G.; Warren, R.F.; Williams, R.J.; Allen, A.A. Effect of Graft Selection on the Incidence of Postoperative Infection in Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2010, 38, 281–286. [Google Scholar] [CrossRef]
- Gorschewsky, O.; Klakow, A.; Riechert, K.; Pitzl, M.; Becker, R. Clinical Comparison of the Tutoplast Allograft and Autologous Patellar Tendon (Bone-Patellar Tendon-Bone) for the Reconstruction of the Anterior Cruciate Ligament. Am. J. Sports Med. 2005, 33, 1202–1209. [Google Scholar] [CrossRef]
- Maletis, G.B.; Chen, J.; Inacio, M.C.S.; Love, R.M.; Funahashi, T.T. Increased Risk of Revision After Anterior Cruciate Ligament Reconstruction with Bone–Patellar Tendon–Bone Allografts Compared with Autografts. Am. J. Sports Med. 2017, 45, 1333–1340. [Google Scholar] [CrossRef]
- Kraeutler, M.J.; Bravman, J.T.; McCarty, E.C. Bone–Patellar Tendon–Bone Autograft Versus Allograft in Outcomes of Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2013, 41, 2439–2448. [Google Scholar] [CrossRef]
- Min, J.H.; Yoon, H.-K.; Oh, H.-C.; Youk, T.; Ha, J.-W.; Park, S.-H. Graft Choice to Decrease the Revision Rate of Anterior Cruciate Ligament Reconstruction: A Nationwide Retrospective Cohort Study. Sci. Rep. 2024, 14, 20004. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.W.; Huston, L.J.; Haas, A.K.; Spindler, K.P.; Nwosu, S.K.; Allen, C.R.; Anderson, A.F.; Cooper, D.E.; DeBerardino, T.M.; Dunn, W.R.; et al. Effect of Graft Choice on the Outcome of Revision Anterior Cruciate Ligament Reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am. J. Sports Med. 2014, 42, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Romanini, E.; D’Angelo, F.; De Masi, S.; Adriani, E.; Magaletti, M.; Lacorte, E.; Laricchiuta, P.; Sagliocca, L.; Morciano, C.; Mele, A. Graft Selection in Arthroscopic Anterior Cruciate Ligament Reconstruction. J. Orthop. Traumatol. 2010, 11, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kraeutler, M.J.; Welton, K.L.; McCarty, E.C.; Bravman, J.T. Revision Anterior Cruciate Ligament Reconstruction. J. Bone Jt. Surg. 2017, 99, 1689–1696. [Google Scholar] [CrossRef]
- Maletis, G.B.; Inacio, M.C.S.; Desmond, J.L.; Funahashi, T.T. Reconstruction of the Anterior Cruciate Ligament. Bone Jt. J. 2013, 95-B, 623–628. [Google Scholar] [CrossRef]
- Kaeding, C.C.; Aros, B.; Pedroza, A.; Pifel, E.; Amendola, A.; Andrish, J.T.; Dunn, W.R.; Marx, R.G.; McCarty, E.C.; Parker, R.D.; et al. Allograft Versus Autograft Anterior Cruciate Ligament Reconstruction. Sports Health A Multidiscip. Approach 2011, 3, 73–81. [Google Scholar] [CrossRef]
- Lamblin, C.J.; Waterman, B.R.; Lubowitz, J.H. Anterior Cruciate Ligament Reconstruction with Autografts Compared with Non-Irradiated, Non-Chemically Treated Allografts. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1113–1122. [Google Scholar] [CrossRef]
- Barber, F.A.; Cowden, C.H.; Sanders, E.J. Revision Rates After Anterior Cruciate Ligament Reconstruction Using Bone–Patellar Tendon–Bone Allograft or Autograft in a Population 25 Years Old and Younger. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 483–491. [Google Scholar] [CrossRef]
- Krych, A.J.; Jackson, J.D.; Hoskin, T.L.; Dahm, D.L. A Meta-Analysis of Patellar Tendon Autograft Versus Patellar Tendon Allograft in Anterior Cruciate Ligament Reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 292–298. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Liu, Y.; Yang, S. Comparison of Clinical Outcomes of Using the Nonirradiated and Irradiated Allograft for Anterior Cruciate Ligament (ACL) Reconstruction: A Systematic Review Update and Meta-Analysis. Medicine 2022, 101, e29990. [Google Scholar] [CrossRef]
- Hamer, A.J.; Stockley, I.; Elson, R.A. Changes in Allograft Bone Irradiated at Different Temperatures. J. Bone Jt. Surg. 1999, 81, 342–344. [Google Scholar] [CrossRef]
- Grieb, T.A.; Forng, R.-Y.; Stafford, R.E.; Lin, J.; Almeida, J.; Bogdansky, S.; Ronholdt, C.; Drohan, W.N.; Burgess, W.H. Effective Use of Optimized, High-Dose (50 kGy) Gamma Irradiation for Pathogen Inactivation of Human Bone Allografts. Biomaterials 2005, 26, 2033–2042. [Google Scholar] [CrossRef]
- Meena, A.; Di Paolo, S.; Grassi, A.; Raj, A.; Farinelli, L.; Hoser, C.; Tapasvi, S.; Zaffagnini, S.; Fink, C. No Difference in Patient Reported Outcomes, Laxity, and Failure Rate after Revision ACL Reconstruction with Quadriceps Tendon Compared to Hamstring Tendon Graft: A Systematic Review and Meta-Analysis. Knee Surg. Sport. Traumatol. Arthrosc. 2023, 31, 3316–3329. [Google Scholar] [CrossRef] [PubMed]
- Grassi, A.; Zicaro, J.P.; Costa-Paz, M.; Samuelsson, K.; Wilson, A.; Zaffagnini, S.; Condello, V. Good Mid-Term Outcomes and Low Rates of Residual Rotatory Laxity, Complications and Failures after Revision Anterior Cruciate Ligament Reconstruction (ACL) and Lateral Extra-Articular Tenodesis (LET). Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; McCormick, J.R.; Credille, K.; Dandu, N.; Wang, Z.; Trasolini, N.A.; Darwish, R.Y.; Chahla, J.; Yanke, A.B. Patient Age and Activity Level, Posterior Tibial Slope, and Use of Allograft Are Significant Risk Factors for Anterior Cruciate Ligament Reconstruction Failure: A Systematic Review. Arthrosc. Sport. Med. Rehabil. 2025, 7, 101075. [Google Scholar] [CrossRef] [PubMed]
- Kentel, M.; Kentel, M.; Korolczuk, K.; Witkowski, J. Comparative Evaluation of Treatment Outcomes of Revision Anterior Cruciate Ligament Reconstruction Using Allograft and Semitendinosus Autograft. J. Clin. Med. 2024, 14, 133. [Google Scholar] [CrossRef]
- Grassi, A.; Kim, C.; Muccioli, G.M.M.; Zaffagnini, S.; Amendola, A. What Is the Mid-Term Failure Rate of Revision ACL Reconstruction? A Systematic Review. Clin. Orthop. Relat. Res. 2017, 475, 2484–2499. [Google Scholar] [CrossRef]
- Carey, J.L.; Dunn, W.R.; Dahm, D.L.; Zeger, S.L.; Spindler, K.P. A Systematic Review of Anterior Cruciate Ligament Reconstruction with Autograft Compared with Allograft. J. Bone Jt. Surg.-Am. Vol. 2009, 91, 2242–2250. [Google Scholar] [CrossRef]
- Shea, K.G.; Carey, J.L.; Richmond, J.; Sandmeier, R.; Pitts, R.T.; Polousky, J.D.; Chu, C.; Shultz, S.J.; Ellen, M.; Smith, A.; et al. The American Academy of Orthopaedic Surgeons Evidence-Based Guideline on Management of Anterior Cruciate Ligament Injuries. J. Bone Jt. Surg. Am. 2015, 97, 672–674. [Google Scholar] [CrossRef]

| Variable | N | % | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Males | 33 | 86.8% | - | - | - | - |
| Females | 5 | 13.2% | - | - | - | - |
| Laterality | ||||||
| Right | 21 | 55.3% | - | - | - | - |
| Left | 17 | 44.7% | - | - | - | - |
| Age | 38 | - | 36.4 | 9.7 | 18 | 53 |
| Height | 38 | - | 174.7 | 7.2 | 153 | 193 |
| Weight | 38 | - | 75.9 | 9.5 | 50 | 93 |
| Body mass index | 38 | 24.8 | 2.7 | 19.3 | 31.5 | |
| Tegner score—PreOp | ||||||
| Lower than a 7 | 22 | 57.9% | - | - | - | - |
| Equal to or higher than 7 | 16 | 42.1% | - | - | - | - |
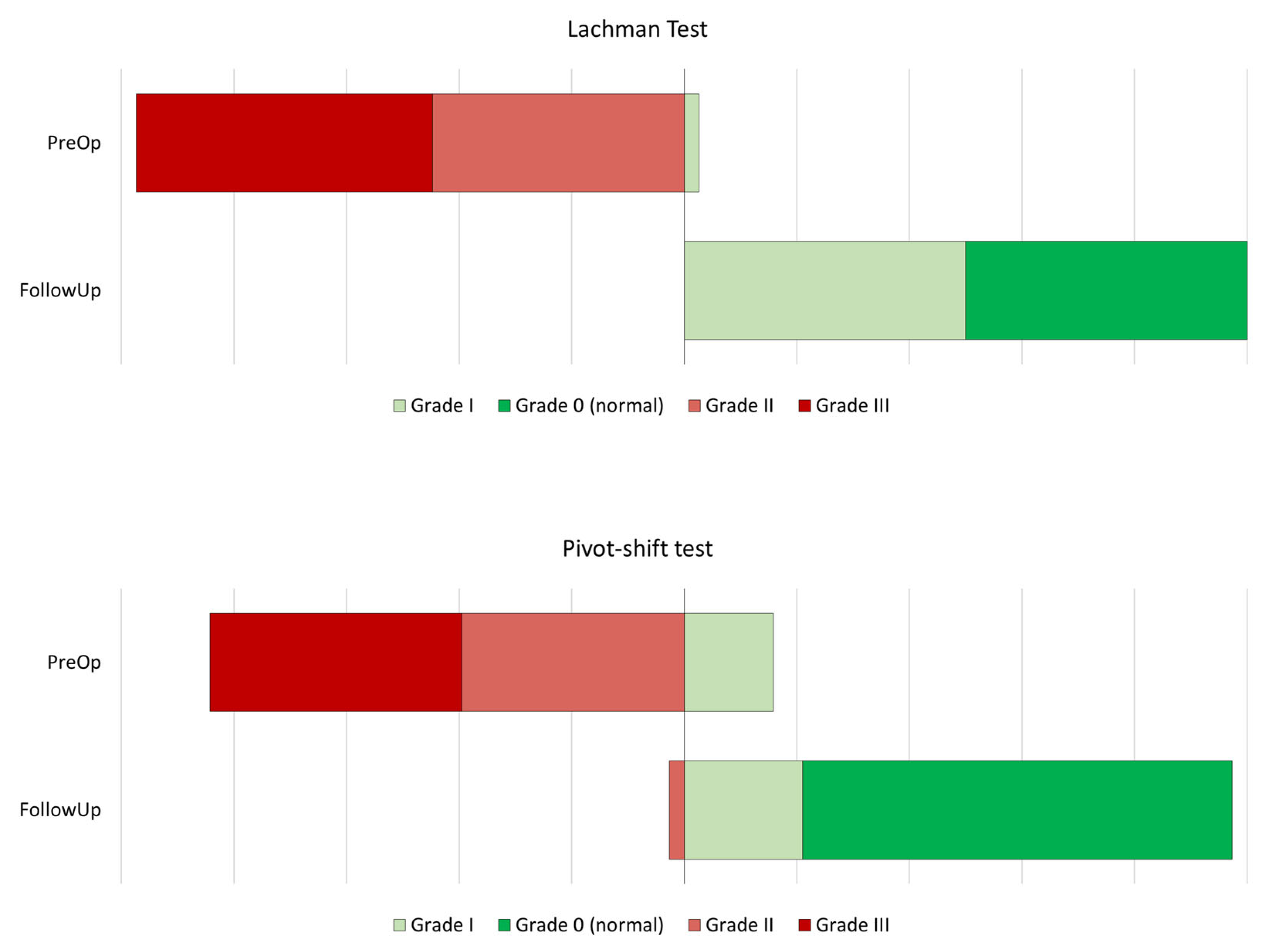
| Lachman test—PreOp | ||||||
| Grade I | 1 | 2.6% | - | - | - | - |
| Grade II | 17 | 44.7% | - | - | - | - |
| Grade III | 20 | 52.6% | - | - | - | - |
| Pivot-shift—PreOp | ||||||
| Grade I | 6 | 15.8% | - | - | - | - |
| Grade II | 15 | 39.5% | - | - | - | - |
| Grade III | 17 | 44.7% | - | - | - | - |
| Variable | N | % | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
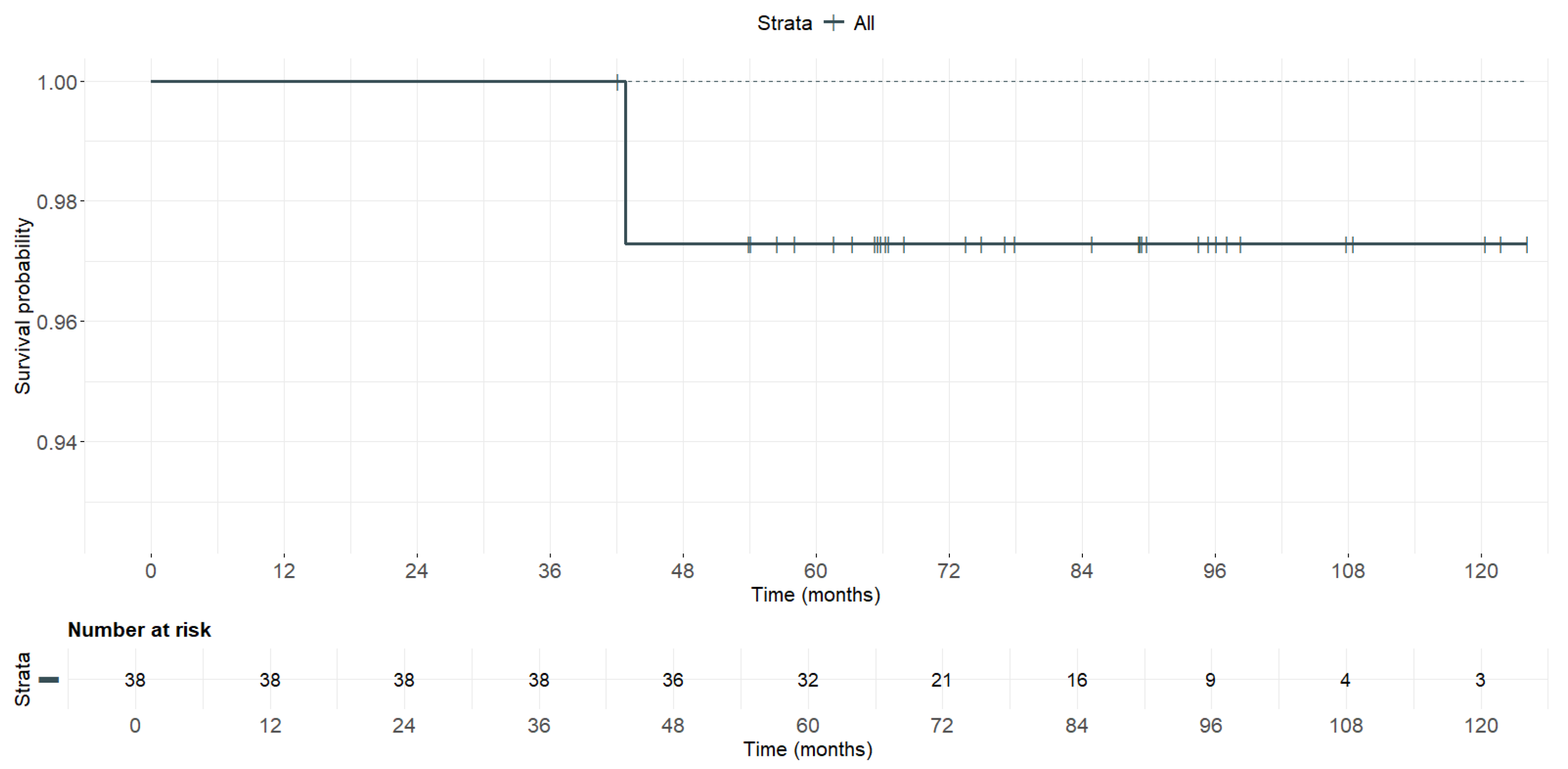
| Follow-up time | 38 | - | 79.3 | 20.5 | 42 | 124 |
| Lachman test—FollowUp | ||||||
| Normal | 19 | 50.0% | - | - | - | - |
| Grade I | 19 | 50.0% | - | - | - | - |
| Pivot-shift—FollowUp | ||||||
| Normal | 29 | 76.3% | - | - | - | - |
| Grade I | 8 | 21.1% | - | - | - | - |
| Grade II | 1 | 2.6% | - | - | - | - |
| IKDC Score—FollowUp | ||||||
| A—Normal | 35 | 92.1% | - | - | - | - |
| B—Nearly normal | 3 | 7.9% | - | - | - | - |
| Subjective IKDC—FollowUp | 38 | - | 0.80 | 0.07 | 0.57 | 0.95 |
| Pain (VAS)—FollowUp | 38 | - | 1.18 | 1.8 | 0 | 7 |
| Single hop test—LSI | 38 | - | 95.8% | 13.0% | 58.9% | 135.0% |
| Triple hop test—LSI | 38 | - | 95.3% | 10.9% | 58.7% | 111.4% |
| Crossover hop test—LSI | 38 | - | 96.7% | 25.4% | 81.3% | 203.7% |
| 6 m timed hop test—LSI | 38 | - | 104.0% | 12.9% | 77.8% | 148.0% |
| Composite YBT score—LSI | 38 | - | 98.7% | 7.9% | 81.8% | 131.5% |
| Return to sport | ||||||
| Same level or higher | 28 | 73.7% | - | - | - | - |
| Could not reach same level | 10 | 26.3% | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestro, A.; Toyos, C.; Rodríguez, N.; Pipa, I.; Lanuza, L.; Machado, F.; Castaño, C.; Maestro, S. The Role of Allografts in Revision ACL Reconstruction. Medicina 2025, 61, 1350. https://doi.org/10.3390/medicina61081350
Maestro A, Toyos C, Rodríguez N, Pipa I, Lanuza L, Machado F, Castaño C, Maestro S. The Role of Allografts in Revision ACL Reconstruction. Medicina. 2025; 61(8):1350. https://doi.org/10.3390/medicina61081350
Chicago/Turabian StyleMaestro, Antonio, Carmen Toyos, Nicolás Rodríguez, Iván Pipa, Lucía Lanuza, Filipe Machado, César Castaño, and Santiago Maestro. 2025. "The Role of Allografts in Revision ACL Reconstruction" Medicina 61, no. 8: 1350. https://doi.org/10.3390/medicina61081350
APA StyleMaestro, A., Toyos, C., Rodríguez, N., Pipa, I., Lanuza, L., Machado, F., Castaño, C., & Maestro, S. (2025). The Role of Allografts in Revision ACL Reconstruction. Medicina, 61(8), 1350. https://doi.org/10.3390/medicina61081350






