Elevated Leukocyte Glucose Index (LGI) Is Associated with Diabetic Ketoacidosis (DKA) Severity and Presence of Microvascular Complications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Study Outcome
2.4. Statistical Analysis
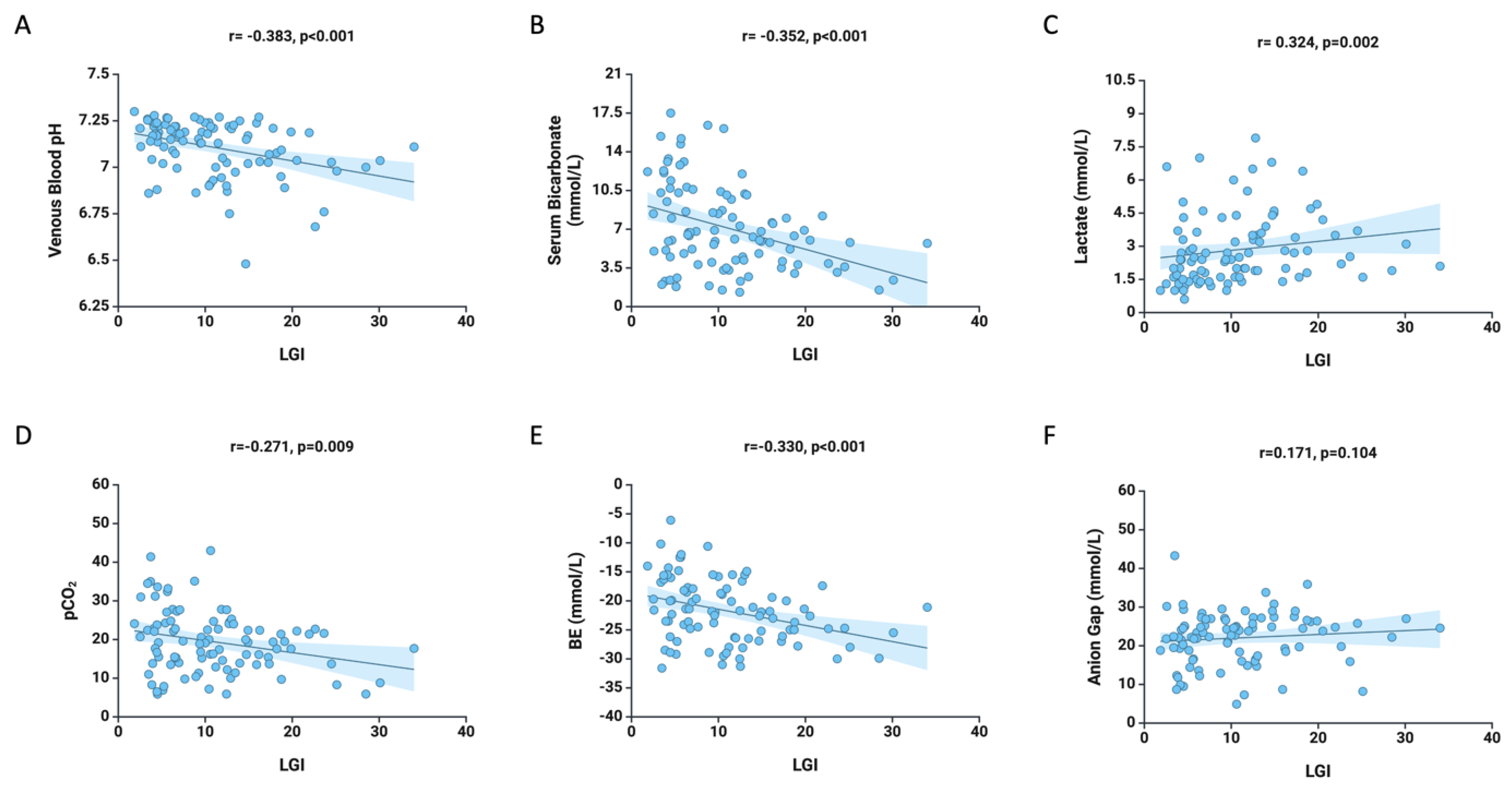
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DKA | Diabetic Ketoacidosis |
| ADA | American Diabetes Association |
| ICU | intensive care unit |
| IL | interleukin |
| SD | Standard Deviation |
| bpm | beats per minute |
| SBP | systolic blood pressure |
| DBP | dystolic blood pressure |
| BUN | blood urea nitrogen |
References
- Calimag, A.P.P.; Chlebek, S.; Lerma, E.V.; Chaiban, J.T. Diabetic Ketoacidosis. Dis. Mon. 2023, 69, 101418. [Google Scholar] [CrossRef]
- Dhatariya, K.K.; Glaser, N.S.; Codner, E.; Umpierrez, G.E. Diabetic Ketoacidosis. Nat. Rev. Dis. Primers 2020, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S158–S178. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Popa, S.G.; Mota, E.; Mitrea, A.; Catrinoiu, D.; Cheta, D.M.; Guja, C.; Hancu, N.; Ionescu-Tirgoviste, C.; Lichiardopol, R.; et al. Prevalence of Diabetes Mellitus and Prediabetes in the Adult Romanian Population: PREDATORR Study. J. Diabetes 2016, 8, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Davis, G.M.; ElSayed, N.A.; Fadini, G.P.; Galindo, R.J.; Hirsch, I.B.; Klonoff, D.C.; McCoy, R.G.; Misra, S.; Gabbay, R.A.; et al. Hyperglycaemic Crises in Adults with Diabetes: A Consensus Report. Diabetologia 2024, 67, 1455–1479. [Google Scholar] [CrossRef]
- Newton, C.A.; Raskin, P. Diabetic Ketoacidosis in Type 1 and Type 2 Diabetes Mellitus: Clinical and Biochemical Differences. Arch. Intern. Med. 2004, 164, 1925–1931. [Google Scholar] [CrossRef]
- Wang, Y.; Desai, M.; Ryan, P.B.; DeFalco, F.J.; Schuemie, M.J.; Stang, P.E.; Berlin, J.A.; Yuan, Z. Incidence of Diabetic Ketoacidosis among Patients with Type 2 Diabetes Mellitus Treated with SGLT2 Inhibitors and Other Antihyperglycemic Agents. Diabetes Res. Clin. Pract. 2017, 128, 83–90. [Google Scholar] [CrossRef]
- Zhong, V.W.; Juhaeri, J.; Mayer-Davis, E.J. Trends in Hospital Admission for Diabetic Ketoacidosis in Adults with Type 1 and Type 2 Diabetes in England, 1998–2013: A Retrospective Cohort Study. Diabetes Care 2018, 41, 1870–1877. [Google Scholar] [CrossRef]
- Tilinca, M.C.; Nania, C.; Pal, S.; Tilinca, R.; Szabo, M.; Nemes-Nagy, E. Evaluation of Metabolic and Hydroelectrolytic Disturbances in Patients Diagnosed with Diabetic Ketoacidosis. Rev. Chim. 2020, 71, 239–243. [Google Scholar] [CrossRef]
- Scutca, A.-C.; Nicoară, D.-M.; Mang, N.; Jugănaru, I.; Brad, G.-F.; Mărginean, O. Correlation between Neutrophil-to-Lymphocyte Ratio and Cerebral Edema in Children with Severe Diabetic Ketoacidosis. Biomedicines 2023, 11, 2976. [Google Scholar] [CrossRef]
- Hoffman, W.H.; Burek, C.L.; Waller, J.L.; Fisher, L.E.; Khichi, M.; Mellick, L.B. Cytokine Response to Diabetic Ketoacidosis and Its Treatment. Clin. Immunol. 2003, 108, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Stentz, F.B.; Umpierrez, G.E.; Cuervo, R.; Kitabchi, A.E. Proinflammatory Cytokines, Markers of Cardiovascular Risks, Oxidative Stress, and Lipid Peroxidation in Patients with Hyperglycemic Crises. Diabetes 2004, 53, 2079–2086. [Google Scholar] [CrossRef]
- Aslan Sirakaya, H.; Sipahioglu, H.; Cetinkaya, A.; Aydin, K. Relationship Between Inflammatory Markers (IL-6, Neutrophil–Lymphocyte Ratio, and C-Reactive Protein-Albumin Ratio) and Diabetic Ketoacidosis Severity: Correlation with Clinical Outcomes. Medicina 2025, 61, 321. [Google Scholar] [CrossRef]
- Aon, M.; Aoun, A.H.; Alshami, A.; Alharbi, A.; Alshammari, F.; Alnajjar, M.; Almutawtah, A.; Bin Naji, B.; Alsaeed, A.; Abdelwahab, O.A. Association of the Systemic Immune-Inflammation Index (SII) and Severity of Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus: A Retrospective Cohort Study. Ann. Med. Surg. 2024, 86, 3865–3872. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Arbanasi, E.M.; Bartus, R.; Mărginean, L.; Cordoș, B.A.; Ciucanu, C.C.; Russu, E. Prognostic Value of Leukocyte-Glycemic Index in Long-Term Evolution of Diabetic Patients with Peripheral Arterial Disease Following Endovascular Treatment. Acta Marisiensis-Ser. Medica 2024, 70, 58–63. [Google Scholar] [CrossRef]
- Danielsson, P.; Truedsson, L.; Eriksson, K.F.; Norgren, L. Inflammatory Markers and IL-6 Polymorphism in Peripheral Arterial Disease with and without Diabetes Mellitus. Vasc. Med. 2005, 10, 191–198. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Zhang, X.-J.; Liu, Y.-M.; Geng, L.-Y.; Guan, L.-Y.; Li, X.-H. Comparison of the Triglyceride Glucose Index and Blood Leukocyte Indices as Predictors of Metabolic Syndrome in Healthy Chinese Population. Sci. Rep. 2021, 11, 10036. [Google Scholar] [CrossRef] [PubMed]
- Aslan, N.; Güneysu, F.; Yürümez, Y.; Güner, N.G.; Akdeniz, S.; Kaner, M. Leukoglycemic Index and Its Prognostic Implications in Diabetic and Nondiabetic Patients with Acute Pulmonary Embolism. Med. Sci. Monit. 2025, 31, e947156. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-Y.; Liu, H.-X.; Cheng, L.-C.; Luo, Y.; Yang, S.-Q.; Chen, X.; Cai, L. Prognostic Value of the Leuko-Glycemic Index in Acute Myocardial Infarction Patients with or without Diabetes. Diabetes Metab. Syndr. Obes. 2022, 15, 1725–1736. [Google Scholar] [CrossRef]
- Kilic, O.; Buber, I.; Kahraman, F. Predicting the Severity of Coronary Artery Disease: Can the Leukocyte Glucose Index Be Used? J. Coll. Physicians Surg. Pak. 2022, 32, 1519–1523. [Google Scholar] [CrossRef]
- Seoane, L.A.; Burgos, L.; Espinoza, J.C.; Furmento, J.F.; Benzadón, M.N.; Vrancic, J.M.; Piccinini, F.; Navia, D. Prognostic Value of the Leuko-Glycaemic Index in the Postoperative Period of Coronary Artery Bypass Grafting. Braz. J. Cardiovasc. Surg. 2021, 36, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Hernández, W.M.; Soto, L.F.; del Rosario-Trinidad, M.; Farfan-Morales, C.N.; de Jesús-González, L.A.; Martínez-Mier, G.; Osuna-Ramos, J.F.; Bastida-González, F.; Bernal-Dolores, V.; del Ángel, R.M.; et al. Leukocyte Glucose Index as a Novel Biomarker for COVID-19 Severity. Sci. Rep. 2022, 12, 14956. [Google Scholar] [CrossRef]
- Nafea, O.E.; Abdelhamid, W.G.; Ibrahim, F. The Role of the Leukocyte Glucose Index in Predicting Clinical Outcomes in Acute Methanol Toxicity. Toxicol. Rep. 2025, 14, 101994. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lee, A.-Y.; Kang, J.-H.; Yu, B.-Y.; Kim, S.-J. Association of Fasting Glucose Level with Neutrophil-Lymphocyte Ratio Compared to Leukocyte Count and Serum C-Reactive Protein. Korean J. Fam. Med. 2018, 39, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, R.; Roshdi Dizaji, S.; Vazirizadeh-Mahabadi, M.; Sarveazad, A.; Forouzannia, S.A. Prognostic Value of the Leuko-Glycemic Index in Acute Myocardial Infarction; a Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2023, 11, e25. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, R.; Aghajani, M.H.; Parandin, R.; Taherpour, N.; Ahmadzadeh, K.; Sarveazad, A. Leuko-Glycemic Index in the Prognosis of Acute Myocardial Infarction; a Cohort Study on Coronary Angiography and Angioplasty Registry. Arch. Acad. Emerg. Med. 2023, 11, e63. [Google Scholar] [CrossRef]
- Peker, T.; Özbek, M.; Boyraz, B.; Aslan, S.F.; Demir, M.; Aslan, B. Prognostic Value of the Leuko-Glycemic Index in Coronary Chronic Total Occlusion Patients. Eur. Res. J. 2023, 9, 1099–1104. [Google Scholar] [CrossRef]
- Demir, M. The Leuko-Glycemic Index Can Predict Multivessel Disease in the Elderly Acute Myocardial Infarction Population? A Retrospective Cohort Study. J. Health Sci. Med./JHSM 2023, 6, 1119–1124. [Google Scholar] [CrossRef]
- Charoenpiriya, A.; Chailurkit, L.; Ongphiphadhanakul, B. Comparisons of Biochemical Parameters and Diabetic Ketoacidosis Severity in Adult Patients with Type 1 and Type 2 Diabetes. BMC Endocr. Disord. 2022, 22, 7. [Google Scholar] [CrossRef]
- Ata, F.; Khan, A.A.; Khamees, I.; Mohammed, B.Z.M.; Barjas, H.H.; Muthanna, B.; Bashir, M.; Kartha, A. Differential Evolution of Diabetic Ketoacidosis in Adults with Pre-Existent versus Newly Diagnosed Type 1 and Type 2 Diabetes Mellitus. BMC Endocr. Disord. 2023, 23, 193. [Google Scholar] [CrossRef]
- Almazrouei, R.; Siddiqua, A.R.; Alnuaimi, M.; Al-Shamsi, S.; Govender, R. Clinical and Biochemical Characteristics of Diabetic Ketoacidosis in Adults with Type 1 or Type 2 Diabetes at a Tertiary Hospital in the United Arab Emirates. Front. Clin. Diabetes Healthc. 2022, 3, 918253. [Google Scholar] [CrossRef]
- Tittel, S.R.; Sondern, K.M.; Weyer, M.; Poeplau, T.; Sauer, B.M.; Schebek, M.; Ludwig, K.-H.; Hammer, F.; Fröhlich-Reiterer, E.; Holl, R.W.; et al. Multicentre Analysis of Hyperglycaemic Hyperosmolar State and Diabetic Ketoacidosis in Type 1 and Type 2 Diabetes. Acta Diabetol. 2020, 57, 1245–1253. [Google Scholar] [CrossRef]
- Kamata, Y.; Takano, K.; Kishihara, E.; Watanabe, M.; Ichikawa, R.; Shichiri, M. Distinct Clinical Characteristics and Therapeutic Modalities for Diabetic Ketoacidosis in Type 1 and Type 2 Diabetes Mellitus. J. Diabetes Its Complicat. 2017, 31, 468–472. [Google Scholar] [CrossRef]
- Emil-Marian, A.; Reka, K.; Adrian, M.; Septimiu, V.; Eliza-Mihaela, A.; Eliza, R. Impact of COVID-19 Pandemic on Vascular Surgery Unit Activity in Central Romania. Front. Surg. 2022, 9, 883935. [Google Scholar] [CrossRef]
- Khan, F.; Paladino, L.; Sinert, R. The Impact of COVID-19 on Diabetic Ketoacidosis Patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102389. [Google Scholar] [CrossRef]
- Stoian, M.; Andone, A.; Boeriu, A.; Bândilă, S.R.; Dobru, D.; Laszlo, S.Ș.; Corău, D.; Arbănași, E.M.; Russu, E.; Stoian, A. COVID-19 and Clostridioides Difficile Coinfection Analysis in the Intensive Care Unit. Antibiotics 2024, 13, 367. [Google Scholar] [CrossRef]
- Palermo, N.E.; Sadhu, A.R.; McDonnell, M.E. Diabetic Ketoacidosis in COVID-19: Unique Concerns and Considerations. J. Clin. Endocrinol. Metab. 2020, 105, 2819–2829. [Google Scholar] [CrossRef]
- Stoian, A.; Bajko, Z.; Stoian, M.; Cioflinc, R.A.; Niculescu, R.; Arbănași, E.M.; Russu, E.; Botoncea, M.; Bălașa, R. The Occurrence of Acute Disseminated Encephalomyelitis in SARS-CoV-2 Infection/Vaccination: Our Experience and a Systematic Review of the Literature. Vaccines 2023, 11, 1225. [Google Scholar] [CrossRef]
- Alamuri, T.T.; Mahesh, S.; Dell’Aquila, K.; Leong, T.J.; Jennings, R.; Duong, T.Q. COVID-19 Associated Ketosis and Diabetic Ketoacidosis: A Rapid Review. Diabetes Obes. Metab. 2023, 25, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Mureșan, A.V.; Russu, E.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Arbănași, E.M.; Voidăzan, S.T. Negative Impact of the COVID-19 Pandemic on Kidney Disease Management—A Single-Center Experience in Romania. J. Clin. Med. 2022, 11, 2452. [Google Scholar] [CrossRef] [PubMed]
- Halmaciu, I.; Arbănași, E.M.; Kaller, R.; Mureșan, A.V.; Arbănași, E.M.; Bacalbasa, N.; Suciu, B.A.; Cojocaru, I.I.; Runcan, A.I.; Grosu, F.; et al. Chest CT Severity Score and Systemic Inflammatory Biomarkers as Predictors of the Need for Invasive Mechanical Ventilation and of COVID-19 Patients’ Mortality. Diagnostics 2022, 12, 2089. [Google Scholar] [CrossRef] [PubMed]
- Arbănași, E.M.; Halmaciu, I.; Kaller, R.; Mureșan, A.V.; Arbănași, E.M.; Suciu, B.A.; Coșarcă, C.M.; Cojocaru, I.I.; Melinte, R.M.; Russu, E. Systemic Inflammatory Biomarkers and Chest CT Findings as Predictors of Acute Limb Ischemia Risk, Intensive Care Unit Admission, and Mortality in COVID-19 Patients. Diagnostics 2022, 12, 2379. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Hălmaciu, I.; Arbănași, E.M.; Kaller, R.; Arbănași, E.M.; Budișcă, O.A.; Melinte, R.M.; Vunvulea, V.; Filep, R.C.; Mărginean, L.; et al. Prognostic Nutritional Index, Controlling Nutritional Status (CONUT) Score, and Inflammatory Biomarkers as Predictors of Deep Vein Thrombosis, Acute Pulmonary Embolism, and Mortality in COVID-19 Patients. Diagnostics 2022, 12, 2757. [Google Scholar] [CrossRef]
- Buicu, A.-L.; Cernea, S.; Benedek, I.; Buicu, C.-F.; Benedek, T. Systemic Inflammation and COVID-19 Mortality in Patients with Major Noncommunicable Diseases: Chronic Coronary Syndromes, Diabetes and Obesity. J. Clin. Med. 2021, 10, 1545. [Google Scholar] [CrossRef]
- Bolla, A.M.; Loretelli, C.; Montefusco, L.; Finzi, G.; Abdi, R.; Ben Nasr, M.; Lunati, M.E.; Pastore, I.; Bonventre, J.V.; Nebuloni, M.; et al. Inflammation and Vascular Dysfunction: The Negative Synergistic Combination of Diabetes and COVID-19. Diabetes/Metab. Res. Rev. 2022, 38, e3565. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Narwaria, M.; Singh, A.; Kumar, S.; Haque, M. Detecting Diabetic Ketoacidosis with Infection: Combating a Life-Threatening Emergency with Practical Diagnostic Tools. Diagnosis 2023, 13, 2441. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Giagulli, V.A.; de Pergola, G.; Guastamacchia, E.; Jirillo, E.; Triggiani, V. Hyperglycemia-Induced Immune System Disorders in Diabetes Mellitus and the Concept of Hyperglycemic Memory of Innate Immune Cells: A Perspective. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 367–370. [Google Scholar] [CrossRef]
- Raya-Cano, E.; Vaquero-Abellán, M.; Molina-Luque, R.; Molina-Recio, G.; Guzmán-García, J.M.; Jiménez-Mérida, R.; Romero-Saldaña, M. Association between Metabolic Syndrome and Leukocytes: Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7044. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Florea, E.; Arbănași, E.-M.; Bartus, R.; Arbănași, E.-M.; Ion, A.P.; Cordoș, B.A.; Halatiu, V.B.; Niculescu, R.; Stoian, A.; et al. Elevated Leukocyte Glucose Index Is Associated with Long-Term Arteriovenous Fistula Failure in Dialysis Patients. J. Clin. Med. 2024, 13, 2037. [Google Scholar] [CrossRef]
- Pattharanitima, P.; Thongprayoon, C.; Kaewput, W.; Qureshi, F.; Qureshi, F.; Petnak, T.; Srivali, N.; Gembillo, G.; O’Corragain, O.A.; Chesdachai, S.; et al. Machine Learning Prediction Models for Mortality in Intensive Care Unit Patients with Lactic Acidosis. J. Clin. Med. 2021, 10, 5021. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The Burden and Risks of Emerging Complications of Diabetes Mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Patients n = 94 | |
|---|---|---|
| Age, mean ± SD | 47.75 ± 18.02 | |
| Male, no. (%) | 63 (67.02%) | |
| Type I Diabetes, no. (%) | 48 (51.06%) | |
| Type II Diabetes, no. (%) | 46 (48.94%) | |
| Height (cm), median (Q1–Q3) | 168 (165–175) | |
| Weight (kg), median (Q1–Q3) | 62 (57–70) | |
| BMI, median (Q1–Q3) | 21.48 (21.32–25.25) | |
| Duration of Diabetes (year), median (Q1–Q3) | 6 (3–15) | |
| Heart Rate, median (Q1–Q3) | 104 (92–120) | |
| Systolic Blood Pressure, median (Q1–Q3) | 128 (115–145) | |
| Diastolic Blood Pressure, median (Q1–Q3) | 78 (70–86) | |
| Severity of DKA | Mild, no. (%) | 15 (15.95%) |
| Moderate, no. (%) | 54 (57.45%) | |
| Severe, no. (%) | 25 (26.60%) | |
| Diabetes-Related Microvascular Complications | Nephropathy, no. (%) | 7 (7.44%) |
| Retinopathy, no. (%) | 16 (17.02%) | |
| Neuropathy, no. (%) | 35 (37.23%) | |
| Laboratory data, median (Q1–Q3) | ||
| WBC | 16.04 (12.24–23.57) | |
| Potassium, mmol/L | 4.67 (4.13–5.6) | |
| Sodium, mmol/L | 134 (131–137) | |
| Clor, mmol/L | 104 (101–110) | |
| Calcium, mmol/L | 1.26 (1.16–1.32) | |
| HbA1c, (%) | 11.2 (10.02–12.47) | |
| Glucose, (mg/dL) | 530 (423–730) | |
| BUN, (mg/dL) | 59.91 (36.15–83.29) | |
| Creatinine, (mg/dL) | 1.71 (1.38–2.2) | |
| Erytrocytes, ×106/uL | 4.7 (4.33–5.17) | |
| Hemoglobin, g/dL | 14.2 (13.12–15.87) | |
| Hematocrit, % | 42.35 (38.9–47.32) | |
| Neutrophils, ×103/uL | 13.94 (9.78–20.34) | |
| Lymphocytes, ×103/uL | 1.42 (1.01–2.38) | |
| Serum albumin, g/dL | 3.54 (3.11–3.81) | |
| Total protein, g/dL | 5.84 (5.33–6.21) | |
| Total cholesterol, mg/dL | 173.15 (138.82–204.15) | |
| Triglycerides, mg/dL | 130.1 (96.35–258.4) | |
| Amylase, U/L | 48 (34–86) | |
| Total bilirubin, mg/dL | 0.46 (0.28–0.66) | |
| ALT, U/L | 20.8 (13–27) | |
| AST, U/L | 22 (16–30) | |
| Venous blood pH | 7.15 (7.02–7.22) | |
| PvCO2 | 19.45 (13.92–24.07) | |
| PvO2 | 55.5 (40.85–102) | |
| Base excess, mmol/L | −21.8 (−26.18–−17.47) | |
| Lactate, mmol/L | 2.4 (1.6–3.6) | |
| Bicarbonate, mmol/L | 6.35 (4.12–10) | |
| Anion gap, mEq/L | 23.4 (18.5–26.4) | |
| LGI | 9.8 (5.28–14.48) | |
| Variables | Mild n = 15 | Moderate n = 54 | Severe n = 25 | p Value | ||
|---|---|---|---|---|---|---|
| 1–2 | 1–3 | 2–3 | ||||
| Age mean ± SD | 41.13 ± 19.01 | 50.05 ± 18.53 | 46.76 ± 15.77 | 0.188 | 0.459 | 0.556 |
| Male no. (%) | 8 (53.33%) | 39 (72.22%) | 16 (64.00%) | 0.171 | 0.506 | 0.461 |
| Type I Diabetes, no. (%) | 9 (60.0%) | 39 (72.22%) | 16 (64.0%) | 0.365 | 0.800 | 0.461 |
| Type II Diabetes, no. (%) | 6 (40.0%) | 15 (27.78%) | 9 (36.0%) | |||
| Height (cm), median (Q1–Q3) | 169 (163.5–173.75) | 168 (165–173.25) | 169 (163.5–175) | 0.960 | 0.863 | 0.771 |
| Weight (kg), median (Q1–Q3) | 65 (60–69) | 63.5 (57.75–70) | 60 (55–65.5) | 0.652 | 0.181 | 0.212 |
| BMI, median (Q1–Q3) | 22.98 (21.32–26.25) | 23.09 (20.42–25.44) | 21.24 (19.49–24.51) | 0.821 | 0.313 | 0.279 |
| Duration of Diabetes (year), median (Q1–Q3) | 6 (2.5–16.5) | 6.5 (2.25–18.75) | 6 (3–13.75) | 0.881 | 0.883 | 0.712 |
| Pulse, median (Q1–Q3) | 101 (94.5–120) | 107 (87–118) | 101 (92–115) | 0.841 | 0.967 | 0.852 |
| SBP, median (Q1–Q3) | 130 (119.5–152.5) | 127 (112–140) | 127 (115–151) | 0.265 | 0.676 | 0.435 |
| DBP, median (Q1–Q3) | 82 (79–86.5) | 73 (70–80) | 80 (72–94) | 0.021 | 0.613 | 0.035 |
| Nephropathy, no. (%) | 3 (20.0%) | 2 (3.70%) | 2 (8.0%) | 0.053 | 0.281 | 0.428 |
| Retinopathy, no. (%) | 2 (13.33%) | 9 (16.67%) | 5 (20.0%) | 0.755 | 0.593 | 0.718 |
| Neuropathy, no. (%) | 5 (33.33%) | 21 (38.89%) | 9 (36.0%) | 0.694 | 0.864 | 0.805 |
| Laboratory data, median (Q1–Q3) | ||||||
| WBC | 12.61 (10.66–16.04) | 15.35 (12.31–23.45) | 20.39 (13.9–31.63) | 0.090 | 0.009 | 0.134 |
| Potassium, mmol/L | 4.56 (4.42–5.09) | 4.89 (4.09–5.6) | 4.77 (4.14–5.6) | 0.595 | 0.571 | 0.903 |
| Sodium, mmol/L | 133 (130–136) | 134 (131–136.75) | 135 (133–138) | 0.542 | 0.100 | 0.138 |
| Chlorine, mmol/L | 104.3 (100.7–108.8) | 102.9 (101–110) | 106 (101–112) | 0.875 | 0.660 | 0.435 |
| Calcium, mmol/L | 1.21 (1.12–1.27) | 1.26 (1.17–1.32) | 1.26 (1.19–1.34) | 0.051 | 0.058 | 0.829 |
| HbA1c, (%) | 11.2 (10.02–12.47) | 11.2 (10.02–12.47) | 11.2 (10.02–12.47) | 0.898 | 0.306 | 0.270 |
| Glucose, (mg/dL) | 504 (407–764) | 515.5 (409–708) | 595.5 (500–739.75) | 0.889 | 0.223 | 0.142 |
| BUN, (mg/dL) | 40.66 (25.55–72.03) | 62.25 (40.4–85.21) | 60.8 (36.6–73.2) | 0.082 | 0.304 | 0.476 |
| Creatinine, (mg/dL) | 1.36 (0.95–2.26) | 1.7 (1.43–2.16) | 1.89 (1.52–2.22) | 0.319 | 0.189 | 0.567 |
| Erythrocytes ×106/uL | 4.53 (4.41–4.85) | 4.84 (4.25–5.21) | 4.65 (4.34–5.36) | 0.589 | 0.643 | 0.979 |
| Hemoglobin, g/dL | 13.8 (13.36–14.35) | 14.3 (13.25–16.02) | 14.8 (12.5–15.8) | 0.314 | 0.474 | 0.805 |
| Hematocrit, % | 41.67 (39.85–42.95) | 42.35 (38.9–46.5) | 42 (38.3–49.21) | 0.434 | 0.378 | 0.807 |
| Neutrophils, ×103/uL | 9.78 (6.60–14.46) | 13.94 (10.22–21.25) | 16.5 (11.5–26.95) | 0.038 | 0.011 | 0.348 |
| Lymphocytes, ×103/uL | 1.49 (1.09–2.62) | 1.28 (0.92–1.81) | 1.9 (1.12–2.32) | 0.280 | 0.874 | 0.280 |
| Serum albumin, g/dL | 3.66 (3.43–3.97) | 3.54 (3.16–3.92) | 3.23 (3.05–3.74) | 0.387 | 0.150 | 0.367 |
| Total protein, g/dL | 5.92 (5.54–6.38) | 6.05 (5.36–6.22) | 5.53 (5.25–5.88) | 0.696 | 0.195 | 0.166 |
| Total cholesterol, mg/dL | 188.1 (166.85–229.17) | 155.1 (131.5–198.1) | 170.4 (142.6–219.4) | 0.102 | 0.314 | 0.512 |
| Triglycerides, mg/dL | 193 (105.35–352.05) | 132.2 (107.4–258.4) | 114 (80.6–165.8) | 0.702 | 0.204 | 0.196 |
| Amylase, U/L | 47.75 (38.75–59) | 44 (30–84) | 68 (41–133.75) | 0.907 | 0.144 | 0.063 |
| Total bilirubin, mg/dL | 0.28 (0.25–0.72) | 0.52 (0.31–0.72) | 0.38 (0.23–0.48) | 0.076 | 0.586 | 0.005 |
| ALT, U/L | 18 (11.45–23.5) | 19 (13–26.75) | 24 (17–40) | 0.540 | 0.036 | 0.037 |
| AST, U/L | 16 (9.65–28.5) | 23 (17.1–29.5) | 20.1 (15.9–42) | 0.045 | 0.077 | 0.969 |
| Venous blood pH | 7.25 (7.19–7.26) | 7.17 (7.11–7.22) | 6.93 (6.87–6.99) | 0.023 | <0.001 | <0.001 |
| PvCO2 | 27.7 (22.25–34.8) | 18.3 (13.85–22.77) | 17.6 (12.2–21.4) | <0.001 | <0.001 | 0.410 |
| PvO2 | 35.4 (29.6–57.45) | 59.5 (43.62–107.77) | 57 (41–87.4) | 0.008 | 0.066 | 0.478 |
| Base excess, mmol/L | −14 (−18.2–−11.3) | −21.25 (−23.8–−17.75) | −27.8 (−29–−25) | 0.003 | <0.001 | <0.001 |
| Lactate, mmol/L | 1.7 (1.6–2.45) | 2.3 (1.6–3.4) | 3.5 (2.5–4.6) | 0.226 | 0.004 | 0.016 |
| Bicarbonate, mmol/L | 12.8 (9.15–15.3) | 6.6 (5.03–9.65) | 3.9 (3.1–5.9) | <0.001 | <0.001 | <0.001 |
| Anion gap, mEq/L | 18.8 (12.6–22.05) | 23.79 (19.3–26.6) | 25 (21.5–27.2) | 0.009 | <0.001 | 0.199 |
| LGI | 5.64 (3.93–9.69) | 9.41 (5.28–13.19) | 12.52 (10.43–17.24) | 0.057 | 0.001 | 0.044 |
| Variables | Cut-Off | AUC | Std. Error | 95% CI | Sensitivity | Specificity | p Value |
|---|---|---|---|---|---|---|---|
| DKA Severity | |||||||
| LGI | 10.43 | 0.688 | 0.066 | 0.565–0.816 | 63.8% | 56.1% | 0.002 |
| Diabetes Microvascular Complications | |||||||
| LGI | 11.07 | 0.700 | 0.080 | 0.543–0.858 | 69% | 64.3% | 0.013 |
| Variables | DKA Severity | Diabetes Microvascular Complications | ||||
|---|---|---|---|---|---|---|
| OR * | 95% CI | p Value | OR * | 95% CI | p Value | |
| WBC | 1.75 | 1.10–2.77 | 0.017 | 1.13 | 0.65–192 | 0.671 |
| Venous blood pH | 0.07 | 0.02–0.21 | <0.001 | 1.52 | 0.76–3.04 | 0.233 |
| PvCO2 | 0.56 | 0.32–0.99 | 0.049 | 0.49 | 0.24–1.03 | 0.059 |
| Base Excess, mmol/L | 0.03 | 0.01–0.14 | <0.001 | 1.32 | 0.82–2.16 | 0.252 |
| Lactate, mmol/L | 1.86 | 1.17–2.96 | 0.009 | 0.79 | 0.43–1.17 | 0.469 |
| Bicarbonate, mmol/L | 0.17 | 0.07–0.42 | <0.001 | 0.67 | 0.35–1.26 | 0.212 |
| Anion gap, mEq/L | 1.69 | 1.01–2.86 | 0.047 | 1.52 | 0.83–2.76 | 0.169 |
| LGI | 1.87 | 1.13–3.13 | 0.016 | 2.16 | 1.20–3.87 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coșarcă, M.C.; Tilinca, R.M.; Lazăr, N.A.; Șincaru, S.V.; Bandici, B.C.; Carașca, C.; Gergő, R.; Mureșan, A.V.; Tilinca, M.C. Elevated Leukocyte Glucose Index (LGI) Is Associated with Diabetic Ketoacidosis (DKA) Severity and Presence of Microvascular Complications. Medicina 2025, 61, 898. https://doi.org/10.3390/medicina61050898
Coșarcă MC, Tilinca RM, Lazăr NA, Șincaru SV, Bandici BC, Carașca C, Gergő R, Mureșan AV, Tilinca MC. Elevated Leukocyte Glucose Index (LGI) Is Associated with Diabetic Ketoacidosis (DKA) Severity and Presence of Microvascular Complications. Medicina. 2025; 61(5):898. https://doi.org/10.3390/medicina61050898
Chicago/Turabian StyleCoșarcă, Mircea Cătălin, Raluca Maria Tilinca, Nicolae Alexandru Lazăr, Suzana Vasilica Șincaru, Bogdan Corneliu Bandici, Cosmin Carașca, Ráduly Gergő, Adrian Vasile Mureșan, and Mariana Cornelia Tilinca. 2025. "Elevated Leukocyte Glucose Index (LGI) Is Associated with Diabetic Ketoacidosis (DKA) Severity and Presence of Microvascular Complications" Medicina 61, no. 5: 898. https://doi.org/10.3390/medicina61050898
APA StyleCoșarcă, M. C., Tilinca, R. M., Lazăr, N. A., Șincaru, S. V., Bandici, B. C., Carașca, C., Gergő, R., Mureșan, A. V., & Tilinca, M. C. (2025). Elevated Leukocyte Glucose Index (LGI) Is Associated with Diabetic Ketoacidosis (DKA) Severity and Presence of Microvascular Complications. Medicina, 61(5), 898. https://doi.org/10.3390/medicina61050898






