Breathless Strength: Ultrasonographic Insights into Expiratory Muscle Dysfunction in Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
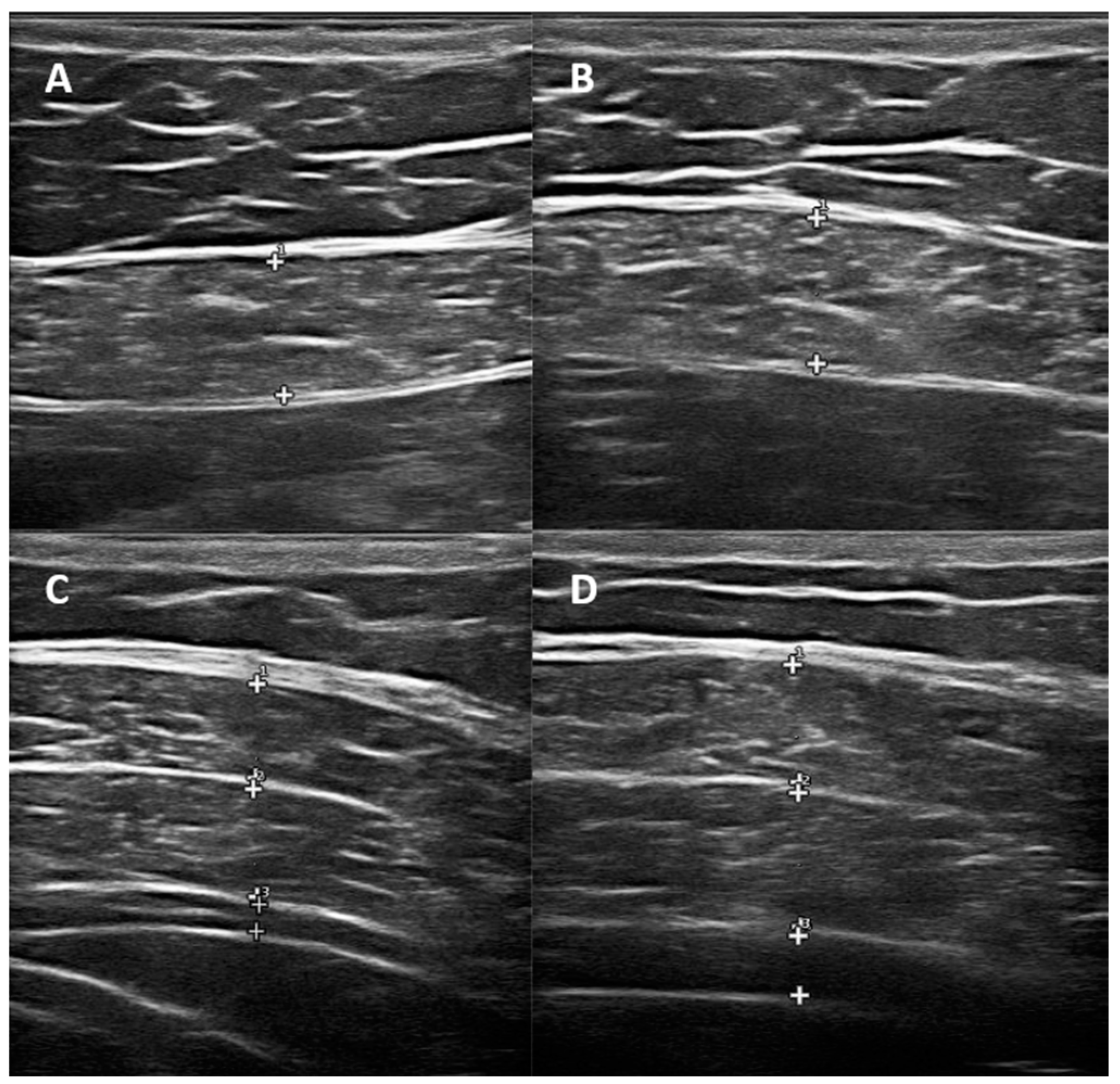
2.2. Ultrasonographic Evaluation
2.3. Respiratory Muscle Strength Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziebart, C.; Jaglal, S.; Guilcher, S.; Matai, L.; Li, P.; Craven, C. Prevalence and Impact of Fractures in Persons with Spinal Cord Injuries: A Population-Based Study Comparing Fracture Rates between Individuals with Traumatic and Nontraumatic Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2024, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Molinares, D.M.; Gater, D.R.; Daniel, S.; Pontee, N.L. Nontraumatic Spinal Cord Injury: Epidemiology, Etiology and Management. J. Pers. Med. 2022, 12, 1872. [Google Scholar] [CrossRef] [PubMed]
- van der Veldt, N.; Faber, W.X.M.; Witteman, B.J.M.; Stolwijk-Swuste, J.M.; Nachtegaal, J. Effective bowel management in spinal cord injury during inpatient rehabilitation: Data from the Dutch spinal cord injury database. Spinal Cord. 2023, 61, 492–498. [Google Scholar] [CrossRef]
- Aras, M.; Altas, M.; Motor, S.; Dokuyucu, R.; Yilmaz, A.; Ozgiray, E.; Seraslan, Y.; Yilmaz, N. Protective effects of minocycline on experimental spinal cord injury in rats. Injury 2015, 46, 1471–1474. [Google Scholar] [CrossRef]
- Josefson, C.; Rekand, T.; Lundgren-Nilsson, A.; Sunnerhagen, K.S. Respiratory complications during initial rehabilitation and survival following spinal cord injury in Sweden: A retrospective study. Spinal Cord. 2021, 59, 659–664. [Google Scholar] [CrossRef]
- Perrouin-Verbe, B.; Lefevre, C.; Kieny, P.; Gross, R.; Reiss, B.; Le Fort, M. Spinal cord injury: A multisystem physiological impairment/dysfunction. Rev. Neurol. 2021, 177, 594–605. [Google Scholar] [CrossRef]
- Pizzolato, C.; Gunduz, M.A.; Palipana, D.; Wu, J.; Grant, G.; Hall, S.; Dennison, R.; Zafonte, R.D.; Lloyd, D.G.; Teng, Y.D. Non-invasive approaches to functional recovery after spinal cord injury: Therapeutic targets and multimodal device interventions. Exp. Neurol. 2021, 339, 113612. [Google Scholar] [CrossRef]
- Bach, J.R.; Burke, L.; Chiou, M. Conventional Respiratory Management of Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 379–395. [Google Scholar] [CrossRef]
- Arango-Cortes, M.L.; Giraldo-Cadavid, L.F.; Latorre Quintana, M.; Forero-Cubides, J.D.; Gonzalez-Bermejo, J. Diaphragm pacing compared with mechanical ventilation in patients with chronic respiratory failure caused by diaphragmatic dysfunction: A systematic review and meta-analysis. Expert. Rev. Respir. Med. 2024, 18, 1101–1111. [Google Scholar] [CrossRef]
- Patel, N.; Chong, K.; Baydur, A. Methods and Applications in Respiratory Physiology: Respiratory Mechanics, Drive and Muscle Function in Neuromuscular and Chest Wall Disorders. Front. Physiol. 2022, 13, 838414. [Google Scholar] [CrossRef]
- Walling, I.; Baumgartner, S.; Patel, M.; Crone, S.A. Electrical stimulation of the sciatic nerve restores inspiratory diaphragm function in mice after spinal cord injury. Front. Neural Circuits 2024, 18, 1480291. [Google Scholar] [CrossRef] [PubMed]
- Ferfeli, S.; Galanos, A.; Dontas, I.A.; Pitidis-Poutous, D.; Triantafyllopoulos, I.K.; Symeonidou, Z.; Tsiamasfirou, D.; Chronopoulos, E. Respiratory Muscle Strength Correlation with Functional Capacity, Quality of Life, Demographics and Co-morbidities in Stroke and Spinal Cord Injury. J. Musculoskelet. Neuronal Interact. 2024, 24, 361–369. [Google Scholar] [PubMed]
- Woods, A.; Gustafson, O.; Williams, M.; Stiger, R. The effects of inspiratory muscle training on inspiratory muscle strength, lung function and quality of life in adults with spinal cord injuries: A systematic review and Meta-analysis. Disabil. Rehabil. 2023, 45, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- de Araujo Morais, L.; Cipriano, G., Jr.; Martins, W.R.; Chiappa, G.R.; Formiga, M.F.; Cipriano, G.F.B. Inspiratory muscle training on quality of life in individuals with spinal cord injury: A systematic review and meta-analysis. Spinal Cord. 2023, 61, 359–367. [Google Scholar] [CrossRef]
- Cruickshank, T.; Flores-Opazo, M.; Tuesta, M.; Reyes, A. Reproducibility of Maximum Respiratory Pressure Assessment: A Systematic Review and Meta-analysis. Chest 2022, 162, 828–850. [Google Scholar] [CrossRef]
- Park, T.S.; Tak, Y.J.; Ra, Y.; Kim, J.; Han, S.H.; Kim, S.H.; Shin, Y.; Shin, M.J.; Kang, J.H. Reference Respiratory Muscle Strength Values and a Prediction Equation Using Physical Functions for Pulmonary Rehabilitation in Korea. J. Korean Med. Sci. 2023, 38, e325. [Google Scholar] [CrossRef]
- Yoshida, R.; Tomita, K.; Kawamura, K.; Setaka, Y.; Ishii, N.; Monma, M.; Mutsuzaki, H.; Mizukami, M.; Ohse, H.; Imura, S. Investigation of inspiratory intercostal muscle activity in patients with spinal cord injury: A pilot study using electromyography, ultrasonography, and respiratory inductance plethysmography. J. Phys. Ther. Sci. 2021, 33, 153–157. [Google Scholar] [CrossRef]
- Hasnakipour, S.; Mosallanezhad, Z.; Rezaeian, T.; Azadi, F.; Noroozi, M. The effect of diaphragmatic breathing exercise with biofeedback on respiratory function in incomplete cervical spinal cord injury: A randomized-controlled study. Am. J. Phys. Med. Rehabil. 2025, 2, 1–7. [Google Scholar] [CrossRef]
- Romero-Morales, C.; Bravo-Aguilar, M.; Ruiz-Ruiz, B.; Almazan-Polo, J.; Lopez-Lopez, D.; Blanco-Morales, M.; Tellez-Gonzalez, P.; Calvo-Lobo, C. Current advances and research in ultrasound imaging to the assessment and management of musculoskeletal disorders. Dis. Mon. 2021, 67, 101050. [Google Scholar] [CrossRef]
- Virto, N.; Rio, X.; Mendez-Zorrilla, A.; Garcia-Zapirain, B. Non invasive techniques for direct muscle quality assessment after exercise intervention in older adults: A systematic review. BMC Geriatr. 2024, 24, 642. [Google Scholar] [CrossRef]
- Yao, X.Y.; Li, H.M.; Sun, B.W.; Zhang, Y.Y.; Feng, J.G.; Jia, J.; Liu, L. Ultrasound assessment of diaphragmatic dysfunction in non-critically ill patients: Relevant indicators and update. Front. Med. 2024, 11, 1389040. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.S.; Kunzweiler, S.; Conley, C.; Kim, K.H.; Adewuyi, A.A.; Mondriguez-Gonzalez, A.; Wolfe, L.F.; Kwasny, M.; Franz, C.K. Predictive Value of Diaphragm Muscle Ultrasound for Ventilator Weaning Outcomes After Cervical Spinal Cord Injury: A Retrospective Case Series. J. Ultrasound Med. 2025, 44, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kilicoglu, M.S.; Yurdakul, O.V.; Celik, Y.; Aydin, T. Investigating the correlation between pulmonary function tests and ultrasonographic diaphragm measurements and the effects of respiratory exercises on these parameters in hemiplegic patients. Top. Stroke Rehabil. 2022, 29, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.F.; Fu, S.N.; Huang, C.; Huang, X.P.; Wong, A.Y.L. Reliability and validity of ultrasonography in evaluating the thickness, excursion, stiffness, and strain rate of respiratory muscles in non-hospitalized individuals: A systematic review. BMC Oral. Health 2023, 23, 959. [Google Scholar] [CrossRef]
- Raab, A.M.; Brinkhof, M.W.G.; Berlowitz, D.J.; Postma, K.; Gobets, D.; Hirschfeld, S.; Hopman, M.T.E.; Huber, B.; Hund-Georgiadis, M.; Jordan, X.; et al. Respiratory function and respiratory complications in spinal cord injury: Protocol for a prospective, multicentre cohort study in high-income countries. BMJ Open 2020, 10, e038204. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, Y.T.; Huang, C.C.; Lin, M.C.; Liaw, M.Y.; Lu, C.H. Effects of Respiratory Muscle Training on Baroreflex Sensitivity, Respiratory Function, and Serum Oxidative Stress in Acute Cervical Spinal Cord Injury. J. Pers. Med. 2021, 11, 377–390. [Google Scholar] [CrossRef]
- Boussuges, A.; Rives, S.; Finance, J.; Bregeon, F. Assessment of diaphragmatic function by ultrasonography: Current approach and perspectives. World J. Clin. Cases 2020, 8, 2408–2424. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, J.; Yang, D.; Gao, F.; Du, L.; Yang, M. Ultrasonographic evaluation of diaphragm thickness and excursion in patients with cervical spinal cord injury. J. Spinal Cord. Med. 2021, 44, 742–747. [Google Scholar] [CrossRef]
- Wang, T.Y.; Park, C.; Zhang, H.; Rahimpour, S.; Murphy, K.R.; Goodwin, C.R.; Karikari, I.O.; Than, K.D.; Shaffrey, C.I.; Foster, N.; et al. Management of Acute Traumatic Spinal Cord Injury: A Review of the Literature. Front. Surg. 2021, 8, 698736. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Xu, Y. Effects of Respiratory Muscle Training on Pulmonary Function in Individuals with Spinal Cord Injury: An Updated Meta-analysis. Biomed. Res. Int. 2020, 2020, 7530498. [Google Scholar] [CrossRef]
- Saeverud, H.A.; Falk, R.S.; Dowrick, A.; Eriksen, M.; Aarrestad, S.; Skjonsberg, O.H. Measuring diaphragm movement and respiratory frequency using a novel ultrasound device in healthy volunteers. J. Ultrasound 2021, 24, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fayssoil, A.; Nguyen, L.S.; Ogna, A.; Stojkovic, T.; Meng, P.; Mompoint, D.; Carlier, R.; Prigent, H.; Clair, B.; Behin, A.; et al. Diaphragm sniff ultrasound: Normal values, relationship with sniff nasal pressure and accuracy for predicting respiratory involvement in patients with neuromuscular disorders. PLoS ONE 2019, 14, e0214288. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Park, J.; Eun, S.D. A preliminary study on the feasibility of community game-based respiratory muscle training for individuals with high cervical spinal cord injury levels: A novel approach. BMC Sports Sci. Med. Rehabil. 2022, 14, 137. [Google Scholar] [CrossRef] [PubMed]
| Variables | Patient (n = 36) | Healthy (n = 30) | p Values |
|---|---|---|---|
| Mean ± SD, n (%) | |||
| Age (Year) | 43.72 ± 15.75 | 45.66 ± 11 | 0.579 |
| Height (Cm) | 170.44 ± 6.65 | 168.9 ± 10.18 | 0.510 |
| Weight (Kg) | 77.38 ± 14.3 | 76.96 ± 15.71 | 0.767 |
| Body Mass index (BMI) | 26.65 ± 4.94 | 26.75 ± 3.84 | 0.862 |
| Gender (M/F) | 24/12 | 18/12 | 0.380 |
| Hypertension (HT) | 4 (11.1%) | 9 (30%) | 0.054 |
| Diabetes Mellitus (DM) | 4 (11.1%) | 4 (13.3%) | 0.537 |
| Coronary Artery Disease (CAD) | 2 (5.6%) | 1 (3.3%) | 0.569 |
| Dyslipidemia | 4 (11.1%) | 2 (6.7%) | 0.428 |
| Occupation | 0.182 | ||
| Officer | 4 (11.1%) | 10 (33.3%) | |
| Retired | 3 (8.3%) | 2 (6.7%) | |
| Worker | 20 (55.6%) | 12 (40%) | |
| Housewife | 9 (25%) | 6 (20%) | |
| Smoking | 0.217 | ||
| None | 18 (50%) | 17 (56.7%) | |
| <10 pack-years | 7 (19.4%) | 1 (3.3%) | |
| 10–20 pack-years | 5 (13.9%) | 7 (23.3%) | |
| >20 pack-years | 6 (16.7%) | 5 (16.7%) | |
| Alcohol Consumption | 8 (22.2%) | 3 (10%) | 0.160 |
| Dominant Side (Right) | 32 (88.9%) | 30 (100%) | 0.082 |
| MIP | 76.27 ± 29 | 91.63 ± 17.3 | 0.007 |
| MEP | 64.52 ± 21.55 | 119.1 ± 26.48 | <0.001 |
| Variables | Patient (n = 36) | Healthy (n = 30) | p Values |
|---|---|---|---|
| Mean ± SD | |||
| RA Rest | 9.07 ± 2.68 | 8.8 ± 2.04 | 0.733 |
| RA Forced | 9.27 ± 2.71 | 9.42 ± 2.31 | 0.743 |
| RA Thickening Ratio | 0.07 ± 0.05 | 0.07 ± 0.05 | 0.003 |
| EO Rest | 5.64 ± 1.71 | 6.43 ± 1.94 | 0.122 |
| EO Forced | 5.76 ± 1.7 | 7.67 ± 2.35 | <0.001 |
| EO Thickening Ratio | 0.03 ± 0.11 | 0.2 ± 0.15 | <0.001 |
| IO Rest | 7.75 ± 2.07 | 8.44 ± 2.38 | 0.254 |
| IO Forced | 8.17 ± 2.75 | 11.45 ± 3.32 | <0.001 |
| IO Thickening Ratio | 0.06 ± 0.14 | 0.36 ± 0.17 | <0.001 |
| TrA Rest | 2.74 ± 0.98 | 3.36 ± 0.95 | 0.007 |
| TrA Forced | 3.24 ± 1.54 | 5.12 ± 2.05 | <0.001 |
| TrA Thickening Ratio | 0.14 ± 0.24 | 0.63 ± 0.3 | <0.001 |
| RA Rest | 9.07 ± 2.68 | 8.8 ± 2.04 | 0.733 |
| RA Forced | 9.27 ± 2.71 | 9.42 ± 2.31 | 0.743 |
| RA Thickening Ratio | 0.07 ± 0.05 | 0.07 ± 0.05 | 0.003 |
| MIP | MEP | ||
|---|---|---|---|
| RA end-expiratory | r | 0.368 | 0.432 |
| p | 0.027 | 0.008 | |
| RA forced | r | 0.287 | 0.384 |
| p | 0.089 | 0.021 | |
| RA thickening ratio | r | −0.276 | 0.065 |
| p | 0.103 | 0.707 | |
| EO end-expiratory | r | 0.271 | 0.311 |
| p | 0.109 | 0.065 | |
| EO forced | r | 0.241 | 0.333 |
| p | 0.157 | 0.047 | |
| EO thickening ratio | r | −0.101 | −0.185 |
| p | 0.559 | 0.281 | |
| IO end-expiratory | r | 0.321 | 0.467 |
| p | 0.056 | 0.004 | |
| IO forced | r | 0.251 | 0.501 |
| p | 0.140 | 0.002 | |
| IO thickening ratio | r | 0.045 | 0.338 |
| p | 0.796 | 0.044 | |
| TrA end-expiratory | r | 0.223 | 0.508 |
| p | 0.191 | 0.002 | |
| TrA forced | r | 0.171 | 0.530 |
| p | 0.319 | 0.001 | |
| TrA thickening ratio | r | 0.062 | 0.428 |
| p | 0.720 | 0.009 |
| Variables | T6 and Above | T7–T12 | p Values |
|---|---|---|---|
| Mean ± SD | |||
| RA Rest | 9.34 ± 2.7 | 8.77 ± 2.66 | 0.822 |
| RA Forced | 9.45 ± 2.68 | 9.07 ± 2.63 | 0.873 |
| RA Thickening Ratio | 0.07 ± 0.05 | 0.06 ± 0.03 | 0.585 |
| EO Rest | 5.71 ± 1.7 | 5.64 ± 1.74 | 0.875 |
| EO Forced | 5.76 ± 1.7 | 5.64 ± 1.7 | 0.876 |
| EO Thickening Ratio | 0.03 ± 0.11 | 0.02 ± 0.1 | 0.797 |
| IO Rest | 7.71 ± 2.07 | 7.75 ± 2.06 | 0.962 |
| IO Forced | 8.17 ± 2.75 | 7.9 ± 2.7 | 0.712 |
| IO Thickening Ratio | 0.06 ± 0.14 | 0.04 ± 0.12 | 0.032 |
| TrA Rest | 2.74 ± 0.98 | 3.36 ± 0.95 | 0.521 |
| TrA Forced | 3.24 ± 1.54 | 3.68 ± 1.91 | 0.494 |
| TrA Thickening Ratio | 0.14 ± 0.24 | 0.18 ± 0.28 | 0.451 |
| RA Rest | 9.34 ± 2.7 | 8.77 ± 2.66 | 0.822 |
| RA Forced | 9.45 ± 2.68 | 9.07 ± 2.63 | 0.873 |
| RA Thickening Ratio | 0.07 ± 0.05 | 0.06 ± 0.03 | 0.585 |
| Variables | Patient (n = 36) | Healthy (n = 30) | p Values |
|---|---|---|---|
| Mean ± SD | |||
| RA Rest | 9.07 ± 2.68 | 8.8 ± 2.04 | 0.733 |
| RA Forced | 9.27 ± 2.71 | 9.42 ± 2.31 | 0.743 |
| RA Thickening Ratio | 0.07 ± 0.05 | 0.07 ± 0.05 | 0.003 |
| EO Rest | 5.64 ± 1.71 | 6.43 ± 1.94 | 0.122 |
| EO Forced | 5.76 ± 1.7 | 7.67 ± 2.35 | <0.001 |
| EO Thickening Ratio | 0.03 ± 0.11 | 0.2 ± 0.15 | <0.001 |
| IO Rest | 7.75 ± 2.07 | 8.44 ± 2.38 | 0.254 |
| IO Forced | 8.17 ± 2.75 | 11.45 ± 3.32 | <0.001 |
| IO Thickening Ratio | 0.06 ± 0.14 | 0.36 ± 0.17 | <0.001 |
| TrA Rest | 2.74 ± 0.98 | 3.36 ± 0.95 | 0.007 |
| TrA Forced | 3.24 ± 1.54 | 5.12 ± 2.05 | <0.001 |
| TrA Thickening Ratio | 0.14 ± 0.24 | 0.63 ± 0.3 | <0.001 |
| Muscle | ICC (95% CI) | Interpretation |
|---|---|---|
| Rectus Abdominis (RA) | 0.94 (0.89–0.97) | Excellent |
| External Oblique (EO) | 0.92 (0.86–0.96) | Excellent |
| Internal Oblique (IO) | 0.95 (0.91–0.98) | Excellent |
| Transversus Abdominis (TrA) | 0.97 (0.93–0.99) | Excellent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutuk, B.; Ones, K.; Dogan, Y.E. Breathless Strength: Ultrasonographic Insights into Expiratory Muscle Dysfunction in Spinal Cord Injury. Medicina 2025, 61, 897. https://doi.org/10.3390/medicina61050897
Kutuk B, Ones K, Dogan YE. Breathless Strength: Ultrasonographic Insights into Expiratory Muscle Dysfunction in Spinal Cord Injury. Medicina. 2025; 61(5):897. https://doi.org/10.3390/medicina61050897
Chicago/Turabian StyleKutuk, Burak, Kadriye Ones, and Yunus Emre Dogan. 2025. "Breathless Strength: Ultrasonographic Insights into Expiratory Muscle Dysfunction in Spinal Cord Injury" Medicina 61, no. 5: 897. https://doi.org/10.3390/medicina61050897
APA StyleKutuk, B., Ones, K., & Dogan, Y. E. (2025). Breathless Strength: Ultrasonographic Insights into Expiratory Muscle Dysfunction in Spinal Cord Injury. Medicina, 61(5), 897. https://doi.org/10.3390/medicina61050897






