Evaluating Heart Rate Variability as a Biomarker for Autonomic Function in Parkinson’s Disease Rehabilitation: A Clustering-Based Analysis of Exercise-Induced Changes
Abstract
1. Introduction
- Total power represents overall autonomic modulation.
- Low-frequency (LF) activity (<0.15 Hz) is primarily linked to baroreflex-mediated autonomic regulation, incorporating both sympathetic and parasympathetic influences.
- High-frequency (HF) activity, on the other hand, is strongly associated with parasympathetic (vagal) activity, reflecting respiratory-related heart rate fluctuations.
- The LF/HF ratio is often interpreted as an index of autonomic balance, though this measure remains debated due to factors such as differences in temporal dynamics between sympathetic and parasympathetic responses and variations in cardiac pacemaker sensitivity.
- Degeneration of brainstem autonomic centers (e.g., dorsal motor nucleus of the vagus, locus coeruleus, and medullary cardiovascular centers), leading to impaired parasympathetic regulation.
- Dysfunction of basal ganglia and limbic circuits, which play a role in autonomic modulation and stress regulation.
- Reduced baroreflex sensitivity and vagal tone, contributing to blood pressure variability, orthostatic hypotension, and cardiovascular instability.
2. Materials and Methods
2.1. Participants
2.1.1. Inclusion Criteria
- Aged between 45 and 75 years;
- Had a diagnosis of Parkinson’s disease, classified as Hoehn and Yahr stages 2–3, indicating moderate disease severity;
- Were stable on PD medications for at least three months prior to the study;
- Could walk independently or with minimal assistance (Unified Parkinson’s Disease Rating Scale—Motor Examination, score ≤ 3 on gait and posture items);
- Had not participated in any structured exercise program or physical activity regimen (e.g., more than three sessions per week, 30–40 min per session) in the past six months or longer;
- Were able to understand and follow instructions in either Arabic or English.
2.1.2. Exclusion Criteria
- Had contraindications to magnetic resonance imaging (MRI), such as metallic implants, claustrophobia, or pacemakers;
- Had significant medical, neurological, or psychiatric conditions unrelated to Parkinson’s disease (e.g., recent strokes, uncontrolled diabetes, or major depressive disorder);
- Displayed severe cognitive impairments (Montreal Cognitive Assessment (MoCA) score < 21), which could hinder their ability to follow instructions;
- Experienced advanced motor complications (Hoehn and Yahr stage > 3) that could compromise their safe participation;
- Were classified as obese (BMI ≥ 30.0 kg/m2), as excessive weight may impact autonomic function, HRV measurements, and exercise tolerance.
2.2. Data Collection
2.3. Statistcal Analysis
3. Results
3.1. Heart Rate Variblity Changes Pre- and Post-Exercise
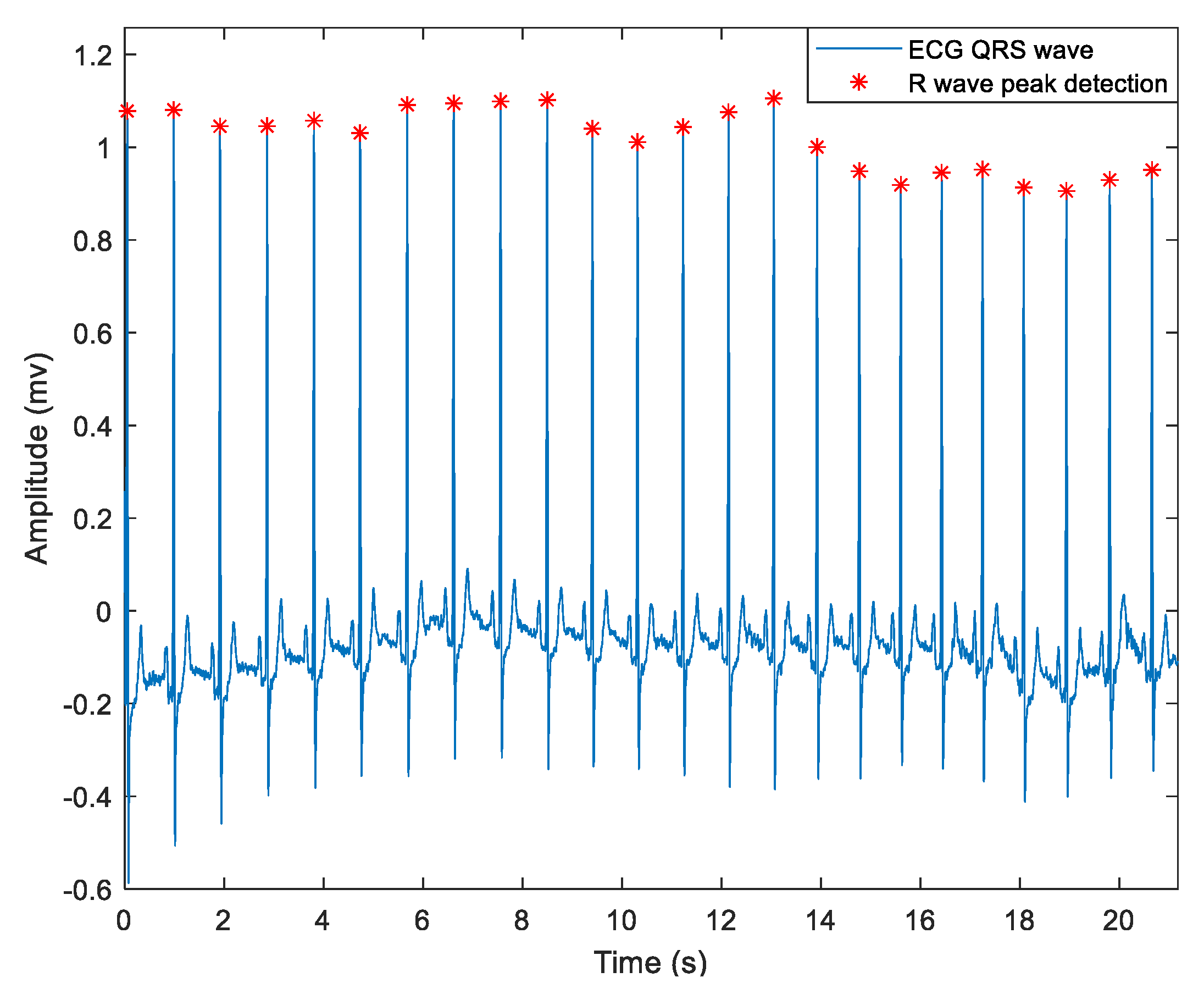
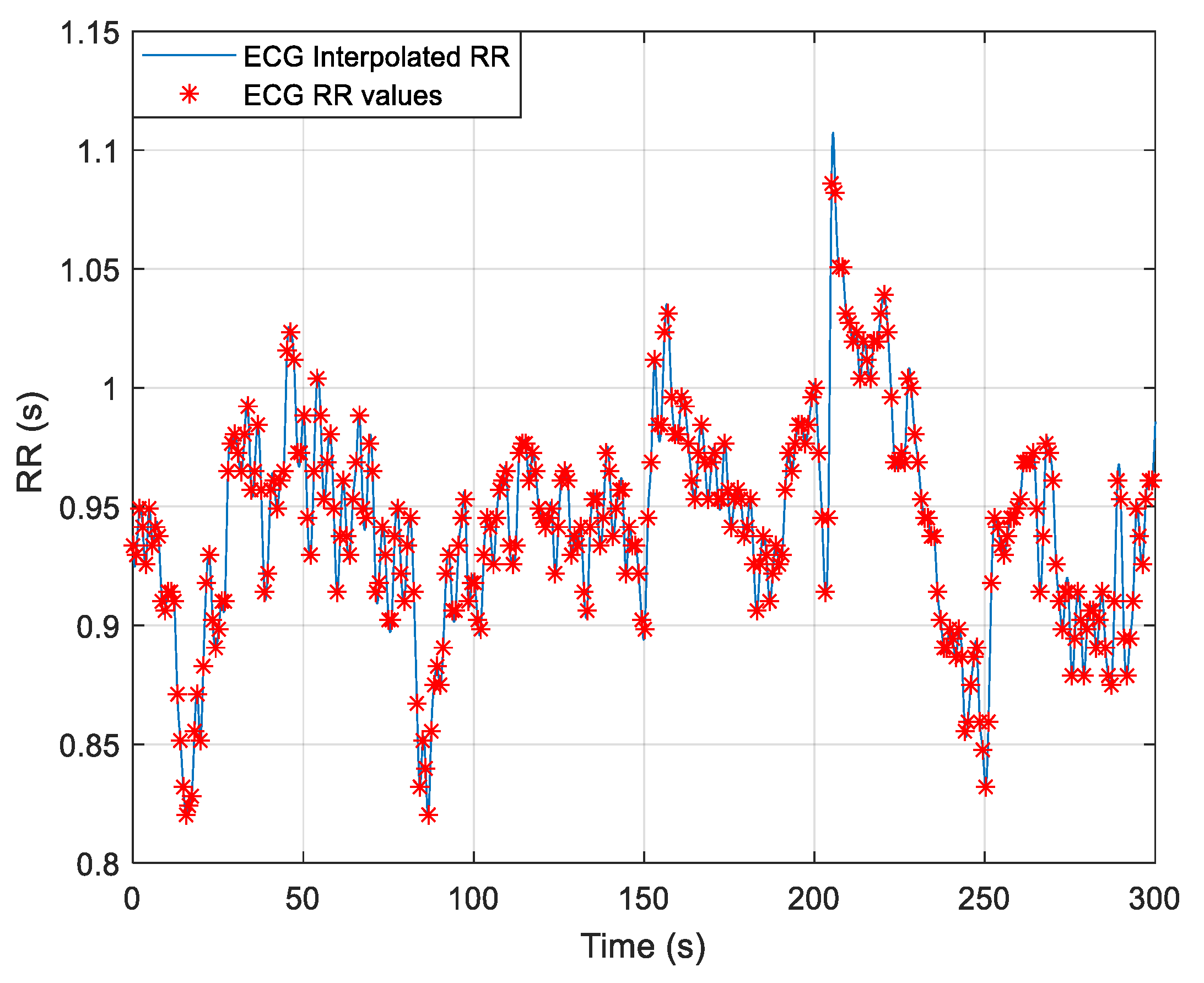
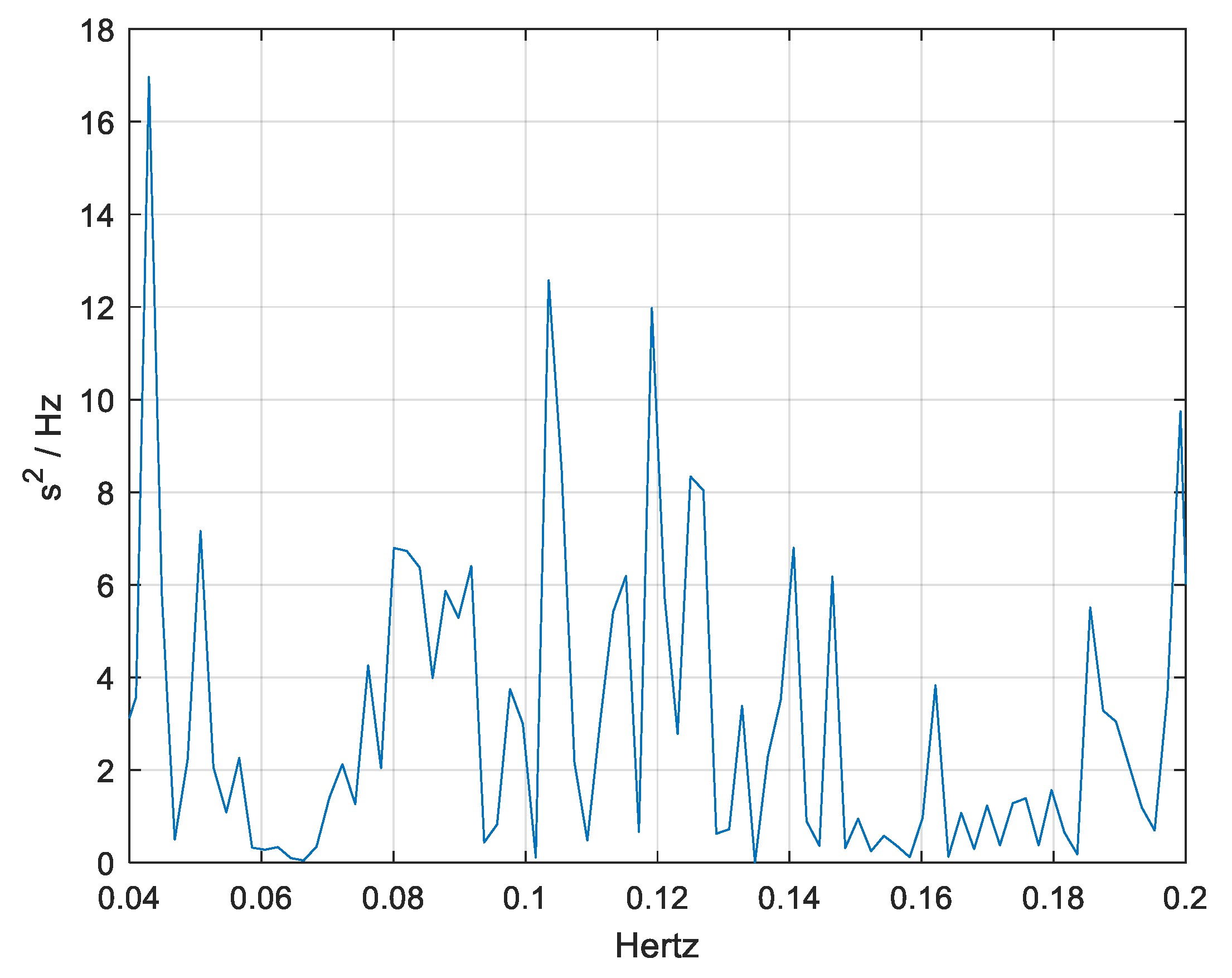
3.2. Frequency-Domain Analysis
3.3. HRV-Based Evidence of Autonomic Adaptation
4. Discussion
4.1. Impact of Exercise on Autonomic Function
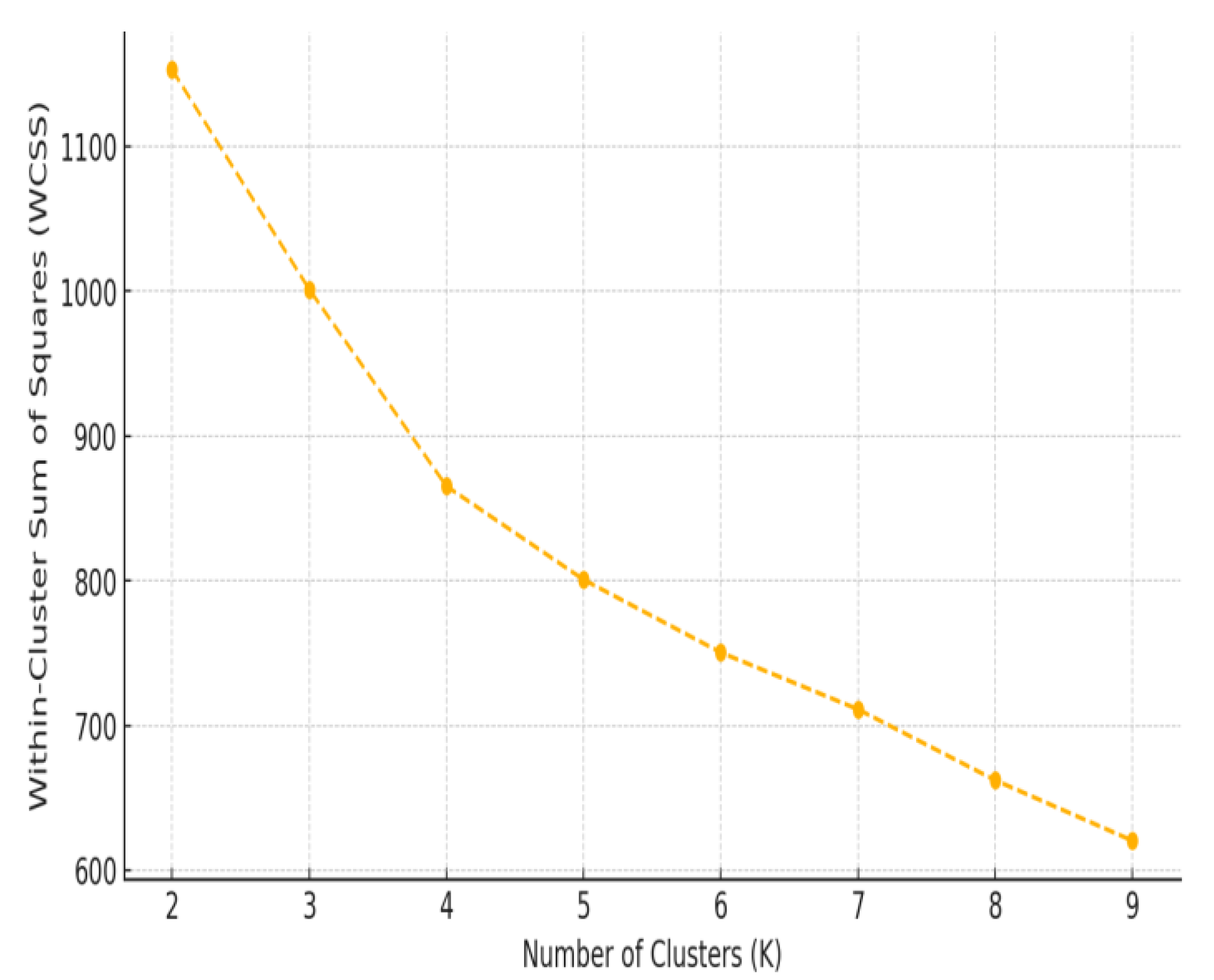
4.2. Heterogeneous HRV Responses: Patient Subgroup Differences
4.3. Clinical and Physiological Implications
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, J.W.; Falace, D.A.; Miller, C.S.; Rhodus, N.L. Neurologic Disorders. In Little and Falace’s Dental Management of the Medically Compromised Patient, 8th ed.; Mosby, Elsevier Inc.: St. Louis, MO, USA, 2013; pp. 494–521. [Google Scholar] [CrossRef]
- Al Rajeh, S.; Bademosi, O.; Ismail, H.; Awada, A.; Dawodu, A.; Al-Freihi, H.; Assuhaimi, S.; Borollosi, M.; Al-Shammasi, S. A community survey of neurological disorders in Saudi Arabia: The Thugbah study. Neuroepidemiology 1993, 12, 164–178. [Google Scholar] [CrossRef]
- Safiri, S.; Noori, M.; Nejadghaderi, S.A.; Mousavi, S.E.; Sullman, M.J.; Araj-Khodaei, M.; Singh, K.; Kolahi, A.A.; Gharagozli, K. The burden of Parkinson’s disease in the Middle East and North Africa region, 1990–2019: Results from the global burden of disease study 2019. BMC Public Health 2023, 23, 107. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, L.; Li, M.; Wen, X.; Zhang, W.; Li, X. Global, regional, national epidemiology and trends of Parkinson’s disease from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. Front. Aging Neurosci. 2025, 16, 1498756. [Google Scholar] [CrossRef]
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, AU, USA, 2018; Chapter 1. [Google Scholar] [CrossRef]
- Lee, H.M.; Koh, S.B. Many Faces of Parkinson’s Disease: Non-Motor Symptoms of Parkinson’s Disease. J. Mov. Disord. 2015, 8, 92–97. [Google Scholar] [CrossRef]
- Khalil, I.; Sayad, R.; Kedwany, A.M.; Sayed, H.H.; Caprara, A.L.F.; Rissardo, J.P. Cardiovascular dysautonomia and cognitive impairment in Parkinson’s disease (Review). Med. Int. 2024, 4, 70. [Google Scholar] [CrossRef]
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, AU, USA, 2018; Chapter 7. [Google Scholar] [CrossRef]
- Currie, A.D.; Wong, J.K.; Okun, M.S. A review of temporal interference, nanoparticles, ultrasound, gene therapy, and designer receptors for Parkinson disease. NPJ Park. Dis. 2024, 10, 195. [Google Scholar] [CrossRef]
- De Masi, C.; Liguori, C.; Spanetta, M.; Fernandes, M.; Cerroni, R.; Garasto, E.; Pierantozzi, M.; Mercuri, N.B.; Stefani, A. Non-motor symptoms burden in motor-fluctuating patients with Parkinson’s disease may be alleviated by safinamide: The VALE-SAFI study. J. Neural Transm. 2022, 129, 1331–1338. [Google Scholar] [CrossRef]
- Tison, F.; Meissner, W.G. Standard strategies for diagnosis and treatment of patients with newly diagnosed Parkinson disease: FRANCE. Neurol. Clin. Pract. 2013, 3, 480–481. [Google Scholar] [CrossRef]
- Fouly, R.T.; Sahli, A.A.; Alqahtani, B.G.; Algosair, I.S.; Garbo, M.S.A.; Rayyani, S.A. Knowledge of Saudi Population About Parkinson Disease. Int. J. Innov. Res. Med. Sci. 2020, 5, 49–51. [Google Scholar] [CrossRef]
- White, D.W.; Raven, P.B. Autonomic neural control of heart rate during dynamic exercise: Revisited. J. Physiol. 2014, 592, 2491–2500. [Google Scholar] [CrossRef]
- Arakaki, X.; Arechavala, R.J.; Choy, E.H.; Bautista, J.; Bliss, B.; Molloy, C.; Wu, D.A.; Shimojo, S.; Jiang, Y.; Kleinman, M.T.; et al. The connection between heart rate variability (HRV), neurological health, and cognition: A literature review. Front. Neurosci. 2023, 17, 1055445. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health. 2017, 5, 258. [Google Scholar] [CrossRef]
- Iniguez, M.; Jimenez-Marin, A.; Erramuzpe, A.; Acera, M.; Tijero, B.; Murueta-Goyena, A.; Del Pino, R.; Fernandez, T.; Carmona-Abellan, M.; Cabrera-Zubizarreta, A.; et al. Heart-brain synchronization breakdown in Parkinson’s disease. NPJ Park. Dis. 2022, 8, 64. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Autonomic Dysfunction in Parkinson’s Disease. Neurotherapeutics 2020, 17, 1464–1479. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef]
- Devos, D.; Kroumova, M.; Bordet, R.; Vodougnon, H.; Guieu, J.D.; Libersa, C.; Destee, A. Heart rate variability and Parkinson’s disease severity. J. Neural Transm. 2003, 110, 997–1011. [Google Scholar] [CrossRef]
- Amara, A.W.; Memon, A.A. Effects of Exercise on Non-Motor Symptoms in Parkinson’s Disease. Clin Ther. 2018, 40, 8–15. [Google Scholar] [CrossRef]
- Santilli, G.; Mangone, M.; Agostini, F.; Paoloni, M.; Bernetti, A.; Diko, A.; Tognolo, L.; Coraci, D.; Vigevano, F.; Vetrano, M.; et al. Evaluation of Rehabilitation Outcomes in Patients with Chronic Neurological Health Conditions Using a Machine Learning Approach. J. Funct. Morphol. Kinesiol. 2024, 9, 176. [Google Scholar] [CrossRef]
- Vargas, A.M.; Bisi, A.; Chiappa, A.S.; Versteeg, C.; Miller, L.E.; Mathis, A. Task-driven neural network models predict neural dynamics of proprioception. Cell 2024, 187, 1745–1761.e19. [Google Scholar] [CrossRef]
- Alonso, A.; Huang, X.; Mosley, T.H.; Heiss, G.; Chen, H. Heart rate variability and the risk of Parkinson disease: The Atherosclerosis Risk in Communities study. Ann Neurol. 2015, 77, 877–883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimojo, G.; Joseph, B.; Shah, R.; Consolim-Colombo, F.M.; De Angelis, K.; Ulloa, L. Exercise activates vagal induction of dopamine and attenuates systemic inflammation. Brain Behav. Immun. 2019, 75, 181–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panassollo, T.R.B.; Mawston, G.; Taylor, D.; Lord, S. Targeting exercise intensity and aerobic training to improve outcomes in Parkinson’s disease. Sport Sci. Health 2024, 20, 287–297. [Google Scholar] [CrossRef]
- Shinoda, L.; Damasceno, L.; Freitas, L.; Campos, R.; Cravo, S.; Scorza, C.A.; Scorza, F.A.; Faber, J. Cardiac and Autonomic Dysfunctions Assessed Through Recurrence Quantitative Analysis of Electrocardiogram Signals and an Application to the 6-Hydroxydopamine Parkinson’s Disease Animal Model. Front. Physiol. 2021, 12, 725218. [Google Scholar] [CrossRef]
- Sabino-Carvalho, J.L.; Fisher, J.P.; Vianna, L.C. Autonomic Function in Patients with Parkinson’s Disease: From Rest to Exercise. Front. Physiol. 2021, 12, 626640. [Google Scholar] [CrossRef]
- Moawad, H. CADASIL and Dementia: Brain Effects and Progression. Verywell Health. 23 September 2024. Available online: https://www.verywellhealth.com/cadasil-8707539 (accessed on 15 February 2025).
- Task Force of the European Society of Cardiology; North American Society of Pacing and Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- American Parkinson Disease Association. The Heart of the Matter: Cardiovascular Effects of Parkinson’s Disease. 14 August 2024. Available online: https://www.apdaparkinson.org/article/heart-and-parkinsons (accessed on 15 February 2025).
- Ruffinazzi, M.; Dusi, V. Central Nervous System Management of Autonomic Cardiovascular Control. In Brain and Heart Dynamics; Govoni, S., Politi, P., Vanoli, E., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Besson, C.; Baggish, A.L.; Monteventi, P.; Schmitt, L.; Stucky, F.; Gremeaux, V. Assessing the clinical reliability of short-term heart rate variability: Insights from controlled dual-environment and dual-position measurements. Sci. Rep. 2025, 15, 5611. [Google Scholar] [CrossRef]
- Olivieri, F.; Biscetti, L.; Pimpini, L.; Pelliccioni, G.; Sabbatinelli, J.; Giunta, S. Heart rate variability and autonomic nervous system imbalance: Potential biomarkers and detectable hallmarks of aging and inflammaging. Ageing Res. Rev. 2024, 101, 102521. [Google Scholar] [CrossRef]
- Li, K.; Cardoso, C.; Moctezuma-Ramirez, A.; Elgalad, A.; Perin, E. Heart Rate Variability Measurement through a Smart Wearable Device: Another Breakthrough for Personal Health Monitoring? Int. J. Environ. Res. Public Health 2023, 20, 7146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fay-Karmon, T.; Galor, N.; Heimler, B.; Zilka, A.; Bartsch, R.P.; Plotnik, M.; Hassin-Baer, S. Home-based monitoring of persons with advanced Parkinson’s disease using smartwatch-smartphone technology. Sci. Rep. 2024, 14, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Demographics | |||||
|---|---|---|---|---|---|
| Age (years) | 60.2 ± 4.5 | ||||
| Sex | 55 Female + 55 Male | ||||
| BMI (kg/m2) | 28.5 ± 2.8 | ||||
| HRV Metric | Time | ECG (Mean ± SD) | t-test p-value | PCA Loadings (Feature Importance) | |
| PC1 | PC2 | ||||
| SD of RR (ms) | Pre-Intervention | 3.4 ± 1.17 | <0.05 | −0.45 | 0.35 |
| Post-Intervention | 4.02 ± 1.56 | 0.20 | 0.59 | ||
| Mean of RR (ms) | Pre-Intervention | 882 ± 13 | 0.5 | - | - |
| Post-Intervention | 890 ± 15 | - | - | ||
| SDRR Coeff. of Variation % | Pre-Intervention | 46 ± 17 | <0.05 | −0.36 | 0.32 |
| Post-Intervention | 41 ± 21 | 0.18 | 0.58 | ||
| VLF (ms2) | Pre-Intervention | 0.56 ± 0.62 | <0.05 | −0.02 | −0.06 |
| Post-Intervention | 0.38 ± 1.44 | 0.08 | 0.13 | ||
|
LF (ms2) | Pre-Intervention | 0.45 ± 0.68 | 0.40 | −0.15 | −0.05 |
| Post-Intervention | 0.37 ± 0.61 | 0.14 | 0.22 | ||
|
HF (ms2) | Pre-Intervention | 0.16 ± 0.09 | 0.33 | −0.12 | −0.09 |
| Post-Intervention | 0.20 ± 0.26 | −0.04 | 0.09 | ||
LF/HF | Pre-Intervention | 2.81 ± 2.30 | <0.05 | 0.58 | −0.01 |
| Post-Intervention | 1.85 ± 8.78 | 0.44 | 0.00 | ||
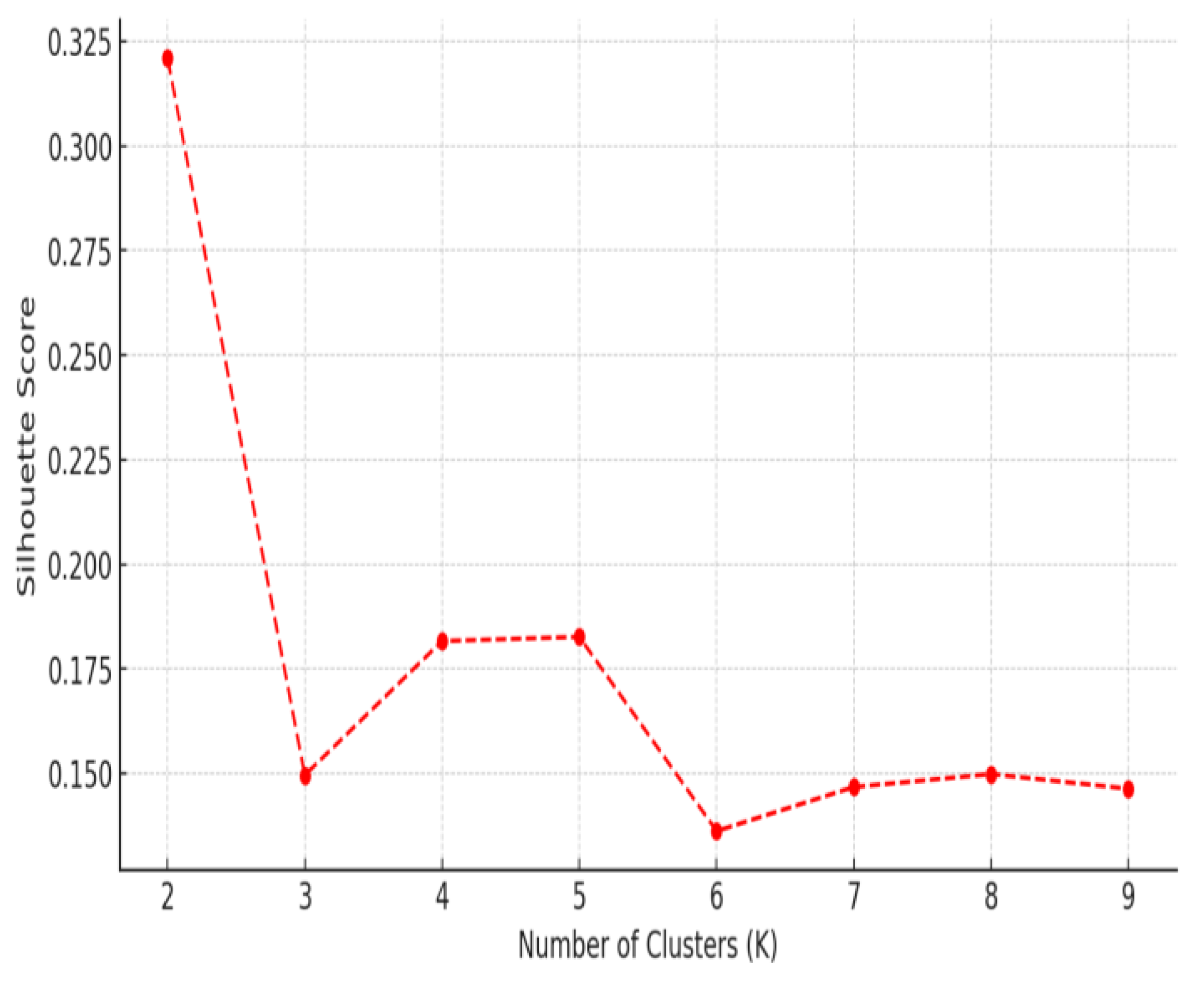
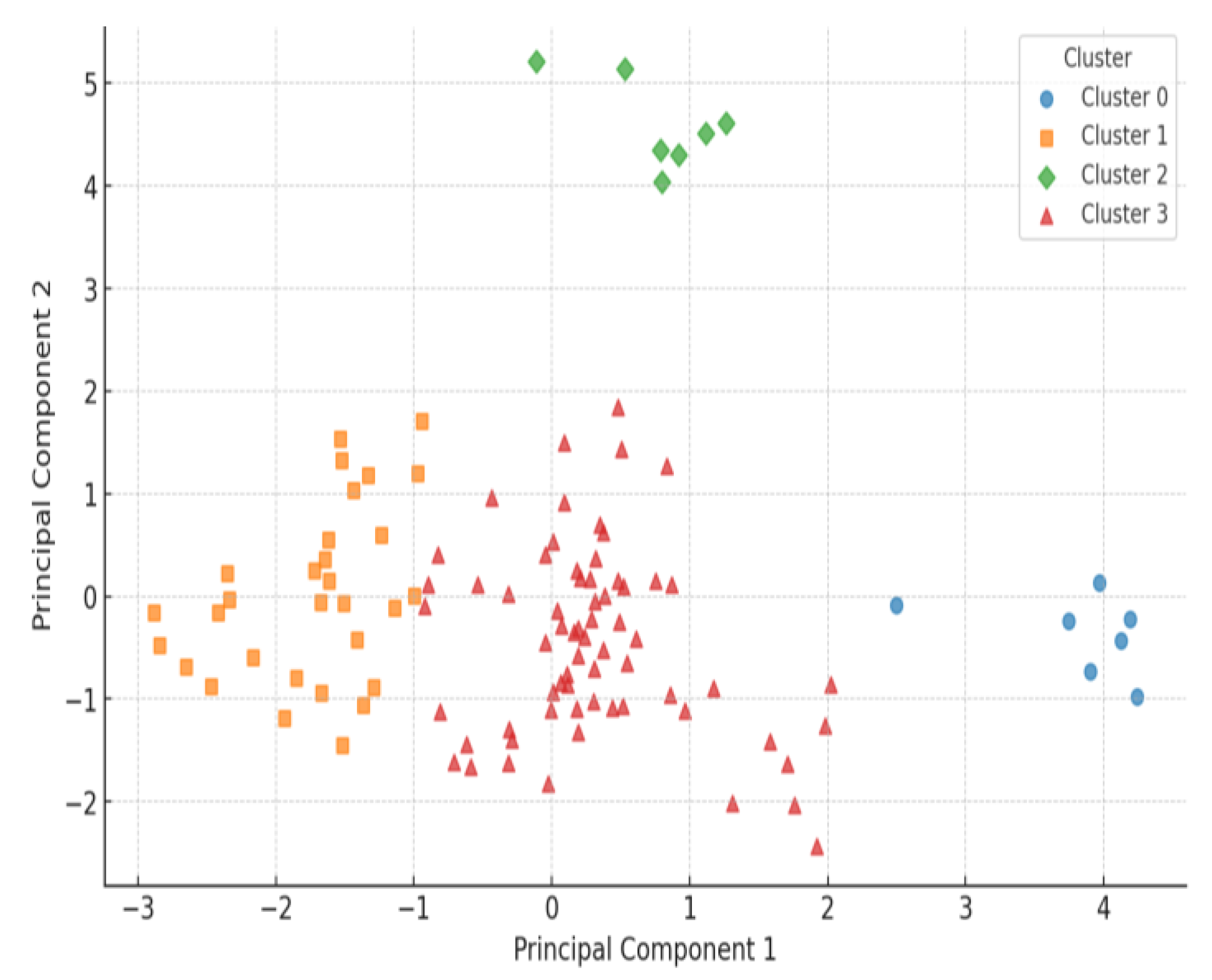
| Cluster | Subgroup Name | Number of Patients (%) |
|---|---|---|
| Cluster 3 | Mixed/Irregular Responders | 66 (60%) |
| Cluster 1 | Moderate HRV Responders | 30 (27%) |
| Cluster 2 | Strong HRV Responders | 7 (6%) |
| Cluster 0 | Low HRV Responders | 7 (6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basri, A.M.; Turki, A.F. Evaluating Heart Rate Variability as a Biomarker for Autonomic Function in Parkinson’s Disease Rehabilitation: A Clustering-Based Analysis of Exercise-Induced Changes. Medicina 2025, 61, 527. https://doi.org/10.3390/medicina61030527
Basri AM, Turki AF. Evaluating Heart Rate Variability as a Biomarker for Autonomic Function in Parkinson’s Disease Rehabilitation: A Clustering-Based Analysis of Exercise-Induced Changes. Medicina. 2025; 61(3):527. https://doi.org/10.3390/medicina61030527
Chicago/Turabian StyleBasri, Ahmed M., and Ahmad F. Turki. 2025. "Evaluating Heart Rate Variability as a Biomarker for Autonomic Function in Parkinson’s Disease Rehabilitation: A Clustering-Based Analysis of Exercise-Induced Changes" Medicina 61, no. 3: 527. https://doi.org/10.3390/medicina61030527
APA StyleBasri, A. M., & Turki, A. F. (2025). Evaluating Heart Rate Variability as a Biomarker for Autonomic Function in Parkinson’s Disease Rehabilitation: A Clustering-Based Analysis of Exercise-Induced Changes. Medicina, 61(3), 527. https://doi.org/10.3390/medicina61030527







