A Narrative Review on Current Status of Conscious Sedation for Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
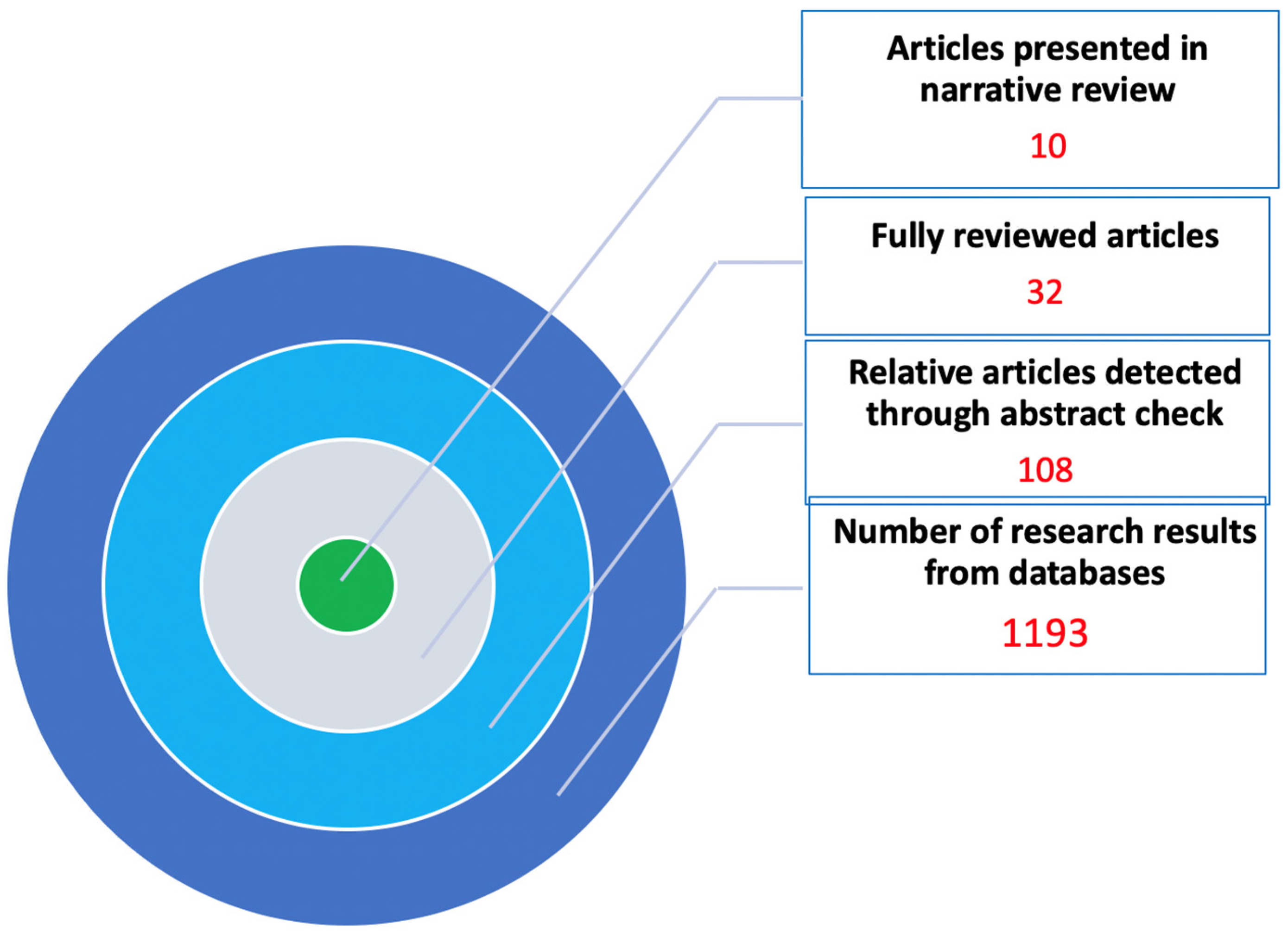
2. Methods
3. Results
4. Discussion
4.1. Is Anesthesiologist-Led Anesthesia Necessary for TAVI?
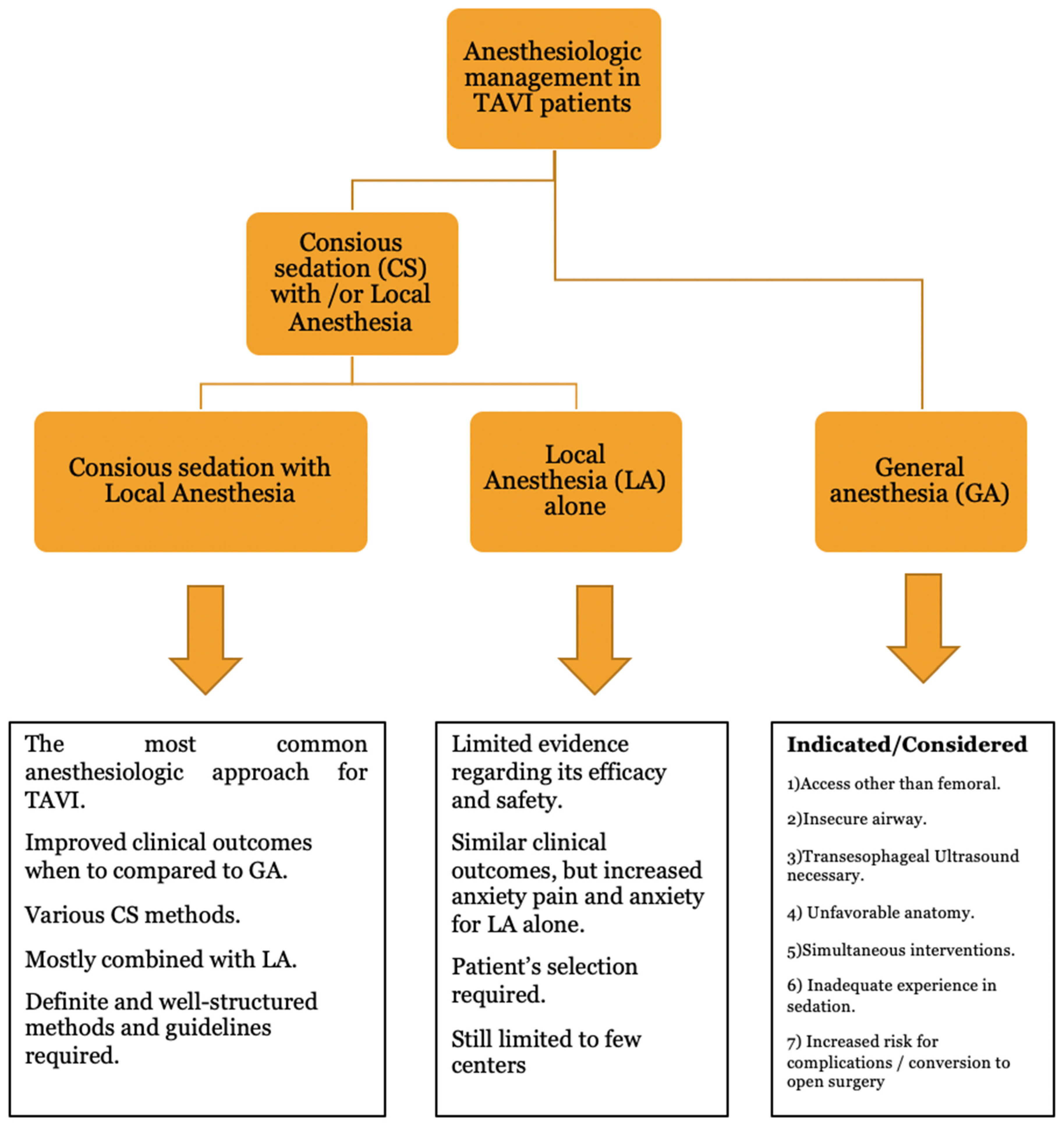
4.2. Type of Anesthesia Selection
4.3. Conscious Sedation (SC) with Local Anesthesia (LA)
4.4. Special Considerations
4.5. Future Developments
4.6. Limitations
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Marsan, N.A.; Barili, F.; Bonaros, N.; ESC/EACTS Scientific Document Group; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2025, ehaf194. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H. Transcatheter Aortic Valve Implantation: Two Decades of a Revolutionary and Ongoing Odyssey. Circulation 2024, 150, 821–822. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.; Allen, C.J.; Aroney, N.; Redwood, S.; Prendergast, B. The Future of Transcatheter Interventions. JACC Case Rep. 2020, 2, 2281–2282. [Google Scholar] [CrossRef] [PubMed]
- İzgi, M.; Halis, A.; Şener, Y.Z.; Şahiner, L.; Kaya, E.B.; Aytemir, K.; Karagöz, A.H. Evaluation of Anaesthetic Approaches in Transcatheter Aortic Valv Implantation Procedures. Turk. J. Anaesthesiol. Reanim. 2023, 51, 427–433. [Google Scholar] [CrossRef]
- Trauzeddel, R.F.; Nordine, M.; Balanika, M.; Bence, J.; Bouchez, S.; Ender, J.; Erb, J.M.; Fassl, J.; Fletcher, N.; Mukherjee, C.; et al. Current Anesthetic Care of Patients Undergoing Transcatheter Aortic Valve Replacement in Europe: Results of an Online Survey. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1737–1746. [Google Scholar] [CrossRef]
- Patel, P.A.; Neuburger, P.J. Ongoing Obstacles for Universal Use of Sedation for Transfemoral Transcatheter Aortic Valve Replacement. J. Cardiothorac. Vasc. Anesth. 2019, 33, 36–38. [Google Scholar] [CrossRef]
- Berkovitch, A.; Finkelstein, A.; Barbash, I.M.; Kornowski, R.; Fefer, P.; Steinvil, A.; Assa, H.V.; Danenberg, H.; Maor, E.; Guetta, V.; et al. Local Anesthesia versus Conscious Sedation among Patients Undergoing Transcatheter Aortic Valve Implantation-A Propensity Score Analysis. J. Clin. Med. 2022, 11, 3134. [Google Scholar] [CrossRef]
- Kinoshita, H.; Yamamoto, M.; Adachi, Y.; Yamaguchi, R.; Takemura, A. Fascia Iliaca Block Reduces Remifentanil Requirement in Conscious Sedation for Transcatheter Aortic Valve Implantation—A Randomized Clinical Trial. Circ. J. 2024, 88, 475–482. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, J.S.; Seo, W.W.; Joung, K.W.; Chin, J.H.; Kim, W.J.; Choi, D.-K.; Choi, I.-C. Effects of remimazolam versus dexmedetomidine on recovery after transcatheter aortic valve replacement under monitored anesthesia care: A propensity score-matched, non-inferiority study. Korean J. Anesthesiol. 2024, 77, 537–545. [Google Scholar] [CrossRef]
- Georgia, N.; Ilias, S.; Panagiotis, D.; Mihalis, A.; Konstantina, R.; Anastasia, A.; Ioannis, A.; Nikolaos, S. Comparative study between sedation and general anesthesia as an anesthesiologic approach for patients treated with TAVR. Which is the best for hemodynamic stability? Hell. J. Cardiol. 2024, 79, 88–91. [Google Scholar] [CrossRef]
- Luzzi, C.; Orlov, D.; Foley, K.; Horlick, E.; Osten, M.; Cusimano, R.J.; Djaiani, G. Choice of anesthesia technique is associated with earlier hospital discharge and reduced costs after transcatheter transfemoral aortic valve implantation. J. Thorac. Dis. 2024, 16, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Feistritzer, H.J.; Kurz, T.; Vonthein, R.; Schröder, L.; Stachel, G.; Eitel, I.; Marquetand, C.; Saraei, R.; Kirchhof, E.; Heringlake, M.; et al. SOLVE-TAVI Investigators. Effect of Valve Type and Anesthesia Strategy for TAVR: 5-Year Results of the SOLVE-TAVI Trial. J. Am. Coll. Cardiol. 2025, 85, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Elsaeidy, A.S.; Ahmad, A.H.M.; Kohaf, N.A.; Aboutaleb, A.; Kumar, D.; Elsaeidy, K.S.; Mohamed, O.S.; Kaye, A.D.; Shehata, I.M. Efficacy and Safety of Ketamine-Dexmedetomidine Versus Ketamine-Propofol Combination for Periprocedural Sedation: A Systematic Review and Meta-analysis. Curr. Pain Headache Rep. 2024, 28, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ooms, J.; de Ronde, M.; van Gorsel, S.; Mattace-Raso, A.M.; Goudzwaard, J.; Mattace-Raso, F.; Kardys, I.; Nuis, R.-J.; Daemen, J.; et al. Anxiety during transcatheter aortic valve replacement under local anesthesia—The ART-VR trial. Cardiovasc. Revascularization Med. 2025, 79, 71–77. [Google Scholar] [CrossRef]
- Mino, T.; Kitaura, A.; Sakamoto, H.; Yoshino, Y.; Tsukimoto, S.; Yuasa, H.; Nakajima, Y. Comparison of Postoperative Nausea and Vomiting Between Sedation with Remimazolam and Dexmedetomidine in Transcatheter Aortic Valve Replacement Patients: A Single-Center Retrospective Observational Study. J. Clin. Med. 2025, 14, 1759. [Google Scholar] [CrossRef]
- Feistritzer, H.J.; Ender, J.; Lauten, P.; Rudolph, T.K.; Rudolph, V.; Geisler, T.; Massberg, S.; Adam, M.; Baldus, S.; Sossalla, S.; et al. DOUBLE-CHOICE Investigators. Peri-interventional Anesthesia Strategies for Transcatheter Aortic Valve Implantation: A Multicenter, Randomized, Controlled, Non-inferiority Trial. Circulation 2025, 152. [Google Scholar] [CrossRef]
- Ghandi, R.; Zwartes, M.; Hordern, M.; Ranchord, A.; Matsis, P.; Sasse, A.; Rama-Chandran, A.; Prescott-Whitaker, G.; Ishver, A.; Chatfield, A. TAVI With Cardiologist-Led Sedation—A Single-Centre Experience of Safety and Cost-Effectiveness in New Zealand. Heart Lung Circ. 2025, 34, S718. [Google Scholar] [CrossRef]
- Norman, S.; Brooks, M.; Wilson, W.; Koshy, A.; Gurvitch, R. Cardiologist -led sedation for TAVI: A single center Australian experience. Heart Lung Circ. 2024, 33, S580. [Google Scholar] [CrossRef]
- Kočka, V.; Nováčková, M.; Kratochvílová, L.; Širáková, A.; Sulženko, J.; Buděšínský, T.; Bystroń, M.; Neuberg, M.; Mašek, P.; Bednář, F.; et al. Nurse-led sedation for transfemoral transcatheter aortic valve implantation seems safe for a selected patient population. Eur. Heart J. Suppl. 2022, 24 (Suppl. B), B23–B27. [Google Scholar] [CrossRef]
- Xie, L.; Lang, Z.; Liu, Y.; Yue, H.; Chen, Q.; Tao, G. Whether monitored anesthesia care is the optimal anesthetic strategy for transcatheter aortic valve implantation surgery? a meta-analysis and systematic review. BMC Anesthesiol. 2024, 24, 429. [Google Scholar] [CrossRef]
- Rosseel, L.; Mylotte, D.; Cosyns, B.; Vanhaverbeke, M.; Zweiker, D.; Teles, R.C.; Angerås, O.; Neylon, A.; Rudolph, T.K.; Wykrzykowska, J.J.; et al. Contemporary European practice in transcatheter aortic valve implantation: Results from the 2022 European TAVI Pathway Registry. Front. Cardiovasc. Med. 2023, 10, 1227217. [Google Scholar] [CrossRef]
- Cheng, D.R. Local or general anesthesia for TAVI surgery? An updated systematic review and meta-analysis. Eur. Heart J. 2021, 42 (Suppl. 1), ehab724.1670. [Google Scholar] [CrossRef]
- Mayr, N.P.; Michel, J.; Bleiziffer, S.; Tassani, P.; Martin, K. Sedation or general anesthesia for transcatheter aortic valve implantation (TAVI). J. Thorac. Dis. 2015, 7, 1518–1526. [Google Scholar] [PubMed]
- Mayr, N.P.; Hapfelmeier, A.; Martin, K.; Kurz, A.; van der Starre, P.; Babik, B.; Mazzitelli, D.; Lange, R.; Wiesner, G.; Tassani-Prell, P. Comparison of sedation and general anaesthesia for transcatheter aortic valve implantation on cerebral oxygen saturation and neurocognitive outcome. Br. J. Anaesth. 2016, 116, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Harjai, K.J.; Bules, T.; Berger, A.; Young, B.; Singh, D.; Carter, R.; Agarwal, S.; Crockett, S.; Mascarenhas, V.; Nawaz, Y.; et al. Efficiency, Safety, and Quality of Life After Transcatheter Aortic Valve Implantation Performed with Moderate Sedation Versus General Anesthesia. Am. J. Cardiol. 2020, 125, 1088–1095. [Google Scholar] [CrossRef]
- Baniani, M.; Dabbagh, A. Primary Report of Anesthesia Methods and Comorbidities in Patients Undergoing Transcatheter Aortic Valve Implementation (TAVI): An Observational Study. Arch. Anesth. Crit. Care 2025, 11, 621–626. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, W.; Wang, X.; Liu, M.; Hei, F.; Guan, Y. General Anesthesia Increased the Risk of Atrial Fibrillation and Acute Kidney Injury in Transcatheter Aortic Valve Replacement. Heart Surg. Forum 2021, 24, E082–E100. [Google Scholar] [CrossRef]
- Aslan, S.; Güner, A.; Demir, A.R.; Yılmaz, E.; Aslan, A.F.; Çelik, Ö.; Uzun, F.; Ertürk, M. Conscious sedation versus general anesthesia for transcatheter aortic valve implantation in patients with severe chronic obstructive pulmonary disease. Perfusion 2023, 38, 186–192. [Google Scholar] [CrossRef]
- Ko, C.C.; Hung, K.C.; Chang, Y.P.; Liu, C.C.; Cheng, W.J.; Wu, J.Y.; Li, Y.-Y.; Lin, T.-C.; Sun, C.-K. Association of general anesthesia exposure with risk of postoperative delirium in patients receiving transcatheter aortic valve replacement: A meta-analysis and systematic review. Sci. Rep. 2023, 13, 16241. [Google Scholar] [CrossRef]
- Goren, O.; Finkelstein, A.; Gluch, A.; Sheinberg, N.; Dery, E.; Matot, I. Sedation or general anesthesia for patients undergoing transcatheter aortic valve implantation—Does it affect outcome? An observational single-center study. J. Clin. Anesth. 2015, 27, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Chen, J.Y.; Hsing, C.H.; Chu, C.C.; Lin, Y.T.; Pang, Y.L.; Teng, I.C.; Chen, I.W.; Sun, C.K. Conscious sedation/monitored anesthesia care versus general anesthesia in patients undergoing transcatheter aortic valve replacement: A meta-analysis. Front. Cardiovasc. Med. 2023, 9, 1099959. [Google Scholar] [CrossRef]
- Heringlake, M.; Berggreen, A.E.; Vigelius-Rauch, U.; Treskatsch, S.; Ender, J.; Thiele, H. Patient Well-being and Satisfaction after General or Local Anesthesia with Conscious Sedation: A Secondary Analysis of the SOLVE-TAVI Trial. Anesthesiology 2023, 139, 701–704. [Google Scholar] [CrossRef]
- Georgia, N.; Anastasia, A.; Aikaterini, D.; Nikolaos, S. Perspective Chapter: Transcatheter Aortic Valve Implantation (TAVI)-Anesthetic Considerations; IntechOpen: London, UK, 2022. [Google Scholar]
- Sawan, M.A.; Calhoun, A.E.; Grubb, K.J.; Devireddy, C.M. Update on Minimalist TAVR Care Pathways: Approaches to Care in 2022. Curr. Cardiol. Rep. 2022, 24, 1179–1187. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [PubMed]
- Schizas, N.; Antonopoulos, C.N.; Patris, V.; Lampropoulos, K.; Kratimenos, T.; Argiriou, M. Current issues on simultaneous TAVR (Transcatheter Aortic Valve Replacement) and EVAR (Endovascular Aneurysm Repair). Clin. Case Rep. 2021, 9, e03929. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Kurz, T.; Feistritzer, H.J.; Stachel, G.; Hartung, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; Lauten, A.; et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: The randomized SOLVE-TAVI trial. Eur. Heart J. 2020, 41, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Mayr, N.P. Transfemoral Transcatheter Aortic Valve Replacement: Conscious Sedation for Everyone? JACC Cardiovasc. Interv. 2020, 13, 1288–1290. [Google Scholar] [CrossRef]
- Jain, A. Awakening the Future: Exploring Awake or Minimalistic Transcatheter Aortic Valve Replacement and the Evolving Role of Sedation Strategies. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1901–1903. [Google Scholar] [CrossRef]
- Fadah, K.; Khalafi, S.; Corey, M.; Sotelo, J.; Farag, A.; Siddiqui, T.; Abolbashari, M. Optimizing Anesthetic Selection in Transcatheter Aortic Valve Replacement: Striking a Delicate Balance between Efficacy and Minimal Intervention. Cardiol. Res. Pract. 2024, 2024, 4217162. [Google Scholar] [CrossRef]
- Chowdhury, S.; Sawires, J.; Weissman, B.; Saju, S.; Lambroussis, C.G. Comparing the Efficacy and Safety of Dexmedetomidine Versus Propofol for Sedation in Adult Patients Undergoing Cardiac Procedures: A Systematic Review. Cureus 2025, 17, e91773. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, L.; Coppolino, F.; Donatiello, V.; Paladini, A.; Sansone, P.; Passavanti, M.B.; Pota, V.; Giaccari, L.G.; Aurilio, C.; Sepolvere, G.; et al. Use of Dexmedetomidine in Transfemoral Transcatheter Aortic Valve Implantation (tf-TAVI) Procedures. Adv. Ther. 2020, 37, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Mayr, N.P.; Wiesner, G.; van der Starre, P.; Hapfelmeier, A.; Goppel, G.; Kasel, A.M.; Hengstenberg, C.; Husser, O.; Schunkert, H.; Tassani-Prell, P. Dexmedetomidine versus propofol-opioid for sedation in transcatheter aortic valve implantation patients: A retrospective analysis of periprocedural gas exchange and hemodynamic support. Can. J. Anaesth. 2018, 65, 647–657. (In English) [Google Scholar] [CrossRef] [PubMed]
- Verolino, G.; Di Mauro, M.; Calderone, D.; Lorusso, R. Major Intraprocedural Complications During Transcatheter Aortic Valve Implantation Requiring Emergent Cardiac Surgery: An Updated Systematic Review. Am. J. Cardiol. 2025, 247, 21–28. [Google Scholar] [CrossRef]
- Hayashida, K.; Lefèvre, T.; Chevalier, B.; Hovasse, T.; Romano, M.; Garot, P.; Mylotte, D.; Uribe, J.; Farge, A.; Donzeau-Gouge, P.; et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc. Interv. 2011, 4, 851–858. [Google Scholar] [CrossRef]
- Eggebrecht, H.; Schmermund, A.; Voigtländer, T.; Kahlert, P.; Erbel, R.; Mehta, R.H. Risk of stroke after transcatheter aortic valve implantation (TAVI): A meta-analysis of 10,037 published patients. EuroIntervention 2012, 8, 129–138. [Google Scholar] [CrossRef]
- Neuburger, P.J.; Pospishil, L.; Ibrahim, H. Anesthetic Management of Conduction Disturbances Following Transcatheter Aortic Valve Replacement: A Review of the 2020 ACC Expert Consensus Decision Pathway. J. Cardiothorac. Vasc. Anesth. 2021, 35, 982–986. [Google Scholar] [CrossRef]
- Martin, J.A.; Carlson, R.E.; Tsai, M.H. The evolution of sedation for transcatheter aortic valve replacement. J. Clin. Anesth. Intensive Care 2021, 2, 15–19. [Google Scholar]
- Neuburger, P.J.; Saric, M.; Huang, C.; Williams, M.R. A Practical Approach to Managing Transcatheter Aortic Valve Replacement with Sedation. Semin. Cardiothorac. Vasc. Anesth. 2016, 20, 147–157. [Google Scholar] [CrossRef]
- Scheuermann, S.; Tan, A.; Govender, P.; Mckie, M.; Pack, J.; Martinez, G.; Falter, F.; George, S.; AKlein, A. High-flow nasal oxygen vs. standard oxygen therapy for patients undergoing transcatheter aortic valve replacement with conscious sedation: A randomised controlled trial. Perioper. Med. 2023, 12, 11. [Google Scholar] [CrossRef]
- Leone, A.; Castiello, D.S.; Angellotti, D.; Mariani, A.; Manzo, R.; Avvedimento, M.; Ilardi, F.; Piccolo, R.; Esposito, G.; Franzone, A. Incidence, predictors, and prognostic impact of temporary left bundle branch block after transcatheter aortic valve replacement. J. Electrocardiol. 2022, 74, 114–115. [Google Scholar] [CrossRef]
- Androutsopoulou, V.; Zotos, P.A.; Xanthopoulos, A.; Boultadakis, E.; Magouliotis, D.; Schizas, N.; Iliopoulos, D.C.; Skoularigis, J.; Athanasiou, T. Individualized Selection of Valve Intervention Strategies in Aortic Disease Is Key for Better Outcomes. J. Pers. Med. 2025, 15, 337. [Google Scholar] [CrossRef]
| Ref | First Author, Year | Study Type | Anesthetic Approach | Number of Patients | Key Findings | Conclusions |
|---|---|---|---|---|---|---|
| [8] | Berkovitch, 2022 | Comparative, retrospective, propensity score analysis | Comparison LA to SC | 1096 |
| LA—only strategy is associated with lower complication rates |
| [9] | Kinoshita, 2022 | RCT | SC (DEX combined with RM or PRO) alone or with ropivacaine FIB | 72 |
| Improved quality of CS for FIB group due to less pain experienced |
| [10] | Kim, 2024 | Comparative, retrospective, propensity score analysis, non-inferiority | Comparison of SC with DEX and remimazolam | 464 |
| Remimazolam was associated with better recovery findings |
| [11] | Georgia, 2024 | Comparative, retrospective | Comparison of GA to SC | 102 |
| SC was associated with better hemodynamic stability during the procedure, fewer complications, and reduced ICU and hospital stay |
| [12] | Luzzi, 2024 | Comparative, retrospective, matched population | Comparison GA to SC | 248 |
| SC is related to shorter hospital stays and lower costs when compared to GA |
| [13] | Feistritzer, 2024 | Multicenter RCT | Comparison of GA to SC | 447 |
| Similar clinical outcomes for both groups, although SC may be associated with lower all-cause mortality |
| [14] | Elsaeidy, 2024 | Systematic review and meta-analysis | Comparison between Ketadex* and Ketofol** | 1429 |
| Ketadex is better combination for periprocedural SC for both adult and pediatric patients who are not at high risk for nausea and vomit |
| [15] | Chatterjee, 2025 | Single center RCT | Comparison between LA alone versus LA + VR | 75 |
| VR implementation did not affect the experienced pain and anxiety |
| [16] | Mino, 2025 | Comparative, retrospective, propensity score analysis | Comparison SC with Remimazolam versus DEX + PR | 177 |
| No significant difference between the two groups regarding remimazolam and DEX+PR |
| [17] | Feistritzer, 2025 | Multicenter, RCT, non-inferiority trial | Comparison between LA and SC | 752 |
| LA was non-inferior to SC in clinical outcomes and safety. LA is related to higher anxiety during the procedure. |
| Databases—Sources of Review | Number of Results | Relative Articles | Fully Reviewed Articles | Articles Presented in Narrative Review |
|---|---|---|---|---|
| PUBMED | 145 | 105 | 22 | 10 |
| EMBASE | 76 | 52 | 16 | 3 |
| COHRANE | 82 | 67 | 19 | 2 |
| GOOGLE SCHOLAR | 890 | 108 | 22 | 10 |
| TOTAL | 1193 | 108 | 32 | 10 |
| DRUGS | BLOOD PRESSURE | HEART RATE | RESPIRATORY DEPRESSION | ANALGESIA | NAUSEA/ VOMITING | SEDATION DOSAGE |
|---|---|---|---|---|---|---|
| PROPOFOL |  |  |  |  |  | 1–2 mg/kg IV for induction 25–150 μg/kg/min infusion |
| KETAMINE |  |  |  |  |  | 0.5–2 mg/kg IV |
| DEXDOR |  |  |  |  |  | 1 μg/kg bolus over 10 min +0.2–0.7 μg/kg/h infusion |
| MIDAZOLAM |  |  |  |  |  | 0.02–0.2 mg/kg IV bolus 0.02–0.1 mg/kg/h infusion |
| REMIMAZOLAM |  |  |  |  |  | 0.1–0.2 mg/kg for induction 1–2 mg/kg/h infusion |
| FENTANYL |  |  |  |  |  | 1–2 μg/kg bolus |
| REMIFENTANIL |  |  |  |  |  | 0.5–1 μg/kg bolus 0.05–0.2 μg/kg/min infusion |
| ETOMIDATE |  |  |  |  |  | 0.2–0.3 mg/kg IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazou, G.; Schizas, N.; Romana, K.N.; Androutsopoulou, V.; Magira, E.; Sarantopoulos, A.; Iliopoulos, D.; Mentzelopoulos, S.D. A Narrative Review on Current Status of Conscious Sedation for Transcatheter Aortic Valve Implantation. Medicina 2025, 61, 1980. https://doi.org/10.3390/medicina61111980
Nazou G, Schizas N, Romana KN, Androutsopoulou V, Magira E, Sarantopoulos A, Iliopoulos D, Mentzelopoulos SD. A Narrative Review on Current Status of Conscious Sedation for Transcatheter Aortic Valve Implantation. Medicina. 2025; 61(11):1980. https://doi.org/10.3390/medicina61111980
Chicago/Turabian StyleNazou, Georgia, Nikolaos Schizas, Konstantina N. Romana, Vasiliki Androutsopoulou, Eleni Magira, Andreas Sarantopoulos, Dimitrios Iliopoulos, and Spyros D. Mentzelopoulos. 2025. "A Narrative Review on Current Status of Conscious Sedation for Transcatheter Aortic Valve Implantation" Medicina 61, no. 11: 1980. https://doi.org/10.3390/medicina61111980
APA StyleNazou, G., Schizas, N., Romana, K. N., Androutsopoulou, V., Magira, E., Sarantopoulos, A., Iliopoulos, D., & Mentzelopoulos, S. D. (2025). A Narrative Review on Current Status of Conscious Sedation for Transcatheter Aortic Valve Implantation. Medicina, 61(11), 1980. https://doi.org/10.3390/medicina61111980







