A Mixed Model of Clinical Characteristics, Strain Elastography and ACR-TIRADS Predicts Malignancy in Small Thyroid Nodules: A Prospective Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
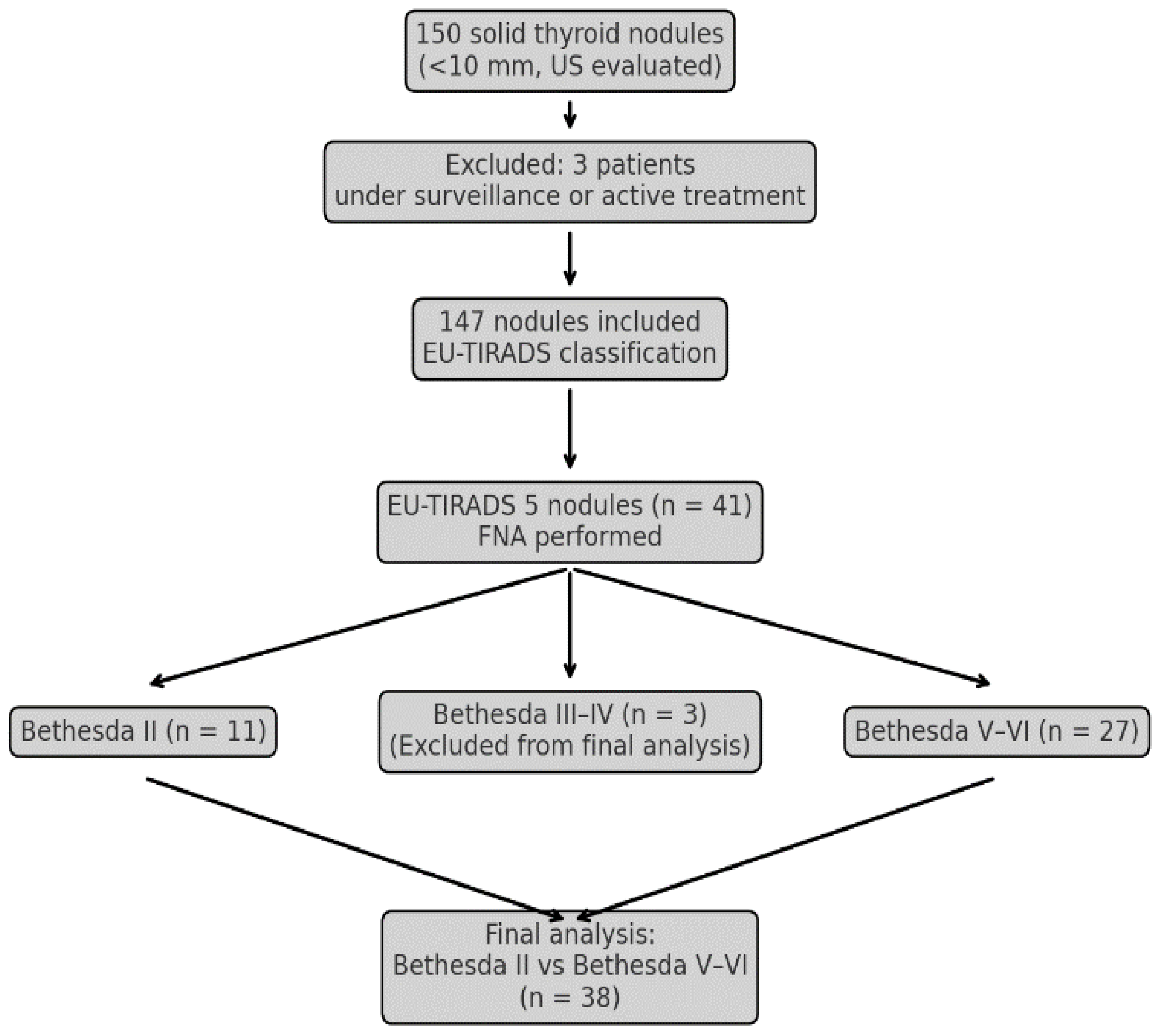
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Logistic Model
3.2. Clinical Model
3.3. Mixed Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, X.; Liu, R.; Gao, L.; Xia, Y.; Jiang, Y. Diagnostic Value of Sonographic Features in Distinguishing Malignant Partially Cystic Thyroid Nodules: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 624409. [Google Scholar] [CrossRef]
- Alexander, E.K.; Doherty, G.M.; Barletta, J.A. Management of thyroid nodules. Lancet Diabetes Endocrinol. 2022, 10, 540–548. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer 2016, 123, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Ito, Y.; Takeuchi, D.; Nakayama, H.; Masaki, C.; Shindo, H.; Teshima, M.; Horiguchi, K.; Yoshida, Y.; Kanai, T.; et al. Indications and Strategy for Active Surveillance of Adult Low-Risk Papillary Thyroid Microcarcinoma: Consensus Statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid 2020, 31, 183–192. [Google Scholar] [CrossRef]
- Jensen, C.B.; Saucke, M.C.; Francis, D.O.; Voils, C.I.; Pitt, S.C. From Overdiagnosis to Overtreatment of Low-Risk Thyroid Cancer: A Thematic Analysis of Attitudes and Beliefs of Endocrinologists, Surgeons, and Patients. Thyroid 2020, 30, 696–703. [Google Scholar] [CrossRef]
- Grani, G.; Sponziello, M.; Pecce, V.; Ramundo, V.; Durante, C. Contemporary thyroid nodule evaluation and management. J. Clin. Endocrinol. Metab. 2020, 105, 2869–2883. [Google Scholar] [CrossRef]
- Yim, Y.; Na, D.G.; Ha, E.J.; Baek, J.H.; Sung, J.Y.; Kim, J.; Moon, W.-J. Concordance of Three International Guidelines for Thyroid Nodules Classified by Ultrasonography and Diagnostic Performance of Biopsy Criteria. Korean J. Radiol. 2020, 21, 108. [Google Scholar] [CrossRef]
- Grani, G.; Lamartina, L.; Ascoli, V.; Bosco, D.; Biffoni, M.; Giacomelli, L.; Maranghi, M.; Falcone, R.; Ramundo, V.; Cantisani, V.; et al. Reducing the Number of Unnecessary Thyroid Biopsies While Improving Diagnostic Accuracy: Toward the “Right” TIRADS. J. Clin. Endocrinol. Metab. 2018, 104, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bojunga, J.; Herrmann, E.; Meyer, G.; Weber, S.; Zeuzem, S.; Friedrich-Rust, M. Real-Time Elastography for the Differentiation of Benign and Malignant Thyroid Nodules: A Meta-Analysis. Thyroid 2010, 20, 1145–1150. [Google Scholar] [CrossRef]
- Trimboli, P.; Guglielmi, R.; Monti, S.; Misischi, I.; Graziano, F.; Nasrollah, N.; Amendola, S.; Morgante, S.N.; Deiana, M.G.; Valabrega, S.; et al. Ultrasound Sensitivity for Thyroid Malignancy Is Increased by Real-Time Elastography: A Prospective Multicenter Study. J. Clin. Endocrinol. Metab. 2012, 97, 4524–4530. [Google Scholar] [CrossRef]
- Sun, J.; Cai, J.; Wang, X. Real-time Ultrasound Elastography for Differentiation of Benign and Malignant Thyroid Nodules. J. Ultrasound Med. 2014, 33, 495–502. [Google Scholar] [CrossRef]
- Brito, J.P.; Gionfriddo, M.R.; Al Nofal, A.; Boehmer, K.R.; Leppin, A.L.; Reading, C.; Callstrom, M.; Elraiyah, T.A.; Prokop, L.J.; Stan, M.N.; et al. The Accuracy of Thyroid Nodule Ultrasound to Predict Thyroid Cancer: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 1253–1263. [Google Scholar] [CrossRef]
- Xu, T.; Gu, J.; Ye, X.; Xu, S.; Wu, Y.; Shao, X.; Liu, D.-Z.; Lu, W.-P.; Hua, F.; Shi, B.-M.; et al. Thyroid nodule sizes influence the diagnostic performance of TIRADS and ultrasound patterns of 2015 ATA guidelines: A multicenter retrospective study. Sci. Rep. 2017, 7, srep43183. [Google Scholar] [CrossRef]
- Zhong, L.; Shi, L.; Lai, J.; Hu, Y.; Gu, L. Combined model integrating clinical, radiomics, BRAFV600E and ultrasound for differentiating between benign and malignant indeterminate cytology (Bethesda III) thyroid nodules: A bi-center retrospective study. Gland. Surg. 2024, 13, 1954–1964. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Angelopoulos, N.; Goulis, D.G.; Chrisogonidis, I.; Livadas, S.; Iakovou, I. Color Doppler ultrasound and real-time elastography in patients with hypothyroidism for the prediction of levothyroxine replacement: A cross-sectional study of 338 patients. J. Ultrasound 2024, 27, 363–373. [Google Scholar] [CrossRef]
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur. J. Clin. Investig. 2009, 39, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Moifo, B.; Roger, J.; Fomekong, S.D.; Djomou, F.; Wankie, E.M. Ultrasonographic prevalence and characteristics of non-palpable thyroid incidentalomas in a hospital-based population in a sub-Saharan country. BMC Med. Imaging 2017, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Suh, C.H.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H. Unnecessary thyroid nodule biopsy rates under four ultrasound risk stratification systems: A systematic review and meta-analysis. Eur. Radiol. 2020, 31, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.-Y.; Chen, X.; Liu, H.; Mao, L.; Chen, J.; He, B.-L.; Zhang, W.-B. Comparison of thyroid nodule FNA rates recommended by ACR TI-RADS, Kwak TI-RADS and ATA guidelines. Eur. J. Radiol. 2022, 148, 110152. [Google Scholar] [CrossRef]
- Seo, H.; Na, D.G.; Kim, J.-H.; Kim, K.W.; Yoon, J.W. Ultrasound-Based Risk Stratification for Malignancy in Thyroid Nodules: A Four-Tier Categorization System. Eur. Radiol. 2015, 25, 2153–2162. [Google Scholar] [CrossRef]
- Papini, E.; Guglielmi, R.; Bianchini, A.; Crescenzi, A.; Taccogna, S.; Nardi, F.; Panunzi, C.; Rinaldi, R.; Toscano, V.; Pacella, C.M. Risk of Malignancy in Nonpalpable Thyroid Nodules: Predictive Value of Ultrasound and Color-Doppler Features. J. Clin. Endocrinol. Metab. 2002, 87, 1941–1946. [Google Scholar] [CrossRef]
- Hekimsoy, İ.; Ertan, Y.; Serin, G.; Karabulut, A.K.; Özbek, S.S. Comparison of ultrasound findings of papillary thyroid carcinoma subtypes based on the 2022 WHO classification of thyroid neoplasms. Front. Endocrinol. 2024, 15, 1434787. [Google Scholar] [CrossRef]
- Shaha, A.R. Controversies in the Management of Thyroid Nodule. Laryngoscope 2000, 110, 183. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; de Souza, J.A.; Cohen, E.E. Thyroid Cancer: Burden of Illness and Management of Disease. J. Cancer 2011, 2, 193–199. [Google Scholar] [CrossRef]
- Dighe, M.; Luo, S.; Cuevas, C.; Kim, Y. Efficacy of thyroid ultrasound elastography in differential diagnosis of small thyroid nodules. Eur. J. Radiol. 2013, 82, e274–e280. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.S.A.E.; Enaba, M.E.; Shareef, M.M.; Hafez, Y.M.; Abbas, I. Comparison the accuracy of thyroid sono-elastography vs. ultrasound-guided fine needle aspiration cytology with thyroid malignancy diagnosis histopathology. Endocr. Regul. 2024, 58, 129–137. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Xin, X.; Wei, X.; Zhang, S.; Li, Y.; Gao, M. Diagnostic value of elastosonography for thyroid microcarcinoma. Ultrasonics 2014, 54, 1945–1949. [Google Scholar] [CrossRef]
- Wang, Y.; Dan, H.J.; Dan, H.Y.; Li, T.; Hu, B. Differential Diagnosis of Small Single Solid Thyroid Nodules using Real-Time Ultrasound Elastography. J. Int. Med. Res. 2010, 38, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, W.; Guo, R. Ultrasonic elastography and conventional ultrasound in the diagnosis of thyroid micro-nodules. Pak. J. Med. Sci. 2019, 35, 1526–1531. [Google Scholar] [CrossRef]
- Li, H.; Peng, Y.; Wang, Y.; Ai, H.; Zhou, X.; Yang, L.; Yan, K.; Xiao, Y.; Liu, L.; Luo, B.; et al. Elastography for the diagnosis of high-suspicion thyroid nodules based on the 2015 American Thyroid Association guidelines: A multicenter study. BMC Endocr. Disord. 2020, 20, 43. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema Steen, J.; Erdogan, M.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid. J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Angell, T.E.; Maurer, R.; Wang, Z.; Kim, M.I.; Alexander, C.A.; Barletta, J.A.; Benson, C.B.; Cibas, E.S.; Cho, N.L.; Doherty, G.M.; et al. A Cohort Analysis of Clinical and Ultrasound Variables Predicting Cancer Risk in 20,001 Consecutive Thyroid Nodules. J. Clin. Endocrinol. Metab. 2019, 104, 5665–5672. [Google Scholar] [CrossRef] [PubMed]
- Kwong, N.; Medici, M.; Angell, T.E.; Liu, X.; Marqusee, E.; Cibas, E.S.; Krane, J.F.; Barletta, J.A.; Kim, M.I.; Larsen, P.R.; et al. The Influence of Patient Age on Thyroid Nodule Formation, Multinodularity, and Thyroid Cancer Risk. J. Clin. Endocrinol. Metab. 2015, 100, 4434–4440. [Google Scholar] [CrossRef]
- Eidt, L.B.; Nunes de Oliveira, C.; Lagos, Y.B.B.D.; Solera, G.L.M.; Izquierdo, R.; Meyer ELde, S.; Mattevi, V.S.; Golbert, L. A prospective comparison of ACR-TIRADS and EU-TIRADS in thyroid nodule assessment for FNA-US. Clin. Endocrinol. 2022, 98, 415–425. [Google Scholar] [CrossRef]
- Shin, A.; Cho, S.; Jang, D.; Abe, S.K.; Saito, E.; Rahman, S.; Islam, R.; Sawada, N.; Shu, X.-O.; Koh, W.-P.; et al. Body Mass Index and Thyroid Cancer Risk: A Pooled Analysis of Half a Million Men and Women in the Asia Cohort Consortium. Thyroid 2022, 32, 306–314. [Google Scholar] [CrossRef]
- Paes, J.; Hua, K.; Nagy, R.J.; Kloos, R.T.; Jarjoura, D.; Ringel, M.D. The Relationship between Body Mass Index and Thyroid Cancer Pathology Features and Outcomes: A Clinicopathological Cohort Study. J. Clin. Endocrinol. Metab. 2010, 95, 4244–4250. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Dionigi, G.; Zhao, Y.S.; Liang, N.; Sun, H. Influence of body mass index on the clinicopathological features of 13,995 papillary thyroid tumors. J. Endocrinol. Investig. 2020, 43, 1283–1299. [Google Scholar] [CrossRef]
- Falstie-Jensen, A.M.; Kjærsgaard, A.; Lorenzen, E.L.; Jensen, J.D.; Reinertsen, K.V.; Dekkers, O.M.; Ewertz, M.; Cronin-Fenton, D.P. Hypothyroidism and the risk of breast cancer recurrence and all-cause mortality—A Danish population-based study. Breast Cancer Res. 2019, 21, 44. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Kӧrmendiné Farkas, D.; Jørgensen, J.O.L.; Cronin-Fenton, D.; Sørensen, H.T. Benign Thyroid Diseases and Risk of Thyroid Cancer: A Nationwide Cohort Study. J. Clin. Endocrinol. Metab. 2018, 103, 2216–2224. [Google Scholar] [CrossRef]
- Paparodis, R.; Livadas, S.; Karvounis, E.; Bantouna, D.; Zoupas, I.; Angelopoulos, N.; Imam, S.; Jaume, J.C. Elevated Preoperative TPO Ab Titers Decrease Risk for DTC in a Linear Fashion: A Retrospective Analysis of 1635 Cases. J. Clin. Endocrinol. Metab. 2023, 109, e347–e355. [Google Scholar] [CrossRef]
- Trimboli, P.; Curti, M.; Colombo, A.; Scappaticcio, L.; Leoncini, A. Combining TSH measurement with TIRADS assessment to further improve the detection of thyroid cancers. Minerva Endocrinol. 2024, 49, 125–131. [Google Scholar] [CrossRef]
- Cappelli, C.; Pirola, I.; Gandossi, E.; Rotondi, M.; Lombardi, D.; Casella, C.; Marini, F.; Saullo, M.; Agosti, B.; Di Lodovico, E.; et al. Could Serum TSH Levels Predict Malignancy in Euthyroid Patients Affected by Thyroid Nodules with Indeterminate Cytology? Int. J. Endocrinol. 2020, 2020, 7543930. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.L.; van Zante, A.; Griffin, A.; Hills, N.K.; Ljung, B.-M. A Large Thyroid Fine Needle Aspiration Biopsy Cohort with Long-Term Population-Based Follow-Up. Thyroid 2021, 31, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Mulita, F.; Plachouri, M.-K.; Liolis, E.; Vailas, M.; Panagopoulos, K.; Maroulis, I. Patient outcomes following surgical management of thyroid nodules classified as Bethesda category III (AUS/FLUS). Endokrynol. Pol. 2021, 72, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, A.; Palermo, A.; Trimboli, P. Cancer prevalence in the subcategories of the indeterminate class III (AUS/FLUS) of the Bethesda system for thyroid cytology: A meta-analysis. J. Endocrinol. Investig. 2021, 44, 1343–1351. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Du, W.; Dai, L.; Fang, Q. Predictors of Malignancy in Thyroid Nodules Classified as Bethesda Category III. Front. Endocrinol. 2022, 13, 806028. [Google Scholar] [CrossRef]
| Bethesda II (n = 11) | Bethesda V–VI (n = 27) | p-Value | |
|---|---|---|---|
| Age (years) | 45.0 ± 13.6 | 47.3 ± 13.3 | ns |
| BMI (kg/m2) | 25.7 ± 4.4 | 26.2 ± 5.4 | ns |
| Diameter (mm) | 7.6 ± 2.0 | 7.8 ± 1.5 | ns |
| AT presence (%) | 0 | 48 | 0.015 * |
| LT4 therapy (%) | 25 | 37 | 0.448 * |
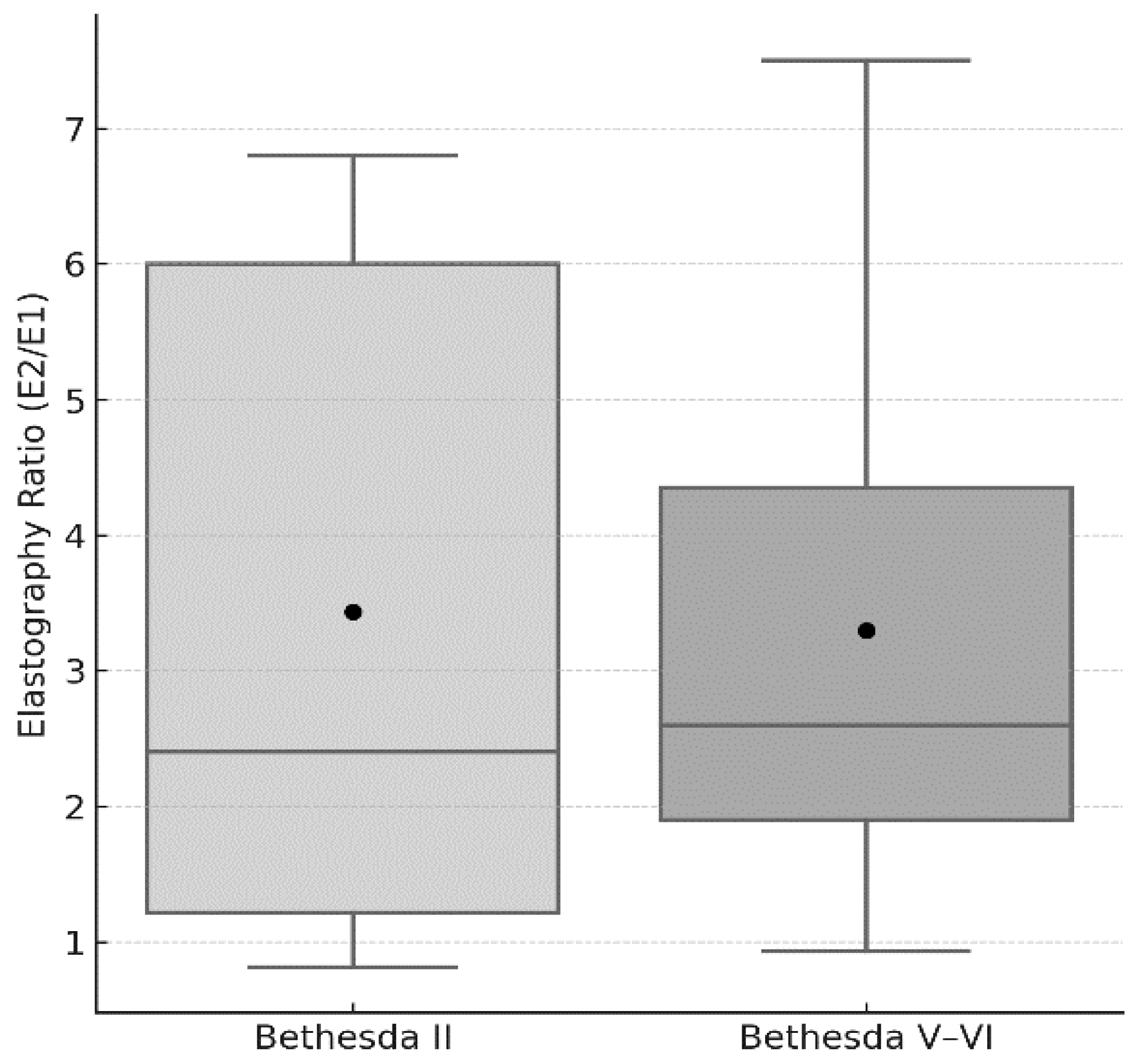
| E2/E1 | 3.2 ± 2.3 | 3.3 ± 1.9 | 0.584 |
| ACR points | 5.3 ± 0.6 | 7.5 ± 1.1 | <0.001 * |
| ACR-category | |||
| 4 | 11 | 2 | <0.001 * |
| 5 | 0 | 25 | <0.001 * |
| Actual Group | Predicted Group | Correct (%) | |
|---|---|---|---|
| no | yes | ||
| Bethesda II | 11 | 0 | 100.0% |
| Bethesda V–VI | 2 | 25 | 92.6% |
| Cases correctly classified | 94.7% | ||
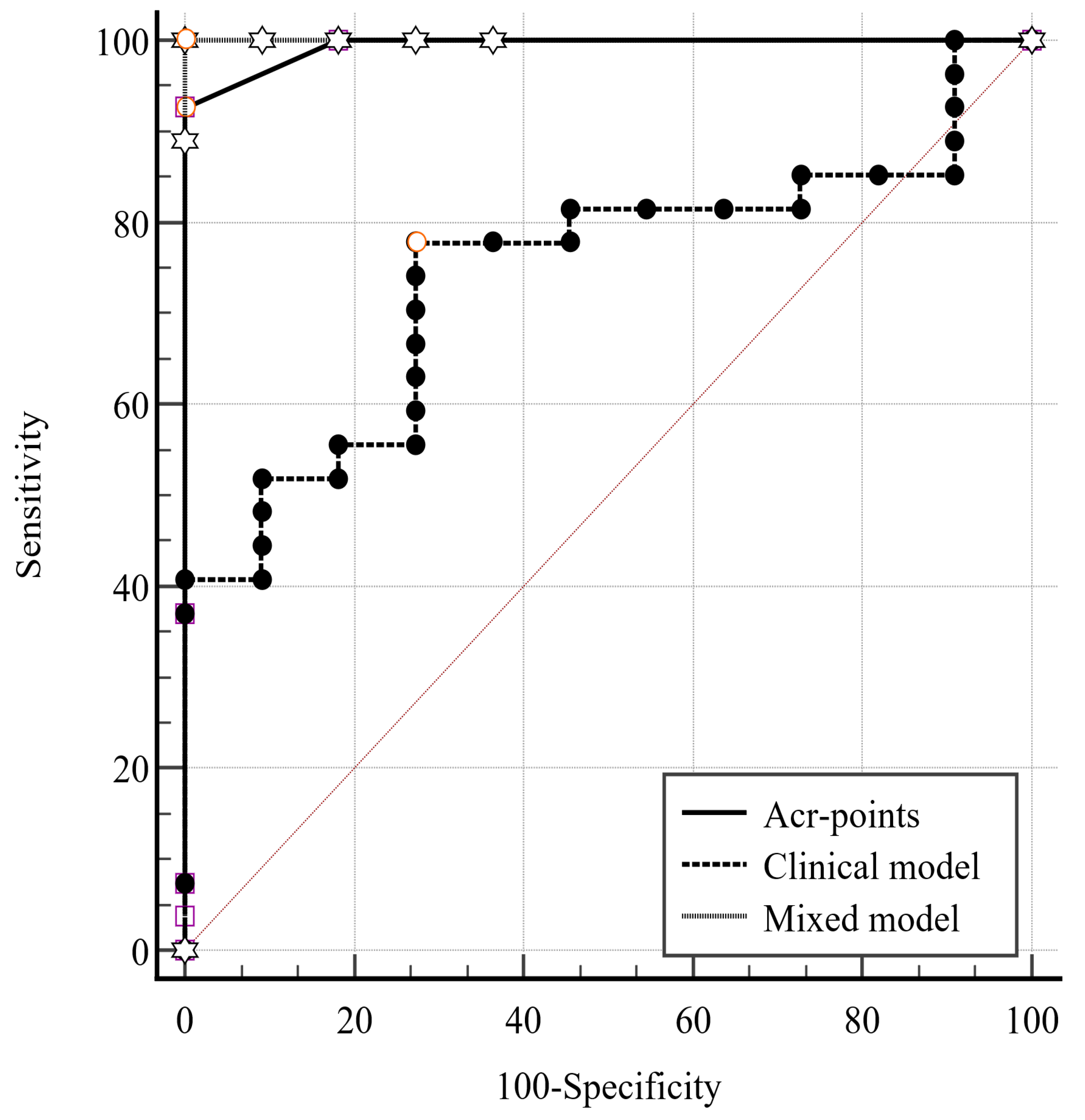
| ACR Points Clinical Model | ACR Points Mixed Model | Clinical Model Mixed Model | |
|---|---|---|---|
| Difference between areas | 0.249 | 0.007 | 0.256 |
| Standard Error | 0.084 | 0.007 | 0.082 |
| 95% Confidence Interval | 0.085–0.414 | −0.006–0.020 | 0.095–0.417 |
| Z statistic | 2.971 | 1.037 | 3.118 |
| p-value | 0.003 | 0.300 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, N.; Petropoulos, E.; Chrisogonidis, I.; Livadas, S.; Paparodis, R.D.; Androulakis, I.; Jaume, J.C.; Goulis, D.G.; Iakovou, I. A Mixed Model of Clinical Characteristics, Strain Elastography and ACR-TIRADS Predicts Malignancy in Small Thyroid Nodules: A Prospective Single-Center Study. Medicina 2025, 61, 1774. https://doi.org/10.3390/medicina61101774
Angelopoulos N, Petropoulos E, Chrisogonidis I, Livadas S, Paparodis RD, Androulakis I, Jaume JC, Goulis DG, Iakovou I. A Mixed Model of Clinical Characteristics, Strain Elastography and ACR-TIRADS Predicts Malignancy in Small Thyroid Nodules: A Prospective Single-Center Study. Medicina. 2025; 61(10):1774. https://doi.org/10.3390/medicina61101774
Chicago/Turabian StyleAngelopoulos, Nikolaos, Emmanouil Petropoulos, Ioannis Chrisogonidis, Sarantis Livadas, Rodis D. Paparodis, Ioannis Androulakis, Juan Carlos Jaume, Dimitrios G. Goulis, and Ioannis Iakovou. 2025. "A Mixed Model of Clinical Characteristics, Strain Elastography and ACR-TIRADS Predicts Malignancy in Small Thyroid Nodules: A Prospective Single-Center Study" Medicina 61, no. 10: 1774. https://doi.org/10.3390/medicina61101774
APA StyleAngelopoulos, N., Petropoulos, E., Chrisogonidis, I., Livadas, S., Paparodis, R. D., Androulakis, I., Jaume, J. C., Goulis, D. G., & Iakovou, I. (2025). A Mixed Model of Clinical Characteristics, Strain Elastography and ACR-TIRADS Predicts Malignancy in Small Thyroid Nodules: A Prospective Single-Center Study. Medicina, 61(10), 1774. https://doi.org/10.3390/medicina61101774







