The Role of the Mediterranean Diet and Alcohol Consumption in Chronic Liver Disease Prevention: A Narrative Review
Abstract
1. Introduction
2. Mediterranean Diet and Global Metabolic Regulation
2.1. The Mediterranean Diet Pattern
2.2. Mediterranean Diet and Health
3. Mediterranean Diet and MASLD
3.1. MASLD: Prevalence, Prevention, and Treatment
3.2. MASLD and Mediterranean Diet: Intervention Trials
3.3. MASLD and Mediterranean Diet: Observational Studies
4. Alcohol Consumption and MASLD
4.1. Wine in the Mediterranean Diet
4.2. Alcohol, a Toxic Substance
4.3. Age-Stratified Recommendations
4.4. Alcohol and MASLD
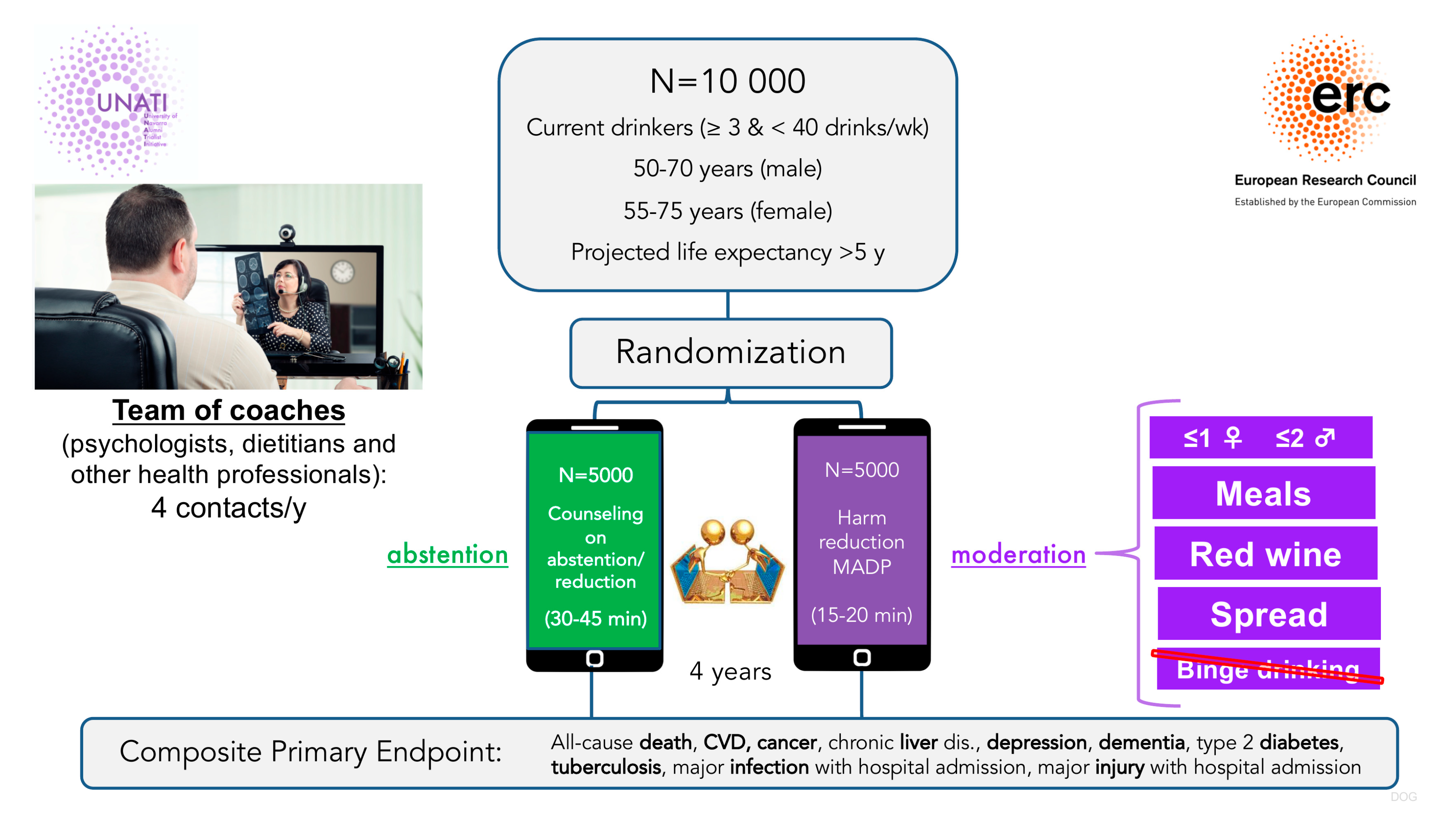
4.5. The UNATI Trial
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MedDiet | Mediterranean diet |
| CVD | Cardiovascular disease |
| RCT | Randomized controlled trial |
| MDS | Mediterranean diet score |
| MR | Mendelian randomization |
| WHO | World Health Organization |
| IARC | International Agency for Research on Cancer |
| UNATI | University of Navarra Alumni Trialist Initiative |
| PREDIMED | PREvencion con Dieta MEDiterranea |
| OS | Oxidative stress |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| BMI | Body mass index |
| NAFLD | Non alcoholic fatty liver disease |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| EVOO | Extra virgin olive oil |
| HbA1c | Glycated hemoglobin |
| FLI | Fatty liver index |
| CYP2E1 | Cytochrome P450 2E1 |
References
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef]
- Keys, A.; Christ, A. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease; Harvard University Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Martínez-González, M.Á.; Hernández, A.H. Effect of the Mediterranean diet in cardiovascular prevention. Rev. Esp. Cardiol. 2024, 77, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [PubMed]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Di Castelnuovo, A.; Bonanni, A.; Costanzo, S.; De Lucia, F.; Pounis, G.; Zito, F.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Adherence to a Mediterranean diet is associated with a better health-related quality of life: A possible role of high dietary antioxidant content. BMJ Open 2013, 3, e003003. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Román-Viñas, B.; Sanchez-Villegas, A.; Guasch-Ferré, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Asp. Med. 2019, 67, 1–55. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021, Erratum in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. https://doi.org/10.1016/j.jacc.2021.02.039. [Google Scholar] [CrossRef]
- Mensah, G.A.; Fuster, V.; Murray, C.J.; Roth, G.A.; Global Burden of Cardiovascular Diseases and Risks Collaborators. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef]
- López-Bueno, R.; Núñez-Cortés, R.; Calatayud, J.; Salazar-Méndez, J.; Petermann-Rocha, F.; López-Gil, J.F.; del Pozo Cruz, B. Global prevalence of cardiovascular risk factors based on the Life’s Essential 8 score: An overview of systematic reviews and meta-analysis. Cardiovasc. Res. 2024, 120, 13–33. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2024, 79, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol. Metab. 2008, 19, 371–379. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- Tierney, A.C.; Roberts, S.K.; George, E.S. Mediterranean diet for the management of metabolic dysfunction-associated steatotic liver disease in non-Mediterranean, Western countries: What’s known and what’s needed? Nutr. Bull. 2024, 49, 444–462. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Zelber-Sagi, S.; Henry, L.; Gerber, L.H. Lifestyle interventions in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 708–722. [Google Scholar] [CrossRef]
- George, E.S.; Reddy, A.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Abbott, G.; Johnson, N.A.; Sood, S.; Roberts, S.K.; Tierney, A.C. Impact of a Mediterranean diet on hepatic and metabolic outcomes in non-alcoholic fatty liver disease: The MEDINA randomised controlled trial. Liver Int. 2022, 42, 1308–1322. [Google Scholar] [CrossRef]
- Haigh, L.; Bremner, S.; Houghton, D.; Henderson, E.; Avery, L.; Hardy, T.; Hallsworth, K.; McPherson, S.; Anstee, Q.M. Barriers and Facilitators to Mediterranean Diet Adoption by Patients with Nonalcoholic Fatty Liver Disease in Northern Europe. Clin. Gastroenterol. Hepatol. 2019, 17, 1364–1371.e3. [Google Scholar] [CrossRef]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Croci, I.; Coombes, J.S.; Sandbakk, S.B.; Keating, S.E.; Nauman, J.; Macdonald, G.A.; Wisloff, U. Non-alcoholic fatty liver disease: Prevalence and all-cause mortality according to sedentary behaviour and cardiorespiratory fitness. The HUNT Study. Prog. Cardiovasc. Dis. 2019, 62, 127–134. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zügel, M.; Steinacker, J.M.; Schumann, U. Association between physical activity and risk of nonalcoholic fatty liver disease: A meta-analysis. Ther. Adv. Gastroenterol. 2017, 10, 701–713. [Google Scholar] [CrossRef]
- Franco, I.; Bianco, A.; Mirizzi, A.; Campanella, A.; Bonfiglio, C.; Sorino, P.; Notarnicola, M.; Tutino, V.; Cozzolongo, R.; Giannuzzi, V.; et al. Physical Activity and Low Glycemic Index Mediterranean Diet: Main and Modification Effects on NAFLD Score. Results from a Randomized Clinical Trial. Nutrients 2020, 13, 66. [Google Scholar] [CrossRef]
- Johnson, N.A.; Sachinwalla, T.; Walton, D.W.; Smith, K.; Armstrong, A.; Thompson, M.W.; George, J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009, 50, 1105–1112. [Google Scholar] [CrossRef]
- Wang, S.-T.; Zheng, J.; Peng, H.-W.; Cai, X.-L.; Pan, X.-T.; Li, H.-Q.; Hong, Q.Z.; Peng, X.E. Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2020, 20, 66. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 2009, 338, b2337. [Google Scholar] [CrossRef] [PubMed]
- Gea, A.; Bes-Rastrollo, M.; Toledo, E.; Garcia-Lopez, M.; Beunza, J.J.; Estruch, R.; Martinez-Gonzalez, M.A. Mediterranean alcohol-drinking pattern and mortality in the SUN (Seguimiento Universidad de Navarra) Project: A prospective cohort study. Br. J. Nutr. 2014, 111, 1871–1980. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Aby, E.S.; Panjawatanan, P.; Lapumnuaypol, K.; Cheungpasitporn, W.; Lukens, F.J.; Harnois, D.M.; Ungprasert, P. Modest alcohol consumption and risk of advanced liver fibrosis in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Ann. Gastroenterol. 2021, 34, 568–574. [Google Scholar] [CrossRef]
- Xiao, J.; Ng, C.H.; Chan, K.E.; Tang, A.S.P.; Teh, R.; Ling, A.H.Z.; Yong, J.N.; Lim, W.H.; Tan, D.J.H.; Tan, C.; et al. Complete alcohol abstinence increases the risk of NAFLD but not severity. A population analysis with transient elastography. Scand. J. Gastroenterol. 2023, 58, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A. Should we remove wine from the Mediterranean diet?: A narrative review. Am. J. Clin. Nutr. 2024, 119, 262–270. [Google Scholar] [CrossRef]
- Vázquez-Ruiz, Z.; Martínez-González, M.A. Addressing Alcohol Use. N. Engl. J. Med. 2025, 393, 207–208. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef]
- Zambrano, A.K.; Cadena-Ullauri, S.; Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Paz-Cruz, E.; Guevara-Ramírez, P.; Frias-Toral, E.; Simancas-Racines, D. Impact of fundamental components of the Mediterranean diet on the microbiota composition in blood pressure regulation. J. Transl. Med. 2024, 22, 417. [Google Scholar] [CrossRef] [PubMed]
- Sarsangi, P.; Salehi-Abargouei, A.; Ebrahimpour-Koujan, S.; Esmaillzadeh, A. Association between Adherence to the Mediterranean Diet and Risk of Type 2 Diabetes: An Updated Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2022, 13, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19, Erratum in Diabetes Care 2018, 41, 2259–2260. https://doi.org/10.2337/dc18-er10. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, A.J.; Suter-Zimmermann, K.; Bucher, H.C.; Shai, I.; Tuttle, K.R.; Estruch, R.; Briel, M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am. J. Med. 2011, 124, 841–851.e2. [Google Scholar] [CrossRef]
- Meir, A.Y.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Arab, A.; Paknahad, Z. The effect of a Mediterranean diet on metabolic parameters in patients with non-alcoholic fatty liver disease: A systematic review of randomized controlled trials. Clin. Nutr. ESPEN 2020, 35, 40–46. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Charlton, M.; Kawaguchi, A.; Yamamura, S.; Nakano, D.; Tsutsumi, T.; Zafer, M.; Torimura, T. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin. Liver Dis. 2021, 41, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Martínez-Urbistondo, D.; Perez-Diaz-Del-Campo, N.; Landecho, M.F.; Martínez, J.A. Alcohol Drinking Impacts on Adiposity and Steatotic Liver Disease: Concurrent Effects on Metabolic Pathways and Cardiovascular Risks. Curr. Obes. Rep. 2024, 13, 461–474. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Commins, I.; Clayton-Chubb, D.; Fitzpatrick, J.A.; George, E.S.; Schneider, H.G.; Phyo, A.Z.Z.; Majeed, A.; Janko, N.; Vaughan, N.; Woods, R.L.; et al. Associations Between MASLD, Ultra-Processed Food and a Mediterranean Dietary Pattern in Older Adults. Nutrients 2025, 17, 1415. [Google Scholar] [CrossRef]
- Konieczna, J.; Fiol, M.; Colom, A.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Soria-Florido, M.T.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Does Consumption of Ultra-Processed Foods Matter for Liver Health? Prospective Analysis among Older Adults with Metabolic Syndrome. Nutrients 2022, 14, 4142. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, S.H.; Mansoori, A.; Hosseinzadeh, M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef]
- Properzi, C.; O’Sullivan, T.A.; Sherriff, J.L.; Ching, H.L.; Jeffrey, G.P.; Buckley, R.F.; Tibballs, J.; MacQuillan, G.C.; Garas, G.; Adams, L.A. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology 2018, 68, 1741–1754. [Google Scholar] [CrossRef]
- Cueto-Galán, R.; Barón, F.J.; Valdivielso, P.; Pintó, X.; Corbella, E.; Gómez-Gracia, E.; Wärnberg, J.; los investigadores del Estudio PREDIMED. Changes in fatty liver index after consuming a Mediterranean diet: 6-year follow-up of the PREDIMED-Malaga trial. Med. Clin. 2017, 148, 435–443. [Google Scholar] [CrossRef]
- Cueto-Galán, R.; Fontalba-Navas, A.; Gutiérrez-Bedmar, M.; Ruiz-Canela, M.; Martínez-González, M.A.; Alves, L.; Babio, N.; Fitó, M.; Ros, E.; Fiol, M.; et al. Adherence to the Mediterranean diet to prevent or delay hepatic steatosis: A longitudinal analysis within the PREDIMED study. Front. Nutr. 2025, 12, 1518082. [Google Scholar] [CrossRef]
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean Diet Rich in Extra-Virgin Olive Oil Is Associated with a Reduced Prevalence of Nonalcoholic Fatty Liver Disease in Older Individuals at High Cardiovascular Risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hennein, R.; Liu, C.; Long, M.T.; Hoffmann, U.; Jacques, P.F.; Lichtenstein, A.H.; Hu, F.B.; Levy, D. Improved Diet Quality Associates with Reduction in Liver Fat, Particularly in Individuals with High Genetic Risk Scores for Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 37, 936–949. [Google Scholar] [CrossRef]
- Khalatbari-Soltani, S.; Marques-Vidal, P.; Imamura, F.; Forouhi, N.G. Prospective association between adherence to the Mediterranean diet and hepatic steatosis: The Swiss CoLaus cohort study. BMJ Open 2020, 10, e040959. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Bertoli, S.; Bedogni, G.; Vignati, L.; Pellizzari, M.; Battezzati, A. Association between Mediterranean Diet and Fatty Liver in Women with Overweight and Obesity. Nutrients 2022, 14, 3771. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Carrasco-Marin, F.; Boonpor, J.; Parra-Soto, S.; Shannon, O.; Malcomson, F.; Phillips, N.; Jain, M.; Deo, S.; Livingstone, K.M.; et al. Association of five diet scores with severe NAFLD incidence: A prospective study from UK Biobank. Diabetes Obes. Metab. 2024, 26, 860–870. [Google Scholar] [CrossRef]
- Li, T.; Zhao, J.; Cao, H.; Han, X.; Lu, Y.; Jiang, F.; Li, X.; Sun, J.; Zhou, S.; Sun, Z.; et al. Dietary patterns in the progression of metabolic dysfunction-associated fatty liver disease to advanced liver disease: A prospective cohort study. Am. J. Clin. Nutr. 2024, 120, 518–527. [Google Scholar] [CrossRef]
- Li, X.; Hur, J.; Cao, Y.; Song, M.; Smith-Warner, S.A.; Liang, L.; Mukamal, K.J.; Rimm, E.B.; Giovannucci, E.L. Moderate alcohol consumption, types of beverages and drinking pattern with cardiometabolic biomarkers in three cohorts of US men and women. Eur. J. Epidemiol. 2023, 38, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Barbería-Latasa, M.; Gea, A.; Martínez-González, M.A. Alcohol, Drinking Pattern, and Chronic Disease. Nutrients 2022, 14, 1954. [Google Scholar] [CrossRef] [PubMed]
- Jani, B.D.; McQueenie, R.; Nicholl, B.I.; Field, R.; Hanlon, P.; Gallacher, K.I.; Mair, F.S.; Lewsey, J. Association between patterns of alcohol consumption (beverage type, frequency and consumption with food) and risk of adverse health outcomes: A prospective cohort study. BMC Med. 2021, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, X.; Zhou, T.; Sun, D.; Shai, I.; Heianza, Y.; Rimm, E.B.; Manson, J.E.; Qi, L. Alcohol Consumption Levels as Compared with Drinking Habits in Predicting All-Cause Mortality and Cause-Specific Mortality in Current Drinkers. Mayo Clin. Proc. 2021, 96, 1758–1769. [Google Scholar] [CrossRef]
- Burton, R.; Sheron, N. No level of alcohol consumption improves health. Lancet 2018, 392, 987–988. [Google Scholar] [CrossRef]
- Naudin, S.; Wang, M.; Dimou, N.; Ebrahimi, E.; Genkinger, J.; Adami, H.-O.; Albanes, D.; Babic, A.; Barnett, M.; Bogumil, D.; et al. Alcohol intake and pancreatic cancer risk: An analysis from 30 prospective studies across Asia, Australia, Europe, and North America. PLoS Med. 2025, 22, e1004590. [Google Scholar] [CrossRef]
- Morford, K.L.; Tetrault, J.M.; O’Connor, P.G. Alcohol and Cancer Risk. JAMA 2025, 334, 908–909. [Google Scholar] [CrossRef]
- Acierno, C.; Barletta, F.; Caturano, A.; Nevola, R.; Sasso, F.C.; Adinolfi, L.E.; Rinaldi, L. Alcohol Consumption and Liver Metabolism in the Era of MASLD: Integrating Nutritional and Pathophysiological Insights. Nutrients 2025, 17, 2229. [Google Scholar] [CrossRef]
- GBD 2020 Alcohol Collaborators. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: A systematic analysis for the Global Burden of Disease Study 2020. Lancet 2022, 400, 185–235, Erratum in Lancet 2022, 400, 358. https://doi.org/10.1016/S0140-6736(22)01389-7. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Review of Evidence on Alcohol and Health; The National Academies Press: Washington, DC, USA, 2025. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Stampfer, M.J.; Rimm, E.B. Genetic instrumental variable analysis: Time to call mendelian randomization what it is. The example of alcohol and cardiovascular disease. Eur. J. Epidemiol. 2020, 35, 93–97. [Google Scholar] [CrossRef]
- van de Luitgaarden, I.A.T.; van Oort, S.; Bouman, E.J.; Schoonmade, L.J.; Schrieks, I.C.; Grobbee, D.E.; van der Schouw, Y.T.; Larsson, S.C.; Burgess, S.; van Ballegooijen, A.J.; et al. Alcohol consumption in relation to cardiovascular diseases and mortality: A systematic review of Mendelian randomization studies. Eur. J. Epidemiol. 2022, 37, 655–669. [Google Scholar] [CrossRef]
- Pan, C.-W.; Abboud, Y.; Chitnis, A.; Zhang, W.; Singal, A.K.; Wong, R.J. Alcohol-Associated Liver Disease Mortality. JAMA Netw. Open 2025, 8, e2514857. [Google Scholar] [CrossRef] [PubMed]
- Lucerón-Lucas-Torres, M.; Saz-Lara, A.; Díez-Fernández, A.; Martínez-García, I.; Martínez-Vizcaíno, V.; Cavero-Redondo, I.; Álvarez-Bueno, C. Association between Wine Consumption with Cardiovascular Disease and Cardiovascular Mortality: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2785. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.S. Long-Term Health Outcomes of Regular, Moderate Red Wine Consumption. Cureus 2023, 15, e46786. [Google Scholar] [CrossRef] [PubMed]


| Metabolic Condition | Benefits of the MedDiet | Key Studies |
|---|---|---|
| Insulin sensitivity | Improved insulin sensitivity; reduced fasting glucose and HbA1c. | [36,37,38] |
| Lipid profile | Reduced triglycerides and LDL-C; increased HDL-C. | [11,21] |
| Body weight | Contributes to weight loss and maintenance. | [39] |
| Inflammation and OS | Improved inflammatory markers and reduced OS. | [8,37,38] |
| Intrahepatic fat | Reduced intrahepatic fat content; improved hepatic biomarkers; prevention/slowing of progression. | [19,21,25,40,41,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbería-Latasa, M.; Martínez-Urbistondo, D.; Martínez-González, M.A. The Role of the Mediterranean Diet and Alcohol Consumption in Chronic Liver Disease Prevention: A Narrative Review. Medicina 2025, 61, 1777. https://doi.org/10.3390/medicina61101777
Barbería-Latasa M, Martínez-Urbistondo D, Martínez-González MA. The Role of the Mediterranean Diet and Alcohol Consumption in Chronic Liver Disease Prevention: A Narrative Review. Medicina. 2025; 61(10):1777. https://doi.org/10.3390/medicina61101777
Chicago/Turabian StyleBarbería-Latasa, María, Diego Martínez-Urbistondo, and Miguel A. Martínez-González. 2025. "The Role of the Mediterranean Diet and Alcohol Consumption in Chronic Liver Disease Prevention: A Narrative Review" Medicina 61, no. 10: 1777. https://doi.org/10.3390/medicina61101777
APA StyleBarbería-Latasa, M., Martínez-Urbistondo, D., & Martínez-González, M. A. (2025). The Role of the Mediterranean Diet and Alcohol Consumption in Chronic Liver Disease Prevention: A Narrative Review. Medicina, 61(10), 1777. https://doi.org/10.3390/medicina61101777






