Abstract
Background and Objectives: The COVID-19 pandemic affects various populations worldwide. The discovery of vaccinations was necessary for the prevention and elimination of the disease. Despite the high importance of these vaccinations, they may cause some complications, such as ocular complications. This study aims to draw attention to the possible complications of the vaccination and highlight its importance. Materials and Methods: Systematic review of the literature from January 2021 to January 2023. A total of 20 published articles were included and reported cases of ocular complications in patients who received COVID-19 vaccines. Results: A total of 243 patients with verified ocular complications following the COVID-19 vaccination were included, ranging in age from 18 to 84 years. The most common ocular complications reported in the current study were ocular inflammatory complications, which represented 47.3%, followed by optic neuritis (24.3%). Retinal artery occlusion, retinal vein occlusion, acute macular neuroretinopathy, and paracentral acute middle maculopathy represented 10.7%. Herpetic ocular infections and herpetic eye disease (14%). Nearly half (42%) of the patients with ocular problems received the Pfizer-BioNTech vaccination. Conclusions: Despite the high importance of the COVID-19 vaccination, it was found that it is associated with the occurrence of some ocular complications. Future projects should come with more extensive prospective studies to further elucidate the underlying mechanisms and risk factors associated with ocular complications following COVID-19 vaccination, thereby enhancing our understanding and guiding appropriate management strategies.
1. Background
The international scientific community has been compelled by the serious COVID-19 pandemic to find treatments and vaccines to control the SARS-CoV-2 virus. On 11 January 2020, the genetic makeup of SARS-CoV-2, the coronavirus that causes COVID-19, was made public. This sparked a surge in global research and development efforts to create a vaccine to prevent this dangerous illness [1]. The COVID-19 vaccination is considered a critical tool for preventing this pandemic and one of the most effective strategies for eliminating it [2]. Different vaccines have been developed in an effort to reduce the morbidity and mortality caused by COVID-19 and stop viral transmission. The U.S. Food and Drug Administration (FDA) authorized the first COVID-19 vaccination, also known as the Pfizer-BioNTech (Pfizer, New York, NY, USA) COVID-19 Vaccine, for emergency use in December 2020. Since then, additional vaccines have been authorized for the prevention of COVID-19 disease that utilize vectors (Ad26.COV2, Janssen Johnson & Johnson; ChAdOx1 nCoV-19/AZD1222, Oxford-AstraZeneca, Oxford, UK), mRNA (mRNA-1273, Moderna, Cambridge, MA, USA), and protein subunits (NVXCoV2373, Novavax, Gaithersburg, MD, USA) [3].
Despite the urgent need and extreme importance of vaccines, which save millions of lives annually, some side effects have been widely reported in many cases. Vaccines are known to promote the development of immunity by inducing immunological responses. Still, they also carry the danger of immune-mediated adverse effects on all human body areas [4]. Little is currently known about potential post-vaccination ocular-specific consequences. Therefore, scientists and eye care medical professionals must look into any possible adverse effects of COVID-19. Previous studies have shown a connection between COVID-19 infection and direct or indirect ocular problems. It is well known that COVID-19 infection can cause conjunctivitis, scleritis, orbital inflammatory illness, phlyctenular keratoconjunctivitis, and retinal involvement [5]. In the current study, we aimed to review and discuss all reported ocular complications following COVID-19 vaccinations.
2. Material and Methods
Research Strategy
We extensively examined the following databases: PubMed, Embase, Medline, Cochrane Central, and Google Scholar, for research on related themes, in order to fully determine the ocular side effects of the COVID-19 vaccination. The literature review covers the period between January 2021 and January 2023.
The following search terms were used to access the databases: (“2019-nCoV” or “nCoV-19” or “nCoV-2019” or “SARS-CoV-2” or “SARS-CoV2” or “SARS2” or “COVID” or “coronavirus” or “coronavirus disease 2019” or “severe acute respiratory syndrome” vaccine) and (“eye” or “ocular” or “oculopathy” or “oculomotor” or “intraocular” or “Ophthalmic”).
Additionally, we looked for other relevant publications in the included studies’ references. The literature review was completed in January 2023. Original articles on ocular complications experienced by COVID-19 vaccination recipients met the inclusion criteria. The case reports were also included in the current study, while studies without any data on ocular complications experienced by patients who received the COVID-19 vaccination were excluded.
We included all patients, regardless of age and sex, who reported experiencing ocular complications after receiving a COVID-19 vaccination.
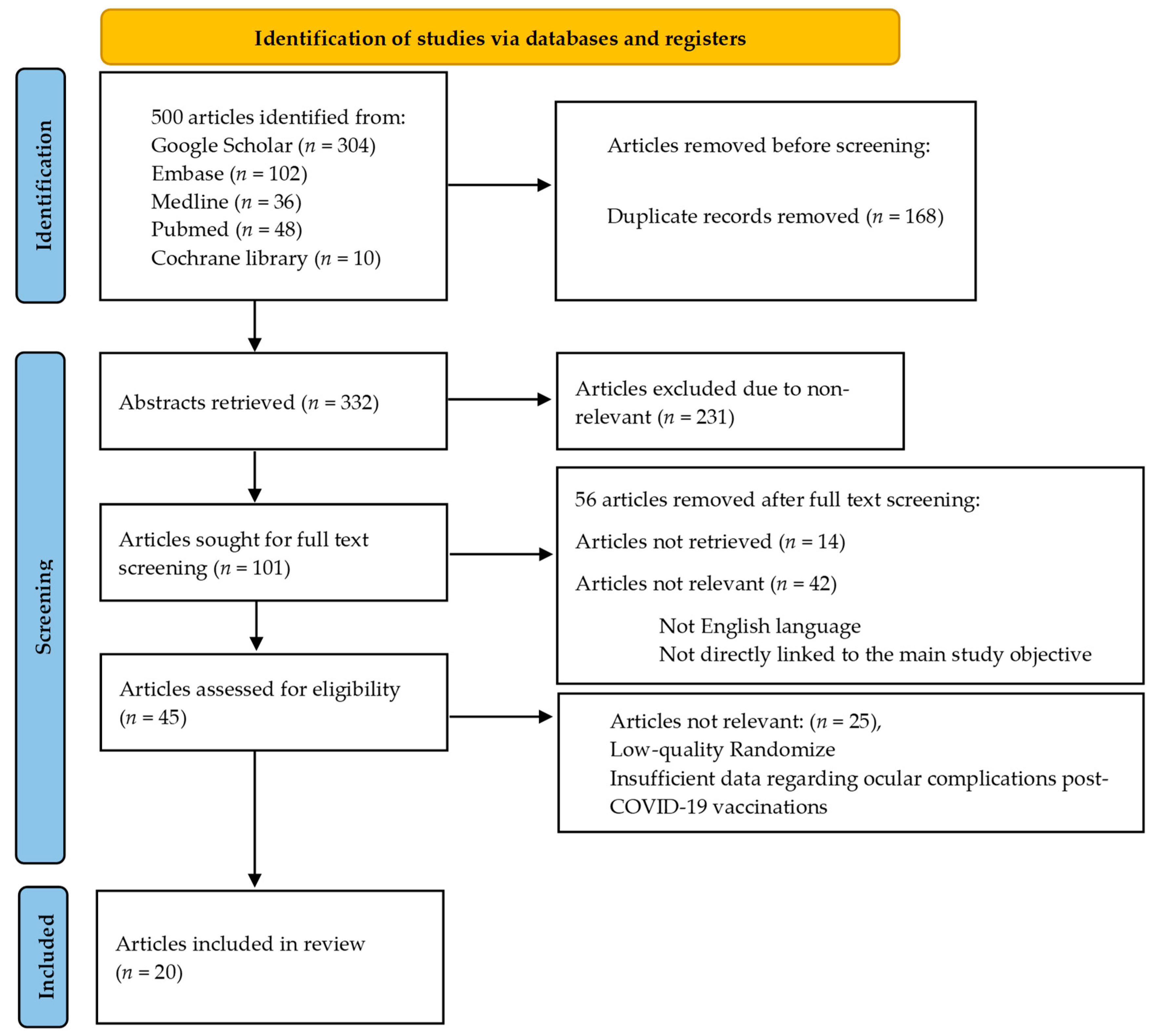
We reviewed 332 abstracts to determine their relevance to ocular complications that may occur after receiving the COVID-19 vaccine. Two authors independently assessed the abstracts and resolved any discrepancies through discussion. We excluded abstracts that were not relevant. Of the remaining articles, 101 were screened in full text, and the authors resolved any differences. Articles that did not have any data regarding ocular complications in COVID-19 vaccine recipients were excluded. Ultimately, 20 articles met our inclusion criteria and were included in our analysis (Figure 1).
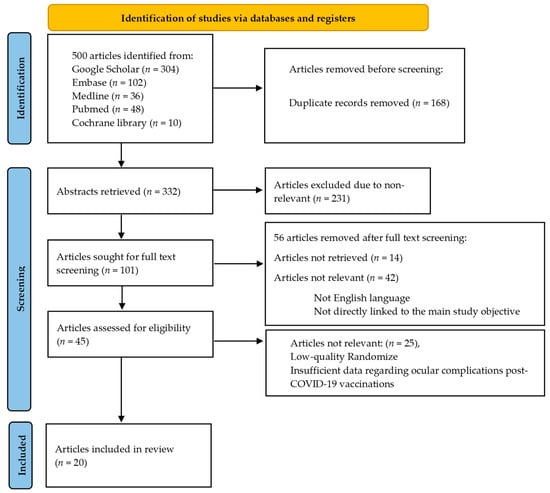
Figure 1.
Shows a flowchart that illustrates the steps involved in retrieving articles.
3. Results
Two hundred forty-three patients with verified ocular complications after receiving the COVID-19 vaccine were assessed across 20 studies from January 2021 to January 2023 (Table 1). A total of 243 cases, ranging in age from 18 to 84 years, with a median age of 50–65 years, with ocular complications post-COVID-19 vaccination, were included in this study. The most commonly reported ocular potential complications were higher in females across all age categories. The most common ocular complications reported in the current study were ocular inflammatory complications, representing 115 patients (47.3%). It includes “uveitis, anterior and posterior uveitis, scleritis, posterior scleritis, choroiditis, keratitis, acute retinal necrosis, and iridocyclitis”. “Optic neuritis” represented 59 patients (24.3%). Herpetic ocular infections and herpetic eye disease represented 34 patients (14%), while retinal artery occlusion, retinal vein occlusion, acute macular neuroretinopathy, and paracentral acute middle maculopathy represented 26 patients (10.7%) (Table 2).

Table 1.
A summary of the published data concerning patients with ocular complications after COVID-19 vaccines.

Table 2.
Distribution of the ocular Complications post-COVID-19 vaccines.
The adverse ocular complications were categorized according to the vaccine manufacturer/name to identify the vaccination type linked to these ocular problems (Table 3). Around 102 (42%) of the patients with ocular adverse events received the Pfizer-BioNTech vaccination, and 69 (28.3%) of the cases received the Astra-Zeneca vaccine.

Table 3.
The most common ocular complications categorization according to the type of vaccine given.
In the current study, it can also be observed that the ocular complications, which include “Uveitis, Anterior uveitis, posterior uveitis, scleritis, choroiditis, keratitis, acute retinal necrosis, and iridocyclitis”, are highly associated with Pfizer vaccines (54.8%), while in relation to “optic neuritis”, they are highly associated with AstraZeneca vaccines (69.5%). Also, it is highly associated with mRNA and adenoviral vector vaccines concerning retinal artery occlusion, retinal vein occlusion, acute macular neuroretinopathy, and paracentral acute middle maculopathy.
4. Discussion
The COVID-19 pandemic has profoundly impacted human health and the global economy. The advent of COVID-19 vaccines, accompanied by regulatory approval and widespread administration worldwide, marks a hopeful milestone in combating the pandemic. On 11 December 2020, the FDA granted emergency use authorization for COVID-19 vaccines, underscoring the significance of vaccines as one of medicine’s most significant achievements [1]. Despite their relatively rare occurrence, this study aimed to assess ocular complications following the COVID-19 vaccination comprehensively. Nevertheless, a diverse range of ocular complications was identified among individuals who received COVID-19 vaccines.
The most prevalent ocular complications reported in this study were classified as “ocular inflammatory complications”, encompassing uveitis, anterior uveitis, posterior uveitis, scleritis, posterior scleritis, choroiditis, keratitis, acute retinal necrosis, and iridocyclitis, collectively accounting for 47.3% of all cases. These findings align with previous literature reviews [26,27], which identified uveitis as the most common ocular complication after receiving vaccines for various conditions. Notably, our study’s predominant subtype of uveitis observed was anterior uveitis, representing 56% of cases. Furthermore, another study also reported scleritis as a common ocular complication following the COVID-19 vaccination, corroborating our findings [28].
Optic neuritis emerged as our investigation’s second-most frequent ocular complication, accounting for approximately 24.3% of cases. This result concurs with prior research [29] linking COVID-19 immunization to various types of optic neuropathy, particularly ischemic optic neuropathy and optic neuritis. Additionally, optic neuropathy was associated with all COVID-19 vaccine subtypes, including mRNA, viral vector, and inactivated viral vaccines. Moreover, a comprehensive review of adverse ocular events from 2010 to 2020 by Cheng and Margo [30] found optic neuritis to be the most frequently reported ocular adverse event across nine distinct vaccines. The exact mechanisms underlying vaccine-induced optic neuritis remain unclear. Still, previous research has proposed the possibility of molecular resemblance between viral proteins and myelin basic proteins and the involvement of epitope dissemination, bystander activation, and superantigen activation [5].
Regarding herpetic ocular infections, which are severe complications and a significant cause of infectious blindness [31], the present study identified them as the third most common ocular complication following COVID-19 vaccinations, accounting for approximately 14% of cases. This observation is consistent with Cohen et al.’s review [15], reporting the occurrence of herpetic ocular infections following SARS-CoV-2 vaccinations. Retinal vascular occlusion was identified as another common ocular complication, aligning with Sonawane, Yadav, Kota, and Singh’s study [32], which reported the development of central retinal vascular complications following COVID-19 vaccinations. Remarkably, all types of COVID-19 vaccines were associated with ocular complications, with the data suggesting a higher frequency of ocular complications among recipients of Pfizer vaccines. This finding is consistent with a literature review by Sonawane et al. [32].
Concerning peripheral ulcerative keratitis, the current review mentioned a single case of post-COVID-19 vaccine-complication peripheral ulcerative keratitis. Our result is consistent with the study of Kuziez et al., who report that the occurrence of herpetic keratitis is common after SARS-CoV-2 vaccination in more than half of all patients [33].
Marginal keratitis as a post-COVID-19 vaccine complication was also reported in one case of our review by Farrell et al. [22]. Approximately 2.5 weeks after receiving the mRNA-1273 vaccine’s initial dosage, a 68-year-old woman complained of increased right eye pain and redness. When her symptoms first appeared, she treated herself with antibiotic eye drops, but nothing changed. Infiltrates of the cornea and peripheral corneal vascularization were present in the right eye during examination, along with a large conjunctival injection. Anomalies of the anterior chamber, discharge, or epithelial defects were nonexistent. It was unimpressive in the left eye. She was prescribed topical anti-biotics and corticosteroids after being found to have marginal keratitis of the right eye, and within a few days, her condition had improved. Concerning the Corneal graft rejection cases, A common and successful solid organ transplant is corneal transplantation [7]. Despite the fact that rejection after immunization is generally thought to be infrequent, the occurrence probably goes unreported. Cornea is considered one of the few tissues in the body with immunological privilege. Immune system entry is blocked by the cornea’s distinct avascular structure and lack of lymphatic tissue. Major Histocompatibility Complexes (MHC) I and II are also expressed at low levels in the corneal layers, which restricts the immune response to antigens. Tregs play a significant part in the downregulation of immunological responses in the cornea. Forkhead box protein 3 (Foxp3), cytotoxic T lymphocyte antigen-4, programmed cell death ligand-1, interleu-kin-10, and transforming growth factor are expressed on the surface of these cells and suppress immune activation by preventing the activity of antigen-presenting cells, CD4+ T [34].
Dendritic cells are present in both the central and peripheral corneas, but because an interleukin-1 receptor antagonist is expressed in the cornea, they are repressed, further excluding the cornea from immune surveillance. The survival of corneal allografts is supported by these pathways and others. It has been speculated that immune system activation and dysregulation following vaccination may put these defenses in jeopardy and expose the immune system to the corneal graft and foreign antigens, triggering rejection [35,36]. A mechanism for acute rejection after SARS-CoV-2 vaccination has been hypothesized, and it involves cross-reactivity between the SARS-CoV-2 antigen and MHC-antigen complexes. The target antigen for the humoral immune response, the spike protein, is encoded by the mRNA molecules BNT162b2 and mRNA-1723, which are lipid-encapsulated. Anti-spike protein titers increase following vaccination. Currently, antibodies that cross-react with molecules from corneal graft donors may trigger an immunological response, resulting in rejection [37].
Based on the corneal responses that have been seen in inflammatory stress situa-tions, another mechanism has been hypothesized. Corneal epithelial cells and dendritic cells express MHC class II and co-stimulatory molecules in response to stress. Such in-flammatory stress may be brought on by the display of donor antigens following im-munization and result in allosensitization [34].
Similar to this, it has been shown that inflammation inside the host bed reduces Foxp3 expression in Tregs and obstructs Treg differentiation, potentially reducing the numerous mechanisms for immunological regulation by Tregs. Additionally, SARS-CoV-2 vaccines induce potent humoral and cellular immunological reactions, including a Th1-biased CD4+ response, as found with previous vaccines. As mediators of corneal transplant rejection, CD4+ Th1 cells might be involved in this situation [38,39].
An immunological response to vaccination adjuvants, which are used to boost the body’s immune response and reduce the frequency and dosage of vaccines required to achieve effective preventive immunity, may be another potential mechanism [16].
At least 34 anecdotal keratoplasty rejection episodes linked to vaccines were dis-covered in a survey of cornea specialists in 2021, but that same study also noted that over the previous 30 years, only four articles describing a total of 12 cases of an association between recent vaccination and corneal transplant rejection had been published [40,41].
Our systematic review is consistent with Shah et al., who report that during the mRNA-1273 vaccination of patients. Four patients who got the vaccination showed signs of rejection two weeks after administration, as described in their case series [42].
Lee and Han described an example of bilateral corneal edema six days after receiving the COVID-19 vaccine; a 55-year-old woman who had undergone successful cataract surgery two months earlier came with an abrupt visual disruption and ocular pain. Her initial 20/20 vision had deteriorated to bilateral 20/30 vision at the time of presentation. A slit-lamp examination indicated mild bilateral corneal edema with a CCT of 580 m and 594 m, respectively, for the right and left eyes. The right eye’s endothelial cell density was 2849/mm2, while the left eye’s was 2778/mm2. Her vision improved to 20/25 bilaterally after two weeks of topical prednisolone therapy, and the corneal edema in the right eye had disappeared with just minor residual edema in the left eye. Her CCTs also improved to 553 m and 579 m in the right and left eyes, respectively [23].
Despite these significant findings, it is essential to acknowledge the limitations of this study. The relatively small sample size of 243 cases from 20 studies may not fully represent the entire vaccinated population, warranting more extensive and diverse samples to derive robust conclusions. Additionally, this study primarily focused on short-term complications, necessitating longer-term follow-up to detect any delayed effects that may emerge over time. Our analysis observed a higher frequency of complications in females across all age categories. However, the exact reasons for this discrepancy require further investigation. Other limitations include the potential for biases in the included studies, such as selection, reporting, and publication biases, which could impact the validity of the overall findings. Furthermore, the heterogeneity of the included studies in terms of design, participants, vaccine types, and complication definitions poses challenges to comparability and generalizability. Moreover, the lack of a control group in most studies hampers the establishment of a direct causal link between vaccination and ocular complications, potentially confounding this study’s outcomes. Reporting inconsistencies in the studies may also lead to incomplete or unreliable data, further affecting this study’s accuracy. The reliance on temporal association to identify ocular complications after vaccination raises concerns about causality, as other factors or pre-existing conditions may influence the outcomes. The variability in COVID-19 vaccine types, including mRNA, viral vector, and inactivated virus, might also introduce confounding variables in the analysis. Additionally, the absence of specific details regarding vaccine dosages from patients experiencing complications could influence the likelihood and severity of ocular adverse events.
5. Conclusions and Recommendations
The invention of the COVID-19 vaccination is one of the most important measures to limit the spread of those dangerous epidemics. Still, it may result in some side effects or complications that can be remedied and dealt with. Overall, the advantages of receiving the COVID-19 vaccine for all populations outweigh the risks of ocular problems that may occur considerably. These uncommon but potential ocular complications following the COVID-19 vaccination should be noted by eye care specialists, especially those that are vision-threatening for individuals with a history of allergic or autoimmune reactions. Doctors must provide intense care and attention to patients complaining of frequent headaches, blurred vision, or eye infections after the COVID-19 vaccination. These occult complications have relied on a temporal association, which does not establish causality. Future studies will be required to determine whether there is any connection between COVID-19 vaccinations of different types and ocular problems. If a patient experiences ocular pain and a change in vision following COVID-19 immunization, ophthalmologists and primary care physicians should be aware of this potential consequence. The ocular complications should be considered in future vaccine safety studies to limit the occurrence of vaccine-related ocular complications. More reports and clinical data are required in order to develop improved guidelines and insights due to the relatively small number of reports per specific phenomenon. The introduction of vaccines should not be discouraged by these similar studies, but producers and independent agencies should continue to monitor the changing data before drawing firm conclusions.
Author Contributions
Conceptualization, E.A.H. and B.M.A.; methodology, E.A.H., A.H.A., B.M.A. and K.M.A.; software, A.H.A., A.J. and Y.M.A.; validation, I.I.A., M.Q.D. and A.J.; formal analysis, A.H.A., B.M.A. and K.M.A.; investigation, E.A.H., I.I.A. and M.Q.D.; resources, E.A.H., K.M.A. and Y.M.A.; data curation, B.M.A., K.M.A. and Y.M.A.; writing—original draft preparation, E.A.H., B.M.A., K.M.A. and Y.M.A.; writing—review and editing, I.I.A., M.Q.D., A.J. and A.H.A.; visualization, I.I.A., M.Q.D., A.H.A. and A.J.; supervision, I.I.A. and M.Q.D.; project administration, E.A.H., B.M.A. and K.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the researchers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Le, T.T.; Andreadakis, Z.; Kumar, A.; Román, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Saeed, B.Q.; Al-Shahrabi, R.; Alhaj, S.S.; Alkokhardi, Z.M.; Adrees, A.O. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int. J. Infect. Dis. 2021, 111, 219–226. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Li, S.; Nakaya, H.I. Systems Vaccinology. Immunity 2010, 33, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Eleiwa, T.K.; Gaier, E.D.; Haseeb, A.; ElSheikh, R.H.; Sallam, A.B.; Elhusseiny, A.M. Adverse Ocular Events following COVID-19 Vaccination. Inflamm. Res. 2021, 70, 1005–1009. [Google Scholar] [CrossRef]
- Yu, S.; Ritterband, D.C.; Mehta, I.D. Acute Corneal Transplant Rejection after Severe Acute Respiratory Syndrome Coronavirus 2 mRNA-1273 Vaccination. Cornea 2022, 41, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; Kim, H.; Lee, J.; Yi, J.; Chung, Y.-R. Retinal vascular occlusions in COVID-19 infection and vaccination: A literature review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1793–1808. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.; Aykaç, S. Optic neuritis after COVID-19 infection: A case report. J. Fr. D’ophtalmologie 2023, 46, e4–e7. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Chandra, A.; Roy, S.; Shrotriya, C. Optic neuritis following COVID-19 vaccination: Coincidence or side-effect?-A case series. Indian J. Ophthalmol. 2022, 70, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.M.; Ning Neo, Y.; Davagnanam, I.; Ashenhurst, M.; Acheson, J.; Abdel-Hay, A.; Alshowaeir, D.; Bakheet, M.; Balaguer, O.; Batra, R.; et al. Post Vaccination Optic Neuritis: Observations from the SARS-CoV-2 Pandemic. SSRN Electron J. 2021; preprint. [Google Scholar] [CrossRef]
- ElSheikh, R.H.; Haseeb, A.; Eleiwa, T.K.; Elhusseiny, A.M. Acute Uveitis following COVID-19 Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 1207–1209. [Google Scholar] [CrossRef]
- Younus, O.; Mulla, U. Posterior Scleritis Following COVID-19 Vaccination: A Case Report. Ocul. Immunol. Inflamm. 2022, 31, 638–640. [Google Scholar] [CrossRef]
- Testi, I.; Agrawal, R.; Pavesio, C.; Brandão-De-Resende, C.; Steeples, L.; Balasubramaniam, B.; McCluskey, P.; Pichi, F.; Agarwal, A.; Herbort, C.; et al. Ocular inflammatory events following COVID-19 vaccination: A multinational case series. J. Ophthalmic Inflamm. Infect. 2022, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Bolletta, E.; Iannetta, D.; Mastrofilippo, V.; De Simone, L.; Gozzi, F.; Croci, S.; Bonacini, M.; Belloni, L.; Zerbini, A.; Adani, C.; et al. Uveitis and Other Ocular Complications Following COVID-19 Vaccination. J. Clin. Med. 2021, 10, 5960. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Olshaker, H.; Fischer, N.; Vishnevskia-Dai, V.; Hagin, D.; Rosenblatt, A.; Zur, D.; Habot-Wilner, Z. Herpetic Eye Disease Following the SARS-CoV-2 Vaccinations. Ocul. Immunol. Inflamm. 2022, 31, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Pan, L.; Guo, H.; Wu, X. Case Report: Associated Ocular Adverse Reactions With Inactivated COVID-19 Vaccine in China. Front. Med. 2022, 8, 823346. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Nahin, S.; Dola, T.A.; Afrin, S.; Hawlader, M.D.H. Retinal hemorrhage of late post-COVID-19 and post-vaccine-related pathogenic mechanisms: A new challenge for ophthalmologist in COVID era. Clin. Case Rep. 2022, 10, e05471. [Google Scholar] [CrossRef] [PubMed]
- Rallis, K.I.; Fausto, R.; Ting, D.S.J.; Al-Aqaba, M.A.; Said, D.G.; Dua, H.S. Manifestation of Herpetic Eye Disease after COVID-19 Vaccine: A UK Case Series. Ocul. Immunol. Inflamm. 2022, 30, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Crnej, A.; Khoueir, Z.; Cherfan, G.; Saad, A. Acute corneal endothelial graft rejection following COVID-19 vaccination. J. Fr. D’ophtalmologie 2021, 44, e445–e447. [Google Scholar] [CrossRef]
- Alam Khan, T.; Sidhu, N.; Khan, L.M.; Sen, S.; Hussain, N.; Tandon, R.M.; Gupta, N.M. Bilateral Immune-Mediated Keratolysis after Immunization with SARS-CoV-2 Recombinant Viral Vector Vaccine. Cornea 2021, 40, 1629–1632. [Google Scholar] [CrossRef]
- Penbe, A. Peripheral Ulcerative Keratitis Secondary to the Inactive COVID-19 Vaccine-CoronaVac. Ocul. Immunol. Inflamm. 2022, 31, 536–540. [Google Scholar] [CrossRef]
- Farrell, D.A.; Deacon, S.; Mauger, T. Marginal keratitis following COVID 19 vaccination. IDCases 2022, 29, e01536. [Google Scholar] [CrossRef]
- Lee, J.Y.; Han, S.B. Case report of transient corneal edema after immunization with adenovirus-vectored COVID-19 vaccine. Medicine 2022, 101, e30041. [Google Scholar] [CrossRef]
- Cunha, L.P.; Atalla, Â.; Costa-Neto, J.d.M.; Costa-Cunha, L.V.F.; Preti, R.C.; Zacharias, L.C.; Monteiro, M.L.R. Multiple attacks of transient monocular visual loss in a previously healthy man: A possible complication after COVID-19 vaccination? Int. J. Retin. Vitr. 2022, 8, 43. [Google Scholar] [CrossRef]
- Lee, D.Y.; Wu, H.; Cheng, K.; Chang, Y. Disc edema in one eye and central serous chorioretinopathy in the other eye shortly after AstraZeneca COVID-19 vaccination. Kaohsiung J. Med. Sci. 2022, 38, 283–285. [Google Scholar] [CrossRef]
- Ferrand, N.; Accorinti, M.; Agarwal, M.; Spartalis, C.; Manni, P.; Stuebiger, N.; Zierhut, M. COVID-19 Vaccination and Uveitis: Epidemiology, Clinical Features and Visual Prognosis. Ocul. Immunol. Inflamm. 2022, 30, 1265–1273. [Google Scholar] [CrossRef]
- Kumari, S.; Anand, R.; Sambyal, B.; Singh, Y.; Rangappa, P.; Jha, S. Ocular adverse effects of COVID-19 vaccines: A systematic review. J. Fam. Med. Prim. Care 2022, 11, 5041–5054. [Google Scholar] [CrossRef]
- Pillar, S.; Weinberg, T.; Amer, R. Posterior ocular manifestations following BNT162b2 mRNA COVID-19 vaccine: A case series. Int. Ophthalmol. 2022, 43, 1677–1686. [Google Scholar] [CrossRef]
- Elnahry, A.G.; Al-Nawaflh, M.Y.; Eldin, A.A.G.; Solyman, O.; Sallam, A.B.; Phillips, P.H.; Elhusseiny, A.M. COVID-19 Vaccine-Associated Optic Neuropathy: A Systematic Review of 45 Patients. Vaccines 2022, 10, 1758. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Margo, C.E. Ocular adverse events following vaccination: Overview and update. Surv. Ophthalmol. 2022, 67, 293–306. [Google Scholar] [CrossRef]
- Koganti, R.; Yadavalli, T.; Shukla, D. Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections. Microorganisms 2019, 7, 429. [Google Scholar] [CrossRef]
- Sonawane, N.; Yadav, D.; Kota, A.; Singh, H. Central retinal vein occlusion post-COVID-19 vaccination. Indian J. Ophthalmol. 2022, 70, 308–309. [Google Scholar] [CrossRef]
- Kuziez, L.; Eleiwa, T.K.; Chauhan, M.Z.; Sallam, A.B.; Elhusseiny, A.M.; Saeed, H.N. Corneal Adverse Events Associated with SARS-CoV-2/COVID-19 Vaccination: A Systematic Review. Vaccines 2023, 11, 166. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chauhan, S.K.; Dana, R. Alloimmunity and Tolerance in Corneal Transplantation. J. Immunol. 2016, 196, 3983–3991. [Google Scholar] [CrossRef]
- Tahvildari, M.; Inomata, T.; Amouzegar, A.; Dana, R. Regulatory T Cell Modulation of Cytokine and Cellular Networks in Corneal Graft Rejection. Curr. Ophthalmol. Rep. 2018, 6, 266–274. [Google Scholar] [CrossRef]
- Dugan, S.P.; Mian, S.I. Impact of vaccination on keratoplasty. Curr. Opin. Ophthalmol. 2022, 33, 296–305. [Google Scholar] [CrossRef]
- Phylactou, M.; Li, J.-P.O.; Larkin, D.F.P. Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. Br. J. Ophthalmol. 2021, 105, 893–896. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Lee, S.-M.; Sung, J.; Niutta, M.; Coassin, M.; Mashaghi, A.; Inomata, T. Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology. J. Clin. Med. 2020, 9, 586. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Lockington, D.M.; Lee, B.; Jeng, B.H.; Larkin, D.F.P.M.; Hjortdal, J. Survey of Corneal Surgeons’ Attitudes regarding Keratoplasty Rejection Risk Associated with Vaccinations. Cornea 2021, 40, 1541–1547. [Google Scholar] [CrossRef]
- Qazi, Y.; Hamrah, P. Corneal Allograft Rejection: Immunopathogenesis to Therapeutics. J. Clin. Cell. Immunol. 2013, 2013, 6. [Google Scholar] [CrossRef]
- Shah, A.P.M.; Dzhaber, D.; Kenyon, K.R.; Riaz, K.M.; Ouano, D.P.; Koo, E.H. Acute Corneal Transplant Rejection after COVID-19 Vaccination. Cornea 2022, 41, 121–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).