Abstract
Background: Children with congenital heart disease (CHD) have impaired pulmonary function both before and after surgery; therefore, pulmonary function assessments are important and should be performed both before and after open-heart surgery. This study aimed to compare pulmonary function between variant pediatric CHD types after open-heart surgery via spirometry. Methods: In this retrospective study, the data for forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and the ratio between FEV1 and FVC (FEV1/FVC) were collected from patients with CHD who underwent conventional spirometry between 2015 and 2017. Results: A total of 86 patients (55 males and 31 females, with a mean age of 13.24 ± 3.32 years) were enrolled in our study. The diagnosis of CHD included 27.9% with atrial septal defects, 19.8% with ventricular septal defects, 26.7% with tetralogy of Fallot, 7.0% with transposition of the great arteries, and 46.5% with other diagnoses. Abnormal lung function was identified by spirometry assessments after surgery. Spirometry was abnormal in 54.70% of patients: obstructive type in 29.06% of patients, restrictive type in 19.76% of patients, and mixed type in 5.81% of patients. More abnormal findings were found in patients who received the Fontan procedure (80.00% vs. 35.80%, p = 0.048). Conclusions: Developing novel therapies to optimize pulmonary function will be critical for improving clinical outcomes.
1. Introduction
Cardiac and pulmonary pathophysiologies are closely interdependent; therefore, congenital heart diseases (CHDs) are frequently associated with respiratory complications, such as abnormalities in pulmonary blood flow that result in impaired lung development [1,2,3,4]. Over 60% of children with CHD have impaired pulmonary function, especially restrictive lung patterns, both before and after surgery [1,2,3,4,5,6]. Reduced lung compliance and higher expiratory airway resistance among children with CHD compared with healthy children were also reported by Yau et al. [7]. However, a spirometry assessment before surgery is often unavailable because the patient is too young or has a hemodynamically unstable condition that is not suitable for the test. The severity and complexity of different CHDs, the different degrees of invasiveness associated with open-heart surgery, the duration of postoperative intensive care, and the frequency of chest surgeries or interventional cardiac catheterizations before and after the primary open-heart surgery might also contribute to a patient’s long-term pulmonary function. Determining associations between these factors and long-term pulmonary function is difficult. We performed this retrospective study to evaluate the spirometry assessment of different types of CHD and compared the pulmonary function of patients with acyanotic CHD and cyanotic CHD and of patients who received the Fontan procedure and other procedures.
2. Material and Methods
2.1. Study Design and Subjects
The study hospital’s institutional review board (IRB) concurred that this retrospective study was a continuous quality improvement initiative to improve patient care and did not require informed consent. This study was approved by the IRB of the study hospital (CMUH109-REC1-024).
This retrospective study was conducted from 1 January 2015 to 31 December 2017 at a regional hospital in central Taiwan. Children (older than 6 years and younger than 18 years) with CHD who underwent open-heart surgery and spirometry assessment at our hospital were enrolled in this study. The pulmonary function testing indicated for the patients enrolled in our study was part of the routine care ordered by their cardiologists (JS Chang and TY Chuang). We excluded patients with acute or chronic disorders, such as respiratory infections, asthma, chronic lung disease, genetic syndromes, and musculoskeletal problems, and those who recently underwent abdominal or thoracic surgery, which is known to affect spirometry performance. No medications affecting spirometry assessments were prescribed to our patients. The parameters measured during the spirometry assessment, including the forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and the ratio between FEV1 and FVC (FEV1/FVC), were collected using a Vitalograph Pneumotrac Type 6800 spirometer (Vitalograph Inc., Lenexa, KS, USA). Three technically acceptable forced expirations were recorded in up to eight attempts.
Lung functional abnormalities were classified as described in Figure 1 [8,9]. Patients with CHD were classified into five groups: atrial septal defect (ASD), ventricular septal defect (VSD), tetralogy of Fallot (TOF), transposition of the great arteries (TGA), and other diagnoses. Patients were classified into two groups: acyanotic CHD and cyanotic CHD. Cyanotic CHD involves heart defects that reduce the amount of oxygen delivered to the rest of the body, whereas acyanotic CHD does not interfere with the amount of oxygen or blood delivered to the rest of the body [10,11]. Patients were also classified into two other groups: those who received the Fontan procedure and those who received other procedures. The spirometry assessment data among these groups were analyzed and compared.
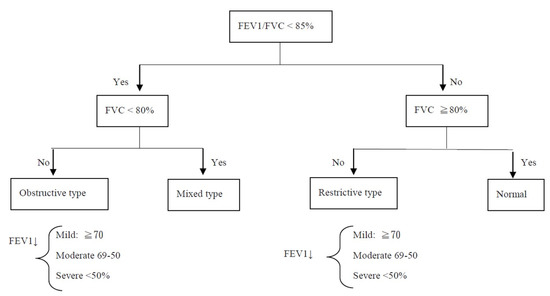
Figure 1.
Interpretation of spirometry results. FVC: Forced vital capacity; FEV1: forced expiratory volume in the first second.
2.2. Analysis
Continuous variables are expressed as the mean ± standard deviation. The distinct demographic variables between the cyanotic and acyanotic cohorts or between the Fontan procedure and other procedures were verified by a chi-squared test and Student’s t-test. When the assumption of the chi-squared test was violated, we used Fisher’s exact test to test the categorical variable.
The collected data were not normally distributed; therefore, analyses were performed using the Wilcoxon test (paired samples). ANOVA was used to compare more than two groups. The SPSS package, version 11 (SPSS, Inc., Chicago, IL, USA), was used for analyses, and p-values less than 0.05 were considered significant for the rejection of the null hypothesis.
3. Results
A total of 86 patients with CHD who received open-heart surgery (mean age 13.24 ± 3.32 years; 55 males and 31 females) and underwent spirometry were enrolled in our study. All enrolled patients underwent spirometry >2 years after surgery (range: 2.1–12 years; median: 7.2 years). The distribution of CHD diagnoses was as follows: 19 (27.9%) had ASD, 17 (19.8%) had VSD, 23 (26.7%) had TOF, 6 (7.0%) had TGA, and 21 (46.5%) had other diagnoses. Among the group with other diagnoses, four (5.65%) had endocardial cushion defect (ECD), three (3.49%) had total anomalous pulmonary venous return (TAPVR), two (2.33%) had coarctation of the aorta (COA), two (2.33%) had double outlet right ventricle (DORV), two (2.33%) had pulmonary atresia, two (2.33%) had pulmonary stenosis, two (2.33%) had tricuspid atresia, two (2.33%) had Ebstein anomaly, one (1.16%) had partial anomalous pulmonary venous return, and one had hypoplastic right heart syndrome. The mean age at operation for the selected type of CHD in our study was 8.1 ± 6.1 years for ASD, 105 ± 15 days for VSD, 16.7 ± 8.5 months for TOF, and 21.3 ± 19.6 days for TGA. Normal spirometry was noted in 39 patients (45.3%), the obstructive type was noted in 25 patients (29.1%), the restrictive type was noted in 17 patients (19.8%), and the mixed type was noted in 5 patients (5.8%; Table 1). Eight patients had a history of lateral thoracotomy for BT shunt, and five patients had a Fontan-type procedure.

Table 1.
Demographic and clinical characteristics of the patients at baseline.
We classified 86 patients into 5 groups: ASD, VSD, TOF, TGA, and other diagnoses (Table 1). The spirometry assessment showed that the patients with ASD and TGA had obstructive-type findings, and patients with VSD, TGA, and others did not have severe findings. In other words, severe pulmonary function problems were only found in patients with ASD and TOF. The results of most identified abnormal pulmonary function tests were mild, except for patients with ASD, which were mostly moderate (Table 1).
Among our cohort, 44 patients were classified as acyanotic CHD, and 42 patients were classified with cyanotic CHD. In the acyanotic group, spirometry was normal in 11 patients, obstructive type in 10 patients, restrictive type in 11 patients, and mixed type in 4 patients. In the cyanotic group, spirometry was normal in 25 patients, obstructive type in 14 patients, and restrictive type in 1 patient, whereas mixed type was not observed.
Comparing acyanotic vs. cyanotic groups, the spirometry assessment results of the acyanotic CHD group showed more significantly abnormal findings than those of the cyanotic group (59.09% vs. 40.48%, p = 0.03), and more restrictive and mixed type results were observed in the acyanotic CHD group than in the cyanotic CHD group (restrictive: 30.56% vs. 2.27%, p < 0.001; mixed: 11.36% vs. 0%, p = 0.046). Most spirometry assessment abnormalities were classified as mild in the acyanotic (38.46%) and cyanotic (58.80%) groups. FEV1/FVC was significantly higher in the cyanotic group (85.71 ± 14.99) than in the acyanotic group (79.38 ± 17.71; p = 0.035). However, FEV1 did not differ significantly between groups (Table 2).

Table 2.
Comparison of spirometry data between the two types of CHD in our patients (acyanotic vs. cyanotic).
Comparing the Fontan vs. other procedure groups, the spirometry assessment results of the Fontan group showed more significantly abnormal findings than those of other procedure groups (80.00% vs. 35.81%, p = 0.005) (Table 3).

Table 3.
Comparison of spirometry data between patients under the Fontan procedure and those under other procedures in our patients.
4. Discussion
Children with CHD generally have normal lung volumes and resistance to airflow when they are infants, but the elastic properties of the lungs become disturbed when the lungs are plethoric due to high pulmonary artery pressure and increased blood flow, resulting in lung stiffness [12]. The elastic properties of the lungs can be altered by the performance of heart surgery [13]. Our study showed that 54.65% of patients with CHD continued to present abnormal pulmonary findings even after surgery, and children with CHD after the Fontan procedure had significantly more abnormal findings than those who received other procedures. However, the obstructive type was observed more frequently than the restrictive type (29.1% vs. 19.8%) in our study, which differs from existing reports in the literature [12,14]. In addition, more patients receiving the Fontan procedure showed abnormal PFT results than patients who had other procedures.
Many factors may contribute to impaired pulmonary function after cardiopulmonary and cardiac bypass surgery, such as postoperative inflammation and hypercortisolemia [15,16,17]. Direct lung injury can be caused by ischemia, and inflammatory responses that follow reperfusion after cardiopulmonary bypass play important roles in lung injury [18,19]. Other contributions to lung dysfunction include alterations of the chest wall’s mechanics due to sternotomy, the presence of postoperative atelectasis, pulmonary edema or pleural effusion, and impaired respiratory efforts due to postoperative pain [20,21]. Phrenic nerve injury has also been reported to induce hemidiaphragmatic paresis or paralysis after the surgical correction of CHD, resulting in poor PFT [22,23,24].
Sulc et al. reported that the surgical repair of ASD did not result in substantial improvements in postoperative spirometry assessments and found only a small decrease in the overall frequency of PFT abnormalities [5,24,25]. Therefore, prominent lung dysfunction is likely caused by events unrelated to perioperative or postoperative factors. In our study, PFT was not performed before surgery in most patients who are typically too young or have severe respiratory conditions that preclude their ability to perform the test; therefore, we were unable to compare spirometry assessment results before and after surgery.
Kurniawan et al. reported that 60.7% of children with left-to-right shunt CHD showed abnormal lung function, among which restrictive lung function was the most commonly observed abnormality [2]. However, in our study, 54.6% of patients with CHD had abnormal lung function, and the obstructive lung pattern was more frequently observed than the restrictive lung pattern. These discrepant outcomes could be due to differences in the CHD distribution between studies in addition to differences in the surgical methods, the times of operation, and the complications after an operation. Our study included a relatively small sample size, indicating the need for further study. In addition, the patients in the study by Kurniawan et al. may not have received definitive defect treatments when they underwent spirometry testing [2,4,26,27,28].
Yau et al. found that children with left-to-right shunt CHD had lower lung compliance and higher expiratory airway resistance than normal children [7], and these results are similar to the findings in our study (59.09%). A review of the literature showed that patients with cyanotic CHD have more restrictive lung patterns after surgery. This phenomenon may be due to these patients having more complex cardiac lesions, such as TGA and tricuspid atresia, which require multiple or multi-staged operations, resulting in further lung volume restriction after surgery [27,28]. However, because of the small number of patients in our study, the percentages of the obstructive pattern and the restrictive pattern were almost the same (52.94% vs. 47.06%). In addition, in our study, CHD patients after the Fontan procedure had significantly more abnormal PFT than patients who received other procedures. The Fontan procedure is used in pediatric patients who possess only a single functional ventricle; those patients had more possibility to progress to heart failure, and their pulmonary function is relatively poorer after the operation than patients who received other procedures.
Some limitations must be noted for our study. First, our study was performed as a retrospective, single-center study with a relatively small number of cases. We compared the results of pulmonary function tests between the four most common types of CHD (ASD, VSD, TOF, and TGA) and found no significant differences, possibly due to the small sample size. The p-values were not significant, possibly due to the low power and limitations of the small subgroup sizes in Table 1.
The spirometry assessment’s timing was at the clinical physician’s discretion, and only patients > 6 years could properly perform forced inspiration and expiration during PFT. Although the timing of spirometry assessments varied between 2.1 and 12 years after surgery in our study, these parameters of pulmonary function were adjusted by the predicted value depending on patient age and height. Second, we did not perform spirometry assessments before heart surgery because most patients underwent heart surgery as young infants and were too young to undergo PFT; therefore, we could not compare lung function before and after heart surgery. Third, some minor residual cardiac lesions, such as valve regurgitation and small septal defect, might also contribute to a patient’s long-term pulmonary function; therefore, these factors should be considered and analyzed in more detail. Fourth, not all patients underwent body plethysmography; therefore, we only collected PFT variables, such as FEV1, FVC, and FEV1/FVC, without the residual lung volume or total lung capacity. Further studies examining residual lung volume and total lung capacity should be performed in the future.
In conclusion, abnormal lung function was identified in 54.6% of children with CHD after surgery and in 40.48% of patients with cyanotic CHD. The obstructive type was more common than the restrictive type (29.1% vs. 19.8%), and abnormal PFT was more commonly identified in patients with CHD who received Fontan-type procedures than in patients who received other procedures. A prospective study that compares the results of pulmonary function tests in different types of CHD and different surgical treatments is needed. The development of novel therapies to optimize pulmonary function under abnormal circulatory conditions after cardiac surgery is crucial for improving clinical outcomes.
Author Contributions
Conceptualization, C.-H.L.; methodology, C.-H.L. and C.-H.C.; validation, T.-C.H.; formal analysis, C.-H.L. and C.-H.C.; investigation, C.-H.L. and C.-H.C.; data curation, C.-H.L., T.-Y.C. and C.-H.C.; writing—original draft preparation, J.-S.C. and C.-H.L.; writing—review and editing, J.-S.C., J.-W.C. and S.-Y.H.; supervision, S.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by funding from China Medical University Hospital, Taichung, Taiwan (grant number: DMR-111-250 and DMR-112-046).
Institutional Review Board Statement
Ethical review and approval were waived for this study (CMUH109-REC1-024).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the Department of Medical Research and the Big Data Center of China Medical University Hospital for performing the statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kurniawan, C.; Murni, K.; Nugroho, S.; Noormanto, N.; Naning, R. Lung function test in children with left-to-right shunt congenital heart disease. Paediatr. Indones. 2018, 58, 165–169. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, R.; Borgia, F.; Diller, G.P.; Inuzuka, R.; Kempny, A.; Martinez-Naharro, A.; Tutarel, O.; Marino, P.; Wustmann, K.; Charalambides, M.; et al. Abnormal lung function in adults with congenital heart disease: Prevalence, relation to cardiac anatomy, and association with survival. Circulation 2013, 127, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, P.M.; Therrien, J.; Veldtman, G.; Warsi, M.A.; Liu, P.; Siu, S.; Williams, W.; Granton, J.; Webb, G. Lung function and aerobic capacity in adult patients following modified Fontan procedure. Heart 2001, 85, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Healy, F.; Hanna, B.D.; Zinman, R. Pulmonary complications of congenital heart disease. Paediatr. Respir. Rev. 2012, 13, 10–15. [Google Scholar] [CrossRef]
- Guerin, S.; Bertille, N.; Khraiche, D.; Bonnet, D.; Goffinet, F.; Lelong, N.; Khoshnood, B.; Delacourt, C. Lung function in children with congenital heart disease. Eur. Respir. J. 2018, 52, PA4676. [Google Scholar]
- Schaan, C.W.; Feltez, G.; Schaan, B.D.; Pelianda, L.U. Functional capacity in children and adolescents with congenital heart disease. Rev. Paul. Pediatr. 2019, 37, 65–72. [Google Scholar] [CrossRef]
- Yau, K.I.; Fang, L.J.; Wu, M.H. Lung mechanics in infants with left-to-right shunt congenital heart disease. Pediatr. Pulmonol. 1996, 21, 42–47. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Moller, J.H.; Hoffman, J.I.E.; Benson, D.W.; Van Hare, G.F.; Wren, C. Pediatric Cardiovascular Medicine, 2nd ed.; Wiley Blackwell: Chichester, UK, 2012. [Google Scholar]
- Rohit, M.; Shrivastava, S. Acyanotic and cyanotic congenital heart disease. Indian J. Pediatr. 2018, 85, 454–460. [Google Scholar] [CrossRef]
- Wallgren, G.; Geubelle, F.; Koch, G. Studies of the mechanics of breathing in children with congenital heart lesions. Acta Paediatrics 1960, 49, 415. [Google Scholar] [CrossRef] [PubMed]
- Howlett, G. Lung mechanics in normal infants and infants with congenital heart disease. Arch. Dis. Child. 1972, 47, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Taylor, A.L.; Sillau, S.H.; Mitchell, M.B.; Rausch, C.M. Restrictive lung function in pediatric patients with structural congenital heart disease. J. Thorac. Cardiovasc. Surg. 2014, 148, 207–211. [Google Scholar] [CrossRef]
- Roncada, G.; Dendale, P.; Linsen, L.; Hendrikx, M.; Hansen, D. Reduction in pulmonary function after CABG surgery is related to postoperative inflammation and hypercortisolemia. Int. J. Clin. Exp. Med. 2015, 15, 10938–10946. [Google Scholar]
- Vargas, F.S.; Terra-Filho, M.; Hueb, W.; Teixeira, L.R.; Cukier, A.; Light, R.W. Pulmonary function after coronary artery bypass surgery. Respir. Med. 1997, 91, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Moore, P.; Jones, C.; Miner, T.J.; Carter, W.R.; Zurcher, R.P.; Lupkas, R.; Edwards, F.H. Effect of internal mammary harvest on postoperative pain and pulmonary function. Ann. Thorac. Surg. 1993, 56, 1107–1109. [Google Scholar] [CrossRef]
- Ovechkin, A.V.; Lominadze, D.; Sedoris, K.C.; Robinson, T.W.; Tyagi, S.C.; Roberts, A.M. Lung ischemia-reperfusion injury: Implications of oxidative stress and platelet-arteriolar wall interactions. Arch. Physiol. Biochem. 2007, 113, 1–12. [Google Scholar] [CrossRef]
- Beckers, P.A.J.; Gielis, J.F.; Van Schil, P.E.; Adriaensen, D. Lung ischemia reperfusion injury: The therapeutic role of dipeptidyl peptidase 4 inhibition. Ann. Transl. Med. 2017, 5, 129. [Google Scholar] [CrossRef]
- Schuller, D.; Morrow, L.E. Pulmonary complications after coronary revascularization. Curr. Opin. Cardiol. 2000, 15, 309–315. [Google Scholar] [CrossRef]
- Taggart, D.P. Respiratory dysfunction after cardiac surgery: Effects of avoiding cardiopulmonary bypass and the use of bilateral internal mammary arteries. Eur. J. Cardiothorac. Surg. 2000, 18, 31–37. [Google Scholar] [CrossRef]
- Al-Ebrahim, K.E.; Elassal, A.A.; Eldib, O.S.; Abdalla, A.; Allam, A.; Al-Ebrahim, E.K.; Abdelmohsen, G.A.; Dohain, A.M.; Al-Radi, O.O. Diaphragmatic palsy after cardiac surgery in adult and pediatric patients. Asian Cardiovasc. Thorac. Ann. 2019, 27, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Agarwala, S.; Mittal, C.M.; Choudhary, S.K.; Airan, B. Diaphragmatic palsy after cardiac surgical procedures in patients with congenital heart. Ann. Pediatr. Cardiol. 2010, 3, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Akbariasbagh, P.; Mirzaghayan, M.R.; Akbariasbagh, N.; Shariat, M.; Ebrahim, B. Risk factors for post-cardiac surgery diaphragmatic paralysis in children with congenital heart disease. J. Tehran Heart Cent. 2015, 10, 134–139. [Google Scholar]
- Rossouw, B. Balancing the heart and the lungs in children with large cardiac shunts. Contin. Med. Educ. 2013, 31, 16–21. [Google Scholar]
- Johnson, J.D.; Theurer, W.M. A stepwise approach to the interpretation of pulmonary function tests. Am. Fam. Physician 2014, 89, 359–366. [Google Scholar]
- Ginde, S.; Bartz, P.J.; Hill, G.D.; Danduran, M.J.; Biller, J.; Sowinski, J.; Tweddell, J.S.; Earing, M.G. Restrictive lung disease is an independent predictor of exercise intolerance in the adult with congenital heart disease. Congenit. Heart Dis. 2013, 8, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Pianosi, P.T.; Johnson, J.N.; Turchetta, A.; Johnson, B.D. Pulmonary function and ventilatory limitation to exercise in congenital heart disease. Congenit. Heart Dis. 2009, 4, 2–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).