Assessment and Correlation of Salivary Ca, Mg, and pH in Smokers and Non-Smokers with Generalized Chronic Periodontitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Characteristics
2.2. Sample Characteristics
2.3. Study Protocol and Clinical Parameters Measured in the Study
2.4. Collection of Salivary Sample and Its Laboratory Analysis
2.5. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. Comparative Analysis of Clinical Parameters between the Study Groups
3.3. Comparative Analysis of Biochemical Parameters between the Study Groups
3.4. Correlational Analysis of Biochemical and Clinical Parameter in the Study Groups
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velsko, I.M.; Overmyer, K.A.; Speller, C.; Klaus, L.; Collins, M.J.; Loe, L.; Frantz, L.A.; Sankaranarayanan, K.; Lewis, C.M.; Martinez, J.B.; et al. The dental calculus metabolome in modern and historic samples. Metabolomics 2017, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Bibi, T.; Khurshid, Z.; Rehman, A.; Imran, E.; Srivastava, K.; Shrivastava, D. Gingival Crevicular Fluid (GCF): A Diagnostic Tool for the Detection of Periodontal Health and Diseases. Molecules 2021, 26, 1208. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Pedersen, A.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral. Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, M.P.M.P.; Picciotti, P.M.; Messana, I.; Fanali, C.; Fiorita, A.; Cabras, T.; Calo, L.; Pisano, E.; Passali, G.C.; Iavarone, F.; et al. Potential application of human saliva as diagnostic fluid. Acta Otorhinolaryngol. Ital. 2011, 31, 347–357. [Google Scholar] [PubMed]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Sonalee, S.; Manpreet, K. A study of analytical indicators of saliva. Ann. Essences Dent. 2012, 4, 9–18. [Google Scholar]
- Al Kawas, S.; Rahim, Z.H.; Ferguson, D.B. Potential uses of human salivary protein and peptide analysis in the diagnosis of disease. Arch. Oral. Biol. 2012, 57, 1–9. [Google Scholar] [CrossRef]
- Tjahajawati, S.; Rafisa, A.; Lestari, E.A. The Effect of Smoking on Salivary Calcium Levels, Calcium Intake, and Bleeding on Probing in Female. Int. J. Dent. 2021, 2021, 2221112. [Google Scholar] [CrossRef]
- Shrivastava, D.; Srivastava, K.C.; Ganji, K.K.; Alam, M.K.; Al Zoubi, I.; Sghaireen, M.G. Quantitative Assessment of Gingival Inflammation in Patients Undergoing Nonsurgical Periodontal Therapy Using Photometric CIELab Analysis. BioMed Res. Int. 2021, 2021, 6615603. [Google Scholar] [CrossRef]
- Giannobile, W.V.; Beikler, T.; Kinney, J.S.; Ramseier, C.A.; Morelli, T.; Wong, D.T. Saliva as a diagnostic tool for periodontal disease: Current state and future directions. Periodontol. 2000 2009, 50, 2–64. [Google Scholar] [CrossRef]
- Acharya, A.; Kharadi, M.D.; Dhavale, R.; Deshmukh, V.L.; Sontakke, A.N. High salivary calcium level associated with periodontal disease in Indian subjects—A pilot study. Oral. Health Prev. Dent. 2011, 9, 195–200. [Google Scholar] [PubMed]
- Sudhir, S. Quantitative evaluation of salivary calcium, phosphorous, protein and Ph in health and diseased periodontium. Ann. Essences Dent. 2010, 2, 21–24. [Google Scholar] [CrossRef]
- Shrivastava, D.; Srivastava, K.C.; Dayakara, J.K.; Sghaireen, M.G.; Gudipaneni, R.K.; Al-Johani, K.; Baig, M.N.; Khurshid, Z. BactericidalActivity of Crevicular Polymorphonuclear Neutrophils in Chronic Periodontitis Patients and Healthy Subjects under the Influence of Areca Nut Extract: An In Vitro Study. Appl. Sci. 2020, 10, 5008. [Google Scholar] [CrossRef]
- Wijaya, T.K.; Susanto, A.; Hendiani, I. Comparison of gingival health status and salivary magnesium levels in smokers and nonsmokers. Sci. Dent. J. 2021, 5, 79. [Google Scholar]
- Rajesh, K.S.; Zareena, S.H.; Kumar, M.A. Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp. Clin. Dent. 2015, 6, 461. [Google Scholar]
- Patel, R.M.; Varma, S.; SuRaGiMath, G.; ZoPe, S. Estimation and comparison of salivary calcium, phosphorous, alkaline phosphatase and pH levels in periodontal health and disease: A cross-sectional biochemical study. J. Clin. Diagn. Res. JCDR 2016, 10, ZC58. [Google Scholar] [CrossRef]
- Sewón, L.; Söderling, E.; Karjalainen, S. Comparative study on mineralization related intraoral parameters in periodontitis affected and periodontitis-free adults. Scand. J. Dent. Res. 1990, 98, 305–312. [Google Scholar] [CrossRef]
- Calsina, G.; Ramón, J.M.; Echeverría, J.J. Effects of smoking on periodontal tissues. J. Clin. Periodontol. 2002, 29, 771–776. [Google Scholar] [CrossRef]
- Nociti, F.H., Jr.; Casati, M.Z.; Duarte, P.M. Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontol. 2000 2015, 67, 187–210. [Google Scholar] [CrossRef]
- Bergstrom, J. Tobacco smoking and supragingival dental calculus. J. Clin. Periodontol. 1999, 26, 541–547. [Google Scholar] [CrossRef]
- Petrovic, M.; Kesic, L.; Obradovic, R.; Savic, Z.; Mihailovic, D.; Obradovic, I.; Avdic-Saracevic, M.; Janjic-Trickovic, O.; Janjic, M. Comparative analysis of smoking influence on periodontal tissue in subjects with periodontal disease. Mater. Sociomed. 2013, 25, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Wasti, A.; Wasti, J.; Singh, R. Estimation of salivary calcium level as a screening tool for the osteoporosis in the post-menopausal women: A prospective study. Indian J. Dent. Res. 2020, 31, 252. [Google Scholar] [PubMed]
- Saha, M.K.; Agrawal, P.; Saha, S.G.; Vishwanathan, V.; Pathak, V.; Saiprasad, S.V.; Dhariwal, P.; Dave, M. Evaluation of correlation between salivary calcium, alkaline phosphatase and osteoporosis-a prospective, comparative and observational study. J. Clin. Diagn. Res. JCDR 2017, 11, ZC63. [Google Scholar] [PubMed]
- Rockenbach, M.I.; Marinho, S.A.; Veeck, E.B.; Lindemann, L.; Shinkai, R.S. Salivary flow rate, pH, and concentrations of calcium, phosphate, and sIgA in Brazilian pregnant and non-pregnant women. Head Face Med. 2006, 2, 44. [Google Scholar] [CrossRef]
- MacGregor, I.D.M.; Edgar, W.M. Calcium and phosphate concentrations and precipitate formation in whole saliva from smokers and non-smokers. J. Periodontal Res. 1986, 21, 429–433. [Google Scholar] [CrossRef]
- Sevon, L.A.; Laine, M.A. Effect of age on flow rate, protein and electrolyte composition of stimulated whole saliva in healthy, non-smoking women. Open Dent. J. 2008, 2, 89–92. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef]
- Ivana, S.; Kristina, P.; Anica, B.; Krunoslav, C.; Kresimir, B.; Kata, R.G. Salivary calcium concentration and periodontal health of young adults in relation to tobacco smoking. Oral. Health Prev. Dent. 2012, 10, 397–403. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Loe, H. The Gingival Index, the Plaque Index and Retention Index systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Haselden, J.N. Metabolic profiling as a tool for understanding mechanisms of toxicity. Toxicol Pathol. 2008, 36, 140–147. [Google Scholar] [CrossRef]
- Kuboniwa, M.; Sakanaka, A.; Hashino, E.; Bamba, T.; Fukusaki, E.; Amano, A. Prediction of periodontal inflammation via metabolic profiling of saliva. J Dent Res. 2016, 95, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Carpenter, G.; So, P.W. Salivary metabolomics: From diagnostic biomarker discovery to investigating biological function. Metabolites 2020, 10, 47. [Google Scholar] [CrossRef]
- Liebsch, C.; Pitchika, V.; Pink, C.; Samietz, S.; Kastenmüller, G.; Artati, A.; Suhre, K.; Adamski, J.; Nauck, M.; Völzke, H.; et al. The saliva metabolome in association to oral health status. J. Dent. Res. 2019, 98, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, I.D. Smoking, saliva and salivation. J. Dent. 1988, 16, 14–17. [Google Scholar] [CrossRef]
- Cichońska, D.; Kusiak, A.; Kochańska, B.; Ochocińska, J.; Świetlik, D. Influence of Electronic Cigarettes on Selected Physicochemical Properties of Saliva. Int. J. Environ. Res. Public. Health 2022, 19, 3314. [Google Scholar] [CrossRef]
- Manea, A.; Nechifor, M. Research on plasma and saliva levels of some bivalent cations in patients with chronic periodontitis (salivary cations in chronic periodontitis). Rev. Med. Chir. Soc. Med. Nat. Iasi 2014, 118, 439–449. [Google Scholar]
- Erdemir, E.O.; Erdemir, A. The detection of salivary minerals in smokers and non-smokers with chronic periodontitis by the inductively coupled plasma-atomic emission spectrophotometry technique. J. Periodontol. 2006, 77, 990–995. [Google Scholar] [CrossRef]
- Sewon, L.A.; Karjalainen, S.M.; Sainio, M.; Seppä, O. Calcium and other salivary factors in periodontitis-affected subjects prior to treatment. J. Clin. Periodontol. 1995, 22, 267–270. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530. [Google Scholar] [CrossRef]
- Sewon, L.A.; Karjalainen, S.M.; Söderling, E.; Lapinlaimu, H.; Simell, O. Association between salivary calcium and oral health. J. Clin. Periodontol 1998, 25, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Hegde, S.; Kashyap, R.; Maiya, A.K. Quantitative assessment of calcium profile in whole saliva from smokers and non-smokers with chronic periodontitis. J. Clin. Diagn. Res. 2015, 9, ZC54–ZC57. [Google Scholar] [CrossRef] [PubMed]
- Kolte, A.P.; Kolte, R.A.; Laddha, R.K. Effect of smoking on salivary composition and periodontal status. J. Indian Soc. Periodontol. 2012, 16, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.V.; Chitkara, N.; Gupta, H.V.; Singh, A.; Gambhir, R.S.; Kaur, H. Comparison ofsalivary calcium level and ph in patients with aggressive periodontitis and healthy individuals: A clinico-biochemical study. Oral. Health Dent. Manag. 2016, 15, 122–126. [Google Scholar]
- Shashikanth, H.; Raghavendra, U.; Naveena, N.; Rajesh, K.S. Assessment of Salivary Composition in Smokers and Non Smokers with Chronic Periodontitis. J. Dent. Med. Sci. 2016, 15, 84–88. [Google Scholar]
- Zuabi, O.; Machtei, E.E.; Ben-Aryeh, H.; Ardekian, L.; Peled, M.; Laufer, D. The effect of smoking and periodontal treatment on salivary composition in patients with established periodontitis. J. Periodontol. 1999, 70, 1240–1246. [Google Scholar] [CrossRef]
- Sewon, L.; Laine, M.; Karjalanien, S.; Dorpguinskania, A.; Lentonen-Veromaa, M. Salivary calcium concentration reflects skeletalosteoporotic changes in heavy smokers. Arch. Oral. Biol. 2004, 49, 335–358. [Google Scholar] [CrossRef]
- Kumar, C.N.; Rao, S.M.; Jethlia, A.; Linganna, C.S.; Bhargava, M.; Palve, D.H. Assessment of salivary thiocyanate levels and pHin the saliva of smokers and nonsmokers with chronic periodontitis—A comparative study. Indian J. Dent. Res. 2021, 32, 74–78. [Google Scholar]
- Rane, M.V.; Suragimath, G.; Varma, S.; Zope, S.A.; Ashwinirani, S.R. Estimation and comparison of salivary calcium levels in healthy controls and patients with generalized gingivitis and chronic periodontitis. J. Oral. Res. Rev. 2017, 9, 12. [Google Scholar]
- Haffajee, A.D.; Socransky, S.S. Relationship of smoking to attachment level profiles. J. Clin. Periodontol. 2001, 28, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Velidandla, S.; Bodduru, R.; Birra, V.; Jain, Y.; Valluri, R.; Ealla, K.K. Distribution of periodontal pockets among smokers and Nonsmokers in patients with chronic periodontitis: A cross-sectional study. Cureus J. Med. Sci. 2019, 11, e5586. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, M.; Ramesh, A.; Dwarakanath, C. Periodontal status in smokers and nonsmokers: A clinical, microbiological, and histopathological study. Int. J. Dent. 2012, 2012, 571590. [Google Scholar] [CrossRef] [PubMed]
- Haber, J.; Wattles, J.; Crowley, M.; Mandell, R. Evidence for cigarette smoking as a major risk factor for periodontitis. J. Periodontol. 1993, 64, 16–23. [Google Scholar] [CrossRef] [PubMed]
| Variable | Categories | Study Group | p Value | |
|---|---|---|---|---|
| Group I (Non-Smoker) n = 105 | Group II (Smoker) n = 105 | |||
| Age (Mean ± SD) | 45.80 ± 3.46 | 42.08 ± 6.19 | 0.573 | |
| Gender † | Male | 57 (54) | 59 (56) | 0.62 |
| Female | 48 (46) | 46 (44) | ||
| Clinical Parameter | Group I | Group II | p Value |
|---|---|---|---|
| Plaque Index | 1.71 ± 0.48 | 1.51 ± 0.34 | 0.109 |
| Gingival Index | 1.55 ± 0.38 | 1.67 ± 0.29 | 0.222 |
| Periodontal Probing Depth | 5.57 ± 1.02 | 6.16 ± 0.77 | 0.025 * |
| Clinical Attachment Level | 5.08 ± 0.73 | 5.70 ± 0.67 | 0.003 ** |
| Parameter | Group I | Group II | p Value |
|---|---|---|---|
| pH | 6.44 ± 0.86 | 6.80 ± 0.91 | 0.160 |
| Calcium | 3.86 ± 1.03 | 5.79 ± 1.76 | 0.000 *** |
| Magnesium | 0.54 ± 0.18 | 0.49 ± 0.24 | 0.413 |
| Ca | Mg | pH | PI | GI | PPD | CAL | Age | Gender ¶ | |
|---|---|---|---|---|---|---|---|---|---|
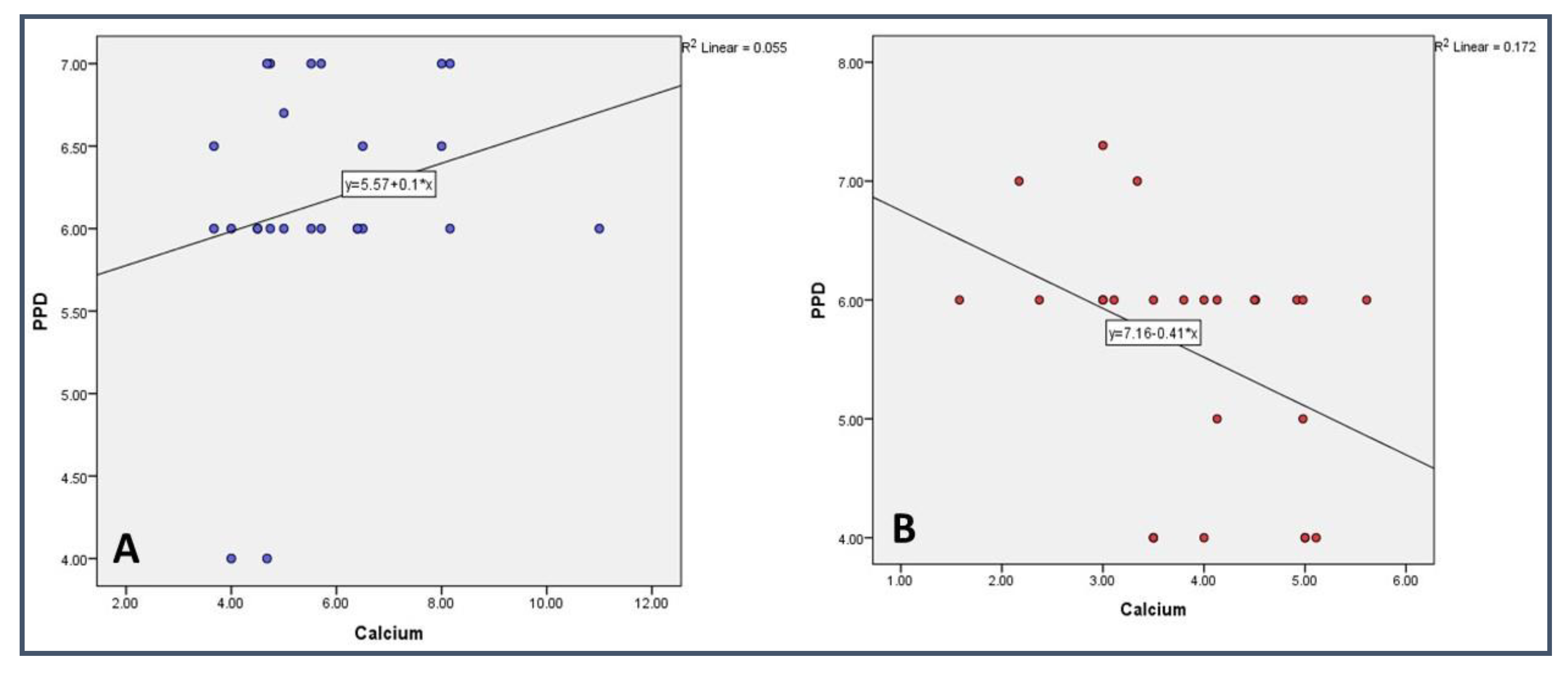
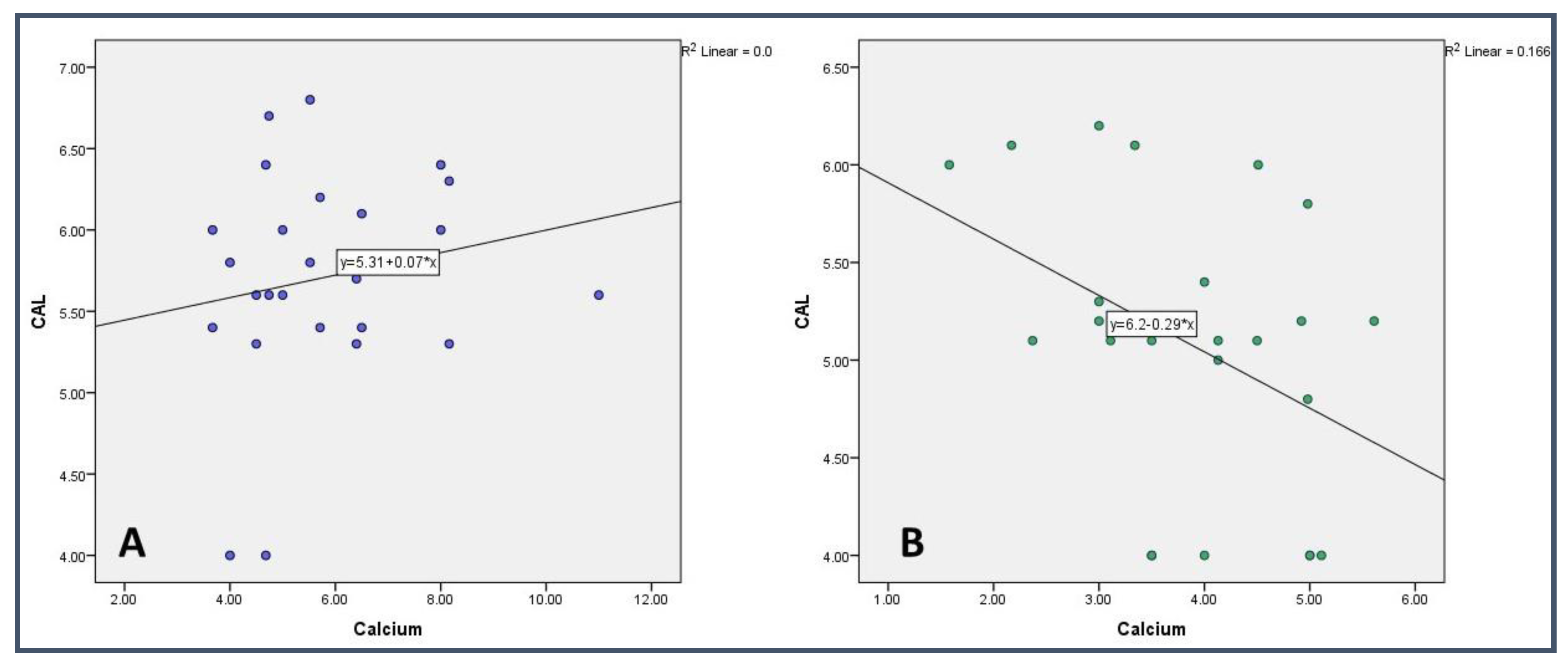
| Ca | - | 0.985 (0.004) | 0.243 (0.237) | 0.978 (−0.006) | 0.994 (0.002) | 0.039 * (−0.415) | 0.043 * (−0.407) | 0.415 (−0.171) | 0.490 (−0.145) |
| Mg | 0.985 (0.004) | - | 0.037 * (−0.419) | 0.306 (0.213) | 0.671 (0.089) | 0.855 (−0.038) | 0.789 (−0.056) | 0.176 (0.280) | 0.853 (−0.039) |
| pH | 0.243 (0.237) | 0.037 * (−0.419) | - | 0.105 (0.332) | 0.068 (0.371) | 0.423 (0.168) | 0.370 (0.187) | 0.616 (0.105) | 0.688 (0.084) |
| PI | 0.978 (−0.006) | 0.306 (0.213) | 0.105 (0.332) | - | 0.000 *** (0.783) | 0.521 (0.135) | 0.570 (0.119) | 0.677 (0.088) | 0.739 (0.070) |
| GI | 0.994 (0.002) | 0.671 (0.089) | 0.068 (0.371) | 0.000 *** (0.783) | - | 0.902 (0.026) | 0.865 (−0.036) | 0.064 (0.376) | 0.711 (0.078) |
| PPD | 0.039 * (−0.415) | 0.855 (−0.038) | 0.423 (0.168) | 0.521 (0.135) | 0.902 (0.026) | - | 0.000 *** (0.937) | 0.834 (0.044) | 0.725 (0.074) |
| CAL | 0.043 * (−0.407) | 0.789 (−0.056) | 0.370 (0.187) | 0.570 (0.119) | 0.865 (−0.036) | 0.000 *** (0.937) | - | 0.791 (−0.056) | 0.669 (0.090) |
| Age | 0.415 (−0.171) | 0.176 (0.280) | 0.616 (0.105) | 0.677 (0.088) | 0.064 (0.376) | 0.834 (0.044) | 0.791 (−0.056) | - | 0.811 (0.050) |
| Gender | 0.490 (−0.145) | 0.853 (−0.039) | 0.688 (0.084) | 0.739 (0.070) | 0.711 (0.078) | 0.725 (0.074) | 0.669 (0.090) | 0.811 (0.050) | - |
| Ca | Mg | pH | PI | GI | PPD | CAL | Age | Gender ¶ | |
|---|---|---|---|---|---|---|---|---|---|
| Ca | - | 0.614 (−0.106) | 0.003 ** (−0.572) | 0.403 (0.175) | 0.343 (0.198) | 0.260 (0.234) | 0.385 (0.182) | 0.058 (0.385) | 0.692 (0.083) |
| Mg | 0.614 (−0.106) | - | 0.488 (−0.145) | 0.580 (0.116) | 0.426 (−0.167) | 0.667 (−0.090) | 0.693 (−0.083) | 0.473 (0.151) | 0.145 (0.300) |
| pH | 0.003 ** (−0.572) | 0.488 (−0.145) | - | 0.089 (0.347) | 0.201 (0.265) | 0.850 (−0.040) | 0.976 (−0.006) | 0.772 (−0.061) | 0.286 (−0.222) |
| PI | 0.403 (0.175) | 0.580 (0.116) | 0.089 (0.347) | - | 0.068 (0.371) | 0.444 (0.160) | 0.327 (0.204) | 0.270 (0.229) | 0.453 (0.157) |
| GI | 0.343 (0.198) | 0.426 (−0.167) | 0.201 (0.265) | 0.068 (0.371) | - | 0.632 (0.101) | 0.492 (0.144) | 0.033 * (0.427) | 0.689 (0.084) |
| PPD | 0.260 (0.234) | 0.667 (−0.090) | 0.850 (−0.040) | 0.444 (0.160) | 0.632 (0.101) | - | 0.000 *** (0.963) | 0.386 (0.181) | 0.098 (−0.338) |
| CAL | 0.385 (0.182) | 0.693 (−0.083) | 0.976 (−0.006) | 0.327 (0.204) | 0.492 (0.144) | 0.000 *** (0.963) | - | 0.388 (0.180) | 0.054 (−0.390) |
| Age | 0.058 (0.385) | 0.473 (0.151) | 0.772 (−0.061) | 0.270 (0.229) | 0.033 * (0.427) | 0.386 (0.181) | 0.388 (0.180) | - | 0.222 (0.253) |
| Gender ¶ | 0.692 (0.083) | 0.145 (0.300) | 0.286 (−0.222) | 0.453 (0.157) | 0.689 (0.084) | 0.098 (−0.338) | 0.054 (−0.390) | 0.222 (0.253) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, S.M.; Gokhale, S.T.; Elagib, M.F.A.; Shrivastava, D.; Nagate, R.R.; Alshmrani, B.A.M.; Alburade, A.M.A.; Alqahtani, F.M.A.; Nagarajappa, A.K.; Natoli, V.; et al. Assessment and Correlation of Salivary Ca, Mg, and pH in Smokers and Non-Smokers with Generalized Chronic Periodontitis. Medicina 2023, 59, 765. https://doi.org/10.3390/medicina59040765
Alqahtani SM, Gokhale ST, Elagib MFA, Shrivastava D, Nagate RR, Alshmrani BAM, Alburade AMA, Alqahtani FMA, Nagarajappa AK, Natoli V, et al. Assessment and Correlation of Salivary Ca, Mg, and pH in Smokers and Non-Smokers with Generalized Chronic Periodontitis. Medicina. 2023; 59(4):765. https://doi.org/10.3390/medicina59040765
Chicago/Turabian StyleAlqahtani, Saad Mohammad, Shankar T. Gokhale, Mohamed Fadul A. Elagib, Deepti Shrivastava, Raghavendra Reddy Nagate, Badar Awadh Mohammad Alshmrani, Abduaziz Mohammed Abdullah Alburade, Fares Mufreh Abdullah Alqahtani, Anil Kumar Nagarajappa, Valentino Natoli, and et al. 2023. "Assessment and Correlation of Salivary Ca, Mg, and pH in Smokers and Non-Smokers with Generalized Chronic Periodontitis" Medicina 59, no. 4: 765. https://doi.org/10.3390/medicina59040765
APA StyleAlqahtani, S. M., Gokhale, S. T., Elagib, M. F. A., Shrivastava, D., Nagate, R. R., Alshmrani, B. A. M., Alburade, A. M. A., Alqahtani, F. M. A., Nagarajappa, A. K., Natoli, V., & Srivastava, K. C. (2023). Assessment and Correlation of Salivary Ca, Mg, and pH in Smokers and Non-Smokers with Generalized Chronic Periodontitis. Medicina, 59(4), 765. https://doi.org/10.3390/medicina59040765









