Resting-State EEG Connectivity at High-Frequency Bands and Attentional Performance Dysfunction in Stabilized Schizophrenia Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Continuous Performance Test and Other Cognitive Tests
2.3. Clinical Measures
2.4. Estimations for EEG Source Localization
2.5. Whole-Brain Electrical Source-Based Functional Connectivity
2.6. Statistical Analyses
3. Results
3.1. Sociodemographic and Clinical Characteristics of the Participants
3.2. Correlation Analyses
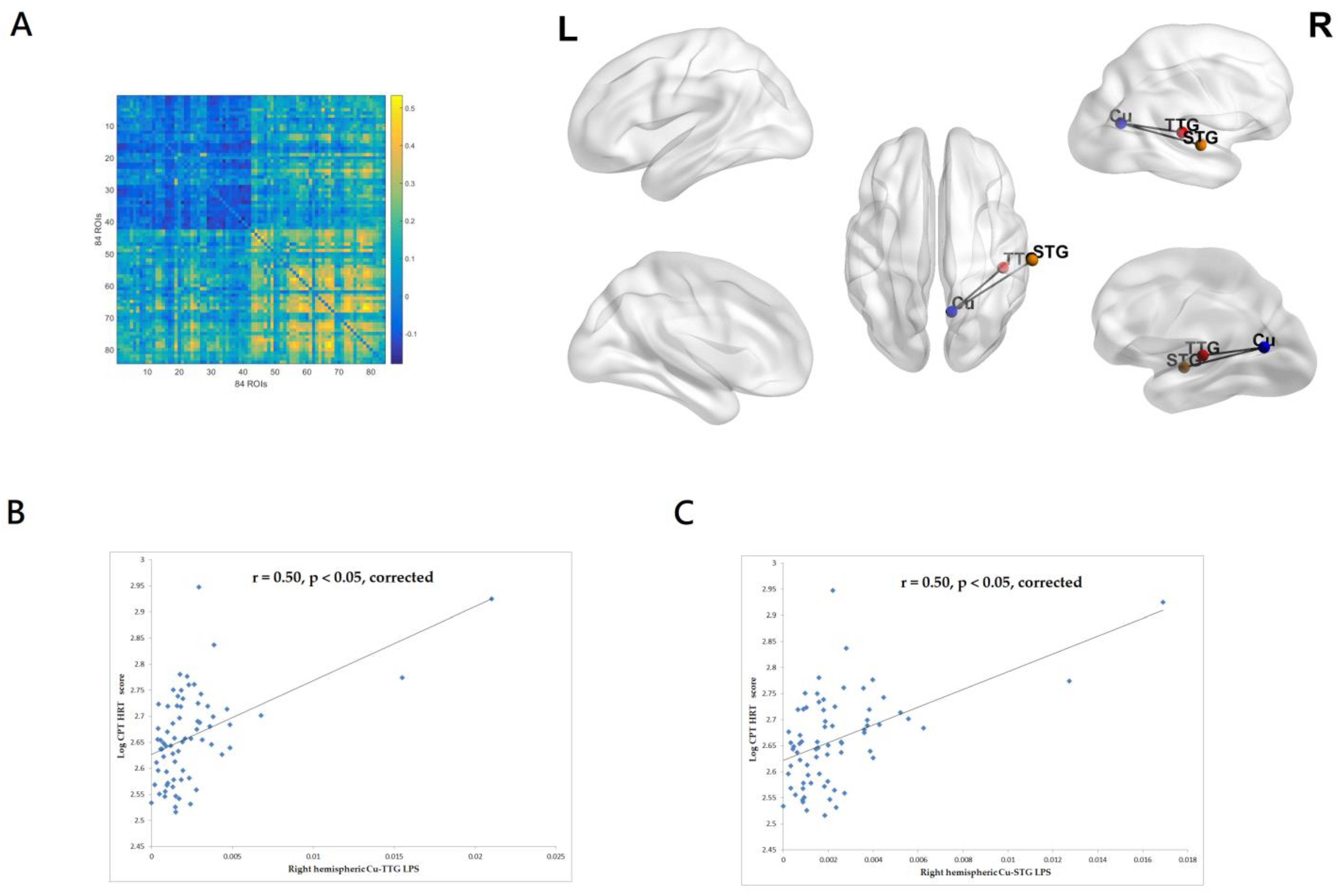
3.2.1. Correlations between CPT-II VAR Score and EEG Source-Based Functional Connectivity
3.2.2. Correlations between CPT-II HRT Score and EEG Source-Based Functional Connectivity
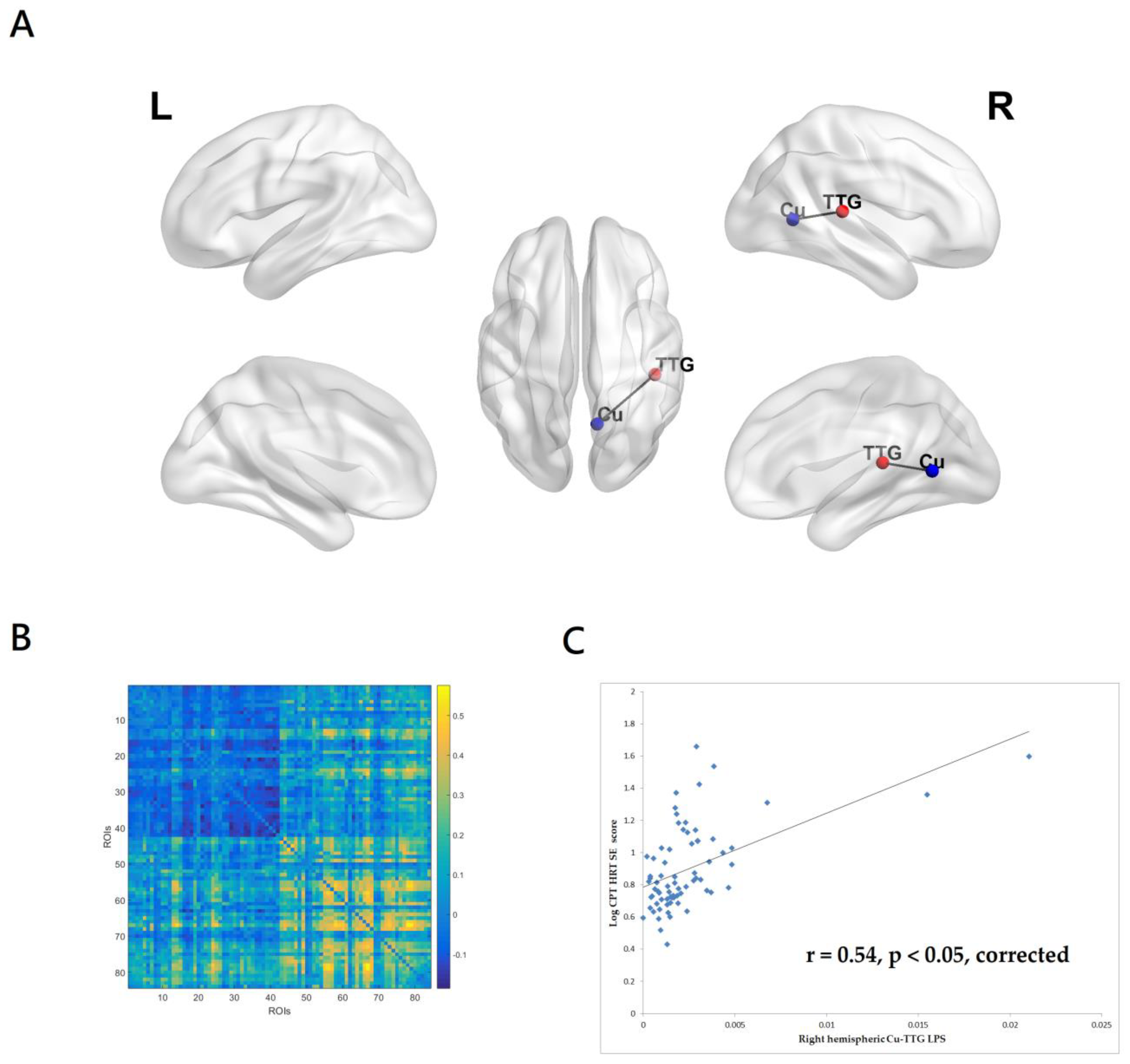
3.2.3. Correlations between CPT-II HRTSE Score and EEG Source-Based Functional Connectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreyenbuhl, J.; Buchanan, R.W.; Dickerson, F.B.; Dixon, L.B. The Schizophrenia Patient Outcomes Research Team (PORT): Updated treatment recommendations 2009. Schizophr. Bull. 2010, 36, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chan, R.; Sun, J.; Yao, J.; Deng, W.; Sun, X.; Liu, X.; Sham, P.C.; Ma, X.; Meng, H.; et al. Reaction time of the Continuous Performance Test is an endophenotypic marker for schizophrenia: A study of first-episode neuroleptic-naive schizophrenia, their non-psychotic first-degree relatives and healthy population controls. Schizophr. Res 2007, 89, 293–298. [Google Scholar] [CrossRef]
- Sanz, J.C.; Gomez, V.; Vargas, M.L.; Marin, J.J. Dimensions of attention impairment and negative symptoms in schizophrenia: A multidimensional approach using the conners continuous performance test in a Spanish population. Cogn. Behav. Neurol. Off. J. Soc. Behav. Cogn. Neurol. 2012, 25, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Rekhi, G.; Saw, Y.E.; Lim, K.; Keefe, R.S.E.; Lee, J. Impact of Cognitive Impairments on Health-Related Quality of Life in Schizophrenia. Brain Sci. 2023, 13, 215. [Google Scholar] [CrossRef]
- Lopez-Luengo, B.; Gonzalez-Andrade, A.; Garcia-Cobo, M. Not All Differences between Patients with Schizophrenia and Healthy Subjects Are Pathological: Performance on the Conners’ Continuous Performance Test. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2016, 31, 983–995. [Google Scholar] [CrossRef]
- Lee, J.; Green, M.F.; Nuechterlein, K.H.; Swerdlow, N.R.; Greenwood, T.A.; Hellemann, G.S.; Lazzeroni, L.C.; Light, G.A.; Radant, A.D.; Seidman, L.J.; et al. The effects of age and sex on cognitive impairment in schizophrenia: Findings from the Consortium on the Genetics of Schizophrenia (COGS) study. PLoS ONE 2020, 15, e0232855. [Google Scholar] [CrossRef] [PubMed]
- Mohn, C.; Torgalsboen, A.K. Details of attention and learning change in first-episode schizophrenia. Psychiatry Res. 2018, 260, 324–330. [Google Scholar] [CrossRef]
- Wang, S.H.; Hsiao, P.C.; Yeh, L.L.; Liu, C.M.; Liu, C.C.; Hwang, T.J.; Hsieh, M.H.; Chien, Y.L.; Lin, Y.T.; Chandler, S.D.; et al. Polygenic risk for schizophrenia and neurocognitive performance in patients with schizophrenia. Genes Brain Behav. 2018, 17, 49–55. [Google Scholar] [CrossRef]
- Lee, P.; Lin, H.Y.; Liu, C.H.; Lu, W.S.; Hsieh, C.L. Relative and Absolute Reliabilities of the Conners’ Continuous Performance Test II in Schizophrenia. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2016, 31, 769–779. [Google Scholar] [CrossRef]
- Hirano, Y.; Uhlhaas, P.J. Current findings and perspectives on aberrant neural oscillations in schizophrenia. Psychiatry Clin. Neurosci. 2021, 75, 358–368. [Google Scholar] [CrossRef]
- Perrottelli, A.; Giordano, G.M.; Brando, F.; Giuliani, L.; Pezzella, P.; Mucci, A.; Galderisi, S. Unveiling the Associations between EEG Indices and Cognitive Deficits in Schizophrenia-Spectrum Disorders: A Systematic Review. Diagnostics 2022, 12, 2193. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, C.; Rosenbrock, H.; Mack, S.R.; Vinisko, R.; Schuelert, N.; Plano, A.; Sussmuth, S.D. Quantitative electroencephalography parameters as neurophysiological biomarkers of schizophrenia-related deficits: A Phase II substudy of patients treated with iclepertin (BI 425809). Transl. Psychiatry 2022, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, I.S.; Pokorny, V.J.; Lynn, P.A.; Klein, S.D.; Sponheim, S.R. Limited consistency and strength of neural oscillations during sustained visual attention in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Bismark, A.W.; Sun, Y.; Zhang, W.; McIlwain, M.; Grootendorst, I.; Light, G.A. Neurophysiological Characterization of Attentional Performance Dysfunction in Schizophrenia Patients in a Reverse-Translated Task. Neuropsychopharmacology 2017, 42, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Brown, H.R.; Siemerkus, J.; Stephan, K.E. The dysconnection hypothesis (2016). Schizophr. Res. 2016, 176, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Daverio, A.; Ferrentino, F.; Santarnecchi, E.; Ciabattini, F.; Monaco, L.; Lisi, G.; Barone, Y.; Di Lorenzo, C.; Niolu, C.; et al. Altered resting-state EEG source functional connectivity in schizophrenia: The effect of illness duration. Front. Hum. Neurosci. 2015, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Hipp, J.F.; Engel, A.K.; Siegel, M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron 2011, 69, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, Y.; Fajnerova, I.; Dvoracek, B.; Bourama, E.; Stamou, I.; Sulcova, K.; Motyl, J.; Horacek, J.; Rodriguez, M.; Spaniel, F. Theoretical Modeling of Cognitive Dysfunction in Schizophrenia by Means of Errors and Corresponding Brain Networks. Front. Psychol. 2018, 9, 1027. [Google Scholar] [CrossRef]
- Vignapiano, A.; Koenig, T.; Mucci, A.; Giordano, G.M.; Amodio, A.; Altamura, M.; Bellomo, A.; Brugnoli, R.; Corrivetti, G.; Di Lorenzo, G.; et al. Disorganization and cognitive impairment in schizophrenia: New insights from electrophysiological findings. Int. J. Psychophysiol. 2019, 145, 99–108. [Google Scholar] [CrossRef]
- Brennan, A.M.; Williams, L.M.; Harris, A.W.F. Intrinsic, task-evoked and absolute gamma synchrony during cognitive processing in first onset schizophrenia. J. Psychiatr. Res. 2018, 99, 10–21. [Google Scholar] [CrossRef]
- Engel, A.K.; Gerloff, C.; Hilgetag, C.C.; Nolte, G. Intrinsic coupling modes: Multiscale interactions in ongoing brain activity. Neuron 2013, 80, 867–886. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Edgar, J.C.; Klook, K.; Siegel, S.J. Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 2012, 62, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Krukow, P.; Jonak, K.; Grochowski, C.; Plechawska-Wojcik, M.; Karakula-Juchnowicz, H. Resting-state hyperconnectivity within the default mode network impedes the ability to initiate cognitive performance in first-episode schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109959. [Google Scholar] [CrossRef] [PubMed]
- Curic, S.; Andreou, C.; Nolte, G.; Steinmann, S.; Thiebes, S.; Polomac, N.; Haaf, M.; Rauh, J.; Leicht, G.; Mulert, C. Ketamine Alters Functional Gamma and Theta Resting-State Connectivity in Healthy Humans: Implications for Schizophrenia Treatment Targeting the Glutamate System. Front. Psychiatry 2021, 12, 671007. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, Y.Y.; Tzeng, N.S.; Kao, Y.C.; Chang, H.A. Adjunct high-frequency transcranial random noise stimulation over the lateral prefrontal cortex improves negative symptoms of schizophrenia: A randomized, double-blind, sham-controlled pilot study. J. Psychiatr. Res. 2021, 132, 151–160. [Google Scholar] [CrossRef]
- Chang, C.C.; Huang, C.C.; Chung, Y.A.; Im, J.J.; Lin, Y.Y.; Ma, C.C.; Tzeng, N.S.; Chang, H.A. Online Left-Hemispheric In-Phase Frontoparietal Theta tACS for the Treatment of Negative Symptoms of Schizophrenia. J. Pers. Med. 2021, 11, 1114. [Google Scholar] [CrossRef]
- Maj, M.; D’Elia, L.; Satz, P.; Janssen, R.; Zaudig, M.; Uchiyama, C.; Starace, F.; Galderisi, S.; Chervinsky, A.; World Health Organization, Division of Mental Health/Global Programme on AIDS. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: A WHO study. Arch. Clin. Neuropsychol. 1993, 8, 123–135. [Google Scholar] [CrossRef]
- Heaton, R.K.; Chelune, G.J.; Talley, J.L. Manual, Wisconsin Card Sorting Test; Psychological Assessment Resources: Odessa, FL, USA, 1993. [Google Scholar]
- Garcia-Alba, J.; Esteba-Castillo, S.; Castellanos Lopez, M.A.; Rodriguez Hidalgo, E.; Ribas Vidal, N.; Moldenhauer Diaz, F.; Novell-Alsina, R. Validation and Normalization of the Tower of London-Drexel University Test 2nd Edition in an Adult Population with Intellectual Disability. Span. J. Psychol. 2017, 20, E32. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef]
- Yeh, T.C.; Huang, C.C.; Chung, Y.A.; Im, J.J.; Lin, Y.Y.; Ma, C.C.; Tzeng, N.S.; Chang, C.C.; Chang, H.A. High-Frequency Transcranial Random Noise Stimulation over the Left Prefrontal Cortex Increases Resting-State EEG Frontal Alpha Asymmetry in Patients with Schizophrenia. J. Pers. Med. 2022, 12, 1667. [Google Scholar] [CrossRef]
- Yeh, T.C.; Huang, C.C.; Chung, Y.A.; Im, J.J.; Lin, Y.Y.; Ma, C.C.; Tzeng, N.S.; Chang, H.A. High-Frequency Transcranial Random Noise Stimulation Modulates Gamma-Band EEG Source-Based Large-Scale Functional Network Connectivity in Patients with Schizophrenia: A Randomized, Double-Blind, Sham-Controlled Clinical Trial. J. Pers. Med. 2022, 12, 1617. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsu, S.H.; Pion-Tonachini, L.; Jung, T.P. Evaluation of Artifact Subspace Reconstruction for Automatic Artifact Components Removal in Multi-Channel EEG Recordings. IEEE Trans Biomed. Eng. 2020, 67, 1114–1121. [Google Scholar] [CrossRef]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. Neuroimage 2019, 198, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- Olbrich, S.; Mulert, C.; Karch, S.; Trenner, M.; Leicht, G.; Pogarell, O.; Hegerl, U. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage 2009, 45, 319–332. [Google Scholar] [CrossRef]
- Cole, M.W.; Bassett, D.S.; Power, J.D.; Braver, T.S.; Petersen, S.E. Intrinsic and task-evoked network architectures of the human brain. Neuron 2014, 83, 238–251. [Google Scholar] [CrossRef]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.H.; Park, H.J.; Lee, J.D.; Kim, H.S.; Chun, J.W.; Son, S.J.; Oh, M.K.; Ku, J.; Lee, H.; Kim, J.J. Regional cerebral blood flow changes and performance deficit during a sustained attention task in schizophrenia: (15) O-water positron emission tomography. Psychiatry Clin. Neurosci. 2012, 66, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, S.; Fan, Y.; Liu, X.; Wang, J.; Lv, Y.; Wang, D.; Wu, D.; Cao, W.; Zou, Q. Acute Tai Chi Chuan exercise enhances sustained attention and elicits increased cuneus/precuneus activation in young adults. Cereb. Cortex 2022, 33, 2969–2981. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, G.; Tian, Y. Increased Temporal Dynamics of Intrinsic Brain Activity in Sensory and Perceptual Network of Schizophrenia. Front. Psychiatry 2019, 10, 484. [Google Scholar] [CrossRef]
- Lee, M.; Sehatpour, P.; Hoptman, M.J.; Lakatos, P.; Dias, E.C.; Kantrowitz, J.T.; Martinez, A.M.; Javitt, D.C. Neural mechanisms of mismatch negativity dysfunction in schizophrenia. Mol. Psychiatry 2017, 22, 1585–1593. [Google Scholar] [CrossRef]
- Thomas, M.L.; Green, M.F.; Hellemann, G.; Sugar, C.A.; Tarasenko, M.; Calkins, M.E.; Greenwood, T.A.; Gur, R.E.; Gur, R.C.; Lazzeroni, L.C.; et al. Modeling Deficits From Early Auditory Information Processing to Psychosocial Functioning in Schizophrenia. JAMA Psychiatry 2017, 74, 37–46. [Google Scholar] [CrossRef]
- Edgar, J.C.; Hunter, M.A.; Huang, M.; Smith, A.K.; Chen, Y.; Sadek, J.; Lu, B.Y.; Miller, G.A.; Canive, J.M. Temporal and frontal cortical thickness associations with M100 auditory activity and attention in healthy controls and individuals with schizophrenia. Schizophr. Res. 2012, 140, 250–257. [Google Scholar] [CrossRef]
- Alkan, E.; Davies, G.; Evans, S.L. Cognitive impairment in schizophrenia: Relationships with cortical thickness in fronto-temporal regions, and dissociability from symptom severity. NPJ Schizophr. 2021, 7, 20. [Google Scholar] [CrossRef]
- Pittman-Polletta, B.R.; Kocsis, B.; Vijayan, S.; Whittington, M.A.; Kopell, N.J. Brain rhythms connect impaired inhibition to altered cognition in schizophrenia. Biol. Psychiatry 2015, 77, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Suzuki, M.; Zhou, S.Y.; Tanino, R.; Hagino, H.; Niu, L.; Kawasaki, Y.; Seto, H.; Kurachi, M. Temporal lobe gray matter in schizophrenia spectrum: A volumetric MRI study of the fusiform gyrus, parahippocampal gyrus, and middle and inferior temporal gyri. Schizophr. Res. 2006, 87, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M.; All, S.D.; Kasi, R.; Berten, S.; Essex, B.; Lathrop, K.L.; Little, D.M. Increased fusiform area activation in schizophrenia during processing of spatial frequency-degraded faces, as revealed by fMRI. Psychol. Med. 2010, 40, 1159–1169. [Google Scholar] [CrossRef]
- Nygard, M.; Eichele, T.; Loberg, E.M.; Jorgensen, H.A.; Johnsen, E.; Kroken, R.A.; Berle, J.O.; Hugdahl, K. Patients with Schizophrenia Fail to Up-Regulate Task-Positive and Down-Regulate Task-Negative Brain Networks: An fMRI Study Using an ICA Analysis Approach. Front. Hum. Neurosci. 2012, 6, 149. [Google Scholar] [CrossRef] [PubMed]
- Palaniyappan, L.; Liddle, P.F. Diagnostic discontinuity in psychosis: A combined study of cortical gyrification and functional connectivity. Schizophr. Bull. 2014, 40, 675–684. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, Y.; Ma, Q.; Zhao, B.; He, X.; Zhu, F.; Wang, W.; Ma, X.; Li, Y. Grey matter volume and its association with cognitive impairment and peripheral cytokines in excited individuals with schizophrenia. Brain Imaging Behav. 2022, 16, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Lalousis, P.A.; Malaviya, A.; Upthegrove, R.; Heinze, K.; Diukova, A.; Auer, D.; Liddle, P.; Mallikarjun, P. Trait related aberrant connectivity in clinically stable patients with schizophrenia: A seed based resting state fMRI study. Brain Imaging Behav. 2022, 16, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gallinat, J. Resting-state brain activity in schizophrenia and major depression: A quantitative meta-analysis. Schizophr. Bull. 2013, 39, 358–365. [Google Scholar] [CrossRef]
- Minzenberg, M.J.; Firl, A.J.; Yoon, J.H.; Gomes, G.C.; Reinking, C.; Carter, C.S. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 2010, 35, 2590–2599. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Aziz, M.Z. Motor imagery BCI classification based on novel two-dimensional modelling in empirical wavelet transform. Electron. Lett. 2020, 56, 1367–1369. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Aziz, M.Z.; Rehman, N.u.; Ding, W.; Xiao, G. Motor Imagery BCI Classification Based on Multivariate Variational Mode Decomposition. IEEE Trans. Emerg. Top. Comput. Intell. 2022, 6, 1177–1189. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Zeming, F.; Rehman, A.U.; Ullah, I.; Li, G.; Xiao, G. Motor Imagery EEG Signals Decoding by Multivariate Empirical Wavelet Transform-Based Framework for Robust Brain–Computer Interfaces. IEEE Access 2019, 7, 171431–171451. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Akbari, H.; Siuly, S.; Li, Y.; Wen, P. Alcoholic EEG signals recognition based on phase space dynamic and geometrical features. Chaos Solitons Fractals 2022, 158, 112036. [Google Scholar] [CrossRef]
- Akbari, H.; Sadiq, M.T.; Payan, M.; Esmaili, S.S.; Baghri, H.; Bagheri, H. Depression detection based on geometrical features extracted from SODP shape of EEG signals and binary PSO. Traitement Du Signal 2022, 38, 13–26. [Google Scholar] [CrossRef]
- Akbari, H.; Sadiq, M.T.; Jafari, N.; Too, J.; Mikaeilvand, N.; Cicone, A.; Serra-Capizzano, S. Recognizing seizure using Poincare plot of EEG signals and graphical features in DWT domain. Bratisl. Lek. Listy 2023, 124, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.M.; Barch, D.M. Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 2016, 61, 108–120. [Google Scholar] [CrossRef] [PubMed]

| Sample 1 N = 36 | Sample 2 N = 36 | Total Sample N = 72 | p Values | |
|---|---|---|---|---|
| Gender (f/m) | 15/21 | 18/18 | 33/39 | 0.48 |
| Age, years old | 43.33 ± 11.83 | 42.47 ± 9.96 | 42.90 ± 10.87 | 0.74 |
| Years of education, years | 13.06 ± 3.00 | 13.56 ± 3.06 | 13.31 ± 3.02 | 0.49 |
| Years since diagnosis, years | 18.97 ± 11.56 | 16.28 ± 10.34 | 17.44 ± 10.97 | 0.37 |
| Chlorpromazine equivalent dose, mg/day | 593.30 ± 304.15 | 613.99 ± 410.85 | 603.64 ± 359.05 | 0.81 |
| PANSS total score | 69.86 ± 9.42 | 73.28 ± 8.51 | 71.57 ± 9.08 | 0.11 |
| PANSS positive subscale | 14.36 ± 4.08 | 16.00 ± 4.46 | 15.18 ± 4.32 | 0.11 |
| PANSS negative subscale | 18.89 ± 3.21 | 19.53 ± 3.71 | 19.21 ± 3.46 | 0.44 |
| PANSS general subscale | 36.61 ± 4.66 | 37.75 ± 4.63 | 37.18 ± 4.65 | 0.30 |
| PSP global scale | 55.36 ± 10.80 | 52.89 ± 10.03 | 53.13 ± 10.42 | 0.32 |
| BCIS-R | 23.58 ± 4.55 | 23.81 ± 4.96 | 23.69 ± 4.73 | 0.96 |
| BCIS-C | 15.00 ± 3.26 | 16.17 ± 3.00 | 15.83 ± 3.13 | 0.37 |
| BCIS R-C index | 8.25 ± 4.16 | 7.64 ± 5.54 | 7.94 ± 4.87 | 0.60 |
| CPT-II | ||||
| d’ | 0.61 ± 0.49 | 0.86 ± 0.59 | 0.74 ± 0.55 | 0.05 |
| OM | 13.00 ± 21.11 | 15.67 ± 31.08 | 14.33 ± 26.41 | 0.67 |
| COM | 17.14 ± 9.69 | 12.75 ± 8.44 | 14.94 ± 9.29 | 0.04 |
| PER | 4.17 ± 7.27 | 3.42 ± 6.67 | 3.79 ± 6.94 | 0.65 |
| HRT | 455.57 ± 100.16 | 478.92 ± 105.17 | 467.25 ± 102.64 | 0.34 |
| HRTSE | 9.65 ± 8.15 | 9.82 ± 8.19 | 9.73 ± 8.11 | 0.93 |
| VAR | 17.64 ± 17.46 | 16.81 ± 18.20 | 17.23 ± 17.72 | 0.85 |
| HRTBC | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.29 |
| HRTISIC | 0.07 ± 0.04 | 0.07 ± 0.04 | 0.06 ± 0.04 | 0.72 |
| CTT1 | 60.08 ± 20.93 | 54.14 ± 25.56 | 57.11 ± 23.39 | 0.28 |
| CTT2 | 105.02 ± 28.10 | 104.96 ± 39.36 | 104.99 ± 33.96 | 0.99 |
| WCST non-perseverative error | 21.22 ± 20.45 | 15.50 ± 16.38 | 18.36 ± 18.62 | 0.52 |
| TOL accuracy | 4.53 ± 2.13 | 3.56 ± 2.32 | 4.04 ± 2.27 | 0.07 |
| TOL time | 234.58 ± 94.30 | 236.03 ± 91.23 | 235.31 ± 92.13 | 0.95 |
| Stroop Interference Test | ||||
| Naming interference tendency | 0.49 ± 0.45 | 0.33 ± 0.32 | 0.41 ± 0.40 | 0.08 |
| Reading interference tendency | 0.28 ± 0.29 | 0.28 ± 0.24 | 0.28 ± 0.26 | 0.84 |
| ROI | Structure | x | y | z | ROI | Structure | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Postcentral Gyrus | −55 | −25 | 50 | 43 | Postcentral Gyrus | 55 | −25 | 50 |
| 2 | Postcentral Gyrus | −45 | −30 | 45 | 44 | Inferior Parietal Lobule | 50 | −30 | 45 |
| 3 | Precentral Gyrus | −35 | −25 | 55 | 45 | Postcentral Gyrus | 40 | −25 | 50 |
| 4 | Precentral Gyrus | −35 | −20 | 50 | 46 | Postcentral Gyrus | 35 | −25 | 50 |
| 5 | Paracentral Lobule | −15 | −45 | 60 | 47 | Paracentral Lobule | 15 | −45 | 60 |
| 6 | Middle Frontal Gyrus | −30 | −5 | 55 | 48 | Middle Frontal Gyrus | 30 | −5 | 55 |
| 7 | Precuneus | −20 | −65 | 50 | 49 | Precuneus | 15 | −65 | 50 |
| 8 | Superior Frontal Gyrus | −20 | 30 | 50 | 50 | Superior Frontal Gyrus | 20 | 25 | 50 |
| 9 | Middle Frontal Gyrus | −30 | 30 | 35 | 51 | Middle Frontal Gyrus | 30 | 30 | 35 |
| 10 | Superior Frontal Gyrus | −25 | 55 | 5 | 52 | Superior Frontal Gyrus | 25 | 55 | 5 |
| 11 | Middle Frontal Gyrus | −20 | 40 | −15 | 53 | Superior Frontal Gyrus | 20 | 45 | −20 |
| 12 | Insula | −40 | −10 | 10 | 54 | Insula | 40 | −5 | 10 |
| 13 | Lingual Gyrus | −10 | −90 | 0 | 55 | Lingual Gyrus | 10 | −90 | 0 |
| 14 | Lingual Gyrus | −15 | −85 | 0 | 56 | Lingual Gyrus | 15 | −85 | 0 |
| 15 | Cuneus | −25 | −75 | 10 | 57 | Cuneus | 25 | −75 | 10 |
| 16 | Fusiform Gyrus | −45 | −20 | −30 | 58 | Fusiform Gyrus | 45 | −20 | −30 |
| 17 | Middle Temporal Gyrus | −60 | −20 | −15 | 59 | Middle Temporal Gyrus | 60 | −15 | −15 |
| 18 | Superior Temporal Gyrus | −55 | −25 | 5 | 60 | Superior Temporal Gyrus | 55 | −20 | 5 |
| 19 | Posterior Cingulate | −5 | −40 | 25 | 61 | Posterior Cingulate | 5 | −45 | 25 |
| 20 | Cingulate Gyrus | −5 | 0 | 35 | 62 | Cingulate Gyrus | 5 | 0 | 35 |
| 21 | Medial Frontal Gyrus | −10 | 20 | −15 | 63 | Subcallosal Gyrus | 5 | 15 | −15 |
| 22 | Parahippocampal Gyrus | −20 | −35 | −5 | 64 | Parahippocampal Gyrus | 20 | −35 | −5 |
| 23 | Parahippocampal Gyrus | −20 | −10 | −25 | 65 | Parahippocampal Gyrus | 20 | −10 | −25 |
| 24 | Posterior Cingulate | −5 | −50 | 5 | 66 | Posterior Cingulate | 5 | −50 | 5 |
| 25 | Posterior Cingulate | −15 | −60 | 5 | 67 | Cuneus | 10 | −60 | 5 |
| 26 | Precuneus | −10 | −50 | 30 | 68 | Precuneus | 10 | −50 | 35 |
| 27 | Anterior Cingulate | −5 | 30 | 20 | 69 | Anterior Cingulate | 5 | 30 | 20 |
| 28 | Anterior Cingulate | −5 | 20 | 20 | 70 | Anterior Cingulate | 0 | 20 | 20 |
| 29 | Parahippocampal Gyrus | −15 | 0 | −20 | 71 | Parahippocampal Gyrus | 15 | 0 | −20 |
| 30 | Parahippocampal Gyrus | −20 | −25 | −20 | 72 | Parahippocampal Gyrus | 25 | −25 | −20 |
| 31 | Parahippocampal Gyrus | −30 | −30 | −25 | 73 | Parahippocampal Gyrus | 30 | −25 | −25 |
| 32 | Fusiform Gyrus | −45 | −55 | −15 | 74 | Fusiform Gyrus | 45 | −55 | −15 |
| 33 | Superior Temporal Gyrus | −40 | 15 | −30 | 75 | Superior Temporal Gyrus | 40 | 15 | −30 |
| 34 | Middle Temporal Gyrus | −45 | −65 | 25 | 76 | Middle Temporal Gyrus | 45 | −65 | 25 |
| 35 | Inferior Parietal Lobule | −50 | −40 | 40 | 77 | Inferior Parietal Lobule | 50 | −45 | 45 |
| 36 | Transverse Temporal Gyrus | −45 | −30 | 10 | 78 | Transverse Temporal Gyrus | 45 | −30 | 10 |
| 37 | Superior Temporal Gyrus | −60 | −25 | 10 | 79 | Superior Temporal Gyrus | 65 | −25 | 10 |
| 38 | Transverse Temporal Gyrus | −60 | −10 | 15 | 80 | Transverse Temporal Gyrus | 60 | −10 | 15 |
| 39 | Precentral Gyrus | −50 | 10 | 15 | 81 | Precentral Gyrus | 55 | 10 | 15 |
| 40 | Inferior Frontal Gyrus | −50 | 20 | 15 | 82 | Inferior Frontal Gyrus | 50 | 20 | 15 |
| 41 | Middle Frontal Gyrus | −45 | 35 | 20 | 83 | Middle Frontal Gyrus | 45 | 35 | 20 |
| 42 | Inferior Frontal Gyrus | −30 | 25 | −15 | 84 | Inferior Frontal Gyrus | 30 | 25 | −15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, T.-C.; Huang, C.C.-Y.; Chung, Y.-A.; Park, S.Y.; Im, J.J.; Lin, Y.-Y.; Ma, C.-C.; Tzeng, N.-S.; Chang, H.-A. Resting-State EEG Connectivity at High-Frequency Bands and Attentional Performance Dysfunction in Stabilized Schizophrenia Patients. Medicina 2023, 59, 737. https://doi.org/10.3390/medicina59040737
Yeh T-C, Huang CC-Y, Chung Y-A, Park SY, Im JJ, Lin Y-Y, Ma C-C, Tzeng N-S, Chang H-A. Resting-State EEG Connectivity at High-Frequency Bands and Attentional Performance Dysfunction in Stabilized Schizophrenia Patients. Medicina. 2023; 59(4):737. https://doi.org/10.3390/medicina59040737
Chicago/Turabian StyleYeh, Ta-Chuan, Cathy Chia-Yu Huang, Yong-An Chung, Sonya Youngju Park, Jooyeon Jamie Im, Yen-Yue Lin, Chin-Chao Ma, Nian-Sheng Tzeng, and Hsin-An Chang. 2023. "Resting-State EEG Connectivity at High-Frequency Bands and Attentional Performance Dysfunction in Stabilized Schizophrenia Patients" Medicina 59, no. 4: 737. https://doi.org/10.3390/medicina59040737
APA StyleYeh, T.-C., Huang, C. C.-Y., Chung, Y.-A., Park, S. Y., Im, J. J., Lin, Y.-Y., Ma, C.-C., Tzeng, N.-S., & Chang, H.-A. (2023). Resting-State EEG Connectivity at High-Frequency Bands and Attentional Performance Dysfunction in Stabilized Schizophrenia Patients. Medicina, 59(4), 737. https://doi.org/10.3390/medicina59040737








