Multi-Target Anticancer Activity of Structurally Diverse Schiff Bases: Insights into Cell-Cycle Arrest, DNA Damage, Metabolic Signaling, and Biomolecular Binding
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Cell Lines
2.3. MTT Assay for Cell Viability
2.4. Cell Cycle Analysis by Flow Cytometry
2.5. Evaluation of ROS Production
2.6. Evaluation of DNA Damage by Comet Assay
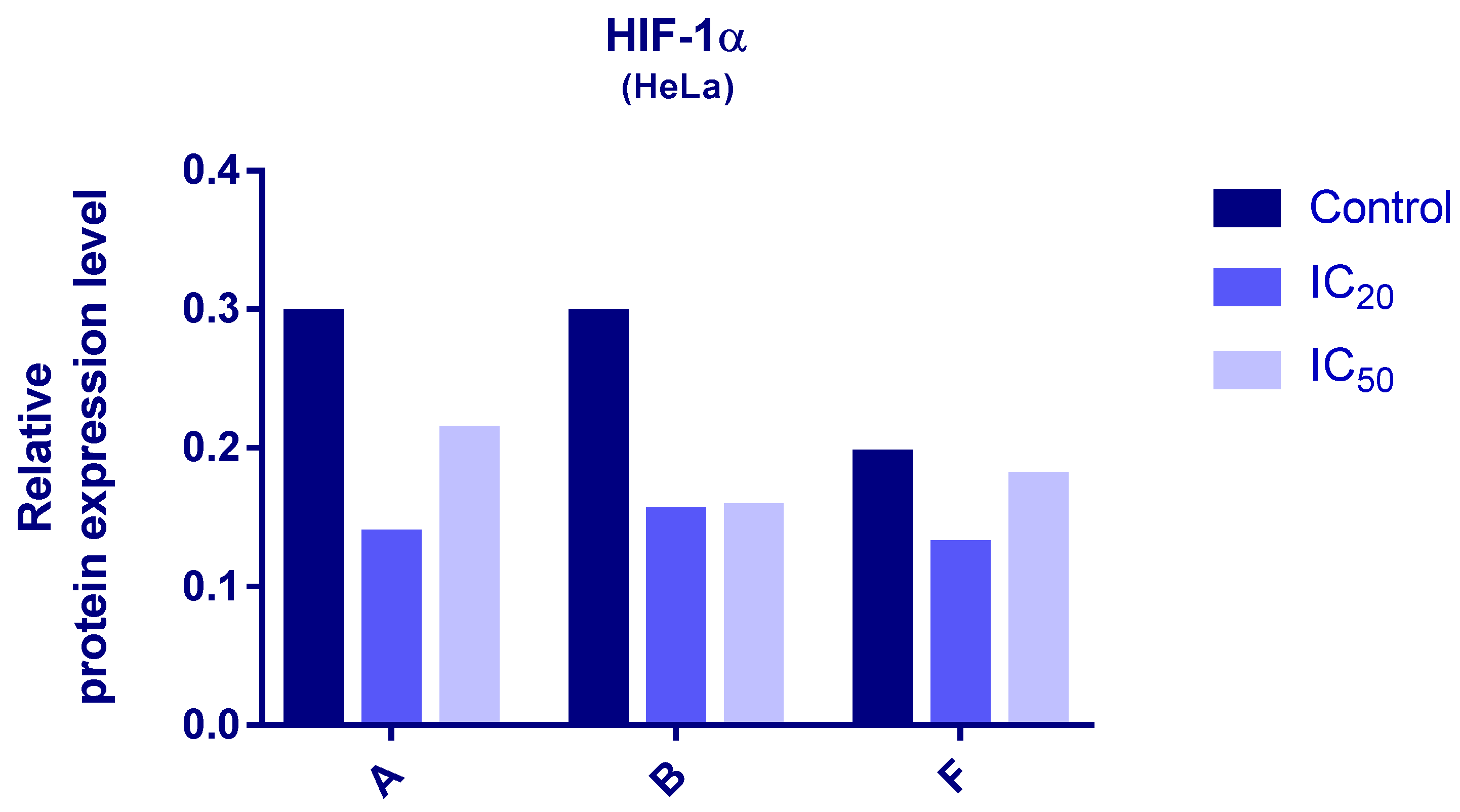
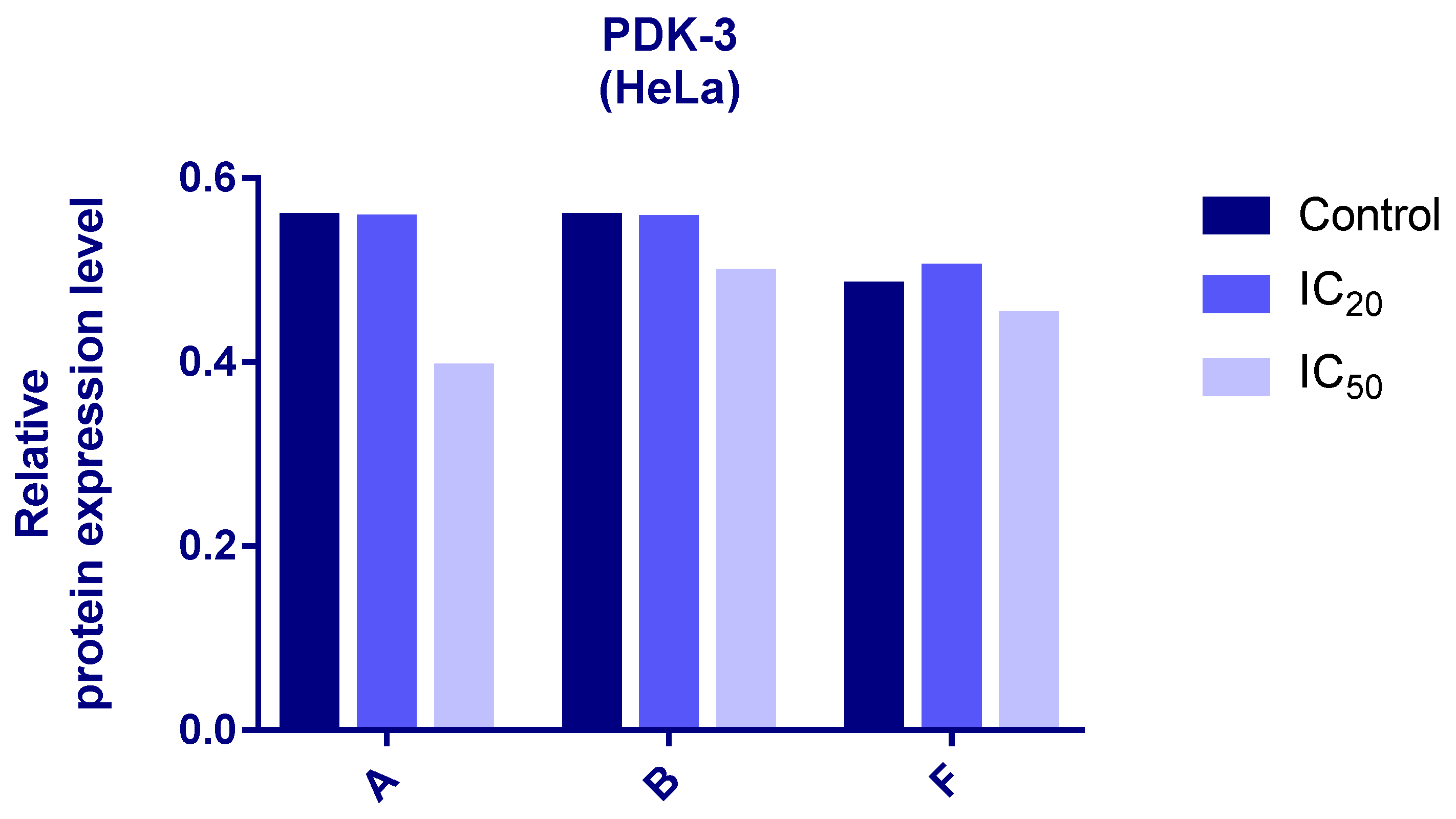
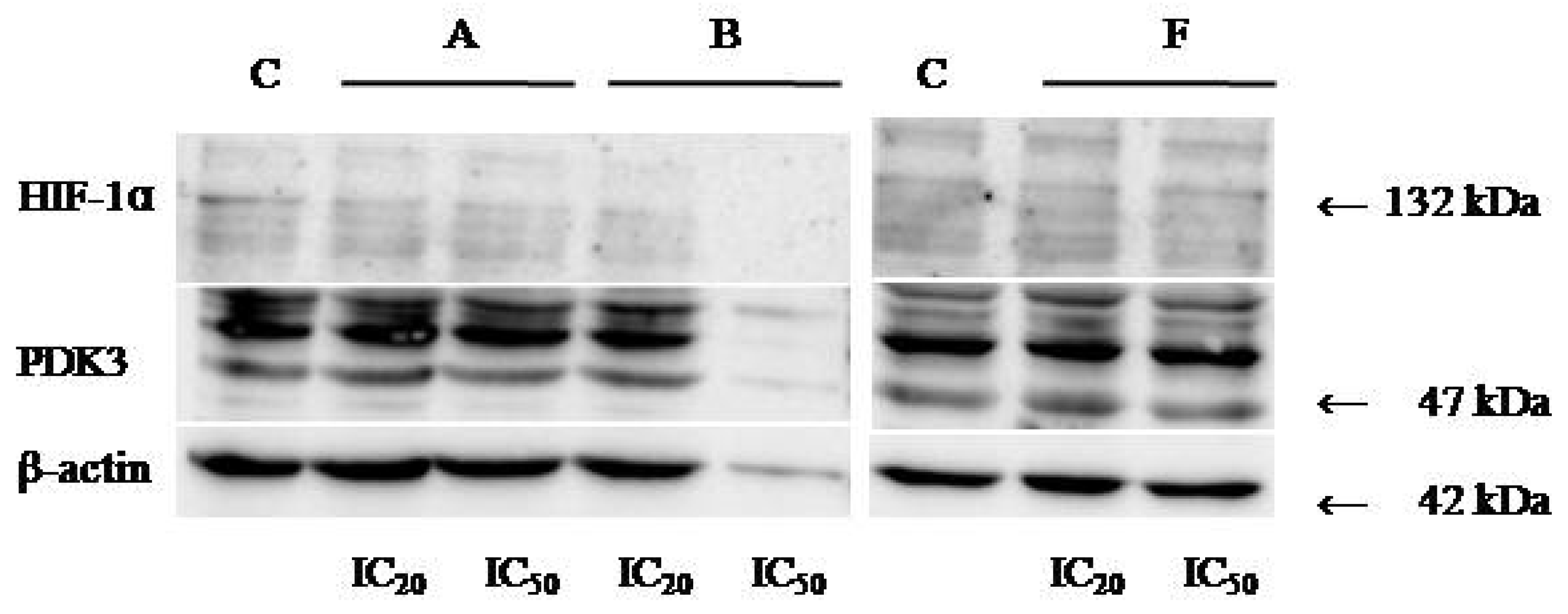
2.7. Evaluation of HIF-1α and PDK-3 Expression by Western Blot
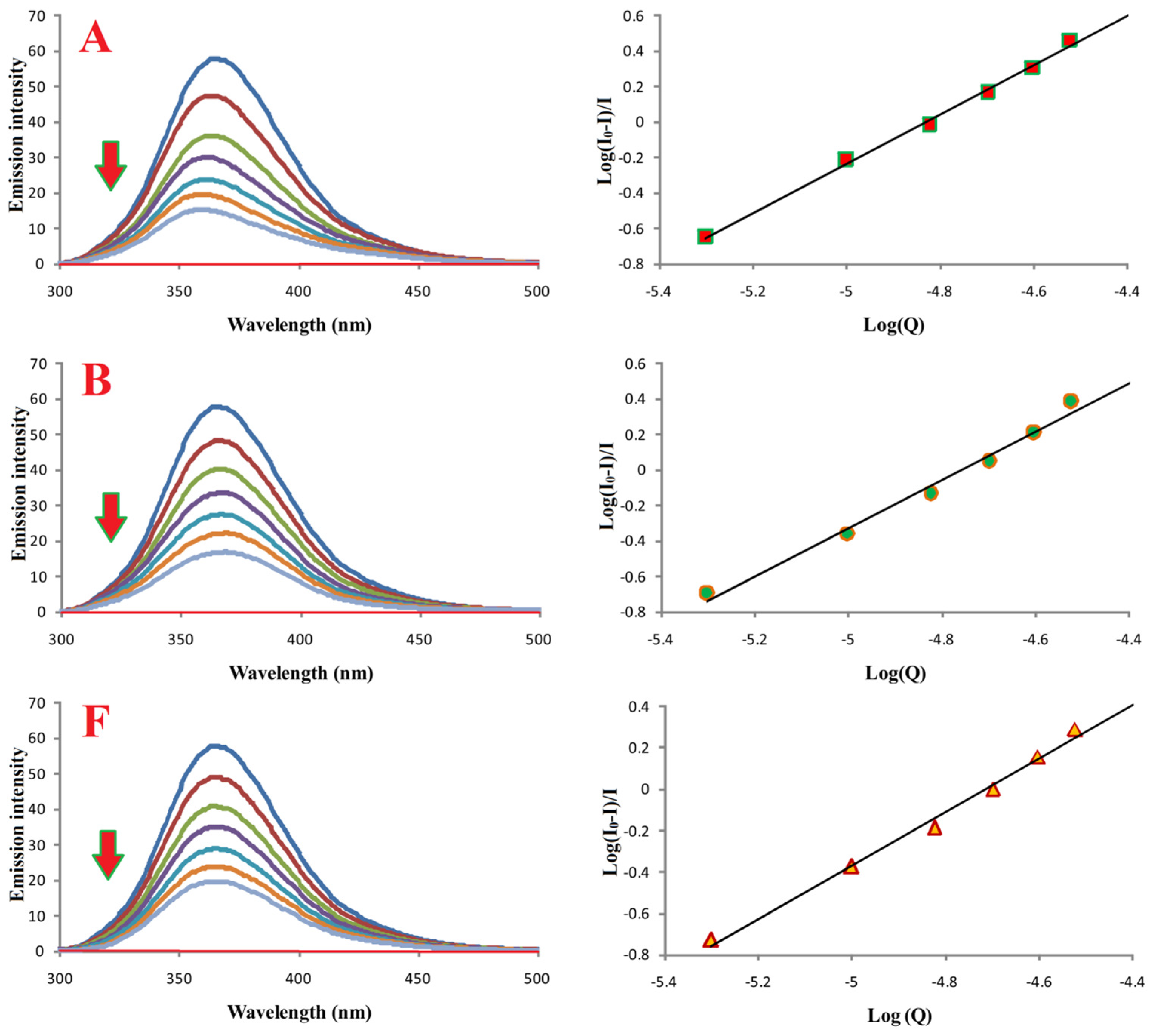
2.8. BSA Fluorescence Binding Study
2.9. Molecular Docking
3. Results
3.1. Anticancer Evaluation
3.2. Cytotoxic Activity
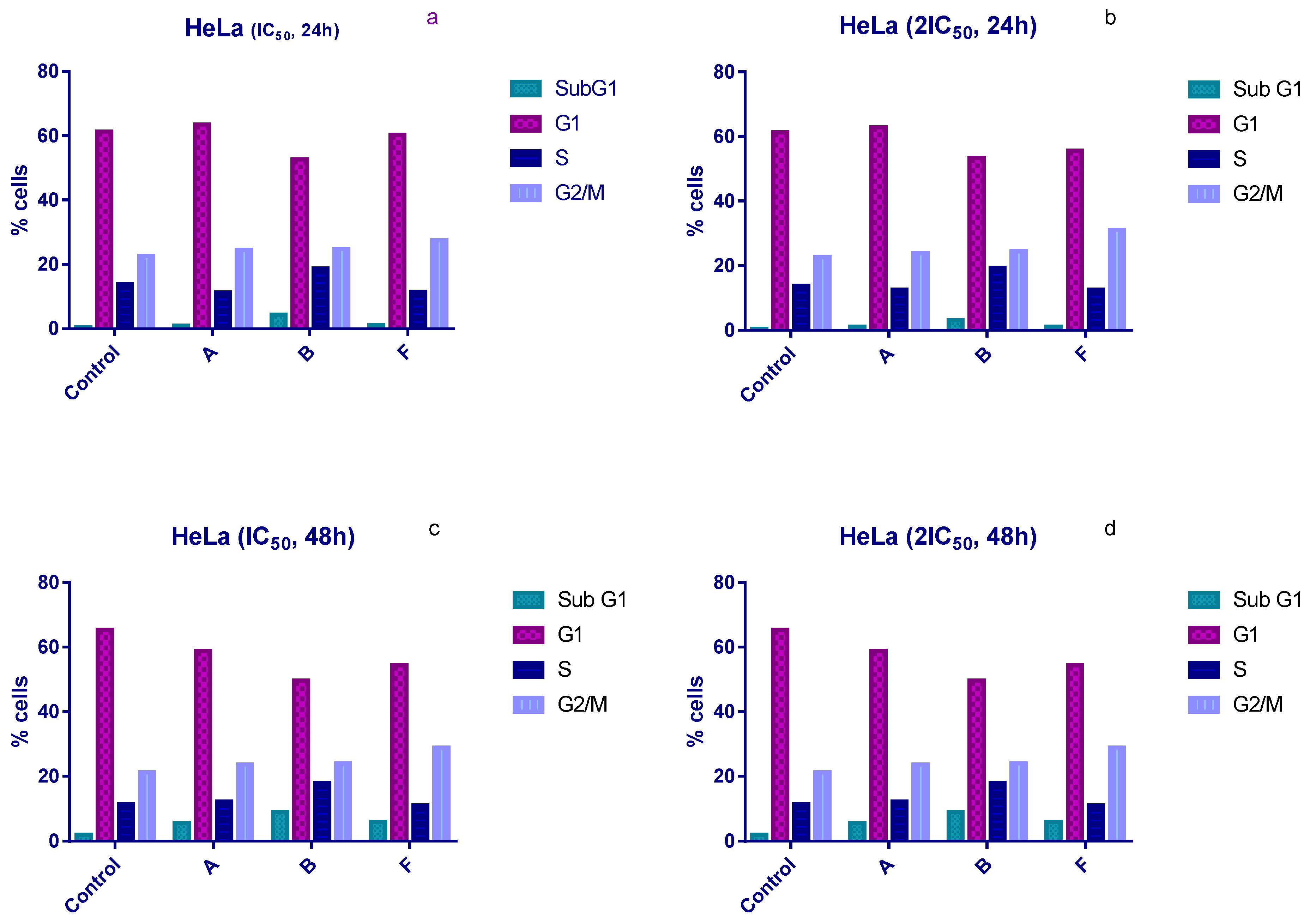
3.3. Cell Cycle Analysis
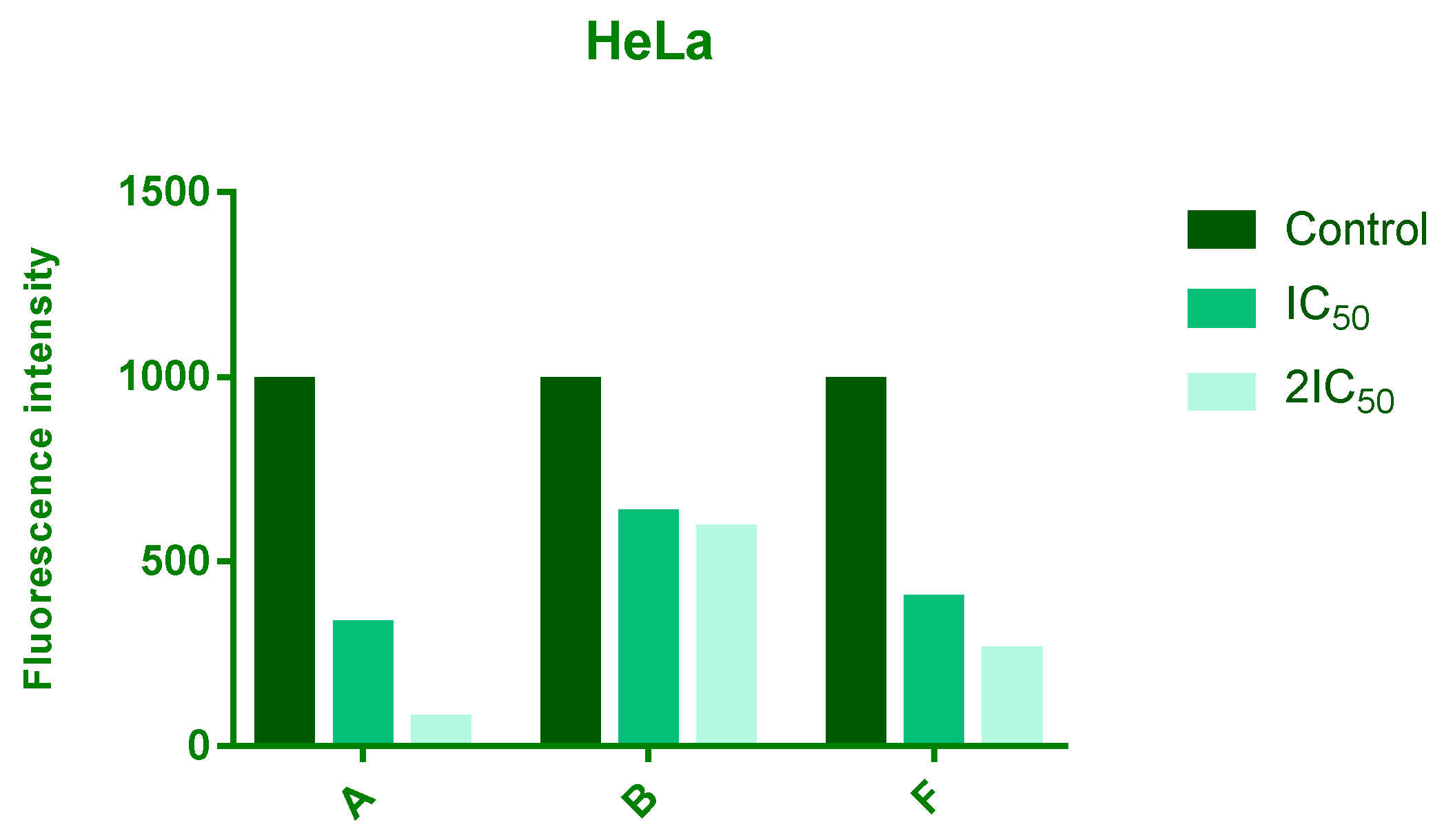
3.4. Analyzes of ROS Level
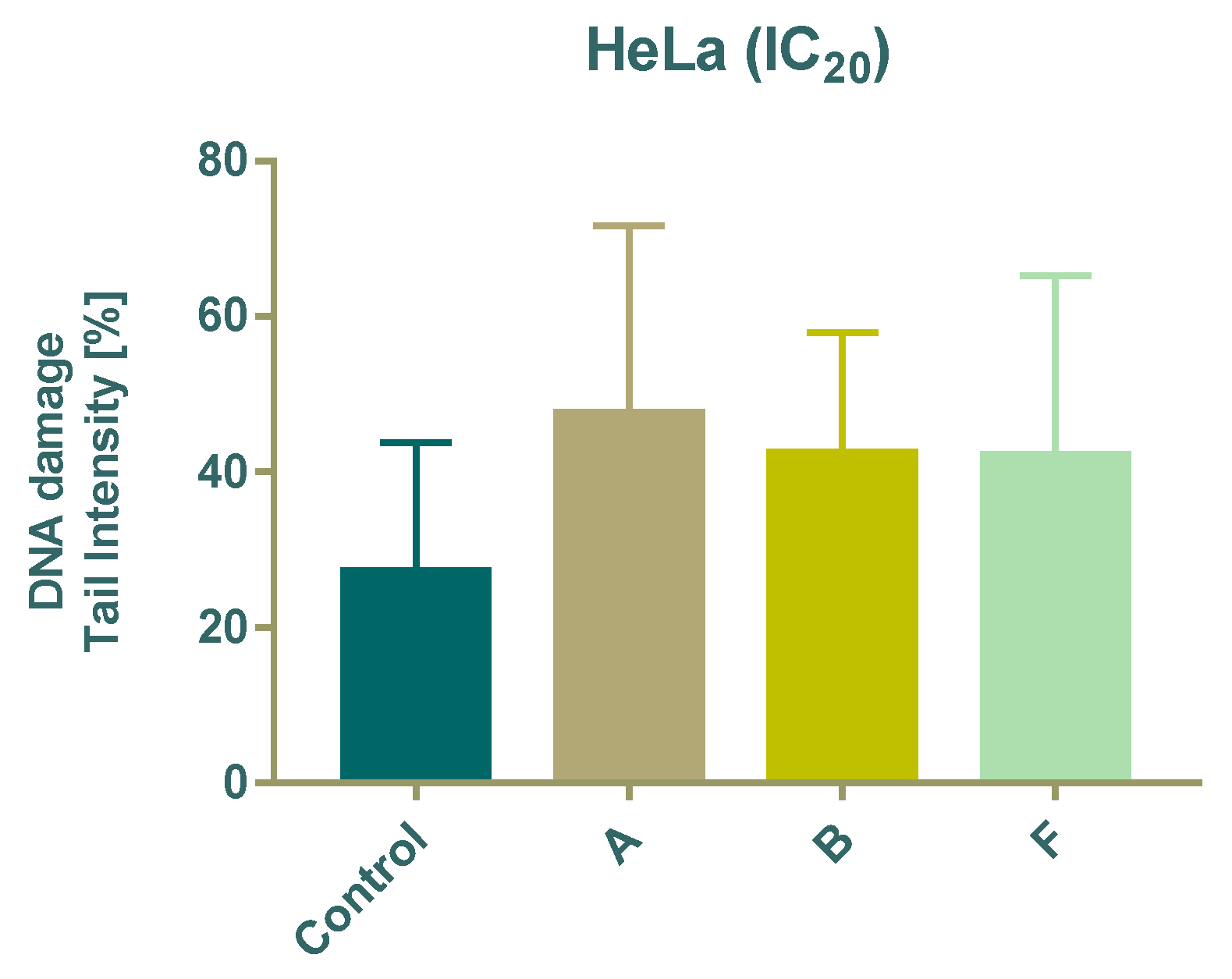
3.5. DNA Damage Analysis
3.6. Western Blot Analyses
3.7. Interactions of Compounds A, B, and F with Bovine Serum Albumin
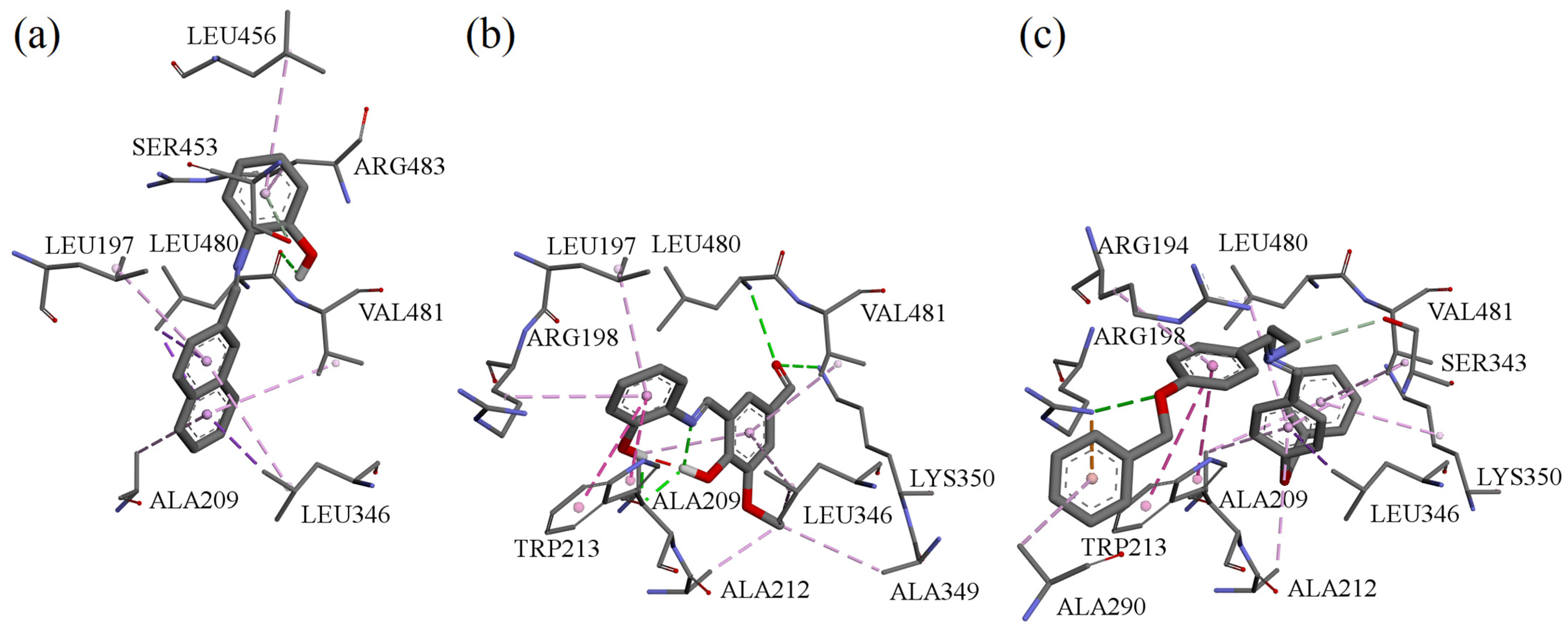
3.8. Molecular Docking of Compounds A, B, and F to Bovine Serum Albumin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, M.K.; Baek, A.R.; Sung, B.; Yang, B.W.; Choi, G.; Park, H.J.; Kim, Y.H.; Kim, M.; Ha, S.; Lee, G.H.; et al. Synthesis, characterization, and anticancer activity of benzothiazole aniline derivatives and their platinum (II) complexes as new chemotherapy agents. Pharmaceuticals 2021, 14, 832. [Google Scholar] [CrossRef]
- Kumari, B.; Kumar, G.; Singh, K.; Mittal, M. Synthesis, structural and antimicrobial studies of transition metal complexes of a novel Schiff base ligand incorporating 1, 2, 4-triazole and 4-(benzyloxy) benzaldehyde moieties. J. Organomet. Chem. 2024, 1010, 123114. [Google Scholar] [CrossRef]
- Kumar, G.; Saroha, B.; Kumar, S.; Kumari, B.; Arya, P.; Raghav, N.; Ghosh, S.; Nassare, V.D. 1,2,3-Triazole-based New aurones as anticancer agents with the capability to target extracellular digestive enzymes. ChemistrySelect 2024, 9, e202403885. [Google Scholar] [CrossRef]
- Kumar, G.; Saroha, B.; Arya, P.; Ghosh, S.; Kumari, B.; Nassare, V.D.; Raghav, N.; Kumar, S. 1,2,3-triazole clubbed and dichloro substituted novel aurones as potential anticancer agents targeting digestive enzymes: Design, synthesis, DFT, ADME and molecular docking studies. J. Mol. Struc. 2025, 1319, 139460. [Google Scholar] [CrossRef]
- Lauria, A.; La Monica, G.; Bono, A.; Martorana, A. Quinoline anticancer agents active on DNA and DNA-interacting proteins: From classical to emerging therapeutic targets. Eur. J. Med. Chem. 2021, 220, 113555–113589. [Google Scholar] [CrossRef]
- Desoize, B.; Madoulet, C. Particular aspects of platinum compounds used at present in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 317−325. [Google Scholar] [CrossRef]
- Ott, I.; Gust, R. Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. (Weinh. Ger.) 2007, 340, 117−126. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Fels, L.M.; Knop, S.; Stolt, H.; Kanz, L.; Bokemeyer, C. A randomized trial comparing the nephrotoxicity of cisplatin/ifosfamide-based combination chemotherapy with or without amifostine in patients with solid tumors. Invest. new Drugs. 2000, 18, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Sastry, J.; Kellie, S.J. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr. Hematol. Oncol. 2005, 22, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum neurotoxicity pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef]
- Lazić, D.; Arsenijević, N.A.; Puchta, R.; Bugarčić, Ž.D.; Rilak, A. DNA binding properties, histidine interaction and cytotoxicity studies of water soluble ruthenium (II) terpyridine complexes. Dalton Trans. 2016, 45, 4633–4646. [Google Scholar] [CrossRef]
- Mowery, P.; Mejia, F.B.; Franceschi, C.L.; Kean, M.H.; Kwansare, D.O.; Lafferty, M.M.; Neerukonda, N.D.; Rolph, C.E.; Truax, N.J.; Pelkey, E.T. Synthesis and evaluation of the anti-proliferative activity of diaryl-3-pyrrolin-2-ones and fused analogs. Bioorg. Med. Chem. Lett. 2016, 27, 191–195. [Google Scholar] [CrossRef]
- Joksimović, N.; Petronijević, J.; Janković, N.; Baskić, D.; Popović, S.; Todorović, D.; Matić, S.; Bogdanović, G.A.; Vraneš, M.; Tot, A.; et al. Synthesis, characterization, anticancer evaluation and mechanisms of cytotoxic activity of novel 3-hydroxy-3-pyrrolin-2-ones bearing thenoyl fragment: DNA, BSA interactions and molecular docking study. Bioorg. Chem. 2019, 88, 102954–102968. [Google Scholar] [CrossRef]
- Joksimović, N.; Janković, N.; Davidović, G.; Bugarčić, Z. 2, 4-Diketo esters: Crucial intermediates for drug discovery. Bioorg. Chem. 2020, 105, 104343–104380. [Google Scholar] [CrossRef]
- Omidi, S.; Kakanejadifard, A. A review on biological activities of Schiff base, hydrazone, and oxime derivatives of curcumin. RSC Adv. 2020, 10, 30186–30202. [Google Scholar] [CrossRef]
- Dhar, D.N.; Taploo, C.L. Schiff bases and their applications. J. Sci. Ind. Res. 1982, 41, 501–506. [Google Scholar]
- Przybylski, P.; Huczynski, A.; Pyta, K.; Brzezinski, B.; Bartl, F. Biological properties of Schiff bases and azo derivatives of phenols. Curr. Org. Chem. 2009, 13, 124–148. [Google Scholar] [CrossRef]
- Bringmann, G.; Dreyer, M.; Faber, J.H.; Dalsgaard, P.W.; Stærk, D.; Jaroszewski, J.W.; Ndangalasi, H.; Mbago, F.; Brun, R.; Christensen, S.B. Ancistrotanzanine C and related 5, 1’-and 7, 3’-coupled Naphthylisoquinoline alkaloids from Ancistrocladus tanzaniensis. J. Nat. Prod. 2004, 67, 743–748. [Google Scholar] [CrossRef] [PubMed]
- ASouza, A.O.D.; Galetti, F.; Silva, C.L.; Bicalho, B.; Parma, M.M.; Fonseca, S.F.; Marsaioli, A.J.; Trindade, A.C.; Gil, R.P.F.; Bezerra, F.S.; et al. Antimycobacterial and cytotoxicity activity of synthetic and natural compounds. Quim. Nova 2007, 30, 1563–1566. [Google Scholar]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Li, P. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr. Res. 2007, 342, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Joksimović, N.; Petronijević, J.; Ćoćić, D.; Janković, N.; Milović, E.; Kosanić, M.; Petrović, N. Synthesis, characterization, biological evaluation, BSA binding properties, density functional theory and molecular docking study of Schiff bases. J. Mol. Struct. 2021, 1244, 130951–130965. [Google Scholar] [CrossRef]
- Gein, V.L.; Rubtsova, D.D.; Gagarina, A.A.; Gein, L.F.; Dmitriev, M.V. Reactions of 5-Aryl-4-(hetaren-2-ylcarbonyl)-3-hydroxy-1-(1,3-thiazol-2-yl)-2,5-dihydro-1H-pyrrol-2-ones with Hydrazine, Phenylhydrazine, and Hydroxylamine. Russ. J. Org. Chem. 2015, 51, 110–115. [Google Scholar] [CrossRef]
- Joksimović, N.; Janković, N.; Petronijević, J.; Baskić, D.; Popovic, S.; Todorović, D.; Zarić, M.; Klisurić, O.; Vraneš, M.; Tot, A.; et al. Synthesis, Anticancer Evaluation and Synergistic Effects with cis platin of Novel Palladium Complexes: DNA, BSA Interactions and Molecular Docking Study. Med. Chem. 2020, 16, 78–92. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ohno, M.; Abe, T. Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6). J. Immunol. Methods 1991, 145, 199–203. [Google Scholar] [CrossRef]
- Ormerod, M.G. Further applications to cell biology. In Flow Cytometry, a Practical Approach; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Chang, H.-Y.; Huang, H.-C.; Huang, T.-C.; Yang, P.-C.; Wang, Y.-C.; Juan, H.-F. Flow cytometric detection of reactive oxygen species. Bio-Protocol 2013, 3, e431. [Google Scholar] [CrossRef]
- Janović, B.S.; Collins, A.R.; Vujčić, Z.M.; Vujčić, M.T. Acidic horseradish peroxidase activity abolishes genotoxicity of common dyes. J. Hazard. Mater. 2017, 321, 576–585. [Google Scholar] [CrossRef]
- Stewart, J.J. MOPAC: A semiempirical molecular orbital program. J. Comput. Aided Mol. Des. 1990, 4, 1–103. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Demehin, A.I.; Oladipo, M.A.; Semire, B. Synthesis, Spectroscopic, Antibacterial and Antioxidant Activities of Pd (Ii) Mixed-Ligand Complexes Containing Tridentate Schiff Bases. Egypt. J. Chem. 2019, 62, 413–426. [Google Scholar]
- Bujacz, A.; Zielinski, K.; Sekula, B. Structural studies of bovine, equine, and leporine serum albumin complexes with naproxen. Proteins 2014, 82, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Donjerkovic, D.; Scott, D.W. Regulation of the G1 phase of the mammalian cell cycle. Cell. Res. 2000, 10, 1–16. [Google Scholar] [CrossRef]
- Boonstra, J.; Moes, M.J. Signal transduction and actin in the regulation of G1-phase progression. Crit. Rev. Eukaryot. Gene Expr. 2005, 15, 255–276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Apostolou, P.; Toloudi, M.; Kourtidou, E.; Mimikakou, G.; Vlachou, I.; Chatziioannou, M.; Papasotiriou, I. Use of the comet assay technique for quick and reliable prediction of in vitro response to chemotherapeutics in breast and colon cancer. J. Biol. Res. 2014, 21, 14. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 and tumor progression: Pathophysiology and therapeutics. Trends Mol. Med. 2002, 8, S62–S67. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, S.C.; Chen, K.F.; Lai, Y.Y.; Tsai, S.J. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J. Biol. Chem. 2008, 283, 28106–28114. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, S.C.; Chien, C.W.; Lin, S.C.; Lee, C.T.; Lin, B.W.; Lee, J.C.; Tsai, S.J. Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer. Am. J. Pathol. 2011, 179, 1405–1414. [Google Scholar] [CrossRef]
- Gopinath, S.; Evangelos, M. Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Front. Oncol. 2013, 3, 38. [Google Scholar] [CrossRef]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ameen, F.; Rehman, S.; Sarwar, T.; Tabisha, M. Studying the interaction of drug/ligand with serum albumin. J. Mol. Liq. 2021, 336, 116200–116215. [Google Scholar] [CrossRef]
- Aguilera-Garrido, A.; Castillo-Santaella, T.; Yang, Y.; Galisteo-González, F.; Gálvez-Ruiza, M.J.; Molina-Bolívar, J.A.; Holgado-Terriza, J.A.; Cabrerizo-Vílchez, M.Á.; Maldonado-Valderrama, J. Applications of serum albumins in delivery systems: Differences in interfacial behaviour and interacting abilities with polysaccharides. Adv. Colloid Interface 2021, 290, 102365–102381. [Google Scholar] [CrossRef] [PubMed]
- Belinskaia, D.A.; Voronina, P.A.; Batalova, A.A.; Goncharov, N.V. Serum albumin. Encyclopedia 2021, 1, 65–75. [Google Scholar] [CrossRef]
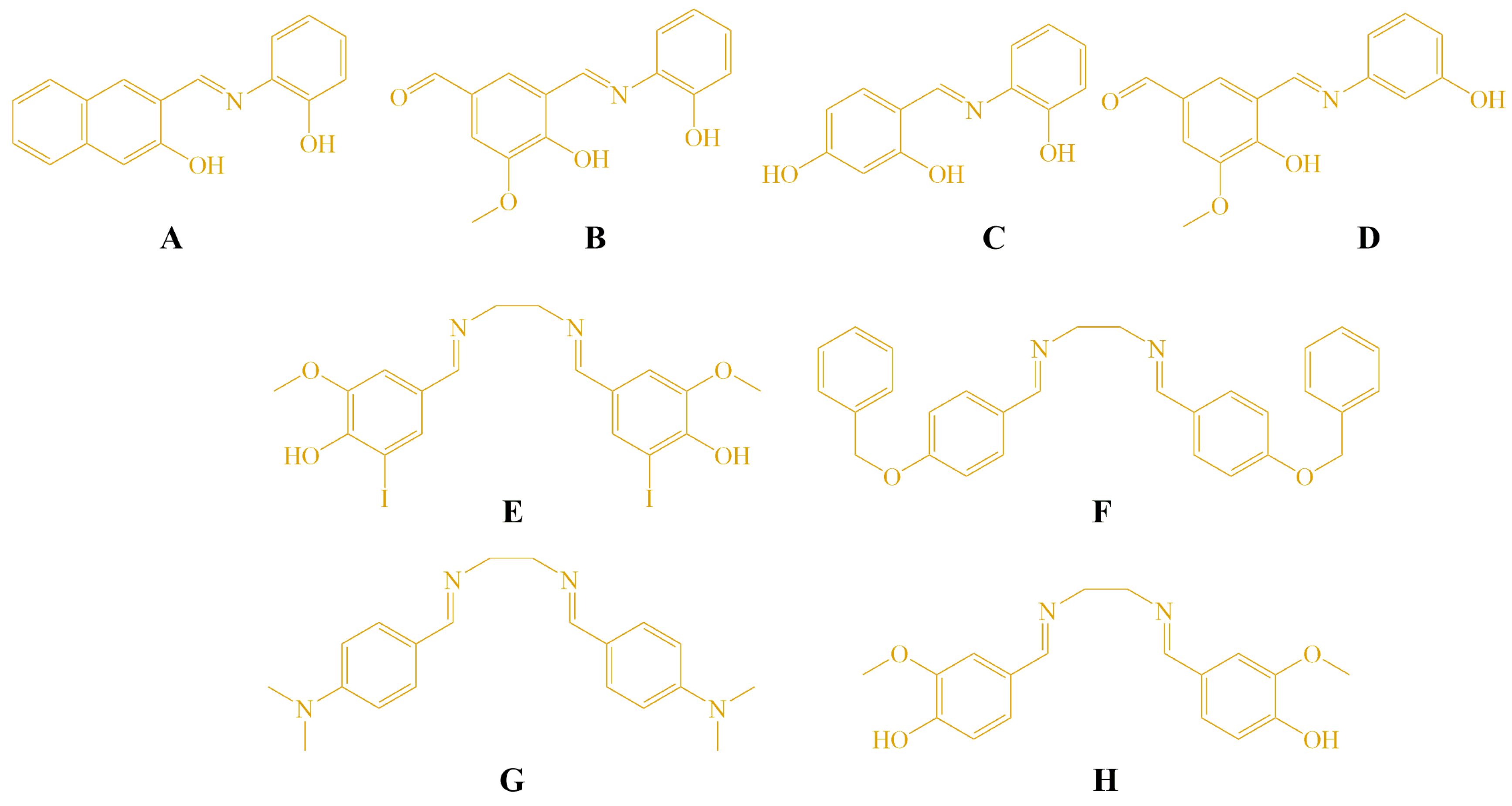
| Compounds | HeLa IC50 (μM) | MRC5 IC50 (μM) | LS 174T IC50 (μM) | A549 IC50 (μM) |
|---|---|---|---|---|
| A | 39.45 ± 0.2 | >200 | 82.35 ± 16.81 | 79.1 ± 4.76 |
| B | 30.78 ± 2.67 | 114.07 ± 19.03 | 107.32 ± 2.23 | 116.03 ± 29.65 |
| C | >200 | >200 | >200 | >200 |
| D | 102.29 ± 1.54 | >200 | >200 | >200 |
| E | 78.22 ± 0.21 | 73.25 ± 6.69 | 146.49 ± 15.61 | >200 |
| F | 55.73 ± 1.4 | 113.84 ± 15.13 | 134.08 ± 39.17 | 116.85 ± 37.26 |
| G | >200 | >200 | >200 | >200 |
| H | 130.68 ± 6.79 | 69.0 ± 2.67 | 118.89 ± 2.18 | 163.23 ± 10.6 |
| cisPt | 4.91 ± 0.74 | 9.35 ± 1.29 | 5.54 ± 1.03 | 13.21 ± 0.89 |
| Compounds | HeLa IC50 (μM) |
|---|---|
| A | 36.12 ± 0.73 |
| B | 26.79 ± 1.5 |
| F | 15.41 ± 1.23 |
| Compound | Ka [M−1] | n | R |
|---|---|---|---|
| A | (5.0 ± 0.2) × 106 | 1.3 | 0.997 |
| B | (2.9 ± 0.2) × 106 | 1.4 | 0.990 |
| F | (1.2 ± 0.2) × 106 | 1.2 | 0.996 |
| Compound | E [kcal mol−1] | |
|---|---|---|
| TRP134 | TRP213 | |
| A | −8.38 | −7.97 |
| B | −7.22 | −8.13 |
| F | −9.42 | −8.97 |
| NPX | −6.54 | −8.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Joksimović, N.; Petronijević, J.; Filipović, I.; Janković, N.; Ilić, B.; Stanojković, T.; Djurić, A. Multi-Target Anticancer Activity of Structurally Diverse Schiff Bases: Insights into Cell-Cycle Arrest, DNA Damage, Metabolic Signaling, and Biomolecular Binding. Curr. Issues Mol. Biol. 2026, 48, 57. https://doi.org/10.3390/cimb48010057
Joksimović N, Petronijević J, Filipović I, Janković N, Ilić B, Stanojković T, Djurić A. Multi-Target Anticancer Activity of Structurally Diverse Schiff Bases: Insights into Cell-Cycle Arrest, DNA Damage, Metabolic Signaling, and Biomolecular Binding. Current Issues in Molecular Biology. 2026; 48(1):57. https://doi.org/10.3390/cimb48010057
Chicago/Turabian StyleJoksimović, Nenad, Jelena Petronijević, Ignjat Filipović, Nenad Janković, Bojana Ilić, Tatjana Stanojković, and Ana Djurić. 2026. "Multi-Target Anticancer Activity of Structurally Diverse Schiff Bases: Insights into Cell-Cycle Arrest, DNA Damage, Metabolic Signaling, and Biomolecular Binding" Current Issues in Molecular Biology 48, no. 1: 57. https://doi.org/10.3390/cimb48010057
APA StyleJoksimović, N., Petronijević, J., Filipović, I., Janković, N., Ilić, B., Stanojković, T., & Djurić, A. (2026). Multi-Target Anticancer Activity of Structurally Diverse Schiff Bases: Insights into Cell-Cycle Arrest, DNA Damage, Metabolic Signaling, and Biomolecular Binding. Current Issues in Molecular Biology, 48(1), 57. https://doi.org/10.3390/cimb48010057







