Circadian Rhythm Disruptions and Cardiovascular Disease Risk: The Special Role of Melatonin
Abstract
1. Introduction
2. Circadian Rhythms and Cardiovascular Function—Molecular Foundations of Circadian Rhythms
2.1. Molecular Basis of Circadian Rhythms
2.2. Blood Pressure Regulation and Peripheral Clocks
2.3. Endothelial Function and Vascular Tone
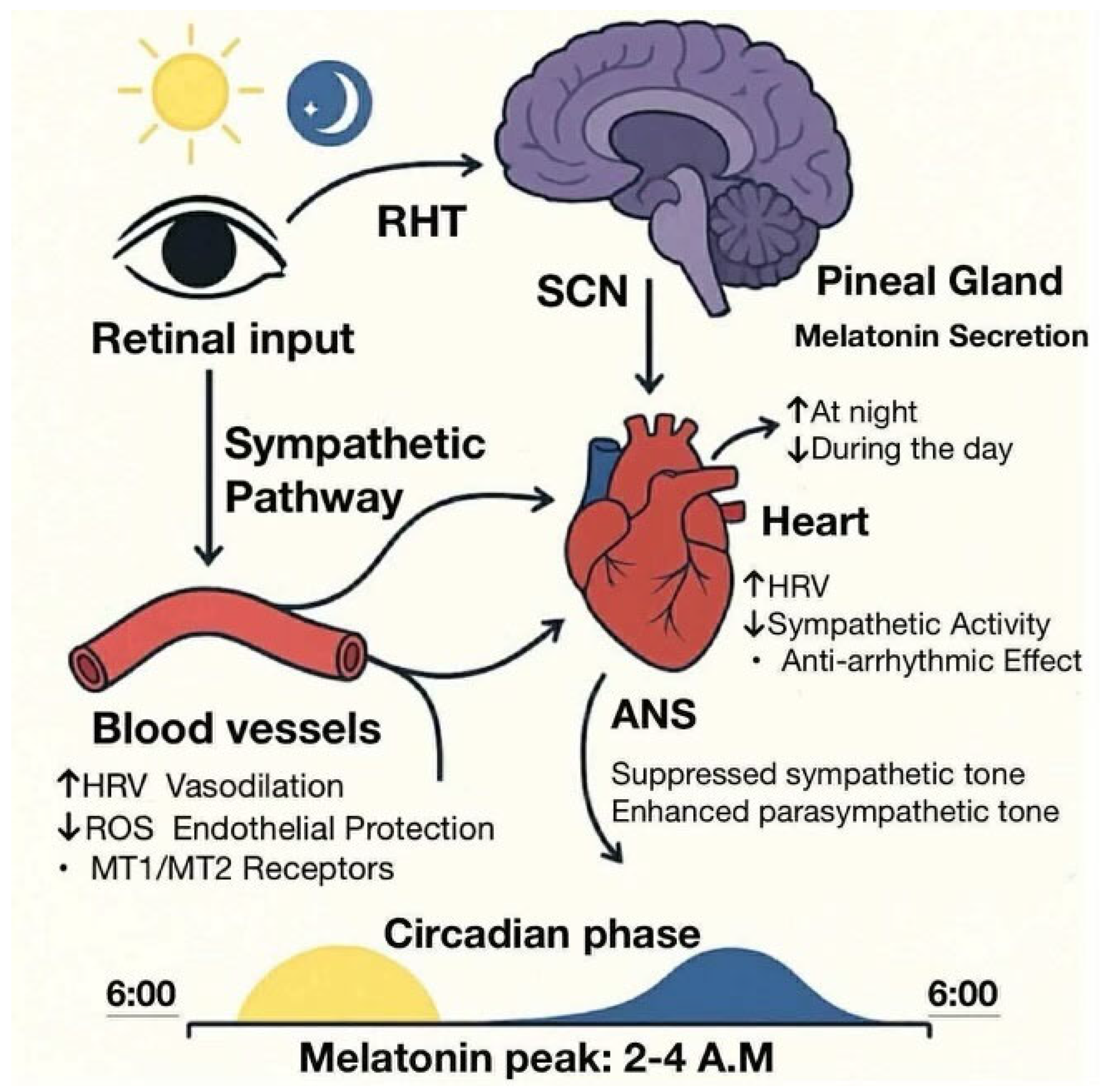
2.4. Heart Rate and Autonomic Nervous System
3. Disruptions of Circadian Rhythms and Cardiovascular Disease Risk
3.1. Shift Work and Sleep Disorders
3.2. Lifestyle and Environmental Factors
3.3. Circadian Disruption and Cardiometabolic Syndromes
4. The Role of Melatonin in Cardiovascular Health
4.1. Melatonin Biosynthesis and Secretion
4.2. Cardioprotective Properties of Melatonin
4.3. Melatonin and Chronotherapy for CVD
5. Clinical and Public Health Implications
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AANAT | arylalkylamine N-acetyltransferase |
| ABPM | ambulatory blood pressure monitoring |
| ACE | angiotensin converting enzyme |
| ALAN | artificial light at night |
| ANS | the autonomic nervous system |
| ARB | angiotensin receptor blocker |
| Arnt | aryl hydrocarbon receptor nuclear translocator |
| BMAL1 | brain and muscle Arnt-like protein 1 |
| BP | blood pressure |
| CAD | coronary artery disease |
| CHD | coronary heart disease |
| CLOCK | circadian locomotor output cycles kaput |
| CRP | C-reactive protein |
| CRY | cryptochrome |
| CVD | cardiovascular disease |
| dLAN | dim light at night |
| DLMO | dim light melatonin onset |
| E-box | enhancer box |
| ET-1 | endothelin-1 |
| GPx | glutathione peroxidase |
| HPA | the hypothalamic–pituitary–adrenal axis |
| HR | heart rate |
| HRV | heart rate variability |
| IL-1β | interleukin-1beta |
| IL-6 | interleukin-6 |
| LDL | low-density lipoprotein |
| LED | light-emitting diode |
| MT1 | melatonin receptor subtype 1 |
| MT2 | melatonin receptor subtype 2 |
| NF-κB | nuclear factor kappa B |
| NO | nitric oxide |
| NR1D1 | nuclear receptor subfamily 1 group D member 1 |
| PER | period (gene) |
| REV-ERBα | reverse orientation c-erbA gene α |
| RNS | reactive nitrogen species |
| RORα | retinoic acid receptor-related orphan receptor alpha |
| ROS | reactive oxygen species |
| SCN | the suprachiasmatic nucleus |
| SOD | superoxide dismutase |
| TNF-α | tumor necrosis factor-alpha |
| TRE | time-restricted eating |
| TTFL | transcriptional–translational feedback loop |
| VSMC | vascular smooth muscle cell |
References
- Drăgoi, C.M.; Nicolae, A.C.; Ungurianu, A.; Margină, D.M.; Grădinaru, D.; Dumitrescu, I.-B. Circadian Rhythms, Chrononutrition, Physical Training, and Redox Homeostasis—Molecular Mechanisms in Human Health. Cells 2024, 13, 138. [Google Scholar] [CrossRef]
- Starnes, A.N.; Jones, J.R. Inputs and Outputs of the Mammalian Circadian Clock. Biology 2023, 12, 508. [Google Scholar] [CrossRef]
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian Rhythm as a Therapeutic Target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef]
- Hesketh, S.J.; Esser, K.A. The Clockwork of Champions: Influence of Circadian Biology on Exercise Performance. Free Radic. Biol. Med. 2024, 224, 78–87. [Google Scholar] [CrossRef]
- Faraci, F.M.; Scheer, F.A.J.L. Hypertension: Causes and Consequences of Circadian Rhythms in Blood Pressure. Circ. Res. 2024, 134, 810–832. [Google Scholar] [CrossRef] [PubMed]
- Sumová, A.; Sládek, M. Circadian Disruption as a Risk Factor for Development of Cardiovascular and Metabolic Disorders—From Animal Models to Human Population. Physiol. Res. 2024, 73, S321–S334. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.; Queiroz, M.; Sena, C.M. Melatonin and Vascular Function. Antioxidants 2024, 13, 747. [Google Scholar] [CrossRef] [PubMed]
- Dobrovinskaya, O.; Alamilla, J.; Olivas-Aguirre, M. Impact of Modern Lifestyle on Circadian Health and Its Contribution to Adipogenesis and Cancer Risk. Cancers 2024, 16, 3706. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G. do Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Blanco, S.; Muñoz-Gallardo, M.d.M.; Hernández, R.; Peinado, M.Á. The Interplay Between Melatonin and Nitric Oxide: Mechanisms and Implications in Stroke Pathophysiology. Antioxidants 2025, 14, 724. [Google Scholar] [CrossRef]
- Joseph, T.T.; Schuch, V.; Hossack, D.J.; Chakraborty, R.; Johnson, E.L. Melatonin: The Placental Antioxidant and Anti-Inflammatory. Front. Immunol. 2024, 15, 1339304. [Google Scholar] [CrossRef]
- Tobeiha, M.; Jafari, A.; Fadaei, S.; Mirazimi, S.M.A.; Dashti, F.; Amiri, A.; Khan, H.; Asemi, Z.; Reiter, R.J.; Hamblin, M.R.; et al. Evidence for the Benefits of Melatonin in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 888319. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Haskologlu, I.C.; Erdag, E. Molecular Links Between Circadian Rhythm Disruption, Melatonin, and Neurodegenerative Diseases: An Updated Review. Molecules 2025, 30, 1888. [Google Scholar] [CrossRef]
- Erren, T.C.; Reiter, R.J. Defining Chronodisruption. J. Pineal Res. 2009, 46, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Heanue, S.; Kanki, M.; Moneghetti, K.J. Circadian Disruption and Its Impact on the Cardiovascular System. Trends Endocrinol. Metab. 2024, S1043-2760(24)00316-3. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Tang, Y.Y.; Zhou, L. Melatonin as an Adjunctive Therapy in Cardiovascular Disease Management. Sci. Prog. 2024, 107, 368504241299993. [Google Scholar] [CrossRef] [PubMed]
- Ewbank, H.; Nagai, M.; Dasari, T.W. Circadian Rhythm And Melatonin In Heart Failure—A Systematic Review. J. Card. Fail. 2025, 31, 222. [Google Scholar] [CrossRef]
- Peng, F.; Li, X.; Xiao, F.; Zhao, R.; Sun, Z. Circadian Clock, Diurnal Glucose Metabolic Rhythm, and Dawn Phenomenon. Trends Neurosci. 2022, 45, 471–482. [Google Scholar] [CrossRef]
- Schrader, L.A.; Ronnekleiv-Kelly, S.M.; Hogenesch, J.B.; Bradfield, C.A.; Malecki, K.M.C. Circadian Disruption, Clock Genes, and Metabolic Health. J. Clin. Investig. 2024, 134, e170998. [Google Scholar] [CrossRef]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian Rhythms, Sleep, and Metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef]
- McLoughlin, S.C.; Haines, P.; FitzGerald, G.A. Clocks and Cardiovascular Function. Methods Enzymol. 2015, 552, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Butler, M.P.; Shea, S.A. Role of the Circadian System in Cardiovascular Disease. J. Clin. Investig. 2018, 128, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Rasouli, M.; Reitz, C.J.; Posen, I.; Margulets, V.; Dhingra, R.; Khatua, T.N.; Thliveris, J.A.; Martino, T.A.; Kirshenbaum, L.A. Mitochondrial Autophagy and Cell Survival Is Regulated by the Circadian Clock Gene in Cardiac Myocytes during Ischemic Stress. Autophagy 2021, 17, 3794–3812. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the Circadian System in Shift Work and Its Health Impact. J. Biol. Rhythms 2022, 37, 3–28. [Google Scholar] [CrossRef]
- Bautista, J.; Ojeda-Mosquera, S.; Ordóñez-Lozada, D.; López-Cortés, A. Peripheral Clocks and Systemic Zeitgeber Interactions: From Molecular Mechanisms to Circadian Precision Medicine. Front. Endocrinol. 2025, 16, 1606242. [Google Scholar] [CrossRef]
- Thakur, A.; Kishore, R. Neurobiology of the Circadian Clock and Its Role in Cardiovascular Disease: Mechanisms, Biomarkers, and Chronotherapy. Neurobiol. Sleep Circadian Rhythm. 2025, 19, 100131. [Google Scholar] [CrossRef]
- Ziegler, K.A.; Engelhardt, S.; Carnevale, D.; McAlpine, C.S.; Guzik, T.J.; Dimmeler, S.; Swirski, F.K. Neural Mechanisms in Cardiovascular Health and Disease. Circ. Res. 2025, 136, 1233–1261. [Google Scholar] [CrossRef]
- Crowthers, R.; Thi Mong Nguyen, T.; Martinez, D. Circadian Disruptions and Their Role in the Development of Hypertension. Front. Neurosci. 2024, 18, 1433512. [Google Scholar] [CrossRef]
- Takeda, N.; Maemura, K. The Role of Clock Genes and Circadian Rhythm in the Development of Cardiovascular Diseases. Cell. Mol. Life Sci. 2015, 72, 3225–3234. [Google Scholar] [CrossRef]
- Webb, A.J.S.; Klerman, E.B.; Mandeville, E.T. Circadian and Diurnal Regulation of Cerebral Blood Flow. Circ. Res. 2024, 134, 695–710. [Google Scholar] [CrossRef]
- Filippone, E.J.; Foy, A.J.; Naccarelli, G.V. Controversies in Hypertension III: Dipping, Nocturnal Hypertension, and the Morning Surge. Am. J. Med. 2023, 136, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.W.; Gillette, M.U. Development of Circadian Neurovascular Function and Its Implications. Front. Neurosci. 2023, 17, 1196606. [Google Scholar] [CrossRef] [PubMed]
- Costello, H.M.; Gumz, M.L. Circadian Rhythm, Clock Genes, and Hypertension: Recent Advances in Hypertension. Hypertension 2021, 78, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Douma, L.G.; Gumz, M.L. Circadian Clock-Mediated Regulation of Blood Pressure. Free Radic. Biol. Med. 2018, 119, 108–114. [Google Scholar] [CrossRef]
- Kanki, M.; Nath, A.P.; Xiang, R.; Yiallourou, S.; Fuller, P.J.; Cole, T.J.; Cánovas, R.; Young, M.J. Poor Sleep and Shift Work Associate with Increased Blood Pressure and Inflammation in UK Biobank Participants. Nat. Commun. 2023, 14, 7096. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, M.-C.; Litrownik, D.; Wayne, P.M.; Yeh, G.Y. Day–Night Patterns in Heart Rate Variability and Complexity: Differences with Age and Cardiopulmonary Disease. J. Clin. Sleep Med. 2023, 19, 873–882. [Google Scholar] [CrossRef]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lanni, C. Molecular Regulations of Circadian Rhythm and Implications for Physiology and Diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef]
- Chen, P.; Sattari, N.; Whitehurst, L.N.; Mednick, S.C. Age-related Losses in Cardiac Autonomic Activity during a Daytime Nap. Psychophysiology 2021, 58, e13701. [Google Scholar] [CrossRef]
- Bonnemeier, H. Chronobiology of Sympathetic Nervous Activity: The Same Old Sun Will Shine in the Morning. Heart Rhythm 2006, 3, 86–87. [Google Scholar] [CrossRef]
- Black, N.; D’Souza, A.; Wang, Y.; Piggins, H.; Dobrzynski, H.; Morris, G.; Boyett, M.R. Circadian Rhythm of Cardiac Electrophysiology, Arrhythmogenesis, and the Underlying Mechanisms. Heart Rhythm 2019, 16, 298–307. [Google Scholar] [CrossRef]
- Lin, J.; Kuang, H.; Jiang, J.; Zhou, H.; Peng, L.; Yan, X.; Kuang, J. Circadian Rhythms in Cardiovascular Function: Implications for Cardiac Diseases and Therapeutic Opportunities. Med. Sci. Monit. 2023, 29, e942215-1–e942215-12. [Google Scholar] [CrossRef]
- Csoma, B.; Bikov, A. The Role of the Circadian Rhythm in Dyslipidaemia and Vascular Inflammation Leading to Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 14145. [Google Scholar] [CrossRef]
- Sweileh, W.M. Analysis and Mapping of Global Research Publications on Shift Work (2012–2021). J. Occup. Med. Toxicol. 2022, 17, 22. [Google Scholar] [CrossRef]
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 2016, 315, 1726–1734. [Google Scholar] [CrossRef]
- Lunde, L.-K.; Skare, Ø.; Mamen, A.; Sirnes, P.A.; Aass, H.C.D.; Øvstebø, R.; Goffeng, E.; Matre, D.; Nielsen, P.; Heglum, H.S.A.; et al. Cardiovascular Health Effects of Shift Work with Long Working Hours and Night Shifts: Study Protocol for a Three-Year Prospective Follow-Up Study on Industrial Workers. Int. J. Environ. Res. Public Health 2020, 17, 589. [Google Scholar] [CrossRef] [PubMed]
- Otamas, A.; Grant, P.J.; Ajjan, R.A. Diabetes and Atherothrombosis: The Circadian Rhythm and Role of Melatonin in Vascular Protection. Diabetes Vasc. Dis. Res. 2020, 17, 147916412092058. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.Y.; Tanz, L.J.; Lawson, C.C.; Schernhammer, E.S.; Vetter, C.; Rich-Edwards, J.W. Night Shift Work and Cardiovascular Disease Biomarkers in Female Nurses. Am. J. Ind. Med. 2020, 63, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, A.; Mareschal, J.; Dibner, C.; Pralong, J.A.; Dorribo, V.; Perrig, S.; Genton, L.; Pichard, C.; Collet, T.-H. The Effects of Shift Work on Cardio-Metabolic Diseases and Eating Patterns. Nutrients 2021, 13, 4178. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian Rhythms and the Molecular Clock in Cardiovascular Biology and Disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Torquati, L.; Mielke, G.I.; Brown, W.J.; Burton, N.W.; Kolbe-Alexander, T.L. Shift Work and Poor Mental Health: A Meta-Analysis of Longitudinal Studies. Am. J. Public Health 2019, 109, e13–e20. [Google Scholar] [CrossRef]
- Alevroudis, I.; Petridou, M.; Sakkou, A.; Kotoulas, S.-C.; Matzolas, S.; Roumelis, P.; Stougianni, M.; Massa, E.; Mouloudi, E. “Restless Nights, Stressed Hearts”: The Link Between Sleep Disorders and Takotsubo Syndrome—A Comprehensive Review. Rev. Cardiovasc. Med. 2025, 26, 28244. [Google Scholar] [CrossRef] [PubMed]
- Gkrinia, E.M.M.; Belančić, A. The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review. Curr. Issues Mol. Biol. 2025, 47, 357. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, D.-B.; Yoon, D.-W.; Yoo, S.-L.; Kim, J. The Effect of Sleep Disruption on Cardiometabolic Health. Life 2025, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Greenlund, I.M.; Carter, J.R. Sympathetic Neural Responses to Sleep Disorders and Insufficiencies. Am. J. Physiol. Circ. Physiol. 2022, 322, H337–H349. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian Rhythms and Metabolic Syndrome. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef]
- Amano, H.; Fukuda, Y.; Yokoo, T.; Yamaoka, K. Interleukin-6 Level among Shift and Night Workers in Japan: Cross-Sectional Analysis of the J-HOPE Study. J. Atheroscler. Thromb. 2018, 25, 1206–1214. [Google Scholar] [CrossRef]
- Brown, D.L.; Feskanich, D.; Sanchez, B.N.; Rexrode, K.M.; Schernhammer, E.S.; Lisabeth, L.D. Rotating Night Shift Work and the Risk of Ischemic Stroke. Am. J. Epidemiol. 2009, 169, 1370–1377. [Google Scholar] [CrossRef]
- Härmä, M.; Gustavsson, P.; Kolstad, H.A. Shift Work and Cardiovascular Disease—Do the New Studies Add to Our Knowledge? Scand. J. Work Environ. Health 2018, 44, 225–228. [Google Scholar] [CrossRef]
- Wang, D.; Ruan, W.; Chen, Z.; Peng, Y.; Li, W. Shift Work and Risk of Cardiovascular Disease Morbidity and Mortality: A Dose–Response Meta-Analysis of Cohort Studies. Eur. J. Prev. Cardiol. 2018, 25, 1293–1302. [Google Scholar] [CrossRef]
- Jeon, B.M.; Kim, S.H.; Shin, S.H. Effectiveness of Sleep Interventions for Rotating Night Shift Workers: A Systematic Review and Meta-Analysis. Front. Public Health 2023, 11, 1187382. [Google Scholar] [CrossRef]
- Cho, Y.; Ryu, S.-H.; Lee, B.R.; Kim, K.H.; Lee, E.; Choi, J. Effects of Artificial Light at Night on Human Health: A Literature Review of Observational and Experimental Studies Applied to Exposure Assessment. Chronobiol. Int. 2015, 32, 1294–1310. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Poggiogalle, E.; Barrea, L.; Tarsitano, M.G.; Garifalos, F.; Liccardi, A.; Pugliese, G.; Savastano, S.; Colao, A.; Colao, A.; et al. Exposure to Artificial Light at Night: A Common Link for Obesity and Cancer? Eur. J. Cancer 2022, 173, 263–275. [Google Scholar] [CrossRef]
- Grubisic, M.; Haim, A.; Bhusal, P.; Dominoni, D.M.; Gabriel, K.M.A.; Jechow, A.; Kupprat, F.; Lerner, A.; Marchant, P.; Riley, W.; et al. Light Pollution, Circadian Photoreception, and Melatonin in Vertebrates. Sustainability 2019, 11, 6400. [Google Scholar] [CrossRef]
- Prabhat, A.; Sami, D.; Ehlman, A.; Stumpf, I.; Seward, T.; Su, W.; Gong, M.C.; Schroder, E.A.; Delisle, B.P. Dim Light at Night Unmasks Sex-Specific Differences in Circadian and Autonomic Regulation of Cardiovascular Physiology. Commun. Biol. 2024, 7, 1191. [Google Scholar] [CrossRef] [PubMed]
- Molcan, L.; Babarikova, K.; Cvikova, D.; Kincelova, N.; Kubincova, L.; Mauer Sutovska, H. Artificial Light at Night Suppresses the Day-Night Cardiovascular Variability: Evidence from Humans and Rats. Pflügers Arch. Eur. J. Physiol. 2024, 476, 295–306. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Huang, Y.; Yu, Y.; Li, J.; Huang, W.; Wan, Y.; Tao, F.; Sun, Y. Physical Activity Alleviates Negative Effects of Bedroom Light Pollution on Blood Pressure and Hypertension in Chinese Young Adults. Environ. Pollut. 2022, 313, 120117. [Google Scholar] [CrossRef]
- Speksnijder, E.M.; Bisschop, P.H.; Siegelaar, S.E.; Stenvers, D.J.; Kalsbeek, A. Circadian Desynchrony and Glucose Metabolism. J. Pineal Res. 2024, 76, e12956. [Google Scholar] [CrossRef]
- Mukherji, A.; Kobiita, A.; Chambon, P. Shifting the Feeding of Mice to the Rest Phase Creates Metabolic Alterations, Which, on Their Own, Shift the Peripheral Circadian Clocks by 12 Hours. Proc. Natl. Acad. Sci. USA 2015, 112, E6683–E6690. [Google Scholar] [CrossRef]
- Imai, S.; Saito, Y.; Kajiyama, S.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Fukui, M. Late-Night-Dinner Deteriorates Postprandial Glucose and Insulin Whereas Consuming Dinner Dividedly Ameliorates Them in Patients with Type 2 Diabetes: A Randomized Crossover Clinical Trial. Asia Pac. J. Clin. Nutr. 2020, 29, 68–76. [Google Scholar] [CrossRef]
- Ezpeleta, M.; Cienfuegos, S.; Lin, S.; Pavlou, V.; Gabel, K.; Tussing-Humphreys, L.; Varady, K.A. Time-Restricted Eating: Watching the Clock to Treat Obesity. Cell Metab. 2024, 36, 301–314. [Google Scholar] [CrossRef]
- O’Byrne, N.A.; Yuen, F.; Butt, W.Z.; Liu, P.Y. Sleep and Circadian Regulation of Cortisol: A Short Review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 178–186. [Google Scholar] [CrossRef]
- McEwen, B.S.; Karatsoreos, I.N. Sleep Deprivation and Circadian Disruption Stress, Allostasis, and Allostatic Load. Sleep Med. Clin. 2022, 17, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Hasenmajer, V.; Sbardella, E.; Sciarra, F.; Simeoli, C.; Pivonello, C.; Ceccato, F.; Pofi, R.; Minnetti, M.; Rizzo, F.; Ferrari, D.; et al. Circadian Clock Disruption Impairs Immune Oscillation in Chronic Endogenous Hypercortisolism: A Multi-Level Analysis from a Multicentre Clinical Trial. eBioMedicine 2024, 110, 105462. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F.A.J.L. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Chellappa, S.L.; Hu, K.; Shea, S.A. Impact of Mental Stress, the Circadian System and Their Interaction on Human Cardiovascular Function. Psychoneuroendocrinology 2019, 103, 125–129. [Google Scholar] [CrossRef]
- Burch, J.B.; Alexander, M.; Balte, P.; Sofge, J.; Winstead, J.; Kothandaraman, V.; Ginsberg, J.P. Shift Work and Heart Rate Variability Coherence: Pilot Study Among Nurses. Appl. Psychophysiol. Biofeedback 2019, 44, 21–30. [Google Scholar] [CrossRef]
- Logan, R.W.; McClung, C.A. Rhythms of Life: Circadian Disruption and Brain Disorders across the Lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef]
- Lecacheur, M.; Ammerlaan, D.J.M.; Dierickx, P. Circadian Rhythms in Cardiovascular (Dys)Function: Approaches for Future Therapeutics. npj Cardiovasc. Health 2024, 1, 21. [Google Scholar] [CrossRef]
- Vetter, C. Circadian Disruption: What Do We Actually Mean? Eur. J. Neurosci. 2020, 51, 531–550. [Google Scholar] [CrossRef]
- Xie, F.; Hu, K.; Fu, R.; Zhang, Y.; Xiao, K.; Tu, J. Association between Night Shift Work and the Risk of Type 2 Diabetes Mellitus: A Cohort-Based Meta-Analysis. BMC Endocr. Disord. 2024, 24, 268. [Google Scholar] [CrossRef]
- Yang, L.; Luo, Y.; He, L.; Yin, J.; Li, T.; Liu, S.; Li, D.; Cheng, X.; Bai, Y. Shift Work and the Risk of Cardiometabolic Multimorbidity Among Patients with Hypertension: A Prospective Cohort Study of UK Biobank. J. Am. Heart Assoc. 2022, 11, e025936. [Google Scholar] [CrossRef]
- Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Dakanalis, A.; Vasios, G.K.; Voulgaridou, G.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. Evaluating the Relationship between Circadian Rhythms and Sleep, Metabolic and Cardiovascular Disorders: Current Clinical Evidence in Human Studies. Metabolites 2023, 13, 370. [Google Scholar] [CrossRef]
- Chaput, J.-P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P. The Role of Insufficient Sleep and Circadian Misalignment in Obesity. Nat. Rev. Endocrinol. 2023, 19, 82–97. [Google Scholar] [CrossRef]
- Hepler, C.; Bass, J. Circadian Mechanisms in Adipose Tissue Bioenergetics and Plasticity. Genes Dev. 2023, 37, 454–473. [Google Scholar] [CrossRef]
- Christie, S.; Vincent, A.D.; Li, H.; Frisby, C.L.; Kentish, S.J.; O’Rielly, R.; Wittert, G.A.; Page, A.J. A Rotating Light Cycle Promotes Weight Gain and Hepatic Lipid Storage in Mice. Am. J. Physiol. Liver Physiol. 2018, 315, G932–G942. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.D.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health. Adv. Nutr. 2019, 10, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, M.; Luo, H. Regulation of Metabolism by Circadian Rhythms: Support from Time-Restricted Eating, Intestinal Microbiota & Omics Analysis. Life Sci. 2024, 351, 122814. [Google Scholar] [CrossRef]
- Villanueva, J.E.; Livelo, C.; Trujillo, A.S.; Chandran, S.; Woodworth, B.; Andrade, L.; Le, H.D.; Manor, U.; Panda, S.; Melkani, G.C. Time-Restricted Feeding Restores Muscle Function in Drosophila Models of Obesity and Circadian-Rhythm Disruption. Nat. Commun. 2019, 10, 2700. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Samanta, S.; Ali, S.A. Impact of Circadian Clock Dysfunction on Human Health. Explor. Neurosci. 2022, 1, 4–30. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Pfeffer, M.; von Gall, C.; Wicht, H.; Korf, H.-W. The Role of the Melatoninergic System in Circadian and Seasonal Rhythms—Insights From Different Mouse Strains. Front. Physiol. 2022, 13, 883637. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.; Arguelles-Prieto, R.; Martinez-Madrid, M.; Reiter, R.; Hardeland, R.; Rol, M.; Madrid, J. Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure. Int. J. Mol. Sci. 2014, 15, 23448–23500. [Google Scholar] [CrossRef] [PubMed]
- Aykan, U.; Güvel, M.C.; Paykal, G.; Uluoglu, C. Neuropharmacologic Modulation of the Melatonergic System. Explor. Neurosci. 2023, 2, 287–306. [Google Scholar] [CrossRef]
- Haim, A.; Zubidat, A.E. Artificial Light at Night: Melatonin as a Mediator between the Environment and Epigenome. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140121. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the Theories of Aging: A Critical Appraisal of Melatonin’s Role in Antiaging Mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef]
- Alam, M.; Abbas, K.; Sharf, Y.; Khan, S. Impacts of Blue Light Exposure From Electronic Devices on Circadian Rhythm and Sleep Disruption in Adolescent and Young Adult Students. Chronobiol. Med. 2024, 6, 10–14. [Google Scholar] [CrossRef]
- Hacışevki, A.; Baba, B. An Overview of Melatonin as an Antioxidant Molecule: A Biochemical Approach. In Melatonin—Molecular Biology, Clinical and Pharmaceutical Approaches; IntechOpen: London, UK, 2018. [Google Scholar]
- Monteiro, K.K.A.C.; Shiroma, M.E.; Damous, L.L.; Simões, M.d.J.; Simões, R.d.S.; Cipolla-Neto, J.; Baracat, E.C.; Soares, J.M., Jr. Antioxidant Actions of Melatonin: A Systematic Review of Animal Studies. Antioxidants 2024, 13, 439. [Google Scholar] [CrossRef]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.-M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef]
- Jang, S.K.; Choi, J.; Lim, H.W.; Kim, H.-G.; Yoo, Y.-M. Comparative Analysis of Melatonin and Polydeoxyribonucleotide: Possible Benefits of Co-Treatment Effects and Potential Synergistic Applicability. Int. J. Mol. Sci. 2025, 26, 5703. [Google Scholar] [CrossRef]
- Pechanova, O.; Paulis, L.; Simko, F. Peripheral and Central Effects of Melatonin on Blood Pressure Regulation. Int. J. Mol. Sci. 2014, 15, 17920–17937. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell. Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef]
- Cook, J.S.; Sauder, C.L.; Ray, C.A. Melatonin Differentially Affects Vascular Blood Flow in Humans. Am. J. Physiol. Circ. Physiol. 2011, 300, H670–H674. [Google Scholar] [CrossRef]
- Masana, M.I.; Doolen, S.; Ersahin, C.; Al-Ghoul, W.M.; Duckles, S.P.; Dubocovich, M.L.; Krause, D.N. MT2 Melatonin Receptors Are Present and Functional in Rat Caudal Artery. J. Pharmacol. Exp. Ther. 2002, 302, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E. The Cardiac Circadian Clock. JACC Basic Transl. Sci. 2023, 8, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- Lempiäinen, P.A.; Ylitalo, A.; Huikuri, H.; Kesäniemi, Y.A.; Ukkola, O.H. Non-Dipping Blood Pressure Pattern Is Associated with Cardiovascular Events in a 21-Year Follow-up Study. J. Hum. Hypertens. 2024, 38, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Kario, K. Nocturnal Blood Pressure and Cardiovascular Disease: A Review of Recent Advances. Hypertens. Res. 2012, 35, 695–701. [Google Scholar] [CrossRef]
- Karolczak, K.; Watala, C. The Mystery behind the Pineal Gland: Melatonin Affects the Metabolism of Cholesterol. Oxidative Med. Cell. Longev. 2019, 2019, 4531865. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Romero, A.; Simko, F.; Dominguez-Rodriguez, A.; Cardinali, D.P. Melatonin Stabilizes Atherosclerotic Plaques: An Association That Should Be Clinically Exploited. Front. Med. 2024, 11, 1487971. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Wang, Z.; Gan, J.; Yu, B.; Lu, B.; Jiang, X. Melatonin as a Therapeutic Agent for Alleviating Endothelial Dysfunction in Cardiovascular Diseases: Emphasis on Oxidative Stress. Biomed. Pharmacother. 2023, 167, 115475. [Google Scholar] [CrossRef]
- Mauer Sutovska, H.; Molcan, L.; Stefanik, P.; Zeman, M. Dark-Phase Melatonin Administration Does Not Reduce Blood Pressure but Induces Changes in Parameters Related to the Control of the Cardiovascular System in Spontaneously Hypertensive Rats. Hypertens. Res. 2025, 48, 2218–2233. [Google Scholar] [CrossRef]
- Festus, I.D.; Spilberg, J.; Young, M.E.; Cain, S.; Khoshnevis, S.; Smolensky, M.H.; Zaheer, F.; Descalzi, G.; Martino, T.A. Pioneering New Frontiers in Circadian Medicine Chronotherapies for Cardiovascular Health. Trends Endocrinol. Metab. 2024, 35, 607–623. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Gu, Y.; Huang, S. Circadian Clocks and Their Role in Kidney and Eye Diseases across Organ Systems. Front. Physiol. 2025, 16, 1583502. [Google Scholar] [CrossRef]
- Lee, E.K.; Poon, P.; Yu, C.; Lee, V.W.; Chung, V.C.; Wong, S.Y. Controlled-release Oral Melatonin Supplementation for Hypertension and Nocturnal Hypertension: A Systematic Review and Meta-analysis. J. Clin. Hypertens. 2022, 24, 529–535. [Google Scholar] [CrossRef]
- Grossman, E.; Laudon, M.; Zisapel, N. Effect of Melatonin on Nocturnal Blood Pressure: Meta-Analysis of Randomized Controlled Trials. Vasc. Health Risk Manag. 2011, 7, 577. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Van Montfrans, G.A.; van Someren, E.J.W.; Mairuhu, G.; Buijs, R.M. Daily Nighttime Melatonin Reduces Blood Pressure in Male Patients with Essential Hypertension. Hypertension 2004, 43, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, X.; Zhang, R.; Ma, B.; Niu, S.; Di, X.; Ni, L.; Liu, C. Melatonin Attenuates Smoking-Induced Atherosclerosis by Activating the Nrf2 Pathway via NLRP3 Inflammasomes in Endothelial Cells. Aging 2021, 13, 11363–11380. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, H.-T.; Lee, D.-C. Melatonin Supplementation for Six Weeks Had No Effect on Arterial Stiffness and Mitochondrial DNA in Women Aged 55 Years and Older with Insomnia: A Double-Blind Randomized Controlled Study. Int. J. Environ. Res. Public Health 2021, 18, 2561. [Google Scholar] [CrossRef]
- Rahbari-Oskoui, F.F.; Abramson, J.L.; Bruckman, A.M.; Chapman, A.B.; Cotsonis, G.A.; Johnson, S.A.; Bliwise, D.L. Nighttime Administration of High-Dose, Sustained-Release Melatonin Does Not Decrease Nocturnal Blood Pressure in African-American Patients: Results from a Preliminary Randomized, Crossover Trial. Complement. Ther. Med. 2019, 43, 157–164. [Google Scholar] [CrossRef]
- Baltatu, O.C.; Senar, S.; Campos, L.A.; Cipolla-Neto, J. Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use. Int. J. Mol. Sci. 2019, 20, 4342. [Google Scholar] [CrossRef]
- Besag, F.M.C.; Vasey, M.J.; Lao, K.S.J.; Wong, I.C.K. Adverse Events Associated with Melatonin for the Treatment of Primary or Secondary Sleep Disorders: A Systematic Review. CNS Drugs 2019, 33, 1167–1186. [Google Scholar] [CrossRef]
- Belloir, J.; Makarem, N.; Shechter, A. Sleep and Circadian Disturbance in Cardiovascular Risk. Curr. Cardiol. Rep. 2022, 24, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Caughey, M.C.; Tanner, R.M.; Booth, J.N.; Diaz, K.M.; Anstey, D.E.; Sims, M.; Ravenell, J.; Muntner, P.; Viera, A.J.; et al. Associations of Blood Pressure Dipping Patterns with Left Ventricular Mass and Left Ventricular Hypertrophy in Blacks: The Jackson Heart Study. J. Am. Heart Assoc. 2017, 6, e004847. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.H.; Messerli, F.H.; Skolnick, A.H.; Newman, J.D.; Berger, J.S.; Bangalore, S. Timing of Antihypertensive Drug Therapy: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Hypertension 2023, 80, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Marhefkova, N.; Sládek, M.; Sumová, A.; Dubsky, M. Circadian Dysfunction and Cardio-Metabolic Disorders in Humans. Front. Endocrinol. 2024, 15, 1328139. [Google Scholar] [CrossRef]
- Chen, Y.; Geng, T.; Xu, X.; Zhang, Z.; Huang, L.; Dong, H.; Yu, H.; Gao, X.; Sun, L. Misalignment Between Circadian Preference and Accelerometer-Derived Sleep-Wake Cycle with Increased Risk of Cardiometabolic Diseases. JACC Adv. 2024, 3, 101406. [Google Scholar] [CrossRef]
- Yeom, J.W.; Park, S.; Lee, H.-J. Managing Circadian Rhythms: A Key to Enhancing Mental Health in College Students. Psychiatry Investig. 2024, 21, 1309–1317. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Geiger-Brown, J.; Scharf, S.M.; Drake, C.L. Shift Work and Shift Work Sleep Disorder. Chest 2017, 151, 1156–1172. [Google Scholar] [CrossRef]
- Lim, D.C.; Najafi, A.; Afifi, L.; Bassetti, C.L.; Buysse, D.J.; Han, F.; Högl, B.; Melaku, Y.A.; Morin, C.M.; Pack, A.I.; et al. The Need to Promote Sleep Health in Public Health Agendas across the Globe. Lancet Public Health 2023, 8, e820–e826. [Google Scholar] [CrossRef]
- Cajochen, C. Thirty-Fifth Annual Meeting of the Society for Light Treatment and Biological Rhythms (SLTBR), 20 June–22 June, Prague, Czech Republic. Clocks Sleep 2024, 6, 690–748. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Fernández, J.R. Influence of Circadian Time of Hypertension Treatment on Cardiovascular Risk: Results of the Mapec Study. Chronobiol. Int. 2010, 27, 1629–1651. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Crespo, J.J.; Domínguez-Sardiña, M.; Otero, A.; Moyá, A.; Ríos, M.T.; Sineiro, E.; Castiñeira, M.C.; Callejas, P.A.; Pousa, L.; et al. Bedtime Hypertension Treatment Improves Cardiovascular Risk Reduction: The Hygia Chronotherapy Trial. Eur. Heart J. 2020, 41, 4565–4576. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Hermida, R.C.; Portaluppi, F. Circadian Mechanisms of 24-Hour Blood Pressure Regulation and Patterning. Sleep Med. Rev. 2017, 33, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Kim, J. Melatonin and Metabolic Disorders: Unraveling the Interplay with Glucose and Lipid Metabolism, Adipose Tissue, and Inflammation. Sleep Med. Res. 2024, 15, 70–80. [Google Scholar] [CrossRef]
- Baranowska, A.; Matyka, K.; Matyszewska, A.; Słowiaczek, A.; Sutkowski, J.; Wilczewska, E.; Wojnar, M. Melatonin—The New Multipotential Drug of the Future? J. Educ. Health Sport 2023, 13, 330–338. [Google Scholar] [CrossRef]
- Pelczyńska, M.; Moszak, M.; Wojciechowska, J.; Płócienniczak, A.; Potocki, J.; Blok, J.; Balcerzak, J.; Zblewski, M.; Bogdański, P. The Role of the Chronotype in Developing an Excessive Body Weight and Its Complications—A Narrative Review. Nutrients 2024, 17, 80. [Google Scholar] [CrossRef]
- Rahman, S.A. Are We Ready to Assess Circadian Phase at Home? Sleep 2015, 38, 849–850. [Google Scholar] [CrossRef][Green Version]
- Mahdi, A.; Watkinson, P.; McManus, R.J.; Tarassenko, L. Circadian Blood Pressure Variations Computed From 1.7 Million Measurements in an Acute Hospital Setting. Am. J. Hypertens. 2019, 32, 1154–1161. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jo, S.-H. Nighttime Administration of Antihypertensive Medication: A Review of Chronotherapy in Hypertension. Korean J. Intern. Med. 2024, 39, 205–214. [Google Scholar] [CrossRef]
- Gubin, D.; Weinert, D.; Stefani, O.; Otsuka, K.; Borisenkov, M.; Cornelissen, G. Wearables in Chronomedicine and Interpretation of Circadian Health. Diagnostics 2025, 15, 327. [Google Scholar] [CrossRef]
- Castaldo, R.; Chappell, M.J.; Byrne, H.; Innominato, P.F.; Hughes, S.; Pescapè, A.; Pecchia, L. Detection of Melatonin-Onset in Real Settings via Wearable Sensors and Artificial Intelligence. A Pilot Study. Biomed. Signal Process. Control 2021, 65, 102386. [Google Scholar] [CrossRef]
- Kervezee, L.; Dashti, H.S.; Pilz, L.K.; Skarke, C.; Ruben, M.D. Using Routinely Collected Clinical Data for Circadian Medicine: A Review of Opportunities and Challenges. PLoS Digit. Health 2024, 3, e0000511. [Google Scholar] [CrossRef]

| Mechanism of Action | Main Molecular Targets/Signaling Pathways | Physiological/Clinical Effect | Type of Evidence | References |
|---|---|---|---|---|
| Antioxidant effect | ROS, hydroxyl radicals, peroxynitrite, SOD, GPx, catalase | Reduction of oxidative stress, endothelial protection, prevention of oxidative vascular damage | Preclinical/Clinical | [98,99] |
| Anti-inflammatory action | NF-κB, pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) | Inflammation reduction, atherosclerosis progression delay, plaque stabilization | Preclinical/Clinical | [100,101] |
| Immunomodulation | Immune cells (macrophages, T lymphocytes) | Maintenance of immune homeostasis, attenuation of low-grade chronic inflammation | Preclinical | [12] |
| Autonomic regulation | Sympathetic nervous system, central nervous system | Blood pressure reduction, vascular resistance decrease, sympathetic activity modulation | Preclinical/Clinical | [102] |
| Receptor-mediated vasodilation | MT1 and MT2 receptors, nitric oxide (NO), ROS | Vasodilation, improved blood flow, enhanced NO bioavailability | Preclinical | [103] |
| Endothelial protection | Anti-inflammatory and antioxidant signaling, endothelial glycocalyx | Improved vascular elasticity, reduced remodeling, enhanced baroreflex function | Preclinical/Clinical | [103] |
| Circadian resynchronization | Circadian blood pressure rhythm, clock gene activity | Restoration of nocturnal blood pressure dip, improved chronobiological regulation in hypertensive patients | Clinical | [106] |
| Regulation of circadian rhythm and HRV | CLOCK, BMAL1, adrenergic receptors, catecholamine rhythmicity, cardiac autonomic regulation | Stabilization of heart rate and blood pressure rhythms, improved HRV, reduced sympathetic overactivation risk | Preclinical/Clinical | [12,28,107,108] |
| Anti-atherosclerotic effect | LDL oxidation, foam cell formation, NF-κB pathway, adhesion molecules, circadian-timed administration | Inhibition of atherosclerosis progression, plaque stabilization, endothelial protection, enhanced effect with nighttime dosing | Preclinical/Clinical | [109,110,111,112] |
| Chronotherapeutic effect | Circadian timing, CLOCK/BMAL1, | Enhanced antihypertensive and endothelial effects when administered at night | Clinical | [111,112,113] |
| Circadian Disruptor | Mechanism of Rhythm Disruption | Physiological Effects | CVD Risk Evidence (Literature-Based) | Evidence-Based Interventions | References |
|---|---|---|---|---|---|
| Shift Work | SCN-peripheral clock desynchronization; disrupted sleep-wake cycles; chronic behavioural misalignment | ↑ sympathetic activity; ↓ HRV; ↑ nocturnal BP; ↑ IL-6, CRP; insulin resistance; Sleep fragmentation | 5–7% ↑ CVD risk per 5 years exposure; 4% ↑ ischemic stroke risk; ↑ CHD in rotating night shifts; dose-response relationship with duration | Optimized shift rotations; limited extended night shifts; bright light during work; sleep hygiene protocols; melatonin administration timing | [12,44,47,48,57,58,59,60,115,129] |
| Artificial Light at Night (ALAN) | Retinohypothalamic tract stimulation; melatonin suppression via SCN inhibition; phase shifts in circadian timing | Loss of nocturnal BP dipping; ↑ resting BP and HR; ↓ parasympathetic recovery; Disrupted glucose tolerance; ↑ cortisol | Attenuated dipping patterns; ↑ cardiovascular morbidity independent of sleep quality; ↑ hypertension risk from bedroom light exposure | Blue light filters; screen-free periods before bedtime; dim red lighting; urban lighting policies; circadian lighting design | [61,64,65,66,93,97,128,131] |
| Mistimed Eating/Late-Night Feeding | Peripheral clock desynchronization; uncoupling from SCN; disrupted metabolic gene expression; feeding-induced phase shifts | ↑ postprandial glucose; ↑ BP; metabolic inflexibility; ↑ visceral adiposity; altered lipid profiles; insulin resistance | ↑ Obesity and CAD risk; metabolic syndrome development; ↑ CVD events in late chronotypes | Time-restricted eating (8–12 h window); consistent meal timing; avoiding eating 3 h before sleep; alignment of feeding with active phase | [67,68,69,70,85,86,87,88,89] |
| Chronic Psychosocial Stress | HPA axis activation; disrupted cortisol rhythm; glucocorticoid receptor dysregulation; SCN-stress system interactions | Sustained hypercortisolemia; ↑ allostatic load; ↓ HRV; ↑ inflammatory markers; Autonomic imbalance; impaired sleep | ↑ CVD vulnerability; time-dependent stress responses; amplified morning cardiovascular events; ↑ atherosclerosis progression | Stress management techniques; cortisol rhythm restoration; circadian-timed stress reduction | [71,72,73,74,75,76,130] |
| Sleep Disruption/Irregular Sleep | Fragmented sleep architecture; reduced sleep efficiency; circadian phase instability; SCN input disruption | ↑ sympathetic tone; ↓ parasympathetic activity; ↑ inflammatory cytokines; Glucose intolerance; ↑ BP variability | Strong predictor of CVD events; ↑ hypertension; ↑ stroke risk; metabolic dysfunction; accelerated atherosclerosis | Sleep hygiene optimization; consistent sleep-wake timing; sleep environment control; cognitive behavioral therapy for insomnia | [51,53,54,60,130] |
| Age-Related Circadian Decline | Pineal gland deterioration; SCN neuronal loss; ↓ photic responsiveness; blunted rhythm amplitude | Progressive melatonin decline; flattened hormonal rhythms; ↓ sleep quality; ↑ fragmentation; metabolic dysregulation | ↑ CVD risk in elderly; accelerated vascular aging; ↑ hypertension prevalence; metabolic syndrome | Melatonin replacement therapy; bright light therapy; activity scheduling; chronotherapy protocols | [44,51,65,73,85,86,93,96,125,131] |
| Urban Light Pollution | Chronic low-level ALAN exposure; disrupted darkness signal; ecological circadian disruption | Suppressed melatonin synthesis; altered sleep patterns; metabolic rhythm disruption; immune dysfunction | Population-level CVD risk increase; environmental circadian disruption; metabolic health impacts | Urban lighting regulations; shielded lighting; reduced brightness standards; circadian-conscious city planning | [63,128,131] |
| Electronic Device Usage | Blue light emission (450 nm); evening circadian phase delays; melatonin suppression; sleep onset disruption | Delayed sleep phase; ↓ sleep quality; ↑ evening alertness; Disrupted morning cortisol rhythm | Association with metabolic dysfunction; ↑ obesity risk; cardiovascular risk markers | Screen time limits; blue light blocking; device-free bedrooms; evening usage restrictions | [93,97,128,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuszkiewicz, J.; Rzepka, W.; Markiel, J.; Porzych, M.; Woźniak, A.; Szewczyk-Golec, K. Circadian Rhythm Disruptions and Cardiovascular Disease Risk: The Special Role of Melatonin. Curr. Issues Mol. Biol. 2025, 47, 664. https://doi.org/10.3390/cimb47080664
Nuszkiewicz J, Rzepka W, Markiel J, Porzych M, Woźniak A, Szewczyk-Golec K. Circadian Rhythm Disruptions and Cardiovascular Disease Risk: The Special Role of Melatonin. Current Issues in Molecular Biology. 2025; 47(8):664. https://doi.org/10.3390/cimb47080664
Chicago/Turabian StyleNuszkiewicz, Jarosław, Wojciech Rzepka, Julia Markiel, Marta Porzych, Alina Woźniak, and Karolina Szewczyk-Golec. 2025. "Circadian Rhythm Disruptions and Cardiovascular Disease Risk: The Special Role of Melatonin" Current Issues in Molecular Biology 47, no. 8: 664. https://doi.org/10.3390/cimb47080664
APA StyleNuszkiewicz, J., Rzepka, W., Markiel, J., Porzych, M., Woźniak, A., & Szewczyk-Golec, K. (2025). Circadian Rhythm Disruptions and Cardiovascular Disease Risk: The Special Role of Melatonin. Current Issues in Molecular Biology, 47(8), 664. https://doi.org/10.3390/cimb47080664







