Extract of Allium Chinense G. Don, a Medicinal Plant, Ameliorates Myocardial Ischemia–Reperfusion Injury by Inhibiting Platelet Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Preparation
2.2. UHPLC-Q-Orbitrap-MS/MS Analysis
2.3. Animals and Treatment
2.4. Platelet Aggregation Rate
2.5. Histology
2.6. Echocardiography
2.7. Serum Myocardial Enzyme
2.8. Flow Cytometry
2.9. ELISA
2.10. Transcriptome Sequencing
2.11. Molecular Docking
2.12. ATP and Lactate
2.13. Western Blot
2.14. Statistical Analysis
3. Results
3.1. Screening and Chemical Characterization by UHPLC-Q-Orbitrap-MS/MS of Anti-Platelet Active Fractions of Ethanol Extract of Allium Chinense
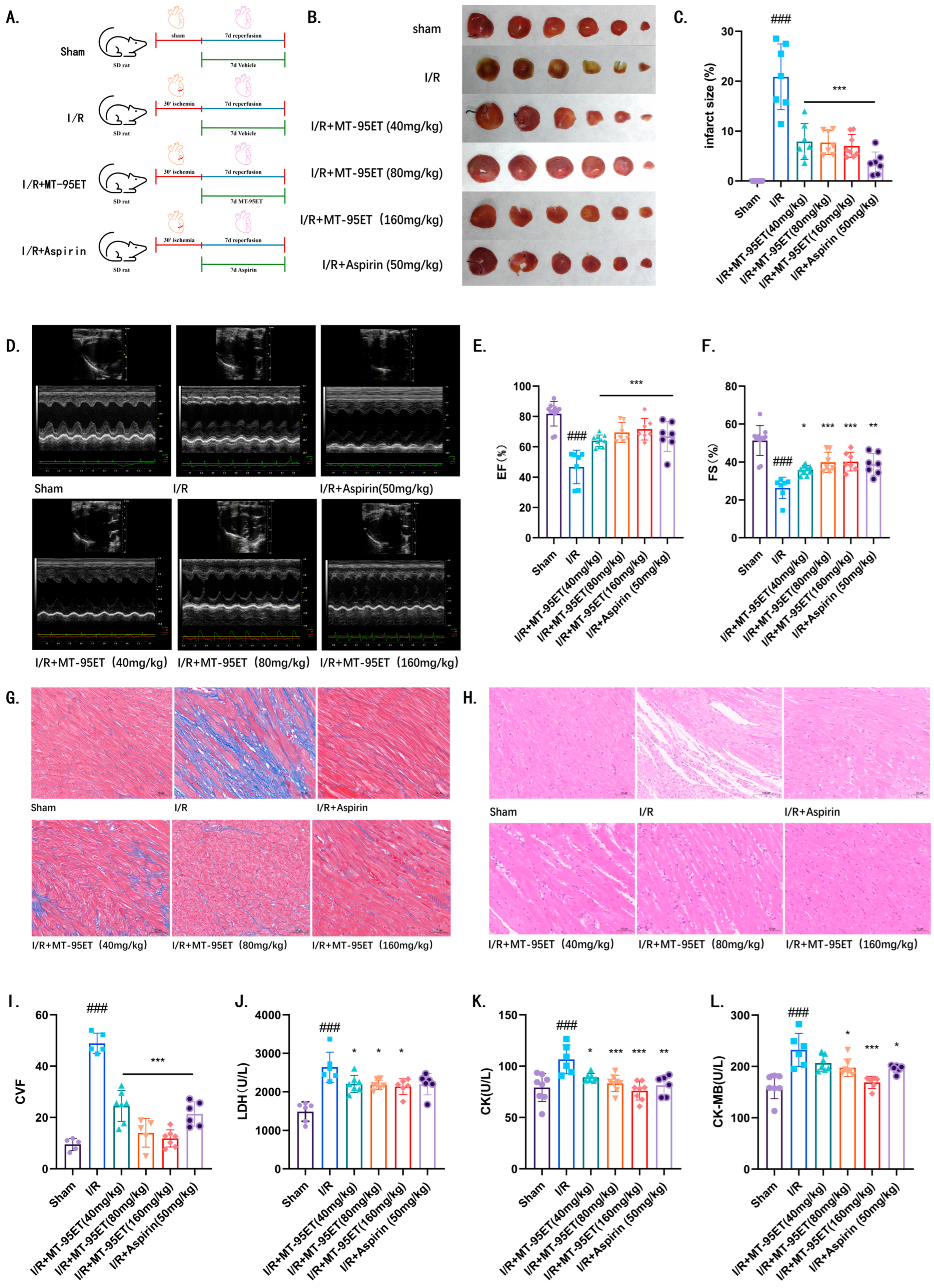
3.2. MT-95ET Alleviates Myocardial Injury and Improves Cardiac Function in I/R SD Rats
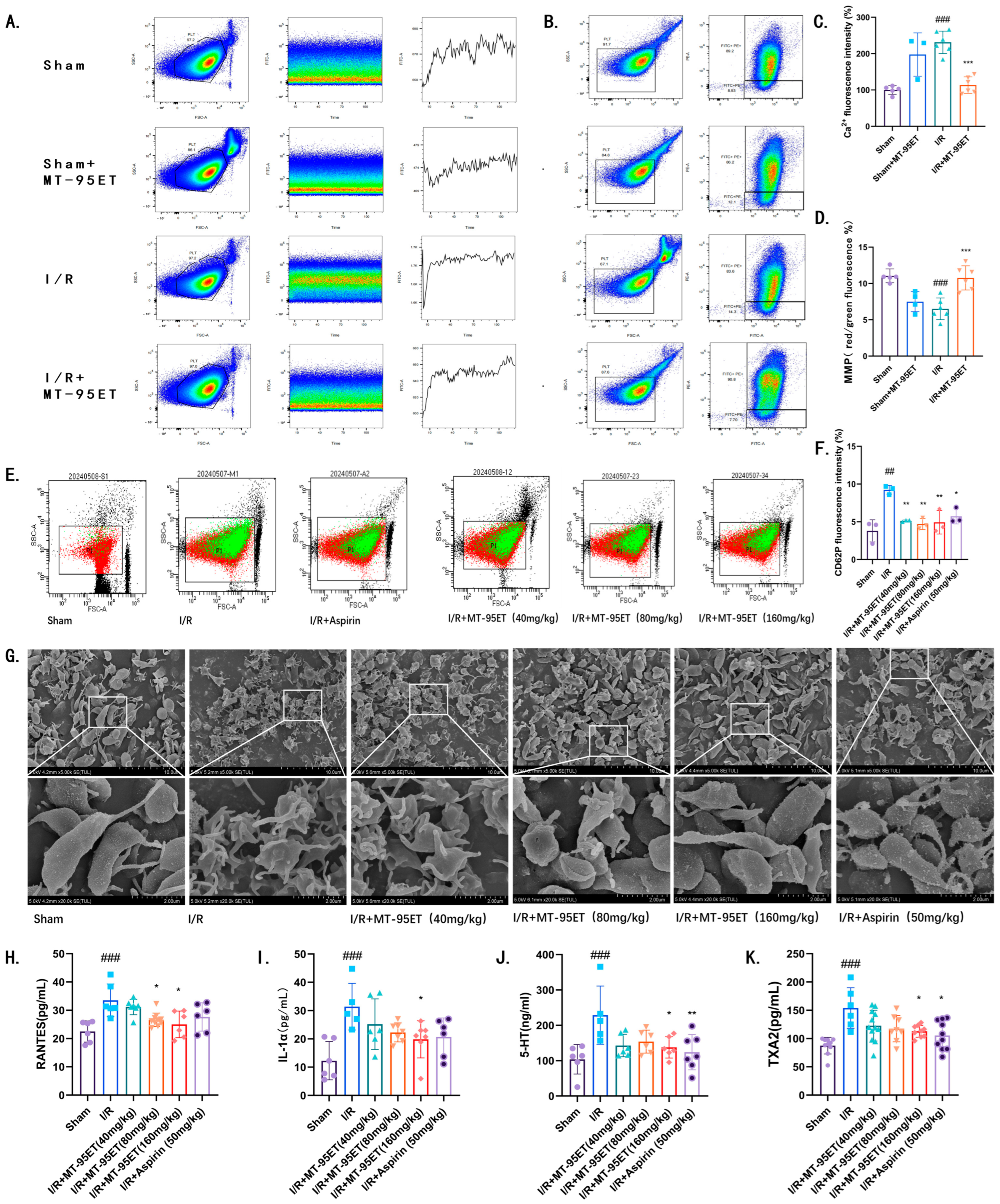
3.3. MT-95ET Plays a Role in Inhibiting I/R-Induced Platelet Activation in Vivo
3.4. MT-95ET Regulates I/R-Induced Platelet Function in Vivo
3.5. Analysis of Transcriptome Sequencing Results
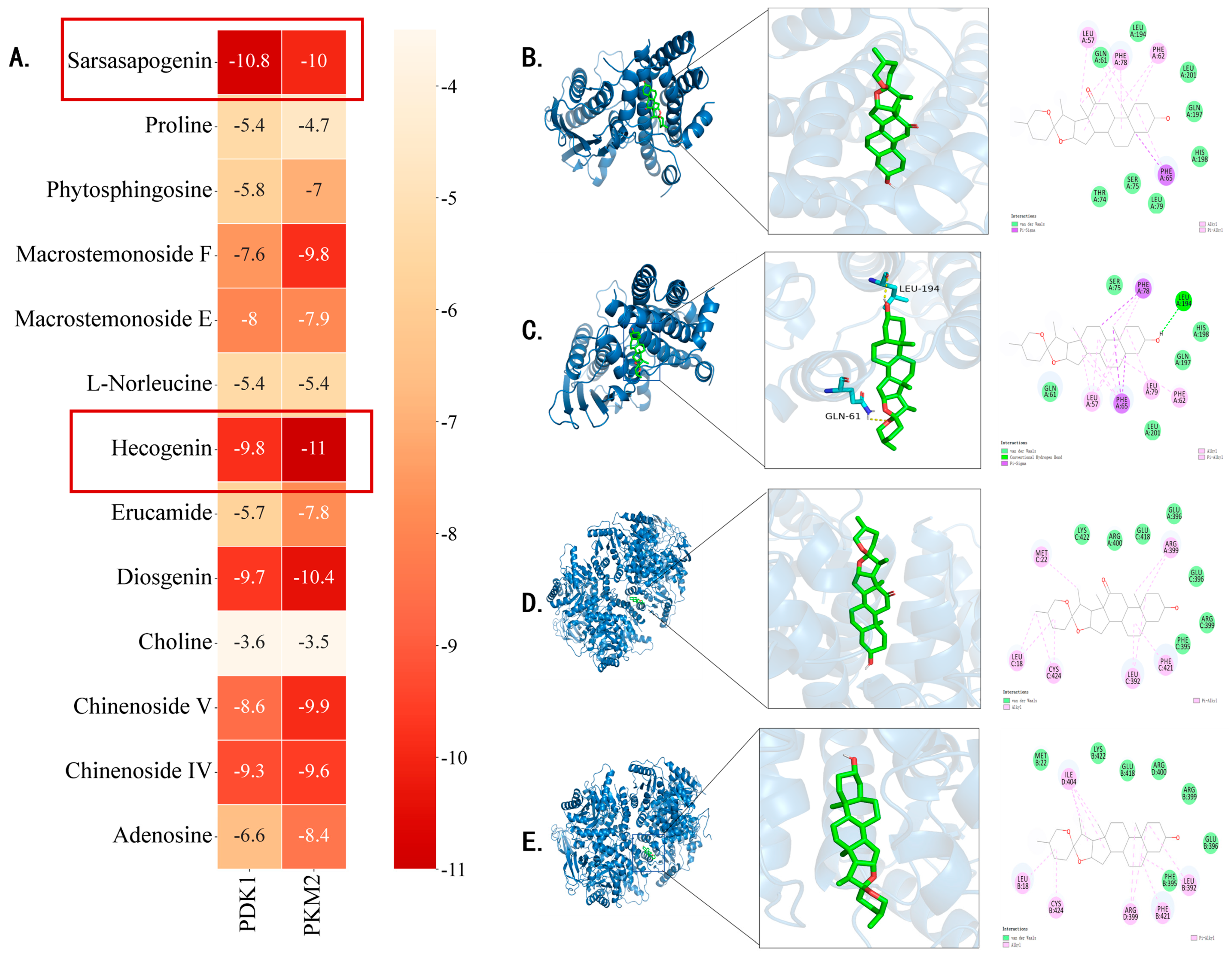
3.6. Analysis of Molecular Docking Results
3.7. MT-95ET Inhibits the Aerobic Glycolysis in Activated Platelets by Regulating the Expression of PKM2 Dimer, and the Phosphorylation of PDK1, PI3K, and GSK3β
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| I/R | ischemia–reperfusion |
| ADP | adenosine diphosphate |
| CAD | coronary artery disease |
| CK | creatine kinase |
| CK-MB | creatine kinase isoenzyme |
| CCL5/RANTES | chemokine ligand 5 |
| EF% | left ventricular ejection fraction |
| ELISA | enzyme-linked immunosorbent assay |
| FS% | left ventricular short-axis shortening rate |
| GO | gene ontology |
| GSK3β | glycogen synthase kinase-3β |
| HE | Hematoxylin–eosin |
| IL-1α | interleukin-1α |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LDH | lactic dehydrogenase |
| MS | mass spectrometry |
| MS1 | primary mass spectrum |
| MS2 | secondary mass spectrum |
| OXPHOS | oxidative phosphorylation |
| PDH | pyruvate dehydrogenase |
| PDK1 | inositol 3-phosphate dependent protein kinase 1 |
| PEP | phosphoenolpyruvate |
| PI3K | phosphatidylinositol 3-kinase |
| PKM2 | pyruvate kinase isozyme typeM2 |
| PPCI | percutaneous coronary intervention |
| SD | Sprague Dawley |
| TCM | traditional Chinese medicine |
| TIC | total ion current chromatograms |
| TXA2 | thromboxane A2 |
| UHPLC-Q-Orbitrap | ultra-high-performance liquid chromatography–quadrupole orbitrap |
| UPLC | ultra-high performance liquid chromatography |
| WB | Western Blot |
References
- Weng, J.; Xiong, S.; Jiang, S.; Zheng, F.; Lin, C.; Zhan, L. Myocardial protective effect and mechanism of pretreatment with Gualou Xiebai Banxia Decoction on rats with myocardial ischemia-reperfusion injury model. Pract. J. Card. Cereb. Pneumal Vasc. Dis. 2023, 31, 43–48+53. [Google Scholar]
- Li, X.; Zhang, H.; Cui, H.; Sui, Y.; Li, Y.; Tang, D. Study on the effects of Gualou Xiebai Banxia Decoction on autophagy and PINK1/Parkin pathway in rats with myocardial ischemia-reperfusion injury. Chin. J. Basic Med. Tradit. Chin. Med. 2020, 26, 1626–1630. [Google Scholar]
- Shi, Y.; Yang, G. Mechanism of PI3K/Akt pathway in myocardium of rats with ischemia-reperfusion protected by pretreatment with Gualou Xiebai Banxia Decoction. Chin. J. Basic Med. Tradit. Chin. Med. 2016, 22, 906–908+921. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Dauerman, H.L.; Ibanez, B. The Edge of Time in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1871–1874. [Google Scholar] [CrossRef]
- Turer, A.T.; Hill, J.A. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am. J. Cardiol. 2010, 106, 360–368. [Google Scholar] [CrossRef]
- Ziegler, M.; Hohmann, J.D.; Searle, A.K.; Abraham, M.K.; Nandurkar, H.H.; Wang, X.; Peter, K. A single-chain antibody-CD39 fusion protein targeting activated platelets protects from cardiac ischaemia/reperfusion injury. Eur. Heart J. 2018, 39, 111–116. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Tian, R.; Zuo, W.; Qian, H.; Wang, L.; Yang, X.; Liu, Z.; Zhang, S. What do we know about platelets in myocardial ischemia-reperfusion injury and why is it important? Thromb. Res. 2023, 229, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Wang, X.; Peter, K. Platelets in cardiac ischaemia/reperfusion injury: A promising therapeutic target. Cardiovasc. Res. 2019, 115, 1178–1188. [Google Scholar] [CrossRef]
- Ou, W.C.; Chen, H.F.; Zhong, Y.; Liu, B.R.; Liu, S.M.; Chen, K.J. Inhibition of platelet activation and aggregation by furostanol saponins isolated from the bulbs of Allium macrostemon Bunge. Am. J. Med. Sci. 2012, 344, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Jin, L.; Li, S.; Zhang, F.; Xu, Q.; Liu, M.; Chen, X.; Liu, X.; Gu, J.; Liu, S.; et al. Allium macrostemon Saponin Inhibits Activation of Platelet via the CD40 Signaling Pathway. Front. Pharmacol. 2020, 11, 570603. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cook, N.R.; Lee, I.M.; Gordon, D.; Gaziano, J.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N. Engl. J. Med. 2005, 352, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Morelli, A.; Donati, A.; Ertmer, C.; Rehberg, S.; Kampmeier, T.; Orecchioni, A.; Di Russo, A.; D’Egidio, A.; Landoni, G.; Lombrano, M.R.; et al. Effects of vasopressinergic receptor agonists on sublingual microcirculation in norepinephrine-dependent septic shock. Crit. Care 2011, 15, R217. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Ghatge, M.; Flora, G.D.; Dhanesha, N.; Jain, M.; Markan, K.R.; Potthoff, M.J.; Lentz, S.R.; Chauhan, A.K. The metabolic enzyme pyruvate kinase M2 regulates platelet function and arterial thrombosis. Blood 2021, 137, 1658–1668. [Google Scholar] [CrossRef]
- Flora, G.D.; Nayak, M.K.; Ghatge, M.; Chauhan, A.K. Metabolic targeting of platelets to combat thrombosis: Dawn of a new paradigm? Cardiovasc. Res. 2023, 119, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Wang, Y.; Li, D.; Zhang, L.; Wang, K.; Luo, X.; Yang, Z.; Wu, Y.; Liu, J. PDK1 regulates platelet activation and arterial thrombosis. Blood 2013, 121, 3718–3726. [Google Scholar] [CrossRef]
- Gawaz, M.; Geisler, T.; Borst, O. Current concepts and novel targets for antiplatelet therapy. Nat. Rev. Cardiol. 2023, 20, 583–599. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- McFadyen, J.; Peter, K. Platelets in the Pathogenesis of Vascular Disease and Their Role as a Therapeutic Target. In Mechanisms of Vascular Disease: A Textbook for Vascular Specialists; Fitridge, R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 233–261. [Google Scholar]
- Wittstein, I.S. Depression, anxiety, and platelet reactivity in patients with coronary heart disease. Eur. Heart J. 2010, 31, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Kuliczkowski, W.; Witkowski, A.; Polonski, L.; Watala, C.; Filipiak, K.; Budaj, A.; Golanski, J.; Sitkiewicz, D.; Pregowski, J.; Gorski, J.; et al. Interindividual variability in the response to oral antiplatelet drugs: A position paper of the Working Group on antiplatelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the Working Group on Thrombosis of the European Society of Cardiology. Eur. Heart J. 2009, 30, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Korte, W.; Cattaneo, M.; Chassot, P.G.; Eichinger, S.; von Heymann, C.; Hofmann, N.; Rickli, H.; Spannagl, M.; Ziegler, B.; Verheugt, F.; et al. Peri-operative management of antiplatelet therapy in patients with coronary artery disease: Joint position paper by members of the working group on Perioperative Haemostasis of the Society on Thrombosis and Haemostasis Research (GTH), the working group on Perioperative Coagulation of the Austrian Society for Anesthesiology, Resuscitation and Intensive Care (ÖGARI) and the Working Group Thrombosis of the European Society for Cardiology (ESC). Thromb. Haemost. 2011, 105, 743–749. [Google Scholar] [CrossRef]
- Chen, H.; Ou, W.; Wang, G.; Wang, N.; Zhang, L.; Yao, X. New steroidal glycosides isolated as CDL inhibitors of activated platelets. Molecules 2010, 15, 4589–4598. [Google Scholar] [CrossRef]
- Feng, H.; Wang, Z.; Wang, C.; Zhu, X.; Liu, Z.; Liu, H.; Guo, M.; Hou, Q.; Chu, Z. Effect of Furostanol Saponins from Allium Macrostemon Bunge Bulbs on Platelet Aggregation Rate and PI3K/Akt Pathway in the Rat Model of Coronary Heart Disease. Evid.-Based Complement. Altern. Med. eCAM 2019, 2019, 9107847. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Lin, X.; Wang, Y.; He, X. A steroidal saponin isolated from Allium chinense simultaneously induces apoptosis and autophagy by modulating the PI3K/Akt/mTOR signaling pathway in human gastric adenocarcinoma. Steroids 2020, 161, 108672. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Wang, Y.; Wang, Z.; He, X. A-24, a steroidal saponin from Allium chinense, induced apoptosis, autophagy and migration inhibition in p53 wild-type and p53-deficient gastric cancer cells. Chem.-Biol. Interact. 2021, 348, 109648. [Google Scholar] [CrossRef]
- Chen, D.Q.; Han, J.; Liu, H.; Feng, K.; Li, P. Targeting pyruvate kinase M2 for the treatment of kidney disease. Front. Pharmacol. 2024, 15, 1376252. [Google Scholar] [CrossRef]
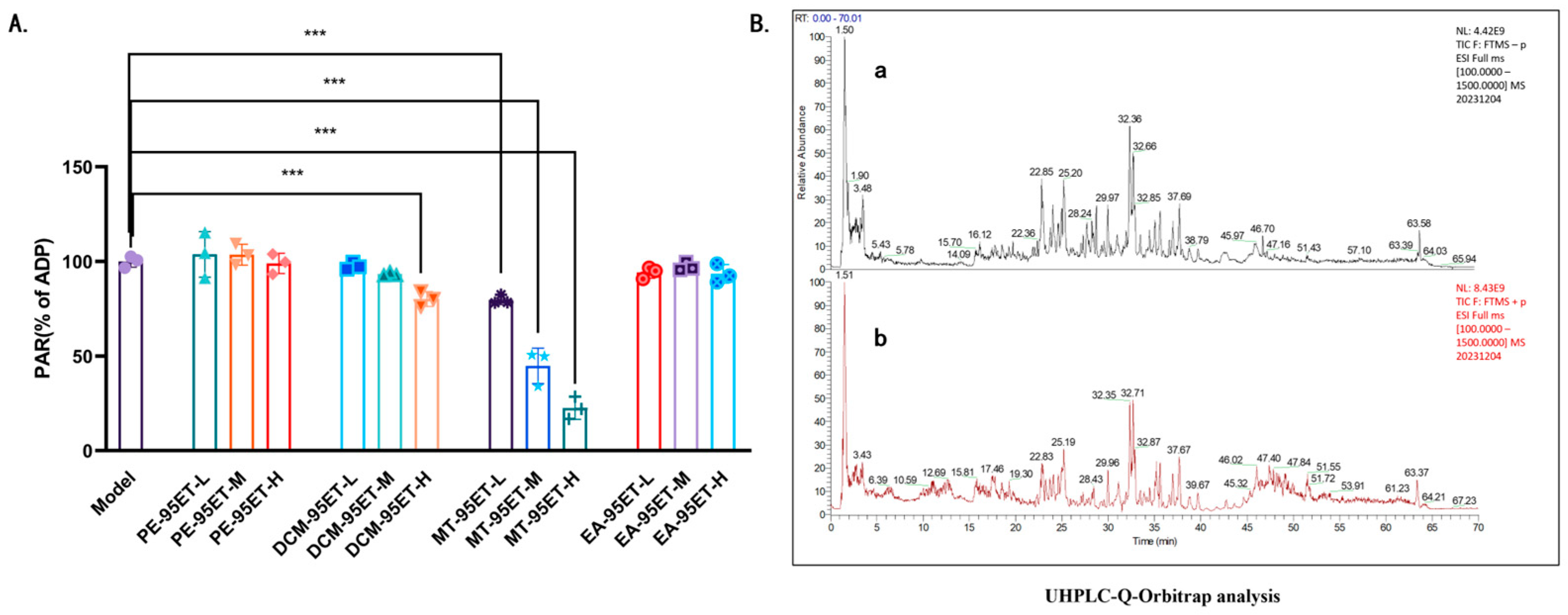



| No. | RT [min] | CAS Number | Name | Formula | Reference Ion | m/z | Annot. DeltaMass [ppm] | Fragment Ions |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.42 | 62-49-7 | Choline | C5H13NO | [M + H]+1 | 104.1071 | 0.55 | 104.1070, 60.0811, 58.0655 |
| 2 | 1.51 | 147-85-3 | Proline | C5H9NO2 | [M + H]+1 | 116.0707 | 1.21 | 116.0706, 70.0653 |
| 3 | 4.29 | 327-57-1 | L-Norleucine | C6H13NO2 | [M + H]+1 | 132.1020 | 0.76 | 86.0965 |
| 4 | 10.01 | 58-61-7 | Adenosine | C10H13N5O4 | [M − H]+1 | 268.1041 | 0.48 | 136.0619, 119.0352, 57.0338 |
| 5 | 27.28 | 151140-39-5 | Macrostemonoside E | C57H94O28 | [M − H]−1 | 1225.5859 | 0.46 | 1063.5342, 901.4816, 739.4290, 577.3757, 161.0457 |
| 6 | 31.29 | 187144-80-5 | Chinenoside IV | C50H80O23 | [M − H]−1 | 1047.5024 | 1.13 | 885.4520, 723.3990, 161.0457 |
| 7 | 32.67 | 170739-22-7 | Chinenoside V | C45H72O19 | [M − H]−1 | 915.4602 | 1.36 | 753.4063, 591.3551, 161.0457 |
| 8 | 33.47 | 512-04-9 | Diosgenin | C27H42O3 | [M + H]+1 | 415.3207 | 0.15 | 415.3205, 271.2057, 253.1951 |
| 9 | 35.29 | 554-62-1 | Phytosphingosine | C18H39NO3 | [M + H]+1 | 318.3003 | 0.12 | 318.3002, 300.2895, 282.2790, 60.0448, 56.0499 |
| 10 | 36.52 | 151215-11-1 | Macrostemonoside F | C45H74O18 | [M − H]−1 | 901.4816 | 2.15 | 739.4288, 577.3756, 161.0457 |
| 11 | 37.78 | 126-19-2 | Sarsasapogenin | C27H44O3 | [M + H]+1 | 417.3362 | −0.34 | 417.3398, 274.2244, 273.2212, 255.2106, 97.0648 |
| 12 | 43.59 | 467-55-0 | Hecogenin | C27H42O4 | [M + H]+1 | 431.3155 | −0.24 | 413.3049, 395.2947, 299.2371, 281.2268 |
| 13 | 51.49 | 112-84-5 | Erucamide | C22H43NO | [M + H]+1 | 338.3417 | −0.21 | 338.3410, 321.3156, 303.3047, 149.1326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, H.; Wang, M.; Wang, Z.; Li, N.; Si, J.; Ye, J. Extract of Allium Chinense G. Don, a Medicinal Plant, Ameliorates Myocardial Ischemia–Reperfusion Injury by Inhibiting Platelet Activation. Curr. Issues Mol. Biol. 2025, 47, 503. https://doi.org/10.3390/cimb47070503
Liu S, Wang H, Wang M, Wang Z, Li N, Si J, Ye J. Extract of Allium Chinense G. Don, a Medicinal Plant, Ameliorates Myocardial Ischemia–Reperfusion Injury by Inhibiting Platelet Activation. Current Issues in Molecular Biology. 2025; 47(7):503. https://doi.org/10.3390/cimb47070503
Chicago/Turabian StyleLiu, Siyuan, Huaxiang Wang, Min Wang, Zhihui Wang, Na Li, Jianyong Si, and Jingxue Ye. 2025. "Extract of Allium Chinense G. Don, a Medicinal Plant, Ameliorates Myocardial Ischemia–Reperfusion Injury by Inhibiting Platelet Activation" Current Issues in Molecular Biology 47, no. 7: 503. https://doi.org/10.3390/cimb47070503
APA StyleLiu, S., Wang, H., Wang, M., Wang, Z., Li, N., Si, J., & Ye, J. (2025). Extract of Allium Chinense G. Don, a Medicinal Plant, Ameliorates Myocardial Ischemia–Reperfusion Injury by Inhibiting Platelet Activation. Current Issues in Molecular Biology, 47(7), 503. https://doi.org/10.3390/cimb47070503





