Unraveling the Contribution of Estrobolome Alterations to Endometriosis Pathogenesis
Abstract
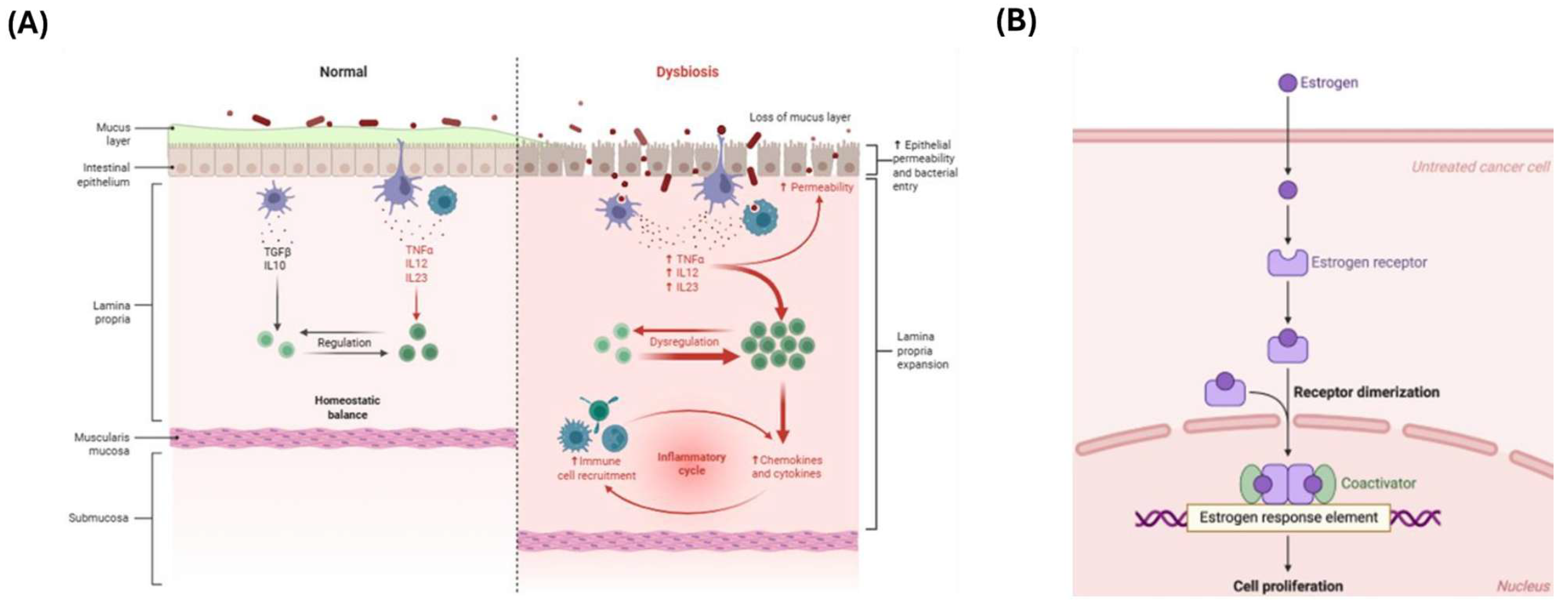
1. Introduction
2. Female Reproductive Tract Microbiota
3. Gut Microbiota and the Estrobolome
3.1. Introduction to the Estrobolome
3.2. Mechanism: β-Glucuronidase Activity
3.3. Influence on Estrogen-Dependent Diseases
3.4. Systemic—Local Microbiota Axis
4. Estrobolome Dysregulation in Endometriosis
5. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mariadas, H.; Chen, J.-H.; Chen, K.-H. The Molecular and Cellular Mechanisms of Endometriosis: From Basic Pathophysiology to Clinical Implications. Int. J. Mol. Sci. 2025, 26, 2458. [Google Scholar] [CrossRef]
- Macer, M.L.; Taylor, H.S. Endometriosis and Infertility: A Review of the Pathogenesis and Treatment of Endometriosis-associated Infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef] [PubMed]
- Lamceva, J.; Uljanovs, R.; Strumfa, I. The Main Theories on the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4254. [Google Scholar] [CrossRef] [PubMed]
- Bloski, T.; Pierson, R. Endometriosis and Chronic Pelvic Pain: Unraveling the Mystery Behind this Complex Condition. Nurs. Womens. Health 2008, 12, 382–395. [Google Scholar] [CrossRef]
- Maroun, P.; Cooper, M.J.W.; Reid, G.D.; Keirse, M.J.N.C. Relevance of gastrointestinal symptoms in endometriosis. Aust. New Zeal. J. Obstet. Gynaecol. 2009, 49, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Uzuner, C.; Mak, J.; El-Assaad, F.; Condous, G. The bidirectional relationship between endometriosis and microbiome. Front. Endocrinol. 2023, 14, 1110824. [Google Scholar] [CrossRef]
- Jiang, I.; Yong, P.J.; Allaire, C.; Bedaiwy, M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021, 22, 5644. [Google Scholar] [CrossRef]
- Szukiewicz, D. Chapter Eight—Aberrant epigenetic regulation of estrogen and progesterone signaling at the level of endometrial/endometriotic tissue in the pathomechanism of endometriosis. In Hormones and Epigenetics; Litwack, G.B., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 122, pp. 193–235. [Google Scholar]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Eaton, S.A.; Sethi, J.K. Immunometabolic Links between Estrogen, Adipose Tissue and Female Reproductive Metabolism. Biology 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, F.M.; Maison, N.; Holtrop, G.; Young, P.; Stevens, V.J.; Ince, J.; Johnstone, A.M.; Lobley, G.E.; Flint, H.J.; Louis, P. Phylogenetic distribution of genes encoding β-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ. Microbiol. 2012, 14, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Simon, C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod. Med. Biol. 2019, 18, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Madere, F.S.; Monaco, C.L. The female reproductive tract virome: Understanding the dynamic role of viruses in gynecological health and disease. Curr. Opin. Virol. 2022, 52, 15–23. [Google Scholar] [CrossRef]
- Punzón-Jiménez, P.; Labarta, E. The impact of the female genital tract microbiome in women health and reproduction: A review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Scott, R.T. Introduction: Microbiome in human reproduction. Fertil. Steril. 2015, 104, 1341–1343. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef]
- Lebeer, S.; Ahannach, S.; Gehrmann, T.; Wittouck, S.; Eilers, T.; Oerlemans, E.; Condori, S.; Dillen, J.; Spacova, I.; Vander Donck, L.; et al. A citizen-science-enabled catalogue of the vaginal microbiome and associated factors. Nat. Microbiol. 2023, 8, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef]
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli Dominance and Vaginal pH: Why is the Human Vaginal Microbiome Unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef] [PubMed]
- Gottschick, C.; Szafranski, S.P.; Kunze, B.; Sztajer, H.; Masur, C.; Abels, C.; Wagner-Döbler, I. Screening of Compounds against Gardnerella vaginalis Biofilms. PLoS ONE 2016, 11, e0154086. [Google Scholar] [CrossRef]
- Navarro, S.; Abla, H.; Delgado, B.; Colmer-Hamood, J.A.; Ventolini, G.; Hamood, A.N. Glycogen availability and pH variation in a medium simulating vaginal fluid influence the growth of vaginal Lactobacillus species and Gardnerella vaginalis. BMC Microbiol. 2023, 23, 186. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lee, H.K. Host and Microbiome Interplay Shapes the Vaginal Microenvironment. Front. Immunol. 2022, 13, 919728. [Google Scholar] [CrossRef] [PubMed]
- Champer, M.; Wong, A.M.; Champer, J.; Brito, I.L.; Messer, P.W.; Hou, J.Y.; Wright, J.D. The role of the vaginal microbiome in gynaecological cancer. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kakkar, V.; Bhushan, I. Crosstalk between Vaginal Microbiome and Female Health: A review. Microb. Pathog. 2019, 136, 103696. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Ma, N.; Mitchell, A.J.; Wu, M.C.; Valint, D.J.; Proll, S.; Reed, S.D.; Guthrie, K.A.; Lacroix, A.Z.; Larson, J.C.; et al. Association between postmenopausal vulvovaginal discomfort, vaginal microbiota, and mucosal inflammation. Am. J. Obstet. Gynecol. 2021, 225, 159.e1–159.e15. [Google Scholar] [CrossRef]
- Srinivasan, S.; Liu, C.; Mitchell, C.M.; Fiedler, T.L.; Thomas, K.K.; Agnew, K.J.; Marrazzo, J.M.; Fredricks, D.N. Temporal Variability of Human Vaginal Bacteria and Relationship with Bacterial Vaginosis. PLoS ONE 2010, 5, e10197. [Google Scholar] [CrossRef]
- Shardell, M.; Gravitt, P.E.; Burke, A.E.; Ravel, J.; Brotman, R.M. Association of Vaginal Microbiota with Signs and Symptoms of the Genitourinary Syndrome of Menopause Across Reproductive Stages. J. Gerontol. Ser. A 2021, 76, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Muzny, C.A.; Josey, W.E. Role of Gardnerella vaginalis in the Pathogenesis of Bacterial Vaginosis: A Conceptual Model. J. Infect. Dis. 2014, 210, 338–343. [Google Scholar] [CrossRef]
- Zeng, Q.; Shu, H.; Pan, H.; Zhang, Y.; Fan, L.; Huang, Y.; Ling, L. Associations of vaginal microbiota with the onset, severity, and type of symptoms of genitourinary syndrome of menopause in women. Front. Cell. Infect. Microbiol. 2024, 14, 1402389. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Srinivasan, S.; Zhan, X.; Wu, M.C.; Reed, S.D.; Guthrie, K.A.; LaCroix, A.Z.; Fiedler, T.; Munch, M.; Liu, C.; et al. Vaginal microbiota and genitourinary menopausal symptoms: A cross-sectional analysis. Menopause 2017, 24, 1160–1166. [Google Scholar] [CrossRef]
- Si, J.; You, H.J.; Yu, J.; Sung, J.; Ko, G. Prevotella as a Hub for Vaginal Microbiota under the Influence of Host Genetics and Their Association with Obesity. Cell Host Microbe 2017, 21, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ang, X.-Y.; Roslan, N.S.; Ahmad, N.; Yusof, S.M.; Abdullah, N.; Nik Ab Rahman, N.N.; Woon, J.-J.; Teh, C.S.-J.; Todorov, S.D.; Liu, G.; et al. Lactobacillus probiotics restore vaginal and gut microbiota of pregnant women with vaginal candidiasis. Benef. Microbes 2023, 14, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor–Positive Female Breast Cancer. JNCI J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar] [CrossRef]
- Trifanescu, O.G.; Trifanescu, R.A.; Mitrica, R.I.; Bran, D.M.; Serbanescu, G.L.; Valcauan, L.; Marinescu, S.A.; Gales, L.N.; Tanase, B.C.; Anghel, R.M. The Female Reproductive Tract Microbiome and Cancerogenesis: A Review Story of Bacteria, Hormones, and Disease. Diagnostics 2023, 13, 877. [Google Scholar] [CrossRef]
- Eriksson, H.; Gustafsson, J.-Å.; Sjövall, J. Steroids in Germfree and Conventional Rats. Eur. J. Biochem. 1969, 9, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef]
- Pollet, R.M.; D’Agostino, E.H.; Walton, W.G.; Xu, Y.; Little, M.S.; Biernat, K.A.; Pellock, S.J.; Patterson, L.M.; Creekmore, B.C.; Isenberg, H.N.; et al. An Atlas of β-Glucuronidases in the Human Intestinal Microbiome. Structure 2017, 25, 967–977.e5. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef] [PubMed]
- Sobstyl, M.; Brecht, P.; Sobstyl, A.; Mertowska, P.; Grywalska, E. The Role of Microbiota in the Immunopathogenesis of Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 5756. [Google Scholar] [CrossRef]
- Miles, S.M.; Hardy, B.L.; Merrell, D.S. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil. Steril. 2017, 107, 813–820.e1. [Google Scholar] [CrossRef]
- Parida, S.; Sharma, D. The Microbiome–Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Hakansson, A.; Molin, G. Gut Microbiota and Inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef]
- Anaf, V.; Simon, P.; El Nakadi, I.; Fayt, I.; Buxant, F.; Simonart, T.; Peny, M.-O.; Noel, J.-C. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum. Reprod. 2000, 15, 1744–1750. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Looijer-van Langen, M.; Hotte, N.; Dieleman, L.A.; Albert, E.; Mulder, C.; Madsen, K.L. Estrogen receptor-β signaling modulates epithelial barrier function. Am. J. Physiol. Liver Physiol. 2011, 300, G621–G626. [Google Scholar] [CrossRef]
- Insenser, M.; Murri, M.; del Campo, R.; Martínez-García, M.Á.; Fernández-Durán, E.; Escobar-Morreale, H.F. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. J. Clin. Endocrinol. Metab. 2018, 103, 2552–2562. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Association of Fecal Microbial Diversity and Taxonomy with Selected Enzymatic Functions. PLoS ONE 2012, 7, e39745. [Google Scholar] [CrossRef]
- Pérez-Prieto, I.; Vargas, E.; Salas-Espejo, E.; Lüll, K.; Canha-Gouveia, A.; Pérez, L.A.; Fontes, J.; Salumets, A.; Andreson, R.; Aasmets, O.; et al. Gut microbiome in endometriosis: A cohort study on 1000 individuals. BMC Med. 2024, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.H.; Wang, Y.-W.; Lu, P.-C.; Wu, H.-M.; Xu, J.-L.; Huang, H.-Y. Gut Microbiome–Estrobolome Profile in Reproductive-Age Women with Endometriosis. Int. J. Mol. Sci. 2023, 24, 16301. [Google Scholar] [CrossRef]
- Paredes, S.; Cantillo, S.; Candido, K.D.; Knezevic, N.N. An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens. Int. J. Mol. Sci. 2019, 20, 5729. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, H.; Tumurbaatar, T.; Oride, A.; Hara, T.; Okada, H.; Kyo, S. Gamma-aminobutyric acidA receptor agonist, muscimol, increases KiSS-1 gene expression in hypothalamic cell models. Reprod. Med. Biol. 2017, 16, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Masumoto, H.; Muto, H.; Kitajima, M.; Masuzaki, H.; Kitawaki, J. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 69–75. [Google Scholar] [CrossRef] [PubMed]

| CST | Dominant Anatomical Site | Dominant Species | Mean pH | Estrogen Levels | Epithelial Glycogen |
|---|---|---|---|---|---|
| I | Lower vagina | L. crispatus | ~4.0 | High | High |
| II | Mid/upper vagina | L. gasseri | ~4.2 | Moderate | Moderate |
| III | Upper vagina/cervix | L. iners | ~4.4 | Variable | Variable |
| IV | Cervix, endometrium | Mixed anaerobes | >5.0 | Low | Low |
| V | Vagina (ascending) | L. jensenii | ~4.2 | Moderate | Moderate |
| Compartment | Dominant Microbial Composition | Influencing Factors | Role and Clinical Implications | References |
|---|---|---|---|---|
| Vagina | Prevalence of Lactobacillus spp. (CST I–III, V); CST IV enriched with anaerobes such as Gardnerella and Atopobium | Estrogen levels, menstrual cycle, age, ethnicity, pregnancy, local estradiol therapy, antibiotic use, contraception, and menopause | Lactobacillus-dominated microbiota supports protective vaginal environment; microbial imbalance associated with infections, menopause-related symptoms, and reduced fertility | [16,18,22,29,30] |
| Endocervix | Lactobacillus iners, Lactobacillus crispatus, Prevotella, Sphingobium, Propionibacterium acnes and Pseudomonas | Menstrual cycle phase, parity (nulliparous vs. multiparous), use of medications (e.g., herbal treatments), and gynecological conditions such as adenomyosis and endometriosis | Maintains protective barrier against pathogens through acidification; microbial imbalance may increase infection risk, impair fertility, and is associated with gynecological disorders | [20] |
| Endometrium | Lactobacillus spp., Gardnerella, Atopobium, Streptococcus, Bifidobacterium, and Prevotella | Estrogen and progesterone levels, intrauterine interventions (e.g., embryo transfer, biopsies), assisted reproductive technologies (ART), local immune and inflammatory status, and gynecological conditions (e.g., endometriosis, cancer) | Balanced endometrial microbiota may support embryo implantation; dysbiosis has been linked to inflammation, implantation failure, and endometrial disorders | [14,46] |
| Fallopian tubes | Bacteroides, Corynebacterium, Lactobacillus, Coprococcus, and Hymenobacter | Shaped by ascending microbial migration, interindividual variability, and anatomical location | Microbial presence detected in healthy fallopian tubes; potential physiological role still under investigation | [20] |
| Ovary/Follicular fluid | Staphylococcus aureus, Streptococcus spp., Enterococcus spp., Lactobacillus spp. and Candida albicans | Underlying gynecological conditions (e.g., endometriosis, PCOS), history of genital tract infections, and invasive gynecological procedures | Microorganisms in follicular fluid associated with reduced fertilization rates, especially in patients with endometriosis or PCOS; possible implications for pregnancy outcomes remain inconclusive | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nannini, G.; Cei, F.; Amedei, A. Unraveling the Contribution of Estrobolome Alterations to Endometriosis Pathogenesis. Curr. Issues Mol. Biol. 2025, 47, 502. https://doi.org/10.3390/cimb47070502
Nannini G, Cei F, Amedei A. Unraveling the Contribution of Estrobolome Alterations to Endometriosis Pathogenesis. Current Issues in Molecular Biology. 2025; 47(7):502. https://doi.org/10.3390/cimb47070502
Chicago/Turabian StyleNannini, Giulia, Francesco Cei, and Amedeo Amedei. 2025. "Unraveling the Contribution of Estrobolome Alterations to Endometriosis Pathogenesis" Current Issues in Molecular Biology 47, no. 7: 502. https://doi.org/10.3390/cimb47070502
APA StyleNannini, G., Cei, F., & Amedei, A. (2025). Unraveling the Contribution of Estrobolome Alterations to Endometriosis Pathogenesis. Current Issues in Molecular Biology, 47(7), 502. https://doi.org/10.3390/cimb47070502







