Neutrophil Extracellular Traps in Pediatric Infections: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Registration and Search Strategy
2.2. Eligibility Criteria
2.3. Collection and Extraction of Data
2.4. Quality Assessment of the Included Studies
2.5. Outcome Measurements
3. Results
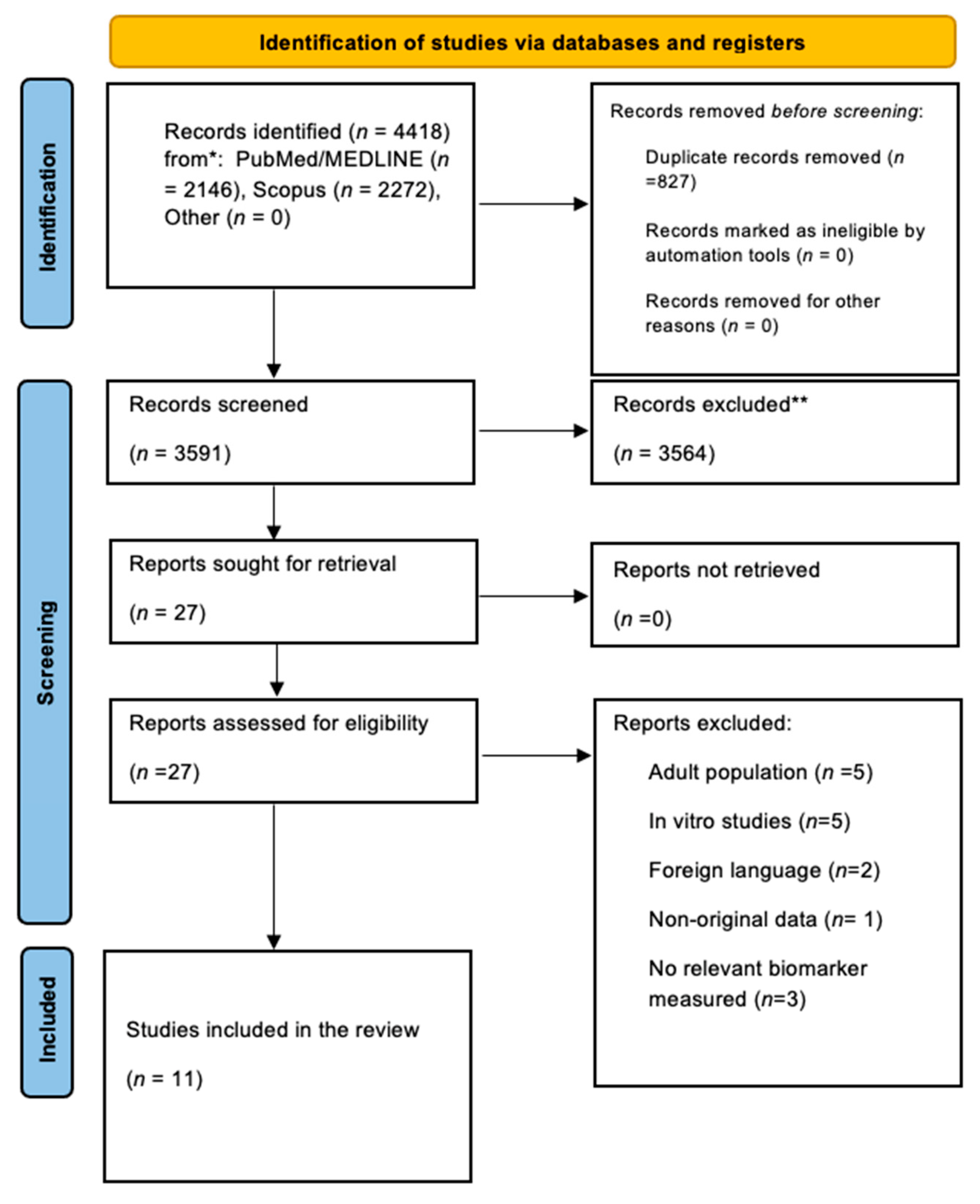
3.1. Search Strategy Results and Study Characteristics
3.2. Quality Assessment of the Included Studies
3.3. Outcomes
3.3.1. Respiratory Infections
3.3.2. Urinary Tract Infections
3.3.3. Central Nervous System Infections
3.3.4. Sepsis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAL | Bronchoalveolar Lavage Fluid |
| CF | Cystic Fibrosis |
| COVID-19 | Coronavirus Disease 2019 |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| ecDNA | Extracellular DNA |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EONS | Early Onset Neonatal Sepsis |
| GA | Gestational Age |
| IF | Immunofluorescence |
| MIS-C | Multisystem Inflammatory Syndrome in Children |
| MPO | Myeloperoxidase |
| mtDNA | Mitochondrial DNA |
| ncDNA | Nuclear DNA |
| NET | Neutrophil Extracellular Traps |
| NOS | Newcastle–Ottawa Scale |
| NR | Not Reported |
| OSF | Open Science Framework |
| PICU | Pediatric Intensive Care Unit |
| PMN | Polymorphonuclear |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| rAOM | Recurrent Acute Otitis Media |
| ROS | Reactive Oxygen Species |
| SEM | Scanning Electron Microscopy |
| UTI | Urinary Tract Infection |
Appendix A
| Database | Search Strategy |
|---|---|
| Pubmed/MEDLINE | (“extracellular traps”[MeSH Terms] OR (“extracellular”[All Fields] AND “traps”[All Fields]) OR “extracellular traps”[All Fields] OR (“neutrophil”[All Fields] AND “extracellular”[All Fields] AND “traps”[All Fields]) OR “neutrophil extracellular traps”[All Fields] OR (“netw spat econ”[Journal] OR “nets”[All Fields])) AND (“child*”[All Fields] OR (“adolescences”[All Fields] OR “adolescency”[All Fields] OR “adolescent”[MeSH Terms] OR “adolescent”[All Fields] OR “adolescence”[All Fields] OR “adolescents”[All Fields] OR “adolescent s”[All Fields]) OR (“infant”[MeSH Terms] OR “infant”[All Fields] OR “infants”[All Fields] OR “infant s”[All Fields]) OR (“infant, newborn”[MeSH Terms] OR (“infant”[All Fields] AND “newborn”[All Fields]) OR “newborn infant”[All Fields] OR “neonatal”[All Fields] OR “neonate”[All Fields] OR “neonates”[All Fields] OR “neonatality”[All Fields] OR “neonatals”[All Fields] OR “neonate s”[All Fields])) AND (“infect”[All Fields] OR “infectability”[All Fields] OR “infectable”[All Fields] OR “infectant”[All Fields] OR “infectants”[All Fields] OR “infected”[All Fields] OR “infecteds”[All Fields] OR “infectibility”[All Fields] OR “infectible”[All Fields] OR “infecting”[All Fields] OR “infection s”[All Fields] OR “infections”[MeSH Terms] OR “infections”[All Fields] OR “infection”[All Fields] OR “infective”[All Fields] OR “infectiveness”[All Fields] OR “infectives”[All Fields] OR “infectivities”[All Fields] OR “infects”[All Fields] OR “pathogenicity”[MeSH Subheading] OR “pathogenicity”[All Fields] OR “infectivity”[All Fields] OR (“communicable diseases”[MeSH Terms] OR (“communicable”[All Fields] AND “diseases”[All Fields]) OR “communicable diseases”[All Fields] OR (“infectious”[All Fields] AND “disease”[All Fields]) OR “infectious disease”[All Fields])) |
| Scopus | TITLE-ABS-KEY (“neutrophil extracellular traps” OR “NETs” AND “child*” OR “adolescent*” OR “infant” OR “neonate” AND “infection” OR “infectious disease”) |
| Truncation (*) was applied to key terms to retrieve all lexical variants and ensure comprehensive search coverage. | |
| Study | Title | Reasons for Exclusion |
|---|---|---|
| Feys 2024 | Lower respiratory tract single-cell RNA sequencing and neutrophil extracellular trap profiling of COVID-19-associatedpulmonary aspergillosis: a single centre, retrospective, observational study | Adult population |
| Zhang 2025 | Screening and Identification of Neutrophil Extracellular Trap-related Diagnostic Biomarkers for Pediatric Sepsis by Machine Learning | In vitro study |
| Byrd 2015 | NETosis in neonates: evidence of a ROS-independent pathway in response to fungal challenge | In vitro study |
| Khaertynov 2020 | The severity of netosis inpatients with neonatal sepsis | Foreign language |
| Dan 2019 | Significance of neutrophil extracellular trap and its markers in the early diagnosis of community-acquired pneumonia in children | Foreign language |
| Cortjens 2016 | Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease | In vitro study |
| Mercado-Evans 2025 | Tamm-Horsfall protein augments neutrophil NETosis during urinary tract infection | In vitro study |
| Grudzinska 2019 | Neutrophils in community-acquired pneumonia: parallels in dysfunction at the extremes of age | No relevant biomarker measured |
| Khan 2019 | Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation | Non original data- Review |
| Muraro 2018 | Respiratory Syncytial Virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation | No relevant biomarker measured |
| Yoo D 2014 | NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes | Adult population |
| Wang 2020 | Excessive Neutrophils and Neutrophil Extracellular Traps in COVID-19 | No relevant biomarker measured |
| Chen 2018 | Neutrophil extracellular traps promote macrophage pyroptosis in sepsis | In vitro study |
| Arruda 2024 | Kinetics of neutrophil extracellular traps and cytokines in oral mucositis and Candida infection | Adult population |
| Krinsky 2023 | NETosis induction reflects COVID-19 severity and long COVID: insights from a 2-center patient cohort study in Israel | Adult population |
| Funchal 2015 | Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils | Adult population |
References
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, F. The Dual Role of Macrophage Extracellular Traps in Host Defense and Disease: Mechanisms and Therapeutic Implications. Biomolecules 2025, 15, 1220. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kronbichler, A.; Park, D.D.Y.; Park, Y.M.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef]
- Wang, W.; Su, J.; Yan, M.; Pan, J.; Zhang, X. Neutrophil extracellular traps in autoimmune diseases: Analysis of the knowledge map. Front. Immunol. 2023, 14, 1095421, Erratum in Front. Immunol. 2023, 14, 1158560. [Google Scholar]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Czaikoski, P.G.; Mota, J.M.S.C.; Nascimento, D.C.; Sônego, F.; Castanheira, F.V.E.S.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Yost, C.C.; Cody, M.J.; Harris, E.S.; Thornton, N.L.; McInturff, A.M.; Martinez, M.L.; Chandler, N.B.; Rodesch, C.K.; Albertine, K.H.; Petti, C.A.; et al. Impaired neutrophil extracellular trap (NET) formation: A novel innate immune deficiency of human neonates. Blood 2009, 113, 6419–6427. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.M.; Corriden, R.; Nizet, V. The Ontogeny of a Neutrophil: Mechanisms of Granulopoiesis and Homeostasis. Microbiol. Mol. Biol. Rev. 2018, 82, e00057-17. [Google Scholar] [CrossRef]
- Lawrence, S.M.; Corriden, R.; Nizet, V. Age-appropriate functions and dysfunctions of the neonatal neutrophil. Front. Pediatr. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Stiel, C.U.; Ebenebe, C.U.; Trochimiuk, M.; Pagarols Raluy, L.; Vincent, D.; Singer, D.; Reinshagen, K.; Boettcher, M. Markers of NETosis Do Not Predict Neonatal Early Onset Sepsis: A Pilot Study. Front. Pediatr. 2020, 7, 555. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Boeddha, N.P.; Ekinci, E.; Emonts, M.; Hazelzet, J.A.; Driessen, G.J.; De Maat, M.P. Neutrophil Extracellular Traps in Children with Meningococcal Sepsis. Pediatr. Crit. Care Med. 2018, 19, e286–e291. [Google Scholar] [CrossRef]
- Lenz, M.; Maiberger, T.; Armbrust, L.; Kiwit, A.; Von der Wense, A.; Reinshagen, K.; Elrod, J.; Boettcher, M. CfDNA and DNases: New Biomarkers of Sepsis in Preterm Neonates—A Pilot Study. Cells 2022, 11, 192. [Google Scholar] [CrossRef]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews Systematic reviews and Meta-Analyses. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Wells, G.A.; Wells, G.; Shea, B.; Shea, B.; O’Connell, D.; Peterson, J.; Welch; Losos, M.; Tugwell, P.; Ga, S.W.; et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://api.semanticscholar.org/CorpusID:79550924 (accessed on 5 September 2025).
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G.; ESH Working Group on CV Risk in Low Resource Settings. Panethnic differences in blood pressure in Europe: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar] [CrossRef]
- Blanchard, L.; Ray, S.; Law, C.; Vega-Salas, M.J.; Bidonde, J.; Bridge, G.; Egan, M.; Petticrew, M.; Rutter, H.; Knai, C. The effectiveness, cost-effectiveness and policy processes of regulatory, voluntary and partnership policies to improve food environments: An evidence synthesis. Public Health Res. 2024, 12, 1–173. [Google Scholar] [CrossRef]
- Appelgren, D.; Enocsson, H.; Skogman, B.H.; Nordberg, M.; Perander, L.; Nyman, D.; Nyberg, C.; Knopf, J.; Muñoz, L.E.; Sjöwall, C.; et al. Neutrophil extracellular traps (NETs) in the cerebrospinal fluid samples from children and adults with central nervous system infections. Cells 2020, 9, 43. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Mohammad, A.F.; Huang, M.; Stephenson, S.T.; Patrignani, J.; Kamaleswaran, R.; Grunwell, J.R. Functional immunophenotyping of blood neutrophils identifies novel endotypes of viral response in preschool children with recurrent wheezing. J. Allergy Clin. Immunol. 2023, 152, 1433–1443. [Google Scholar] [CrossRef]
- Seery, V.; Raiden, S.C.; Algieri, S.C.; Grisolía, N.A.; Filippo, D.; De Carli, N.; Di Lallaf, S.; Cairolib, H.; Chiolog, M.J.; Meregalli, C.N.; et al. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. EBioMedicine 2021, 67, 103357. [Google Scholar] [CrossRef] [PubMed]
- Thornton, R.B.; Wiertsema, S.P.; Kirkham, L.A.S.; Rigby, P.J.; Vijayasekaran, S.; Coates, H.L.; Richmond, P.C. Neutrophil Extracellular Traps and Bacterial Biofilms in Middle Ear Effusion of Children with Recurrent Acute Otitis Media—A Potential Treatment Target. PLoS ONE 2013, 8, e53837. [Google Scholar] [CrossRef] [PubMed]
- King, P.T.; Dousha, L.; Clarke, N.; Schaefer, J.; Carzino, R.; Sharma, R.; Wan, K.L.; Anantharajah, A.; O’Sullivan, K.; Lu, Z.X.; et al. Phagocyte extracellular traps in children with neutrophilic airway inflammation. ERJ Open Res. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Carmona-Rivera, C.; Zhang, Y.; Dobbs, K.; Markowitz, T.E.; Dalgard, C.L.; Oler, A.J.; Claybaugh, D.R.; Draper, D.; Truong, M.; Delmonte, O.M.; et al. Multicenter analysis of neutrophil extracellular trap dysregulation in adult and pediatric COVID-19. JCI Insight. 2022, 7, e160332. [Google Scholar] [CrossRef]
- Martínez-Alemán, S.; Bustamante, A.E.; Jimenez-Valdes, R.J.; González, G.M.; Sánchez-González, A. Pseudomonas aeruginosa isolates from cystic fibrosis patients induce neutrophil extracellular traps with different morphologies that could correlate with their disease severity. Int. J. Med. Microbiol. 2020, 310, 151451. [Google Scholar] [CrossRef]
- Krivošíková, K.; Šupčíková, N.; Gaál Kovalčíková, A.; Janko, J.; Pastorek, M.; Celec, P.; Podracká, Ľ.; Tóthová, Ľ. Neutrophil extracellular traps in urinary tract infection. Front. Pediatr. 2023, 11, 1154139. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, Z.S.; Sweezey, N.; Grasemann, H.; Palaniyar, N. Progression of cystic fibrosis lung disease from childhood to adulthood: Neutrophils, neutrophil extracellular trap (NET) formation, and NET degradation. Genes 2019, 10, 183. [Google Scholar] [CrossRef]
- Mercado-Evans, V.; Branthoover, H.; Chew, C.; Serchejian, C.; Saltzman, A.B.; Mejia, M.E.; Zulk, J.J.; Cornax, I.; Nizet, V.; Patras, K.A. Tamm-Horsfall protein augments neutrophil NETosis during urinary tract infection. JCI Insight 2025, 10, e180024. [Google Scholar] [CrossRef]
- Byrd, A.S.; O’brien, X.M.; Laforce-Nesbitt, S.S.; Parisi, V.; Hirakawa, M.P.; Bliss, J.M.; Reichner, J.S. NETosis in neonates: Evidence of a ROS-independent pathway in response to fungal challenge. J. Infect. Dis. Adv. Access 2015, 4, 634–639. [Google Scholar]
- Zhang, G.; Zhang, K. Screening and Identification of Neutrophil Extracellular Trap-related Diagnostic Biomarkers for Pediatric Sepsis by Machine Learning. Inflammation 2025, 48, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Mikacenic, C.; Moore, R.; Dmyterko, V.; West, T.E.; Altemeier, W.A.; Liles, W.C.; Lood, C. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit. Care 2018, 22, 358. [Google Scholar] [CrossRef]
- Stoimenou, M.; Tzoros, G.; Skendros, P.; Chrysanthopoulou, A. Methods for the Assessment of NET Formation: From Neutrophil Biology to Translational Research. Int. J. Mol. Sci. 2022, 23, 15823. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cavanaugh, L.; Leung, H.; Yan, F.; Ahmadi, Z.; Chong, B.H.; Passam, F. Quantification of NETs-associated markers by flow cytometry and serum assays in patients with thrombosis and sepsis. Int. J. Lab. Hematol. 2018, 40, 392–399. [Google Scholar] [CrossRef]
- Henneck, T.; Krüger, C.; Nerlich, A.; Langer, M.; Fingerhut, L.; Bonilla, M.C.; Meurer, M.; von den Berg, S.; de Buhr, N.; Branitzki-Heinemann, K.; et al. Comparison of NET quantification methods based on immunofluorescence microscopy: Hand-counting, semi-automated and automated evaluations. Heliyon 2023, 9, e16982. [Google Scholar] [CrossRef]
- Retter, A.; Singer, M.; Annane, D. “The NET effect”: Neutrophil extracellular traps—A potential key component of the dysregulated host immune response in sepsis. Crit. Care 2025, 29, 59. [Google Scholar] [CrossRef]
- Espiritu, A.; O’Sullivan, K.M. A Web of Challenges: The Therapeutic Struggle to Target NETs in Disease. Int. J. Mol. Sci. 2025, 26, 4773. [Google Scholar] [CrossRef]
- Tonello, S.; Vercellino, N.; D’Onghia, D.; Fracchia, A.; Caria, G.; Sola, D.; Tillio, P.A.; Sainaghi, P.P.; Colangelo, D. Extracellular Traps in Inflammation: Pathways and Therapeutic Targets. Life 2025, 15, 627. [Google Scholar] [CrossRef] [PubMed]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, C.S.; Veras, F.P.; dos Santos Ramos, A.; Gomes, G.F.; Rodrigues Lemes, R.M.; Arruda, E.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q. PAD4 inhibition impacts immune responses in SARS-CoV-2 infection. Mucosal Immunol. 2025, 18, 861–873. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Protocol Number | Country | Study Design | Sample Size (n) | Population | Mean/ Median Age | Infection Context | Sample Type | NET Assessment |
|---|---|---|---|---|---|---|---|---|---|
| Appelgren et al., 2020 ([24]) | 2010/106 | Sweden | Observational | 111 children (subset) | Children & adults with CNS infections | 10y [IQR: 5–15] | CNS infections (LNB, others) | CSF | DNA/MPO, elastase assays |
| Carmona-Rivera et al., 2022 ([29]) | NCT04582903, NCT03394053 and NCT0361080 | Italy/Chile/USA | Observational | 250 | COVID-19, MIS-C | Chile MIS-C cohort 6 y [IQR: 3–11] Italian MIS-C cohort 5.8y [IQR: 0.3–12] Italian CLL cohort 13 y [IQR: 9–15] US CLL cohort 17y [IQR: 14–19.5] | COVID-19 and MIS-C | Serum, skin biopsies | NET remnants, degradation assays |
| Fitzpatrick et al., 2023 ([25]) | NR | USA | Observational | 52 | Preschool children with recurrent wheezing | No sensitization group 34.7 months (14.6) Sensitization group 34.3 months (12.1) | Viral analog stimulation | Blood neutrophils | Extracellular DNA, MPO release |
| Hoppenbrouwers et al., 2018 ([15]) | 2015–49 | The Netherlands | Observational | 60 | PICU patients with meningococcal sepsis | 2 years and 10 months [IQR, 21 months to 9 years]) | Meningococcal sepsis | Serum | MPO-DNA ELISA, in vitro assays |
| King et al., 2021 ([28]) | AREST CF protocol | Australia | Observational | 76 | Children with CF or chronic cough | CF group 4.1 y [IQR: 1.8–6.0] Non-CF group 7 y [IQR: 1.8–6.7] | Airway inflammation | BAL fluid | Confocal microscopy, NE activity |
| Krivošíková et al., 2023 ([31]) | NR | Slovakia | Observational + mouse model | 148 | Children with febrile UTI | UTI group 0.8 y [IQR: 0.3–1.3] Control group 2.4 y [IQR: 0.5–5.2] | Urinary tract infection | Serum and urine | ecDNA, ncDNA, mtDNA, MPO, cathelicidin |
| Lenz et al., 2022 ([16]) | NCT02567305 | Germany | Observational | 31 | Preterm neonates with suspected sepsis | EONS group 2 days (1) Control group 1.5 days (0.71) | Neonatal sepsis | Plasma | cfDNA, DNase I, nucleosomes, NE, H3Cit |
| Martínez-Alemán et al., 2020 ([30]) | NM11015 and MB16-0002 | Mexico | Pilot observational | 14 | Cystic fibrosis patients | 11 y | Pseudomonas aeruginosa | In vitro assays | NET morphology, microscopy |
| Seery et al., 2021 ([26]) | 1226/20 and 1720/20 | Argentina | Observational | 243 | Children with COVID-19 or MIS-C | Median 9 y | COVID-19 | Plasma | cfDNA, citH3 ELISA |
| Stiel et al., 2020 ([14]) | PV5374 | Germany | Observational | 491 | Neonates at birth | Infection group GA
40.14 [IQR: 37.71–40.29 weeks]
Control group GA 39.43 [IQR: 38.86–40.86 weeks] | Neonatal early-onset sepsis | Cord blood | cfDNA, NE, MPO |
| Thornton et al., 2013 ([27]) | 1295/EP | Australia | Observational | 24 | Children with rAOM | 17.9 m [IQR: 9.7–36.0] | Recurrent acute otitis media | Middle ear effusion | IF microscopy |
| Author (Year) | Selection | Comparability | Outcome | Total Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Sample | Sample Size | Non-Respondents | Ascertainment of Exposure | Comparability | Assessment of Outcome | Statistical Test | ||||
| Appelgren et al., 2020 ([24]) | * | * | * | * | * | * | 6/10 | |||
| Carmona-Rivera et al., 2022 ([29]) | * | * | ** | ** | ** | * | 9/10 | |||
| Fitzpatrick et al., 2023 ([25]) | * | * | * | * | * | * | 6/10 | |||
| King et al., 2021 ([28]) | * | * | ** | * | ** | * | 8/10 | |||
| Krivošíková et al., 2023 ([31]) | * | * | * | ** | * | ** | * | 9/10 | ||
| Lenz et al., 2021 ([16]) | * | * | * | * | * | * | 6/10 | |||
| Seery et al., 2021 ([26]) | * | * | ** | * | * | * | 7/10 | |||
| Thornton et al., 2013 ([27]) | * | * | * | * | * | * | 6/10 | |||
| Author (Year) | Case Definition Adequate | Representativeness of Cohort | Selection of Controls/Non-Exposed | Ascertainment of Exposure | Control for Main Confounder | Control for Additional Confounder | Assessment of Outcome | Follow-Up Long Enough | Adequacy of Follow-Up | Total Score |
| Hoppenbrouwers et al., 2018 ([15]) | * | * | * | * | * | * | * | 7/9 | ||
| Martínez-Alemán et al., 2020 ([30]) | * | * | * | * | * | * | * | 7/9 | ||
| Stiel et al., 2020 ([14]) | * | * | * | * | * | 5/9 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoimeni, A.; Gkiourtzis, N.; Karatisidou, V.; Charitakis, N.; Makedou, K.; Tramma, D.; Panagopoulou, P. Neutrophil Extracellular Traps in Pediatric Infections: A Systematic Review. Curr. Issues Mol. Biol. 2025, 47, 999. https://doi.org/10.3390/cimb47120999
Stoimeni A, Gkiourtzis N, Karatisidou V, Charitakis N, Makedou K, Tramma D, Panagopoulou P. Neutrophil Extracellular Traps in Pediatric Infections: A Systematic Review. Current Issues in Molecular Biology. 2025; 47(12):999. https://doi.org/10.3390/cimb47120999
Chicago/Turabian StyleStoimeni, Anastasia, Nikolaos Gkiourtzis, Vera Karatisidou, Nikolaos Charitakis, Kali Makedou, Despoina Tramma, and Paraskevi Panagopoulou. 2025. "Neutrophil Extracellular Traps in Pediatric Infections: A Systematic Review" Current Issues in Molecular Biology 47, no. 12: 999. https://doi.org/10.3390/cimb47120999
APA StyleStoimeni, A., Gkiourtzis, N., Karatisidou, V., Charitakis, N., Makedou, K., Tramma, D., & Panagopoulou, P. (2025). Neutrophil Extracellular Traps in Pediatric Infections: A Systematic Review. Current Issues in Molecular Biology, 47(12), 999. https://doi.org/10.3390/cimb47120999









