Abstract
The complex bidirectional relationship between diabetes mellitus (DM) and oral cancer (OC) denotes that metabolic dysfunction and malignancy intersect at molecular, cellular, and systemic levels. This state-of-the-art review analyzes the most recent literature data on the multiple interconnected pathways linking DM and OC, including hyperinsulinemia/IGF-1 signaling, chronic hyperglycemia-induced cellular damage, persistent inflammation, immune dysfunction, and oral microbiota dysbiosis. These mechanisms create a permissive environment for oral carcinogenesis while simultaneously impairing the body’s natural tumor surveillance systems. Key molecular networks explored include the PI3K/AKT/mTOR pathway, AGE-RAGE interactions, NF-κB signaling, the p53 tumor suppressor pathway, and HIF-mediated responses. Clinical evidence demonstrates that patients with diabetes have higher OC prevalence (250 per 100,000 patients) and significantly increased mortality (HR of 2.09) compared to non-diabetics. The review highlights metformin as the most promising anti-diabetic agent for OC management, showing anti-tumor effects through mTOR inhibition. Novel therapeutics, such as GLP-1 agonists, particularly semaglutide, may be helpful but require further clinical validation. Understanding the shared molecular pathways enables the development of integrated therapeutic strategies that target both conditions simultaneously, and it supports effective screening programs, personalized prevention strategies, and optimized multidisciplinary management approaches for this high-risk patient population.
1. Introduction
Oral cancer ranks as the sixth most prevalent cancer globally, affecting approximately 650,000 individuals annually [1]. The incidence of oral cancer has escalated by 49% over the past decade, according to the Mouth Cancer Foundation (available at https://www.mouthcancerfoundation.org/mouth-cancer-facts-and-figures, accessed on 1 November 2025). Still, two-thirds of patients are diagnosed at an advanced stage of disease, with a 5-year survival rate of 50% or less [2]. Notably, ten-year survival rates exhibit considerable variability, ranging from 18% to 57%, and are contingent on the cancer’s specific location and the timeliness of diagnosis [3]. It presents substantial mortality and morbidity challenges, particularly in Southeast Asia, South Asia, and certain regions of Europe, where incidence rates are highest [4,5,6,7]. The primary risk factors associated with oral cancer include tobacco and alcohol consumption; however, emerging research suggests that obesity may also be a significant contributing factor to the increased risk of developing this disease [8].
Obesity is also a significant risk factor for developing diabetes mellitus (DM), as it leads to insulin resistance and impairs the function of insulin-producing cells; when excess body fat accumulates, particularly around the abdomen, it raises levels of fatty acids and other substances that decrease the body’s sensitivity to insulin [9]. Affecting over 400 million people worldwide, DM has emerged as a potential risk factor for various cancers [10]. The relationship between diabetes and cancer has been recognized for over a century. Still, recent epidemiological and molecular studies have provided more concrete evidence for this association, particularly regarding oral malignancies [11,12,13].
The presence of DM encompasses increased cancer risk, altered treatment outcomes, and heightened complications, making OC a particular burden for diabetic patients [13,14]. Patients with DM have a higher risk of OC development than non-diabetic patients (Odds Ratio = 1.41); a pooled prevalence of 0.25% (250 per 100,000 patients) in diabetic patients was reported [15,16]. The association is influential for precancerous lesions; leukoplakia reveals a pooled prevalence of 2.49% among diabetic patients [15]. A Danish population-based cohort study found that the incidence of OC was increased 2-fold in the diabetic population younger than 50 years [17]. Austrian data revealed that 59.9% of OC patients had glucose metabolism disorder compared to 36.5% in controls [18]. In Austria, numerous people have diabetes (7–11% of the total population), with OC being the tenth most common malignancy [18]. In Hungary, oral cavity cancer shows the second-highest age-standardized mortality rate across European nations, with approximately 3000 new diagnoses and more than 1600 deaths annually [19].
Patients with OC and DM had significantly higher mortality than controls, with an HR of 2.09 (95%CI = 1.36–3.22) [15]. Diabetic patients with oral squamous cell carcinoma (OSCC) tend to have lower overall survival, recurrence-free survival, and cancer-specific survival compared with non-diabetics [20]. The negative impact persists even in less aggressive tumor stages (stage I and II), with diabetic patients who were not prescribed adjuvant therapy showing a significantly higher recurrence rate than non-diabetic patients [20]. DM significantly impacts the OC development, metastasis, and prognosis. This influence may be attributed to several associated factors (hyperglycemia, hyperinsulinemia, insulin resistance, and chronic inflammation) [21]. The bidirectional relationship is further complicated by periodontal disease negatively impacting glycemic control and potentially contributing to diabetes complications [19]. Effective management of DM in patients undergoing OC treatment requires a multidisciplinary approach. It should include a diabetes specialist, educator, dietician, pharmacist, and psychosocial support professional, who collaborate closely with the oncology care team. Such comprehensive management is essential for preventing diabetes-related complications and ultimately improving patient outcomes [22,23].
Significant clinical and epidemiological studies, along with their corresponding findings, are summarized in Table 1.

Table 1.
The impact of DM on OC.
Considering the potentially harmful effects of oral cancer, all these findings are worrying. The evidence clearly establishes OC as a significant burden for diabetic patients, characterized by increased incidence, worse prognosis, and heightened treatment complications [15]. Ongoing research is examining pathogenic pathways that explain the association between diabetes and OC [17]. As long as it remains unknown why patients with diabetes are at higher risk for oral cancer, targeted prevention and treatment programs are unlikely to succeed [17]. Therefore, this comprehensive analysis examines the most recent findings regarding key molecular pathways involved in both diseases, their dysregulation patterns, cross-talk mechanisms, and therapeutic implications. It examines the progress of current and emerging molecular targets, clinical significations, therapeutic approaches, and clinical developments that could enrich management strategies for patients with these interconnected conditions.
2. Biological Mechanisms That Correlate Diabetes Mellitus with Oral Cancer
Beyond incidence, DM also significantly impacts the prognosis of patients with oral cancer. Studies have consistently shown that: (a) patients with OC and DM have higher mortality compared to non-diabetic patients with oral cancers; (b) diabetic patients tend to have lower overall survival, recurrence-free survival, and cancer-specific survival; (c) even in less aggressive tumor stages (stages I and II), diabetic patients demonstrate worse outcomes [17,20,21].
The association between DM and OC involves multiple, interconnected biological mechanisms (Figure 1).
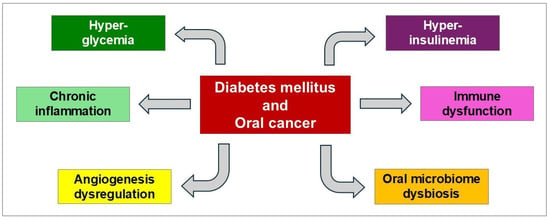
Figure 1.
Biological pathways shared by DM and OC [27].
2.1. Hyperglycemia-Mediated Effects
High glucose concentrations directly impact cancer cells through: (a) metabolic reprogramming (providing enhanced energy substrate for rapidly dividing cancer cells) [28]; (b) oxidative stress (hyperglycemia increases reactive oxygen species (ROS) production through mitochondrial metabolism, leading to DNA damage and mutations); (c) signaling pathway activation (high glucose activates various pathways controlling proliferation, migration, invasion, and recurrence); (d) hyperglycemic memory (cancer cells exposed to hyperglycemic conditions maintain permanently activated oncogenic pathways even after glucose normalization, suggesting epigenetic modifications that persist beyond the initial metabolic insult) [29,30,31,32,33].
2.2. Hyperinsulinemia
Insulin resistance (IR) results in compensatory hyperinsulinemia. This elevated insulin level may directly promote the proliferation of OC cells. High insulin levels can also promote tumor cell growth by stimulating DNA synthesis and inhibiting apoptosis, thereby facilitating cancer cell invasion and metastasis [34,35,36].
2.3. Chronic Inflammation
DM has increasingly emerged as a condition distinguished by chronic low-grade systemic inflammation. This persistent inflammatory state is predominantly driven by metabolic stresses arising from hyperglycemia and insulin resistance. Chronic inflammation plays a pivotal role in facilitating carcinogenesis through various intricate mechanisms. Inflammatory cytokines can instigate oxidative stress and DNA damage, significantly elevating the risk of mutagenesis and genomic instability. Moreover, these mediators not only promote cellular proliferation but also inhibit apoptosis and stimulate angiogenesis, thereby creating a microenvironment highly conducive to tumor development and growth. Within oral tissues, inflammatory signaling can alter the behavior of keratinocytes and immune cells, thereby enhancing the potential for malignant transformation, particularly in combination with other carcinogenic factors such as tobacco or alcohol [12,15,37,38,39,40].
2.4. Immune Dysfunction
Patients with DM often encounter a range of health challenges due to compromised immune surveillance mechanisms, altered cytokine profiles, and a reduced capacity for OC cells recognition and elimination [13,41].
2.5. Angiogenesis Dysregulation
The pro-angiogenic state associated with DM may significantly influence the onset and progression of various complications related to diabetes. This altered physiological condition not only impacts vascular integrity but also contributes to the initiation and aggressive behavior of oral neoplastic lesions. Enhanced angiogenesis can foster a favorable microenvironment for OC development, potentially leading to more severe health outcomes in individuals with DM [42,43].
2.6. Oral Microbiota Dysbiosis
Recent research has revealed a complex, bidirectional relationship between oral pathogens and systemic diseases, particularly DM and OC [44,45,46]. For example, periodontal disease can significantly disrupt glycemic control, making it more difficult for individuals to manage their blood sugar levels effectively. This deterioration in oral health not only affects the mouth but also contributes to systemic issues, as hyperglycemia creates an environment conducive to the proliferation of harmful oral pathogens. These pathogens thrive in high-glucose conditions, perpetuating a destructive cycle where poor oral health exacerbates DM outcomes, leading to further complications. In addition to this reciprocal relationship, specific strains of oral bacteria have been identified as producers of carcinogenic metabolites. These compounds, along with the chronic inflammation triggered by oral pathogens, can initiate cellular changes that lead to malignant transformation [47].
3. Molecular Pathways in Diabetes Mellitus and Oral Cancer
All the abovementioned data indicate that DM and OC share multiple biological and molecular pathways, forming a complex network of pathophysiological interactions. Having previously established the epidemiological association and biological mechanisms linking DM to OC (hyperglycemia- and hyperinsulinemia-mediated effects, chronic inflammation, immune dysfunction, angiogenesis dysregulation, and oral microbiota dysbiosis), this section provides a detailed molecular description of the key pathways involved, including their normal physiological functions, alterations in diabetes and oral cancer, and points of convergence between both diseases. Understanding these molecular details is essential for identifying therapeutic targets and developing biomarker-based risk stratification strategies.
3.1. AGE-RAGE Interactions: Molecular Mechanisms and Implications in Diabetes and Oral Cancer
In diabetes, hyperglycemia-induced signaling pathways lead to insulin resistance and β-cell dysfunction [48]. High blood glucose levels accelerate the formation and accumulation of advanced glycation end products (AGEs) in tissues and the bloodstream. They result from a glucose non-enzymatic reaction (glycation) with proteins, lipids, or nucleic acids [49]. The AGE-RAGE signaling pathway is a complex molecular cascade triggered when AGEs bind their cognate receptors (RAGEs) [50]. This binding initiates various downstream signaling pathways, leading to cellular responses that contribute to diseases such as diabetes and its complications, including OC [51]. RAGEs are overexpressed in OC associated with increased invasive and metastatic activity. Key downstream effects include the activation of inflammatory and profibrotic pathways, such as NF-κB, MAPK, and JNK, increased oxidative stress, and alterations in PI3K/AKT signaling [52,53,54]. Through angiogenesis and transcription factor (TF) activation, the AGE-RAGE interaction initiates DM complications, OC progression, invasion, and metastases [55]. Thus, the AGE-RAGE axis serves as a critical molecular bridge between diabetes and oral cancer, facilitating inflammatory and oncogenic processes through complex signaling networks [56] (Table 2).

Table 2.
AGE-RAGE Interactions: molecular mechanisms and implications in diabetes and oral cancer.
3.2. Insulin/IGF-1 System in Diabetes Mellitus and Oral Cancer
Insulin-like growth factors (IGF-1 and IGF-2) are polypeptide hormones that share a high degree of structural similarity with insulin, the key regulator of glucose metabolism. IGF-1, in particular, is notable for its strong affinity for its specific receptors, known as IGF-1 receptors (IGF-1R) [57]. The primary site of IGF-1 production is the liver, where hepatocytes synthesize and secrete it into the bloodstream. The production of IGF-1 is predominantly regulated by growth hormone (GH), which stimulates its synthesis in response to physiological needs such as growth and tissue repair [44].
Under physiological conditions, IGF-1 binds to both IGF-1R and the insulin receptor (IR), forming the IGF-1/insulin receptor complex. This interaction facilitates complex signaling dynamics that maintain metabolic homeostasis, highlighting the critical interplay between the insulin/IGF-1/GH signaling pathways in ensuring proper growth and metabolic function across diverse tissues [57,58].
IGFs are essential regulators of cell growth and exhibit complex relationships with oral cancer. Several studies suggest increased expression of IGF-2 and the IGF-1 receptor in oral tumors, suggesting a potential role in OC proliferation; other research has demonstrated no significant difference in IGF-1 expression levels between healthy tissues and those affected by OC [59,60,61]. Upon binding to IGF-1R, IGF-1 initiates the activation of two critical intracellular signaling pathways: the p38 mitogen-activated protein kinase (MAPK), protein kinase B (AKT), and the phosphoinositide 3-kinase (PI3K) pathways (Table 2). These pathways are essential for various tumor cellular processes (proliferation, differentiation, and survival) [55,62,63]. Insulin/IGF-1 signaling contributes to obesity-associated cancer risk [64,65,66]. Understanding these mechanisms can potentially lead to better OC management (Table 3).

Table 3.
Insulin/IGF-1 System in Diabetes and Oral Cancer.
3.3. Core Molecular Pathways
The most essential molecular pathways shared between DM and OC are the PI3K/AKT/mTOR pathway, the inflammatory pathway, the p53 tumor suppressor network, the HIF-mediated pathway, and the oral microbiome pathway.
3.3.1. PI3K/AKT/mTOR Signaling Network
The PI3K/AKT/mTOR pathway represents a central hub for metabolic regulation and cellular growth control, making it essential in both DM and OC pathogenesis [68,69] (Table 4). The phosphoinositol 3-kinase (P13K) and mechanistic target of rapamycin (mTOR) play essential roles in cell homeostasis and metabolism [70,71]. The PI3K/Akt/mTOR signaling pathway is dysregulated in DM [70,72]. It mediates OC progression and chemotherapy resistance [73,74].
PTEN is a key tumor suppressor that helps control cell growth, survival, and division. It does this by dephosphorylating PIP3 (phosphatidylinositol (3,4,5)-trisphosphate), thereby counteracting PI3K/Akt signaling. PTEN plays essential roles in cell movement, adhesion, and maintaining genomic stability [75]. Therefore, PTEN is vital for maintaining the structural and functional integrity of pancreatic islets, which produce insulin and regulate insulin signaling and glucose metabolism. When PTEN is dysfunctional, it can lead to type 2 DM. Changes in PTEN activity can hinder insulin’s ability to manage glucose uptake and balance in tissues such as the liver, muscle, and pancreas [76]. On the other hand, mutations that reduce PTEN activity may increase insulin sensitivity, but they also raise the risk of obesity and cancer [77]. PTEN loss contributes to OC development and progression [78] (Table 4)

Table 4.
Architecture and functions of the molecular pathways shared by DM and OC.
Table 4.
Architecture and functions of the molecular pathways shared by DM and OC.
| Molecular Players | Normal Function | DM Implications | OC Implications | References |
|---|---|---|---|---|
| PI3K/AKT/mTOR signaling network | ||||
| PI3K |
|
|
| [79,80] |
| Protein kinase B (AKT) |
|
|
| [79,81] |
| mTOR |
|
|
| [82] |
| PTEN |
|
|
| [83] |
| Inflammatory pathway | ||||
| NLRP3 |
|
|
| [84,85] |
| ASC |
|
|
| [86] |
| Caspase-1 |
|
|
| [87] |
| IL-1β |
|
|
| [87] |
| IL-18 |
|
|
| [88,89] |
| p53 tumor suppressor network | ||||
| p53 |
|
|
| [90] |
| MDM2 |
|
|
| [91] |
| p21 |
|
|
| [92,93] |
| BAX/BAK |
|
|
| [94,95] |
| Hypoxia-Inducible Factor (HIF) pathway | ||||
| HIF-1α |
|
|
| [96,97] |
| HIF-2α |
|
|
| [98,99] |
| VEGF |
|
|
| [43,100] |
| GLUT1 |
|
|
| [101] |
| Oral microbiome pathway | ||||
| Microbial sp. | Pathway targets | Diabetes implications | OC implications | References |
| Fusobacterium nucleatum | TLR4/NF-κB, Wnt/β-catenin | Inflammation | Tumor promotion | [102,103] |
| Porphyromonas gingivalis | NLRP3, PI3K/AKT | Insulin resistance | DNA damage, tumor induction, and progression | [103,104] |
| Candida albicans | NF-κB, complement | Immune dysfunction | DNA damage | [103,105] |
3.3.2. Inflammatory Pathway
Inflammatory mediators create a permissive environment for both metabolic dysfunction and tumor development (Table 4). NOD-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) has a complex role in the innate immune system and cell metabolism [106]. The NLRP3 inflammasome serves as a molecular platform that detects metabolic stress and pathogen-associated signals, triggering inflammatory responses that lead to DM complications and the progression of OC [107].
In diabetes, persistent hyperglycemia and metabolic dysregulation lead to chronic NLRP3 activation in pancreatic β-cells, adipose tissue, and vasculature [107]. Moreover, metabolic stressors such as hyperglycemia, hyperlipidemia, and free fatty acids can trigger inflammasome activation [39]. Free fatty acids and advanced glycation end products, mitochondrial dysfunction, and oxidative stress also activate these mechanisms (Table 4).
In OSCC, NLRP3 contributes to cancer cell proliferation, epithelial–mesenchymal transition (EMT), and enhanced metastatic potential by activating NF-κB and STAT3 signaling pathways [108]. Carcinogen exposure, DNA damage, tumor microenvironment factors, oral microbiota dysbiosis, and bacterial components activate NLRP3 inflammasome signaling [109].
Apoptosis-associated speck-like protein containing a CARD (ASC) is a central adaptor protein for inflammasome formation [110]. The ASC inflammasome stands as a pivotal player in the onset of diabetes, intricately linking chronic inflammation to the troubling phenomena of insulin resistance and pancreatic dysfunction. Acting as a vital adapter protein, it skillfully assembles with notable partners such as NLRP3 and procaspase-1 to detect metabolic stress signals—including free fatty acids, cholesterol, and islet amyloid polypeptide —substances all too common in the landscape of diabetes. This assembly triggers the activation of pro-inflammatory cytokines, particularly IL-1β and IL-18, which not only fuel insulin resistance but also damage pancreatic beta cells and intensify the complications associated with DM [111,112]. Studies using OSCC cell lines have shown that high ASC expression levels enhance cell migration and invasion. In vivo experiments have confirmed that ASC overexpression promotes the metastasis of OSCC cells [113]. Caspases and inflammatory cytokines IL1β and IL18 are overexpressed in DM and OC development [114,115,116,117] (Table 4)
3.3.3. p53 Tumor Suppressor Network
The p53 tumor suppressor pathway integrates multiple stress signals and coordinates cellular responses, including DNA repair, cell cycle arrest, and apoptosis [118]. The p53 mutation (TP53) is responsible for the onset and progression of both DM and OC [119,120,121,122]. Metformin selectively induces apoptosis in cells lacking functional p53 by activating the AMPK pathway, thereby coordinating metabolic stress response and the DNA damage response to hyperglycemia-induced oxidative stress [90]. This pathway also influences cell cycle checkpoint control, DNA repair coordination, and the induction of apoptosis in damaged cells (Table 4). Moreover, dysregulation of the MDM2-p53 pathway affects mitochondrial metabolism and contributes to inflammation and cellular damage in DM and OC [123,124]. The p21 protein is a cell cycle inhibitor [125]. p21 significantly influences the development of diabetes mellitus through multiple mechanisms. It plays an essential role in the function of pancreatic β-cells, which produce insulin to regulate blood sugar levels. In individuals with type 2 DM, p21 levels are often elevated in the pancreas, contributing to inflammation and reducing insulin production [126]. In OC patients, high p21 levels are associated with metastases [127,128]. In a healthy cell, the Bcl-2 proteins BAX and BAK are inactive and spread throughout the cytosol. When stress signals are received, they are transduced and transported to the outer mitochondrial membrane, where they form pores that release pro-apoptotic factors, such as cytochrome c. This release initiates a cascade of events leading to programmed cell death (apoptosis) [129]. In DM, both proteins induce pancreatic cell death [130], while in OC, they induce tumor cell apoptosis [131] (Table 4).
3.3.4. Hypoxia-Inducible Factor (HIF) Pathways
HIF pathways coordinate cellular responses to hypoxia and metabolic stress, playing substantial roles in both diabetic complications and cancer progression [99,132] (Table 4). HIF-1α drives metabolic reprogramming in both diabetes and cancer, promoting aerobic glycolysis. In diabetes, elevated glucose levels induce HIF-1α activity, leading to a switch from oxidative to glycolytic metabolism and a Warburg-like metabolic phenotype. Vascular Endothelial Growth Factor (VEGF) is significantly higher in oral cancer. It plays a key role in promoting tumor growth, proliferation, and metastasis by stimulating the formation of new blood vessels (angiogenesis). Elevated levels of VEGF in DM are linked with DM retinopathy [133], while in OSCC, they have been associated with lymph node metastasis and a poorer prognosis [134]. Research indicates that changes in glucose metabolism occur early in the development of oral cancer. The notable increase in GLUT1 expression [135] is closely linked to lymph node status and is associated with poor outcomes and reduced survival in patients with OC.
3.3.5. Oral Microbiome Pathway
The oral microbiome comprises over 700 bacterial species, with periodontal pathogens playing crucial roles in both local and systemic pathology [136]. Emerging evidence reveals bidirectional relationships between oral pathogens and systemic diseases, particularly DM and OC (Figure 1) [102,137,138]. Therefore, periodontal health is a modifiable risk factor for both conditions [139]. Periodontal disease affects glycemic control while hyperglycemia promotes pathogen proliferation, creating a vicious cycle [140]. Pathogenic oral bacteria produce carcinogenic metabolites and promote chronic inflammation that drives malignant transformation; the neoplastic tissues are rich in most of them [141].
The essential bacterial and fungal pathogens associated with the induction and progression of OC in patients with diabetes are illustrated in Table 4.
Gram-Negative Bacteria
Gram-negative periodontal pathogenic bacteria, P. gingivalis and F. nucleatum, secrete lipopolysaccharides (LPS), which subsequently induce the release of pro-inflammatory cytokines. Among these cytokines are tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP) [103]. These inflammatory mediators have been shown to impair insulin signaling, increase insulin resistance, and exacerbate hyperglycemia. Furthermore, persistent periodontal infections have been strongly correlated with elevated hemoglobin A1c (HbA1c), a widely recognized key indicator of long-term glycemic control [142]. These Gram-negative periodontal pathogens also play a pivotal role in promoting carcinogenesis [143].
P. gingivalis disrupts the body’s insulin signaling pathways, leading to insulin resistance and potentially increasing the risk of DM [144,145]. It potentiates stem-like properties of oral squamous cell carcinoma by modulating SCD1-dependent lipid synthesis via the NOD1/KLF5 axis [146]. P. gingivalis can promote OC development by inducing epithelial–mesenchymal transition (EMT), altering the tumor immune microenvironment, protecting cancer cells from macrophage attack, and developing drug resistance [147,148,149,150]. It has shown high development in patients with diabetes and OC [151].
F. nucleatum accelerates cancer development via the adhesin FadA, triggering the Wnt/β-catenin signaling pathway. Bacterial lipopolysaccharides stimulate TLR4, inducing inflammation and activating NF-κB, while microbial metabolites modulate host pathway activity (Table 3) [152]. F. nucleatum possesses the remarkable ability to suppress the activity of natural killer (NK) cells while simultaneously drawing myeloid-derived suppressor cells (MDSCs) to the site of infection. This intricate interplay can indirectly foster the emergence of cancer. The pivotal factor in this process is the bacterial virulence factor FAP2, which adeptly binds to and obstructs the NK receptor TIGIT. As a result, the immune system’s powerful NK cells are unable to mount an effective attack on tumor cells, allowing cancer to gain a foothold [153].
C. albicans
DM patients exhibit increased susceptibility to C. albicans oral infections, attributed to higher salivary glucose levels, reduced salivary flow, microvascular degeneration, generalized immunosuppression, and impaired neutrophil candidacidal activity [154,155]. Recent studies reported a high occurrence of this opportunistic fungus in periodontal disease; its hyphae were found in the connective tissue of periodontal patients in association with highly invasive anaerobic bacteria, such as P. gingivalis [156]. C. albicans can directly lead to OC development through carcinogenic enzymes and other compounds (nitrosamines, acetaldehyde) that can cause DNA damage and inhibit DNA repair mechanisms, thus inducing genetic mutations and chromosomal abnormalities [157]. By indirectly binding glutathione, acetaldehyde increases the ROS levels, promotes chronic inflammation, and causes mitochondrial damage [158]. C. albicans infection promotes the expression of interleukin-17A (IL-17A) and its receptor (IL-17RA) in OC cells and macrophages. Therefore, the increased IL-17A/IL-17RA signaling activates macrophages, promoting the release of inflammatory cytokines and thereby enhancing the proliferation of OC cells. [159]. C. albicans upregulates the expression of programmed death-ligand 1 (PD-L1) in OC cells, leading to an inhibition of T cell activation and proliferation [160]. Moreover, recent studies reported that, by inducing lipid droplet formation, C. albicans biofilm may contribute to the development and progression of OC and decrease the efficacy of chemotherapeutic drugs [161].
3.3.6. NF-κB Transcriptional Network
NF-κB serves as a central transcriptional hub coordinating inflammatory responses, metabolic regulation, and cancer progression. Hyperglycemia, insulin resistance, and AGE/RAGE signaling activate the IKK complex, thereby amplifying the production of pro-inflammatory cytokines [54,56]. On the other hand, F. nucleatum promotes cancer progression by stimulating TLR4/MyD88 signaling to activate NF-κB, oncogenic pathways, and resistance to apoptosis [162].
Fan et al. identified eight significant genes that were immunologically related cross-talk genes related to OC and T2DM: C1QC (Complement C1q subcomponent, C chain), NOS2 (Nitric Oxide Synthase 2), ABCD1 (ATP-Binding Cassette Sub-Family D Member 1), PD1A4 (PD-1 (Programmed Cell Death Protein 1) Auxiliary Protein 4), ALOX15 (Arachidonate Lipoxygenase 15), CSE1L (Chromosome Segregation 1-Like), IL1RN (Interleukin 1 Receptor Antagonist), and PSMC4 (Proteasome 26S Subunit ATPase 4) [163].
A noteworthy study explored the impact of the LDL (low-density lipoprotein) receptor-related protein 1B polymorphism in diabetic patients diagnosed with OC. It revealed a significant association between the minor allele of rs10496915 and larger tumor size among these patients. Additionally, the heterozygous genotype and specific combinations of minor alleles of rs6742944 were associated with lymph node metastasis and more advanced clinical stages of this neoplastic disease [164]. These findings shed light on the complex ways in which genetic variations influence oral cancer characteristics in individuals with diabetes, thereby enhancing our understanding of this intricate relationship.
A recent study identified four genes (CYP2C19, NLRP3, PVT1, and APP) that appear central to DM’s influence on HNSCC via the protein–protein interaction (PPI) network [165].
3.4. Molecular Biomarkers with Clinical Significance in Diabetes and Oral Cancer
Due to their key functions, numerous molecular biomarkers involved in various interconnected pathways of diabetes and OC can be helpful in clinical practice, including diagnosis, prognosis, and monitoring of therapy [117,166,167,168] (Table 5).

Table 5.
Molecular biomarkers with clinical applications in OC and DM.
4. Oral Cancer Treatment in Diabetes Patients
Oral squamous cell carcinoma (OSCC) patients with higher preoperative HbA1c levels had more extended hospitalization and worse survival outcomes [169,170]. While high fasting insulin levels are a significant risk factor for OSCC and are associated with an increased risk of developing the disease, they could be used for the direct early detection of OC [13,172,201]. However, the focus remains on lifestyle changes and monitoring for cancer development in those with signs of insulin resistance [172]. Elevated levels of the protein IGF2BP2 are associated with increased cell proliferation, enhanced cell invasion and metastasis, and altered tumor-infiltrating immune cells in OSCC [202].
Given these metabolic considerations, anti-diabetic drugs are the most promising medications for the treatment of patients with DM and OC [203].
4.1. Metformin (Biguanides)
A meta-analysis of 22 randomized controlled trials comprising 5943 participants with various cancer types [204]. It found that the pooled hazard ratios were not statistically significant for progression-free survival (HR, 0.97; 95% CI, 0.82–1.15) and overall survival (HR, 0.98; 95% CI, 0.86–1.13) in patients with cancer between the metformin and control groups [204]. However, metformin appears to be the most promising among the anti-diabetic agents for treating oral cancer. There is evidence from both in vitro and in vivo studies for anti-tumor effects, especially in OSCC.
In vitro and xenograft model experiments revealed that metformin inhibited the growth and metastasis of OSCC cells, with metformin-restrained tumorigenesis accompanied by a substantial decrease in both Aurora-A and Late SV40 Factor (LSF) expressions [205]. It reduced OSCC progression by regulating RNA alternative splicing, promoting NUBP2 splicing to increase the canonical full-length isoform, thereby inhibiting cancer cell proliferation [206]. Moreover, metformin enhanced cisplatin cytotoxicity and reversed chemoresistance in OSCC by inhibiting the NF-κB/HIF-1α signal axis and downregulating hypoxia-regulated genes, potentially serving as a chemosensitizer for cisplatin-based regimens [207].
Clinical trial results suggest that patients with diabetes treated with metformin had a lower incidence of head and neck cancer, improved overall survival, and cancer-specific survival compared to both non-diabetic patients and patients not treated with metformin [208]. There remains high heterogeneity among the study results.
An open-label Phase IIa clinical trial in 35 individuals with oral premalignant lesions (mild to moderate dysplasia) demonstrated that 60% of participants exhibited histological responses after 3 months of metformin treatment, including 17% with complete responses. It occurred concomitant with reduced cell proliferation and modulation of the mTOR pathway, without affecting circulating glucose or C-peptide levels, and independently of participants’ BMI. A significant correlation was found between decreased mTOR activity and both histological (p = 0.04) and clinical (p = 0.01) responses [209]. This mechanism was further explored, and results suggested that metformin prevented the development of OSCC by significantly reducing the size and number of carcinogen (4-nitroquinoline-1-oxide, 4NQO)-induced oral premalignant lesions and preventing their spontaneous conversion into OSCC in mice in vivo [210].
A study by Madera et al. demonstrated that high OCT3 expression in oral premalignant and malignant lesions enables metformin to inhibit mTOR signaling, with specific effects on mTORC1 and OSCC progression, both in vitro and in vivo. It provides a mechanistic basis for patient stratification in metformin chemoprevention trials [211].
A cohort study with propensity score analysis included 138 patients with T2DM and OSCC who underwent radical surgical treatment and investigated the impact of T2DM and metformin on recurrence and survival outcomes. Results showed that T2DM was associated with a higher risk of OSCC recurrence; however, metformin treatment significantly reduced this increased recurrence risk compared to diabetic patients not receiving metformin, both before (p = 0.005) and after (p = 0.002) propensity score matching. Apparently, metformin treatment can counteract the negative prognostic impact of T2DM on OSCC recurrence [212].
Metformin treatment reduced the proliferative capacity of OSCC cells by decreasing mTOR and AKT activity, activating AMPK, and suppressing cancer stemness gene-expression programs, while enhancing their commitment to terminal differentiation. The findings revealed that metformin’s anti-tumor effects are concentration-dependent and occur at physiologically relevant doses, providing mechanistic evidence that metformin directly targets carcinoma-initiating cells and supporting its potential for stratified patient selection in OSCC prevention and treatment trials [213].
Based on all available evidence, metformin could serve as a potential adjuvant therapy in HNSCC [214].
4.2. GLP-1 Agonists
Obesity is increasingly recognized as a significant cause of cancer in the United States and around the world. However, no medications have been proven to reduce the cancer risk associated with obesity. Recent studies aimed to address this gap by evaluating GLP-1 receptor agonists, widely prescribed to treat diabetes, obesity, and related conditions. The findings suggest that GLP1-RAs may modestly reduce the risk of obesity-related cancers and lower overall mortality [215,216]. While the reported results are encouraging, further studies are needed to establish a clear cause-and-effect relationship.
4.2.1. Semaglutide
A recent study by Wang et al. explored the potential use of semaglutide as a treatment for oral squamous cell carcinoma and the mechanisms underlying its benefits [217]. The study utilized 10 samples of OSCC tissue, 10 samples of normal oral epithelial tissue, and CFPAC-1 cells (human pancreatic cancer cells). In cell culture experiments, semaglutide demonstrated dose-dependent anticancer activity, reducing proliferation markers (Ki-67 and PCNA) and inhibiting epithelial–mesenchymal transition, as well as OSCC cell proliferation [217]. Additionally, semaglutide increased E-cadherin expression and decreased Vimentin expression, thereby suppressing migration, invasion, and epithelial–mesenchymal transition in OSCC cells. In xenograft mouse models, semaglutide significantly reduced tumor volume and weight by increasing E-cadherin, Bax, and GLP-1R expression and decreasing Ki-67, PCNA, Vimentin, and Bcl-xL [217]. It confirms the in vitro findings. Also, semaglutide induced specific activation of the P38 MAPK pathway, leading to increased apoptosis in OSCC tissue [217]. The authors note that clinical validation is essential to confirm therapeutic potential and long-term safety, and future work should explore the combination of semaglutide with existing OSCC treatments, such as chemotherapy or immunotherapy [217].
4.2.2. Liraglutide
Liraglutide has shown anti-tumor effects in several cancer types (hepatic, prostate, pancreatic, breast, colorectal, thyroid, lung, and endometrial) [218,219,220,221,222,223,224,225,226,227,228,229,230]. However, Liu et al. found that liraglutide promoted breast cancer through NOX4/ROS/VEGF pathway activation, directly contradicting other breast cancer studies [224]. There is no published research on its use specifically in OCs. The concentration-dependent effects and contradictory findings in different cancer types suggest caution is needed, and clinical trials would be necessary before considering it for OC treatment.
While semaglutide shows promising preclinical activity against OSCC via p38 MAPK-mediated apoptosis, and liraglutide demonstrates anticancer effects in multiple malignancies (with notable contradictions), the complete absence of clinical data specific to OC precludes any therapeutic recommendations supporting GLP-1 agonist use for oral cancer.
4.2.3. SGLT Inhibitors
SGLT2 inhibitors have emerged as promising agents in the battle against cancer, demonstrating notable anticancer effects across various malignancies, including breast, liver, pancreatic, thyroid, prostate, and lung cancers. Their efficacy is attributed to several mechanisms: they induce mitochondrial membrane instability, inhibit key signaling pathways such as β-catenin and PI3K/AKT/mTOR, promote cell cycle arrest and apoptosis, and downregulate oxidative phosphorylation [231,232].
Recently, an in vitro study on two OSCC cell lines (SCCL-MT1 and UPSI-SCC-131) reported that Empafliglozin (Emp), an SGLT2 inhibitor, effectively suppresses SLC2A3 and NLRP3 expression, resulting in a notable reduction in tumor cell proliferation and migration [233]. By disrupting glucose metabolism and alleviating chronic inflammation through NLRP3 inhibition, Emp has the potential to lessen the aggressive behavior of OSCC. These insights not only highlight Emp’s promise as a therapeutic agent for OSCC but also underscore the necessity for further research to confirm its clinical efficacy [233].
On the other hand, research indicates that SGLT1 (not SGLT2) is expressed in oral squamous cell carcinoma, correlates with tumor differentiation and a poor prognosis, and may be theoretically relevant for OC [234,235,236]. Still, no clinical trials have been conducted.
4.3. Molecular Biomarkers as Therapeutic Targets in Diabetes and Oral Cancer
The intersection of diabetes and OC represents a critical frontier in precision medicine, where shared molecular pathways offer numerous other therapeutic opportunities (Table 6).

Table 6.
Molecular biomarkers as therapeutic targets in DM and OC.
5. Methodological Limitations and Further Research
While the epidemiological literature consistently demonstrates associations between DM and oral cancer, significant methodological limitations limit causal inference. There is heterogeneity across studies concerning diabetes classification (self-report versus biochemical confirmation), disease duration, treatment regimens, and glycemic control, variables that can influence both metabolic status and cancer risk.
The majority of mechanistic data linking diabetes to oral carcinogenesis derives from in vitro experiments using cancer cell lines exposed to supraphysiological glucose concentrations or from xenograft models that may not recapitulate the chronic, heterogeneous metabolic milieu of diabetes in humans.
Critical evidence gaps include: the absence of longitudinal studies measuring proposed mediators (insulin, IGF-1, inflammatory cytokines, AGEs) before OC diagnosis; limited comparative analyses of pathway activation in oral tumor tissues from diabetic versus non-diabetic patients; and insufficient integration of genetic susceptibility factors that may independently predispose individuals to both conditions. The temporal relationship remains unclear: do diabetes-related molecular changes precede malignant transformation, or do both conditions share common upstream causes, such as chronic inflammation or mitochondrial dysfunction?
While specific pathogens (P. gingivalis, F. nucleatum, C. albicans) are consistently associated with both conditions, establishing causality versus consequence remains challenging. Diabetic hyperglycemia creates favorable conditions for pathogen proliferation; however, periodontal disease itself may worsen glycemic control, creating a bidirectional relationship.
Considering the limitations mentioned above, we suggest further research in these directions:
- Prospective longitudinal studies measuring proposed molecular mediators (insulin, IGF-1, inflammatory cytokines, AGEs) before cancer diagnosis to establish temporal causality rather than correlation.
- Direct validation of pathway dysregulation (PI3K/AKT/mTOR, HIF-1α, NF-κB) in human oral tissues comparing diabetic versus non-diabetic patients while controlling for confounding factors like smoking and alcohol use.
- Microbiome intervention trials testing whether periodontal treatment or antimicrobial strategies reduce cancer incidence in diabetic populations, combined with metagenomic analyses that identify functional pathways beyond taxonomic associations.
- Mendelian randomization and genetic studies to strengthen causal inference and identify shared susceptibility loci.
- Biomarker discovery and validation for early detection, specifically in diabetic populations.
- Randomized controlled trials evaluating enhanced screening protocols and integrated diabetes-dental care models to determine whether intensified surveillance or glycemic optimization strategies could reduce OC morbidity and mortality in this high-risk population.
6. Materials and Methods
A comprehensive search of recent literature was conducted across multiple electronic databases, including PubMed/MEDLINE, Embase, Cochrane Library, and Web of Science, from January 2010 to September 2025. The search strategy employed a combination of terms and keywords related to “oral cancer,” “diabetes mellitus,” “molecular biomarkers,” “HIF-1α,” “metabolic pathways,” “inflammatory markers,” “oral microbiota”, “metformin,” antidiabetic drugs,” “GLP-1 receptor agonists,” “SGLT2 inhibitors,” and “therapeutic targets.” Two independent reviewers screened titles and abstracts, with full-text articles retrieved for potentially eligible studies. Reference lists of included studies and relevant systematic reviews were manually searched to identify additional studies.
Due to the heterogeneity in study designs, populations, interventions, and outcomes, a state-of-the-art synthesis approach was employed rather than quantitative meta-analysis. The synthesis was organized thematically around:
- Molecular mechanisms: Grouped by biomarker categories.
- Therapeutic interventions: Organized by drug class with emphasis on mechanism of action and clinical evidence.
- Clinical implications: Integration of molecular insights with therapeutic potential.
For studies reporting quantitative outcomes, effect sizes were extracted and presented with 95% confidence intervals where available.
7. Conclusions and Clinical Implications
The present review highlights that the analyzed literature data overwhelmingly support DM as a significant risk factor for OC development. Multiple biological pathways, including hyperinsulinemia, hyperglycemia, chronic inflammation, and immune dysfunction, contribute to this association through complex molecular mechanisms. This relationship is powerful in specific populations and is associated with worse clinical outcomes. Significant clinical implications may involve (a) risk recognition (diabetic patients should be recognized as a high-risk population for oral cancer), (b) enhanced screening (more frequent and thorough OC screening is warranted in diabetic patients), (c) optimal diabetes management (reasonable glycemic control may help reduce OC risk), (d) metformin consideration (metformin therapy may provide additional protective benefits beyond glucose control) and (e) multidisciplinary care (coordination between diabetes specialists and oral health professionals is essential).
The growing evidence establishing diabetes as a risk factor for oral cancer has important implications for clinical practice, public health policy, and future research directions. Healthcare providers caring for patients with diabetes should be aware of this association and implement appropriate screening and prevention strategies, as follows: (1) maintaining an optimal body weight, (2) avoiding destructive habits (alcohol consumption and smoking), (3) regular dental check-ups for diabetic patients, (4) early intervention strategies, and (5) a multidisciplinary approach to managing diabetes and oral health.
Author Contributions
Conceptualization, V.E., V.P. and M.I.N.; methodology, M.I.N., A.M.N. and S.M.A.; investigation, V.E., V.P., M.I.N., A.M.N., S.M.A. and E.A.O.; resources, V.E., V.P., M.I.N., A.M.N., S.M.A. and E.A.O.; writing—original draft preparation, V.E., V.P., M.I.N., A.M.N., S.M.A. and E.A.O.; writing—review and editing, V.E. and V.P.; project administration, V.E. and M.I.N.; funding acquisition, V.P. and E.A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This paper has no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
During the preparation of this manuscript, the authors used Grammarly Premium to improve English clarity, https://app.grammarly.com/, accessed on 31 October 2025. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OC | Oral cancer |
| OSCC | Oral squamous cell carcinoma |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| DM | Diabetes mellitus |
| HR | Hazard ratio |
| IL | Interleukin |
| IGF | Insulin-like growth factor |
| IRS | Insulin-Receptor substrate |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | Protein kinase B |
| mTOR | Mammalian Target of Rapamycin |
| PTEN | Phosphatase and tensin homolog deleted on chromosome 10 |
| TNF | Tumor necrosis factor |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| p-53 | A stress-sensitive tumor suppressor protein |
| p-21 | A protein that regulates the cell cycle |
| MDM2 | Murine Double Minute 2–an oncogene |
| BAK | Bcl-2 homologous antagonist/killer |
| BAX | family of proteins involved in inducing cell death |
| VEGF | Vascular Endothelial Growth Factor |
| GLUT | Glucose Transporter |
| CRP | C-Reactive Protein |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| TH1/TH2 | Type 1/2 T helper cells |
| PD-1/PD-L1 | An immune checkpoint pathway where the receptor PD-1 on immune cells interacts with its ligand PD-L1, primarily found on cancer cells, to suppress T cell activity and enable cancer to evade the immune system. |
| CCLCX1 | A chemokine that functions as a signaling molecule to recruit immune cells, particularly neutrophils, to sites of infection and inflammation |
| HbA1c | Glycated hemoglobin |
| IGFBP3 | Insulin-like Growth Factor Binding Protein-3. |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| MDA | Malonyl Dialdehyde |
| ADIPOQ | Adiponectin gene |
| TCF7L2 | Transcription factor 7-like 2 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| AGE | advanced glycation end products |
| RAGE | Receptors for AGE |
| IFN-γ | Interferon γ |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HIF-2α | Hypoxia-inducible factor 2-alpha |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| RNS | Reactive nitrogen species |
| CFPAK-1 | A pancreatic adenocarcinoma cell line |
| PCNA | Proliferating Cell Nuclear Antigen |
| Ki67 | Antigen Kiel 67 |
| Bcl-x | B-cell lymphoma-X |
| JNK | c-Jun N-terminal kinase |
| MAPK | p38 mitogen-activated protein kinase |
| AP-1 | Activator Protein-1 |
| IL-6 | Interleukin 6 |
| JAK2 | Janus kinase 2 |
| STAT3 | signal transducer and activator of transcription 3 |
| Emp | Empagliflozine |
References
- Rodríguez-Molinero, J.; Migueláñez-Medrán, B.d.C.; Puente-Gutiérrez, C.; Delgado-Somolinos, E.; Martín Carreras-Presas, C.; Fernández-Farhall, J.; López-Sánchez, A.F. Association between Oral Cancer and Diet: An Update. Nutrients 2021, 13, 1299. [Google Scholar] [CrossRef]
- Saka-Herrán, C.; Jané-Salas, E.; Mari-Roig, A.; Estrugo-Devesa, A.; López-López, J. Time-to-Treatment in Oral Cancer: Causes and Implications for Survival. Cancers 2021, 13, 1321. [Google Scholar] [CrossRef] [PubMed]
- Senevirathna, K.; Jayawardana, N.; Udumalagala Gamage, C.; Perera, U.; Jayasinghe, R.; Seneviratne, B. Use of Oral Squamous Cell Carcinoma: A Discussion Paper. Int. Health Trends Perspect. 2023, 3, 1–15. [Google Scholar] [CrossRef]
- Huber, M.A.; Tantiwongkosi, B. Oral and Oropharyngeal Cancer. Med. Clin. N. Am. 2014, 98, 1299–1321. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Living with Oral Cancer: Epidemiology with Particular Reference to Prevalence and Life-Style Changes That Influence Survival. Oral Oncol. 2010, 46, 407–410. [Google Scholar] [CrossRef]
- Garavello, W.; Bertuccio, P.; Levi, F.; Lucchini, F.; Bosetti, C.; Malvezzi, M.; Negri, E.; La Vecchia, C. The Oral Cancer Epidemic in Central and Eastern Europe. Int. J. Cancer 2010, 127, 160–171. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, R.; Acharya, A.K.; Patthi, B.; Goud, V.; Reddy, S.; Garg, A.; Singla, A. Changing Trends in Oral Cancer—A Global Scenario. Nepal J. Epidemiol. 2017, 6, 613–619. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Chen, X.; Wang, C.; Zhang, J.; Ren, X.; Wang, Y.; Tao, X.; Li, H.; Song, M.; et al. Diet-Induced Obesity Accelerates Oral Carcinogenesis by Recruitment and Functional Enhancement of Myeloid-Derived Suppressor Cells. Cell Death Dis. 2021, 12, 946. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why Does Obesity Cause Diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Elian, V.; Popovici, V.; Karampelas, O.; Pircalabioru, G.G.; Radulian, G.; Musat, M. Risks and Benefits of SGLT-2 Inhibitors for Type 1 Diabetes Patients Using Automated Insulin Delivery Systems—A Literature Review. Int. J. Mol. Sci. 2024, 25, 1972. [Google Scholar] [CrossRef]
- Abhinav, R.P.; Williams, J.; Livingston, P.; Anjana, R.M.; Mohan, V. Burden of Diabetes and Oral Cancer in India. J. Diabetes Complicat. 2020, 34, 107670. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H. Oral Cancer in Taiwan: Is Diabetes a Risk Factor? Clin. Oral Investig. 2013, 17, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Vegh, A.; Banyai, D.; Ujpal, M.; Somogyi, K.S.; Biczo, Z.; Kammerhofer, G.; Nemeth, Z.; Hermann, P.; Payer, M.; Vegh, D. Prevalence of Diabetes and Impaired Fasting Glycemia in Patients with Oral Cancer: A Retrospective Study in Hungary. Anticancer Res. 2022, 42, 109–113. [Google Scholar] [CrossRef]
- Goutzanis, L.; Vairaktaris, E.; Yapijakis, C.; Kavantzas, N.; Nkenke, E.; Derka, S.; Vassiliou, S.; Acil, Y.; Kessler, P.; Stavrianeas, N.; et al. Diabetes May Increase Risk for Oral Cancer through the Insulin Receptor Substrate-1 and Focal Adhesion Kinase Pathway. Oral Oncol. 2007, 43, 165–173. [Google Scholar] [CrossRef]
- Ramos-Garcia, P.; Roca-Rodriguez, M.d.M.; Aguilar-Diosdado, M.; Gonzalez-Moles, M.A. Diabetes Mellitus and Oral Cancer/Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis. Oral Dis. 2021, 27, 404–421. [Google Scholar] [CrossRef]
- Gong, Y.; Wei, B.; Yu, L.; Pan, W. Type 2 Diabetes Mellitus and Risk of Oral Cancer and Precancerous Lesions: A Meta-Analysis of Observational Studies. Oral Oncol. 2015, 51, 332–340. [Google Scholar] [CrossRef]
- Verhulst, M.J.L.; Loos, B.G.; Gerdes, V.E.A.; Teeuw, W.J. Evaluating All Potential Oral Complications of Diabetes Mellitus. Front. Endocrinol. 2019, 10, 56. [Google Scholar] [CrossRef]
- Remschmidt, B.; Pau, M.; Gaessler, J.; Zemann, W.; Jakse, N.; Payer, M.; Végh, D. Diabetes Mellitus and Oral Cancer: A Retrospective Study from Austria. Anticancer Res. 2022, 42, 1899–1903. [Google Scholar] [CrossRef]
- Ghanem, A.S.; Nagy, A.C. Oral Health’s Role in Diabetes Risk: A Cross-Sectional Study with Sociodemographic and Lifestyle Insights. Front. Endocrinol. 2024, 15, 1342783. [Google Scholar] [CrossRef]
- Wu, C.-H.; Wu, T.-Y.; Li, C.-C.; Lui, M.-T.; Chang, K.-W.; Kao, S.-Y. Impact of Diabetes Mellitus on the Prognosis of Patients with Oral Squamous Cell Carcinoma: A Retrospective Cohort Study. Ann. Surg. Oncol. 2010, 17, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Zhang, T.; Cai, L.; Dai, E.; He, J. Diabetes and Its Potential Impact on Head and Neck Oncogenesis. J. Cancer 2020, 11, 583–591. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Cho, J.; Rothman, J.; Zeng, C.; Wilson, M.; Kern, L.M.; Tamimi, R.M.; Safford, M.M. Diabetes and Cancer Co-Management: Patient-Reported Challenges, Needs, and Priorities. Support. Care Cancer 2023, 31, 145. [Google Scholar] [CrossRef]
- Shahid, R.K.; Ahmed, S.; Le, D.; Yadav, S. Diabetes and Cancer: Risk, Challenges, Management and Outcomes. Cancers 2021, 13, 5735. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Z.; Zhang, L. Impact of Diabetes on the Prognosis of Patients with Oral and Oropharyngeal Cancer: A Meta-Analysis. J. Diabetes Investig. 2024, 15, 1140–1150. [Google Scholar] [CrossRef]
- Priyadharsini, J.V.; Pandi, A. A Review on Diabetes and Oral Cancer: Molecular Links and Implications. Mol. Biol. Res. Commun. 2025, 14, 109–113. [Google Scholar] [CrossRef]
- De Falco, V.; Vitale, P.; Brancati, C.; Cicero, G.; Auriemma, A.; Addeo, R. Prognostic Value of Diabetes and Metformin Use in a Real-Life Population of Head and Neck Cancer Patients. Front. Med. 2023, 10, 1252407. [Google Scholar] [CrossRef]
- Vinay Deshmukh, C.; Suresh Dodamani, A.; Dilip Mistry, V.; Bhikaji Nepale, M.; Sakharam Patil, S.; Sanjay Ahirrao, S. Biological Mechanisms Linking Diabetes Mellitus and Oral Carcinogenesis: A Review. Acta Sci. Dent. Sci. 2025, 9, 9–14. [Google Scholar]
- Kim, G.-J.; Han, K.-D.; Joo, Y.-H. Association of Metabolic Syndrome with the Risk of Head and Neck Cancer: A 10-Year Follow-Up Study of 10 Million Initially Healthy Individuals. Cancers 2023, 15, 4118. [Google Scholar] [CrossRef]
- Adnan, Y.; Ali, S.M.A.; Awan, M.S.; Zahid, N.; Awan, M.O.; Afzal Kayani, H.; Farooqui, H.A. Body Mass Index and Diabetes Mellitus May Predict Poorer Overall Survival of Oral Squamous Cell Carcinoma Patients: A Retrospective Cohort from a Tertiary-Care Centre of a Resource-Limited Country. Clin. Med. Insights Oncol. 2022, 16, 11795549221084832. [Google Scholar] [CrossRef]
- Ranc, K.; Jørgensen, M.E.; Friis, S.; Carstensen, B. Mortality after Cancer among Patients with Diabetes Mellitus: Effect of Diabetes Duration and Treatment. Diabetologia 2014, 57, 927–934. [Google Scholar] [CrossRef]
- Végh, D.; Bányai, D.; Hermann, P.; Németh, Z.; Ujpál, M. Type-2 Diabetes Mellitus and Oral Tumors in Hungary: A Long-Term Comparative Epidemiological Study. Anticancer Res. 2017, 37, 1853–1857. [Google Scholar] [CrossRef]
- Duan, W.; Shen, X.; Lei, J.; Xu, Q.; Yu, Y.; Li, R.; Wu, E.; Ma, Q. Hyperglycemia, a Neglected Factor during Cancer Progression. BioMed Res. Int. 2014, 2014, 461917. [Google Scholar] [CrossRef]
- Ramteke, P.; Deb, A.; Shepal, V.; Bhat, M.K. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers 2019, 11, 1402. [Google Scholar] [CrossRef]
- Harborg, S.; Kjærgaard, K.A.; Thomsen, R.W.; Borgquist, S.; Cronin-Fenton, D.; Hjorth, C.F. New Horizons: Epidemiology of Obesity, Diabetes Mellitus, and Cancer Prognosis. J. Clin. Endocrinol. Metab. 2024, 109, 924–935. [Google Scholar] [CrossRef]
- Park, J.B.; Moon, G.H.; Cho, A.; Kwon, M.; Park, J.W.; Yi, E.C.; Kim, H.; Fukuda, J.; Kwak, C.; Ko, Y.G.; et al. Neddylation of Insulin Receptor Substrate Acts as a Bona Fide Regulator of Insulin Signaling and Its Implications for Cancer Cell Migration. Cancer Gene Ther. 2024, 31, 599–611. [Google Scholar] [CrossRef]
- Zhang, X.; Varma, S.; Yee, D. Suppression of Insulin Receptor Substrate 1 Inhibits Breast Cancer Growth In Vitro and in Female Athymic Mice. Endocrinology 2023, 164, bqac214. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P. State of Evidence on Oral Health Problems in Diabetic Patients: A Critical Review of the Literature. J. Clin. Med. 2021, 10, 5383. [Google Scholar] [CrossRef]
- Abhinav, R.P.; Williams, J.; Bennett, C.; Livingston, P.; Jebarani, S.; Pradeepa, R.; Anjana, R.M.; Mohan, V. Frequency and Association of Self-Reported Oral Cancer among Individuals with Type 2 Diabetes at a Tertiary Care Diabetes Centre in South India—A Retrospective Study. J. Diabetes Complicat. 2022, 36, bqab103. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Receptor for AGE (RAGE): Signaling Mechanisms in the Pathogenesis of Diabetes and Its Complications. Ann. N. Y. Acad. Sci. 2011, 1243, 88–102. [Google Scholar] [CrossRef]
- Dobrucki, I.T.; Miskalis, A.; Nelappana, M.; Applegate, C.; Wozniak, M.; Czerwinski, A.; Kalinowski, L.; Dobrucki, L.W. Receptor for Advanced Glycation End-products: Biological Significance and Imaging Applications. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1935. [Google Scholar] [CrossRef]
- Mekala, M.R.; Bangi, B.B.; Jayalatha, N.; Lebaka, R.R.; Nadendla, L.K.; Ginjupally, U. Association of Diabetes with Oral Cancer-an Enigmatic Correlation. Asian Pac. J. Cancer Prev. 2020, 21, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, Y. The Impact of VEGF on Cancer Metastasis and Systemic Disease. Semin. Cancer Biol. 2022, 86, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The Role of VEGF in Cancer-Induced Angiogenesis and Research Progress of Drugs Targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Pisano, M.; Giordano, F.; Sangiovanni, G.; Capuano, N.; Acerra, A.; D’Ambrosio, F. The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiol. Res. 2023, 14, 1862–1878. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chung, S.W.; Auh, Q.-S.; Hong, S.-J.; Lee, Y.-A.; Jung, J.; Lee, G.-J.; Park, H.J.; Shin, S.-I.; Hong, J.-Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef]
- Shaalan, A.; Lee, S.; Feart, C.; Garcia-Esquinas, E.; Gomez-Cabrero, D.; Lopez-Garcia, E.; Morzel, M.; Neyraud, E.; Rodriguez-Artalejo, F.; Streich, R.; et al. Alterations in the Oral Microbiome Associated with Diabetes, Overweight, and Dietary Components. Front. Nutr. 2022, 9, 914715. [Google Scholar] [CrossRef]
- Rai, A.K.; Panda, M.; Das, A.K.; Rahman, T.; Das, R.; Das, K.; Sarma, A.; Kataki, A.C.; Chattopadhyay, I. Dysbiosis of Salivary Microbiome and Cytokines Influence Oral Squamous Cell Carcinoma through Inflammation. Arch. Microbiol. 2021, 203, 137–152. [Google Scholar] [CrossRef]
- Hu, X.; Wu, J.; Xiong, H.; Zeng, L.; Wang, Z.; Wang, C.; Huang, D.; Zhang, T.; Peng, Y.; Chen, W.; et al. Type 2 Diabetes Mellitus Promotes the Proliferation, Metastasis, and Suppresses the Apoptosis in Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2022, 51, 483–492. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Ilea, A.; Băbţan, A.M.; Boşca, B.A.; Crişan, M.; Petrescu, N.B.; Collino, M.; Sainz, R.M.; Gerlach, J.Q.; Câmpian, R.S. Advanced Glycation End Products (AGEs) in Oral Pathology. Arch. Oral Biol. 2018, 93, 22–30. [Google Scholar] [CrossRef]
- Haque, E.; Kamil, M.; Hasan, A.; Irfan, S.; Sheikh, S.; Khatoon, A.; Nazir, A.; Mir, S.S. Advanced Glycation End Products (AGEs), Protein Aggregation and Their Cross Talk: New Insight in Tumorigenesis. Glycobiology 2020, 30, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Palati, S.; Ramani, P.; Sekaran, S. Receptors of Advanced Glycation End Products in Oral Squamous Cell Carcinoma: A Systematic Review. Tumor Discov. 2023, 2, 244. [Google Scholar] [CrossRef]
- Ko, S.-Y.; Ko, H.-A.; Shieh, T.-M.; Chi, T.-C.; Chen, H.-I.; Chen, Y.-T.; Yu, Y.-H.; Yang, S.-H.; Chang, S.-S. Advanced Glycation End Products Influence Oral Cancer Cell Survival via Bcl-Xl and Nrf-2 Regulation in Vitro. Oncol. Lett. 2017, 13, 3328–3334. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-Y.; Ko, H.-A.; Shieh, T.-M.; Chang, W.-C.; Chen, H.-I.; Chang, S.-S.; Lin, I.-H. Cell Migration Is Regulated by AGE-RAGE Interaction in Human Oral Cancer Cells In Vitro. PLoS ONE 2014, 9, e110542. [Google Scholar] [CrossRef]
- Huang, B.; Lang, X.; Li, X. The Role of IL-6/JAK2/STAT3 Signaling Pathway in Cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, Y.; Shi, L.; Li, L.; Zhang, D.; Gong, Z.; Wu, Q. Activation and Modulation of the AGEs-RAGE Axis: Implications for Inflammatory Pathologies and Therapeutic Interventions—A Review. Pharmacol. Res. 2024, 206, 107282. [Google Scholar] [CrossRef]
- Nijenhuis-Noort, E.C.; Berk, K.A.; Neggers, S.J.C.M.M.; van der Lely, A.J. The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin. Endocrinol. Metab. 2024, 39, 83–89. [Google Scholar] [CrossRef]
- LeRoith, D.; Scheinman, E.; Bitton-Worms, K. The Role for Insulin and Insulin-like Growth Factors in the Increased Risk of Cancer in Diabetes. Rambam Maimonides Med. J. 2011, 2, e0043. [Google Scholar] [CrossRef]
- Chen, P.; Lin, C.; Yang, S.; Chang, Y. Oral Submucous Fibrosis Stimulates Invasion and Epithelial-mesenchymal Transition in Oral Squamous Cell Carcinoma by Activating MMP-2 and IGF-IR. J. Cell. Mol. Med. 2021, 25, 9814–9825. [Google Scholar] [CrossRef]
- Zhi, X.; Lamperska, K.; Golusinski, P.; Schork, N.J.; Luczewski, L.; Golusinski, W.; Masternak, M.M. Expression Levels of Insulin-like Growth Factors 1 and 2 in Head and Neck Squamous Cell Carcinoma. Growth Horm. IGF Res. 2014, 24, 137–141. [Google Scholar] [CrossRef]
- Brady, G.; Crean, S.; Naik, P.; Kapas, S. Upregulation of IGF-2 and IGF-1 Receptor Expression in Oral Cancer Cell Lines. Int. J. Oncol. 2007, 31, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Al-Samerria, S.; Radovick, S. The Role of Insulin-like Growth Factor-1 (IGF-1) in the Control of Neuroendocrine Regulation of Growth. Cells 2021, 10, 2664. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Insulin/IGF-1 Signaling Promotes Immunosuppression via the STAT3 Pathway: Impact on the Aging Process and Age-Related Diseases. Inflamm. Res. 2021, 70, 1043–1061. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, X.; Wang, Y.; Sun, G.; Zhang, J.; Li, Z. Obesity and Endocrine-Related Cancer: The Important Role of IGF-1. Front. Endocrinol. 2023, 14, 1093257. [Google Scholar] [CrossRef]
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2021, 10, 615375. [Google Scholar] [CrossRef]
- Kilvert, A.; Fox, C. Hyperinsulinaemia and Cancer Risk: Cause and Effect? Pract. Diabetes 2020, 37, 223–227a. [Google Scholar] [CrossRef]
- Bowers, L.W.; Rossi, E.L.; O’Flanagan, C.H.; deGraffenried, L.A.; Hursting, S.D. The Role of the Insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. Front. Endocrinol. 2015, 6, 77. [Google Scholar] [CrossRef]
- Starzyńska, A.; Sejda, A.; Adamska, P.; Marvaso, G.; Sakowicz-Burkiewicz, M.; Adamski, Ł.; Jereczek-Fossa, B. Prognostic Value of the PIK3CA, AKT, and PTEN Mutations in Oral Squamous Cell Carcinoma: Literature Review. Arch. Med. Sci. 2021, 17, 207–217. [Google Scholar] [CrossRef]
- Dey, S.; Singh, A.K.; Singh, A.K.; Rawat, K.; Banerjee, J.; Agnihotri, V.; Upadhaya, D. Critical Pathways of Oral Squamous Cell Carcinoma: Molecular Biomarker and Therapeutic Intervention. Med. Oncol. 2022, 39, 30. [Google Scholar] [CrossRef]
- Rehim, A.A.; Rohoma, K.; Elwafa, R.A.; Dabees, H.; Amin, N.G. The Relation of Mtor with Diabetic Complications and Insulin Resistance in Patients with Type 2 Diabetes Mellitus. Atherosclerosis 2022, 355, 204. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Maffei, A.; Lembo, G.; Carnevale, D. PI3Kinases in Diabetes Mellitus and Its Related Complications. Int. J. Mol. Sci. 2018, 19, 4098. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Sekar, R.; Dhayasankar, P.S.; Ali, E.M.; Abdelsalam, S.A.; Balaraman, S.; Chellappan, B.V.; Metwally, A.M.; Abdallah, B.M. PI3K/AKT Signaling Pathway Mediated Autophagy in Oral Carcinoma—A Comprehensive Review. Int. J. Med. Sci. 2024, 21, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Devaraji, M.; Thanikachalam, P.V. Targeting the MTOR Pathway: A New Horizon in Oral Cancer Treatment. Oral Oncol. Rep. 2024, 11, 100619. [Google Scholar] [CrossRef]
- Chow, L.M.L.; Baker, S.J. PTEN Function in Normal and Neoplastic Growth. Cancer Lett. 2006, 241, 184–196. [Google Scholar] [CrossRef]
- Li, Y.Z.; Di Cristofano, A.; Woo, M. Metabolic Role of PTEN in Insulin Signaling and Resistance. Cold Spring Harb. Perspect. Med. 2020, 10, a036137. [Google Scholar] [CrossRef]
- Heald, R. PTEN Link between Cancer and Diabetes. Lancet Oncol. 2012, 13, e417. [Google Scholar] [CrossRef]
- Squarize, C.H.; Castilho, R.M.; Abrahao, A.C.; Molinolo, A.; Lingen, M.W.; Gutkind, J.S. PTEN Deficiency Contributes to the Development and Progression of Head and Neck Cancer. Neoplasia 2013, 15, 461–471. [Google Scholar] [CrossRef]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK Signalling: Protein Kinases in Glucose Homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in Cancer: Mechanisms and Advances in Clinical Trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Chang, H.-C.; Yang, C.-C.; Loi, L.-K.; Hung, C.-H.; Wu, C.-H.; Lin, Y.-C. Interplay of P62-MTORC1 and EGFR Signaling Promotes Cisplatin Resistance in Oral Cancer. Heliyon 2024, 10, e28406. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Alzohairy, M.; Babiker, A.Y.; Rizvi, M.A.; Elkarimahmad, H.G. Clinicopathological Significance of PTEN and Bcl2 Expressions in Oral Squamous Cell Carcinoma. Int. J. Clin. Exp. Pathol. 2012, 5, 965–971. [Google Scholar] [PubMed]
- Sharma, B.R.; Kanneganti, T.-D. NLRP3 Inflammasome in Cancer and Metabolic Diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, X.; Zhang, H.; Li, S.; Yang, P.; Tan, Q. Relevance of NLRP3 Inflammasome-Related Pathways in the Pathology of Diabetic Wound Healing and Possible Therapeutic Targets. Oxid. Med. Cell. Longev. 2022, 2022, 9687925. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Y.; Xie, R.; Ning, Z. Metabolomics Uncovers the Diabetes Metabolic Network: From Pathophysiological Mechanisms to Clinical Applications. Front. Endocrinol. 2025, 16, 1624878. [Google Scholar] [CrossRef]
- Tylutka, A.; Walas, Ł.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and Age-Related Diseases: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Feng, Y.; He, R.; Xu, X.; Xie, Y.; Shi, X.; Zhou, M.; Pan, S.; Wang, M.; et al. Regulatory B Cells Induced by Pancreatic Cancer Cell-Derived Interleukin-18 Promote Immune Tolerance via the PD-1/PD-L1 Pathway. Oncotarget 2018, 9, 14803–14814. [Google Scholar] [CrossRef]
- Cirella, A.; Olivera, I.; Luri-Rey, C.; Bolaños, E.; Berraondo, P.; Melero, I. Interleukin-18 in Cancer Immunology and Immunotherapy. Expert Opin. Ther. Targets 2023, 27, 1035–1042. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Mao, Y.; Gao, W.; Duan, S. Targeting the P53 Signaling Pathway in Cancers: Molecular Mechanisms and Clinical Studies. MedComm 2023, 4, e288. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chao, C.-H. P53-Mediated Indirect Regulation on Cellular Metabolism: From the Mechanism of Pathogenesis to the Development of Cancer Therapeutics. Front. Oncol. 2022, 12, 895112. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, K.; Dong, Z. The Role of P21-Activated Kinases in Cancer and Beyond: Where Are We Heading? Front. Cell Dev. Biol. 2021, 9, 641381. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Jiang, M.; Zhou, J.; Liang, H.; Xia, H.; Chen, G. LincRNA-p21 Inhibits the Progression of Non-small Cell Lung Cancer via Targeting MiR-17-5p. Oncol. Rep. 2018, 41, 789–800. [Google Scholar] [CrossRef]
- Stevens, M.; Oltean, S. Modulation of the Apoptosis Gene Bcl-x Function Through Alternative Splicing. Front. Genet. 2019, 10, 804. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Blanc, M.; Vallée, D.; Keller, H.; Bailly-Maitre, B. BAX Inhibitor-1: Between Stress and Survival. FEBS J. 2020, 287, 1722–1736. [Google Scholar] [CrossRef]
- Durrani, I.A.; Bhatti, A.; John, P. The Prognostic Outcome of ‘Type 2 Diabetes Mellitus and Breast Cancer’ Association Pivots on Hypoxia-Hyperglycemia Axis. Cancer Cell Int. 2021, 21, 351. [Google Scholar] [CrossRef]
- He, F.; Xiao, H.; Cai, Y.; Zhang, N. ATF5 and HIF1α Cooperatively Activate HIF1 Signaling Pathway in Esophageal Cancer. Cell Commun. Signal. 2021, 19, 53. [Google Scholar] [CrossRef]
- Hallis, S.P.; Kim, S.K.; Lee, J.-H.; Kwak, M.-K. Association of NRF2 with HIF-2α-Induced Cancer Stem Cell Phenotypes in Chronic Hypoxic Condition. Redox Biol. 2023, 60, 102632. [Google Scholar] [CrossRef]
- Lee, S.-H.; Golinska, M.; Griffiths, J.R. HIF-1-Independent Mechanisms Regulating Metabolic Adaptation in Hypoxic Cancer Cells. Cells 2021, 10, 2371. [Google Scholar] [CrossRef]
- Mabeta, P.; Steenkamp, V. The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 15585. [Google Scholar] [CrossRef]
- Yu, M.; Yongzhi, H.; Chen, S.; Luo, X.; Lin, Y.; Zhou, Y.; Jin, H.; Hou, B.; Deng, Y.; Tu, L.; et al. The Prognostic Value of GLUT1 in Cancers: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 43356–43367. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xiao, L.; Gong, T.; Liu, J.; Li, Y.; Zhou, X.; Li, Y.; Zheng, X. Role of Oral Microbiome in Oral Oncogenesis, Tumor Progression, and Metastasis. Mol. Oral Microbiol. 2023, 38, 9–22. [Google Scholar] [CrossRef]
- Murray, P.E.; Coffman, J.A.; Garcia-Godoy, F. Oral Pathogens’ Substantial Burden on Cancer, Cardiovascular Diseases, Alzheimer’s, Diabetes, and Other Systemic Diseases: A Public Health Crisis—A Comprehensive Review. Pathogens 2024, 13, 1084. [Google Scholar] [CrossRef]
- Yao, Y.; Shen, X.; Zhou, M.; Tang, B. Periodontal Pathogens Promote Oral Squamous Cell Carcinoma by Regulating ATR and NLRP3 Inflammasome. Front. Oncol. 2021, 11, 722797. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Y.; Chen, F. Cross-Cohort Microbiome Analysis of Salivary Biomarkers in Patients with Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 816526. [Google Scholar] [CrossRef]
- Coll, R.; O’Neill, L.; Schroder, K. Questions and Controversies in Innate Immune Research: What Is the Physiological Role of NLRP3? Cell Death Discov. 2016, 2, 16019. [Google Scholar] [CrossRef]
- Gora, I.M.; Ciechanowska, A.; Ladyzynski, P. Nlrp3 Inflammasome at the Interface of Inflammation, Endothelial Dysfunction, and Type 2 Diabetes. Cells 2021, 10, 314. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Casili, G.; Basilotta, R.; Lanza, M.; Filippone, A.; Raciti, G.; Puliafito, I.; Colarossi, L.; Esposito, E.; Paterniti, I. NLRP3 Inflammasome Inhibitor BAY-117082 Reduces Oral Squamous Cell Carcinoma Progression. Int. J. Mol. Sci. 2021, 22, 11108. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiang, Z.; Liu, Y.; Zhu, L.; Xiao, L.; Du, Z.; Cai, R.; Qiang, S. Diverse Functions of NLRP3 Inflammasome in PANoptosis and Diseases. Cell Death Discov. 2025, 11, 389. [Google Scholar] [CrossRef]
- Agrawal, I.; Jha, S. Comprehensive Review of ASC Structure and Function in Immune Homeostasis and Disease. Mol. Biol. Rep. 2020, 47, 3077–3096. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a Link between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.W.; Dixit, V.D. Mechanisms of Disease: Inflammasome Activation and the Development of Type 2 Diabetes. Front. Immunol. 2013, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Chang, K.P.; OuYang, C.N.; Kao, H.K.; Hsueh, C.; Chen, L.C.; Cheng, H.Y.; Liang, Y.; Liou, W.; Liang, C.; et al. ASC Contributes to Metastasis of Oral Cavity Squamous Cell Carcinoma. Oncotarget 2016, 7, 50074–50085. [Google Scholar] [CrossRef]
- Silva, F.; Padín-Iruegas, M.; Caponio, V.; Lorenzo-Pouso, A.; Saavedra-Nieves, P.; Chamorro-Petronacci, C.; Suaréz-Peñaranda, J.; Pérez-Sayáns, M. Caspase 3 and Cleaved Caspase 3 Expression in Tumorogenesis and Its Correlations with Prognosis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 11937. [Google Scholar] [CrossRef]
- Shih, L.-C.; Tsai, C.-W.; Sun, K.-T.; Hsu, H.-M.; Shen, T.-C.; Tsai, Y.-T.; Chang, W.-S.; Lin, M.-L.; Wang, Y.-C.; Gong, C.-L.; et al. Association of Caspase-8 Genotypes with Oral Cancer Risk in Taiwan. In Vivo 2019, 33, 1151–1156. [Google Scholar] [CrossRef]
- Lutz, V.; Hellmund, V.M.; Picard, F.S.R.; Raifer, H.; Ruckenbrod, T.; Klein, M.; Bopp, T.; Savai, R.; Duewell, P.; Keber, C.U.; et al. IL18 Receptor Signaling Regulates Tumor-Reactive CD8+ T-Cell Exhaustion via Activation of the IL2/STAT5/MTOR Pathway in a Pancreatic Cancer Model. Cancer Immunol. Res. 2023, 11, 421–434. [Google Scholar] [CrossRef]
- Brinkmann, O.; Kastratovic, D.A.; Dimitrijevic, M.V.; Konstantinovic, V.S.; Jelovac, D.B.; Antic, J.; Nesic, V.S.; Markovic, S.Z.; Martinovic, Z.R.; Akin, D.; et al. Oral Squamous Cell Carcinoma Detection by Salivary Biomarkers in a Serbian Population. Oral Oncol. 2011, 47, 51–55. [Google Scholar] [CrossRef]
- Lacroix, M.; Riscal, R.; Arena, G.; Linares, L.K.; Le Cam, L. Metabolic Functions of the Tumor Suppressor P53: Implications in Normal Physiology, Metabolic Disorders, and Cancer. Mol. Metab. 2020, 33, 2–22. [Google Scholar] [CrossRef]
- Darawadi, B. Role of Tumor Suppressor P53 Family in Glucose Metabolism in Association with Diabetes. Adv. Cancer Chemother. Pharmacol. 2023, 1, 1–7. [Google Scholar] [CrossRef]
- Lahalle, A.; Lacroix, M.; De Blasio, C.; Cissé, M.Y.; Linares, L.K.; Le Cam, L. The P53 Pathway and Metabolism: The Tree That Hides the Forest. Cancers 2021, 13, 133. [Google Scholar] [CrossRef]
- Schuch, L.F.; De Arruda, J.A.A.; Viana, K.S.S.; Caldeira, P.C.; Abreu, M.H.N.G.; Bernardes, V.F.; de Aguiar, M.C.F. DNA Damage-Related Proteins in Smokers and Non-Smokers with Oral Cancer. Braz. Oral Res. 2022, 36, e027. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S. Immunohistochemistry Examination to Reveal the Pathogenesis of Oral Squamous Cell Carcinoma. Int. J. Ecophysiol. 2023, 4, 5–25. [Google Scholar] [CrossRef]
- Saito, R.; Rocanin-Arjo, A.; You, Y.-H.; Darshi, M.; Van Espen, B.; Miyamoto, S.; Pham, J.; Pu, M.; Romoli, S.; Natarajan, L.; et al. Systems Biology Analysis Reveals Role of MDM2 in Diabetic Nephropathy. J. Clin. Investig. 2016, 1, e87877. [Google Scholar] [CrossRef]
- Yanamoto, S.; Kawasaki, G.; Yoshitomi, I.; Mizuno, A. P53, Mdm2, and P21 Expression in Oral Squamous Cell Carcinomas: Relationship with Clinicopathologic Factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 94, 593–600. [Google Scholar] [CrossRef]
- Gartel, A.L.; Serfas, M.S.; Tyner, A.L. P21-Negative Regulator of the Cell Cycle. Exp. Biol. Med. 1996, 213, 138–149. [Google Scholar] [CrossRef]
- Elmitwalli, O.; Darwish, R.; Al-Jabery, L.; Algahiny, A.; Roy, S.; Butler, A.E.; Hasan, A.S. The Emerging Role of P21 in Diabetes and Related Metabolic Disorders. Int. J. Mol. Sci. 2024, 25, 13209. [Google Scholar] [CrossRef]
- Tatemoto, Y.; Osaki, T.; Yoneda, K.; Yamamoto, T.; Ueta, E.; Kimura, T. Expression of P53 and P21 Proteins in Oral Squamous Cell Carcinoma: Correlation with Lymph Node Metastasis and Response to Chemoradiotherapy. Pathol. Res. Pract. 1998, 194, 821–830. [Google Scholar] [CrossRef]
- Parvathy, M.; Sreeja, S.; Kumar, R.; Pillai, M.R. Potential Role of P21 Activated Kinase 1 (PAK1) in the Invasion and Motility of Oral Cancer Cells. BMC Cancer 2016, 16, 293. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial Performance in Apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- White, S.A.; Zhang, L.S.; Pasula, D.J.; Yang, Y.H.C.; Luciani, D.S. Bax and Bak Jointly Control Survival and Dampen the Early Unfolded Protein Response in Pancreatic β-Cells under Glucolipotoxic Stress. Sci. Rep. 2020, 10, 10986. [Google Scholar] [CrossRef]
- Alam, M.; Kashyap, T.; Mishra, P.; Panda, A.K.; Nagini, S.; Mishra, R. Role and Regulation of Proapoptotic Bax in Oral Squamous Cell Carcinoma and Drug Resistance. Head Neck 2019, 41, 185–197, Erratum in Head Neck 2025, 47, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, J.; Osborne, O.; Oh, D.Y.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; et al. Increased Adipocyte O2 Consumption Triggers HIF-1α, Causing Inflammation and Insulin Resistance in Obesity. Cell 2014, 157, 1339–1352. [Google Scholar] [CrossRef]
- Mahdy, R.A.; Nada, W.M.; Hadhoud, K.M.; El-Tarhony, S.A. The Role of Vascular Endothelial Growth Factor in the Progression of Diabetic Vascular Complications. Eye 2010, 24, 1576–1584. [Google Scholar] [CrossRef]
- Stîngǎ, A.C.; Mǎrgǎritescu, O.; Stîngǎ, A.S.; Pirici, D.; Ciurea, R.; Bunget, A.; Cruce, M. VEGFR1 and VEGFR2 Immunohistochemical Expression in Oral Squamous Cell Carcinoma: A Morphometric Study. Rom. J. Morphol. Embryol. 2011, 52, 1269–1275. [Google Scholar]
- Elian, V.; Popovici, V.; Steriade, A.T.; Radulian, G.; Ozon, E.A.; Moroșan, E.; Musat, M. Molecular Biomarkers and Therapeutic Approach of Patients with Diabetes and Obstructive Sleep Apnea. Int. J. Mol. Sci. 2025, 26, 10234. [Google Scholar] [CrossRef]
- Mahuli, A.V.; Sagar, V.; Kumar, A.; Mahuli, S.A.; Kujur, A. A Systematic Review and Meta-Analysis Assessing the Role of Oral Health as a Risk Factor in Oral Cancer. Cureus 2023, 15, e39786. [Google Scholar] [CrossRef]
- Wei, K.; Ma, Y.; Xu, J.; Zheng, H.; Xue, L.; Chu, Y.; Shi, Y.; Sun, Z.; Sun, Q. Potential Changes in Microorganisms and Metabolites Associated with Oral Cancer: A Preliminary Study. BMC Cancer 2025, 25, 611. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Lalla, E.; Papapanou, P.N. Diabetes Mellitus and Periodontitis: A Tale of Two Common Interrelated Diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef]
- Polak, D.; Shapira, L. An Update on the Evidence for Pathogenic Mechanisms That May Link Periodontitis and Diabetes. Nat. Rev. Endocrinol. 2018, 45, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, Y.; Li, X.; Wang, X.; Lu, L.; Li, C.; Pan, Y.; Wang, S. Association between Periodontitis and HbA1c Levels in Non-Diabetic Patients: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 2649. [Google Scholar] [CrossRef] [PubMed]
- Mivehchi, H.; Eskandari-Yaghbastlo, A.; Pour Bahrami, P.; Elhami, A.; Faghihinia, F.; Nejati, S.T.; Kazemi, K.S.; Nabi Afjadi, M. Exploring the Role of Oral Bacteria in Oral Cancer: A Narrative Review. Discov. Oncol. 2025, 16, 242. [Google Scholar] [CrossRef]
- Jia, S.; Li, X.; Du, Q. Host Insulin Resistance Caused by Porphyromonas gingivalis-Review of Recent Progresses. Front. Cell. Infect. Microbiol. 2023, 13, 1209381. [Google Scholar] [CrossRef]
- Song, Y.; Kwon, J.-J.; Na, H.S.; Kim, S.Y.; Shin, S.-H.; Chung, J. High Glucose Condition Aggravates Inflammatory Response Induced by Porphyromonas gingivalis in THP-1 Macrophages via Autophagy Inhibition. BMC Immunol. 2024, 25, 69. [Google Scholar] [CrossRef]
- Zang, W.; Geng, F.; Liu, J.; Wang, Z.; Zhang, S.; Li, Y.; Lu, Z.; Pan, Y. Porphyromonas gingivalis Potentiates Stem-like Properties of Oral Squamous Cell Carcinoma by Modulating SCD1-Dependent Lipid Synthesis via NOD1/KLF5 Axis. Int. J. Oral Sci. 2025, 17, 15. [Google Scholar] [CrossRef]
- Song, J.M.; Woo, B.H.; Lee, J.H.; Yoon, S.; Cho, Y.; Kim, Y.-D.; Park, H.R. Oral Administration of Porphyromonas gingivalis, a Major Pathogen of Chronic Periodontitis, Promotes Resistance to Paclitaxel in Mouse Xenografts of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2494. [Google Scholar] [CrossRef]
- Guo, Z.; Jing, S.; Jumatai, S.; Gong, Z. Porphyromonas gingivalis Promotes the Progression of Oral Squamous Cell Carcinoma by Activating the Neutrophil Chemotaxis in the Tumour Microenvironment. Cancer Immunol. Immunother. 2023, 72, 1523–1539. [Google Scholar] [CrossRef]
- Yamada, C.; Ho, A.; Nusbaum, A.; Xu, R.; Davey, M.E.; Nichols, F.; Mao, C.; Movila, A. Inhibitory Effect of Porphyromonas gingivalis -derived Phosphoethanolamine Dihydroceramide on Acid Ceramidase Expression in Oral Squamous Cells. J. Cell. Mol. Med. 2023, 27, 1290–1295. [Google Scholar] [CrossRef]
- Wen, L.; Mu, W.; Lu, H.; Wang, X.; Fang, J.; Jia, Y.; Li, Q.; Wang, D.; Wen, S.; Guo, J.; et al. Porphyromonas gingivalis Promotes Oral Squamous Cell Carcinoma Progression in an Immune Microenvironment. J. Dent. Res. 2020, 99, 666–675. [Google Scholar] [CrossRef]
- Bachtiar, B.M.; Bachtiar, E.W.; Kusumaningrum, A.; Sunarto, H.; Soeroso, Y.; Sulijaya, B.; Apriyanti, E.; Fragrantia Theodorea, C.; Putra Pratomo, I.; Yudhistira; et al. Porphyromonas gingivalis Association with Inflammatory Markers and Exosomal MiRNA-155 in Saliva of Periodontitis Patients with and without Diabetes Diagnosed with COVID-19. Saudi Dent. J. 2023, 35, 61–69. [Google Scholar] [CrossRef]
- Wei, W.; Li, J.; Tang, B.; Deng, Y.; Li, Y.; Chen, Q. Metabolomics and Metagenomics Reveal the Impact of Γδ T Inhibition on Gut Microbiota and Metabolism in Periodontitis-Promoting OSCC. mSystems 2024, 9, e0077723. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Candida Sp. Infections in Patients with Diabetes Mellitus. J. Clin. Med. 2019, 8, 76. [Google Scholar] [CrossRef]
- Mohammed, L.; Jha, G.; Malasevskaia, I.; Goud, H.K.; Hassan, A. The Interplay Between Sugar and Yeast Infections: Do Diabetics Have a Greater Predisposition to Develop Oral and Vulvovaginal Candidiasis? Cureus 2021, 18, e13407. [Google Scholar] [CrossRef] [PubMed]
- Suresh Unniachan, A.; Krishnavilasom Jayakumari, N.; Sethuraman, S. Association between Candida Species and Periodontal Disease: A Systematic Review. Curr. Med. Mycol. 2020, 6, 63–68. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Dmitrović, B.; Juzbašić, M.; Matijević, T.; Bekić, S.; Erić, S.; Flam, J.; Belić, D.; Petek Erić, A.; et al. A Putative Role of Candida albicans in Promoting Cancer Development: A Current State of Evidence and Proposed Mechanisms. Microorganisms 2023, 11, 1476. [Google Scholar] [CrossRef]
- Mizumoto, A.; Ohashi, S.; Hirohashi, K.; Amanuma, Y.; Matsuda, T.; Muto, M. Molecular Mechanisms of Acetaldehyde-Mediated Carcinogenesis in Squamous Epithelium. Int. J. Mol. Sci. 2017, 18, 1943. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Wu, W.; Zhang, W.; Li, L.; Liu, Q.; Yan, Z. Candida albicans Promotes Oral Cancer via IL-17A/IL-17RA-Macrophage Axis. mBio 2023, 14, e0044723. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Zhang, W.; Wu, S.; Yan, Z. Candida albicans Induces Upregulation of Programmed Death Ligand 1 in Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2022, 51, 444–453. [Google Scholar] [CrossRef]
- Marin-Dett, F.H.; Campanella, J.E.M.; Trovatti, E.; Bertolini, M.C.; Vergani, C.E.; Barbugli, P.A. Extracellular Lipids of Candida albicans Biofilm Induce Lipid Droplet Formation and Decreased Response to a Topoisomerase I Inhibitor in Dysplastic and Neoplastic Oral Cells. J. Appl. Oral Sci. 2022, 30, e20220319. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kitamura, N.; Yoshida, K.; Yamamoto, T.; Ozaki, K.; Kudo, Y. Involvement of Fusobacterium Species in Oral Cancer Progression: A Literature Review Including Other Types of Cancer. Int. J. Mol. Sci. 2020, 21, 6207. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, J.; Shi, J.; Chen, L.; Long, J.; Zhang, S.; Liu, S. Genetic Cross-Talk between Oral Squamous Cell Carcinoma and Type 2 Diabetes: The Potential Role of Immunity. Dis. Markers 2022, 2022, 6389906. [Google Scholar] [CrossRef]
- Chen, L.-C.; Lo, Y.-S.; Ho, H.-Y.; Lin, C.-C.; Chuang, Y.-C.; Chang, W.-C.; Hsieh, M.-J. LDL Receptor-Related Protein 1B Polymorphisms Associated with Increased Risk of Lymph Node Metastasis in Oral Cancer Group with Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 3963. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Han, B.; Zhao, Y.; Hou, L.; Fang, J.; Lian, M. The Association between Diabetes and Head and Neck Squamous Cell Carcinoma: Evidence from Clinical Cohort and Bioinformatics Analyses. Front. Genet. 2025, 16, 1660012. [Google Scholar] [CrossRef]
- Mojumder, D.; Paul, S.; Podder, A. Molecular Biomarkers for Diagnosis of Oral Cancer: An Overview. Int. J. Hum. Health Sci. 2022, 6, 11–16. [Google Scholar] [CrossRef]
- Thomaidou, A.C.; Batsaki, P.; Adamaki, M.; Goulielmaki, M.; Baxevanis, C.N.; Zoumpourlis, V.; Fortis, S.P. Promising Biomarkers in Head and Neck Cancer: The Most Clinically Important MiRNAs. Int. J. Mol. Sci. 2022, 23, 8257. [Google Scholar] [CrossRef]
- Piotrowski, I.; Zhu, X.; Saccon, T.D.; Ashiqueali, S.; Schneider, A.; de Carvalho Nunes, A.D.; Noureddine, S.; Sobecka, A.; Barczak, W.; Szewczyk, M.; et al. Mirnas as Biomarkers for Diagnosing and Predicting Survival of Head and Neck Squamous Cell Carcinoma Patients. Cancers 2021, 13, 3980. [Google Scholar] [CrossRef]
- Tay, Z.Y.; Kao, H.K.; Lien, K.H.; Hung, S.Y.; Huang, Y.; Tsang, N.M.; Chang, K.P. The Impact of Preoperative Glycated Hemoglobin Levels on Outcomes in Oral Squamous Cell Carcinoma. Oral Dis. 2020, 26, 1449–1458. [Google Scholar] [CrossRef]
- Vegh, A.; Vegh, D.; Banyai, D.; Kammerhofer, G.; Biczo, Z.; Voros, B.; Ujpal, M.; Peña-Cardelles, J.F.; Yonel, Z.; Joob-Fancsaly, A.; et al. Point-of-Care HbA1c Measurements in Oral Cancer and Control Patients in Hungary. In Vivo 2022, 36, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. Hyperinsulinaemia in Cancer. Nat. Rev. Cancer 2020, 20, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Insulin Resistance: The Increased Risk of Cancers. Curr. Oncol. 2024, 31, 998–1027. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Saini, S.; Singhal, P.; Mathur, A.; Sinha, M. The Diagnostic and Prognostic Utility of Insulin Growth Factor of Squamous Cell Carcinoma in Oral Cavity. Tzu Chi Med. J. 2021, 33, 160–164. [Google Scholar] [CrossRef]
- Shah, K.H.; Odedra, S.P.; Subramanyam, R.V.; Shah, V.S.; Pillai, J.P.; Dholabhai, P.N. Estimation and Correlation of Serum C-Reactive Protein in Patients with Oral Squamous Cell Carcinoma. J. Indian Acad. Oral Med. Radiol. 2025, 37, 30–34. [Google Scholar] [CrossRef]
- Mengji, A.; Yaga, U.; Besta, R.; Soankamble, S. C-Reactive Protein: An Inflammatory Biomarker in Oral Cancer. J. Indian Acad. Oral Med. Radiol. 2015, 27, 565. [Google Scholar] [CrossRef]
- Vinocha, A.; Grover, R.; Deepak, R. Clinical Significance of Interleukin-6 in Diagnosis of Lung, Oral, Esophageal, and Gall Bladder Carcinomas. J. Cancer Res. Ther. 2018, 14, 758. [Google Scholar] [CrossRef]
- Deepthi, G.; Nandan, S.R.K.; Kulkarni, P.G. Salivary Tumour Necrosis Factor-α as a Biomarker in Oral Leukoplakia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 2087–2093. [Google Scholar] [CrossRef]
- Brundha, M.P.; Raveendran, S.R.; Rajeshkar, N. Salivary Tumour Necrosis Factor-Alpha and Receptor for Advanced Glycation End Products as Prognostic and Predictive Markers for Recurrence in Oral Squamous Cell Carcinoma—A Pilot Study. Eur. J. Clin. Exp. Med. 2023, 21, 36–43. [Google Scholar] [CrossRef]
- Piva, M.R.; DE Souza, L.B.; Martins-Filho, P.R.S.; Nonaka, C.F.W.; De Santana SANTOS, T.; De Souza Andrade, E.S.; Piva, D. Role of Inflammation in Oral Carcinogenesis (Part II): CD8, FOXP3, TNF-α, TGF-β and NF-ΚB Expression. Oncol. Lett. 2013, 5, 1909–1914. [Google Scholar] [CrossRef][Green Version]
- Prieto-Correa, J.R.; Bologna-Molina, R.; González-González, R.; Molina-Frechero, N.; Soto-Ávila, J.J.; Isiordia-Espinoza, M.; Márquez, M.C.; Verdín, S.L. DNA Oxidative Damage in Oral Cancer: 8-Hydroxy-2′-Deoxyguanosine Immunoexpression Assessment. Med. Oral Patol. Oral Cir. Bucal 2020, 28, e530–e538. [Google Scholar] [CrossRef]
- Mohideen, K.; Sudhakar, U.; Balakrishnan, T.; Almasri, M.A.; Al-Ahmari, M.M.; Al Dira, H.S.; Suhluli, M.; Dubey, A.; Mujoo, S.; Khurshid, Z.; et al. Malondialdehyde, an Oxidative Stress Marker in Oral Squamous Cell Carcinoma—A Systematic Review and Meta-Analysis. Curr. Issues Mol. Biol. 2021, 43, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.R.; Babu, S.; Kumari, S.; Shetty, P.; Hegde, S.H.; Castelino, R. Status of Salivary Lipid Peroxidation in Oral Cancer and Precancer. Indian J. Med. Paediatr. Oncol. 2014, 35, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Zhao, X.; Zhang, J.; Zhang, Y. The Adipokines in Oral Cancer Pathogenesis and Its Potential as a New Therapeutic Approach. Naunyn Schmiedeberg’s Arch. Pharmacol. 2025, 398, 9623–9639. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Yousefian, M.; Mohammadpour, H.; Razavi, A.E.; Talaei, S.; Sheykhbahaei, N. Long Non-Coding RNAs PVT1, CCAT2, and TCF7L2, and MiR-33 and c-Myc Expression in Oral Squamous Cell Carcinoma and Oral Lichen Planus Patients. J. Cranio-Maxillofac. Surg. 2025, 53, 1197–1204. [Google Scholar] [CrossRef]
- Heianza, Y.; Xue, Q.; Ma, H.; Bray, G.; Sacks, F.; Qi, L. 1257-P: Changes in Circulating Pancreatic Islet-Specific MicroRNA-375 Levels and Improvements in Insulin Sensitivity and Glucose Metabolism in Response to Weight-Loss Diets with Different Compositions of Macronutrients—The POUNDS Lost Trial. Diabetes 2023, 72, 1257-P. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Hao, W. Post-Chemotherapy MiR-146a Expression and Its Prognostic Potential in Oral Cancer Patients. Trop. J. Pharm. Res. 2021, 20, 2205–2211. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Yu, C.-C.; Lu, M.-Y.; Chao, S.-C.; Liao, Y.-W.; Yu, C.-H.; Lee, Y.-H. MiR-146a Participates in the Regulation of Cancer Stemness of Oral Carcinoma Cells. J. Dent. Sci. 2023, 18, 503–509. [Google Scholar] [CrossRef]
- Dioguardi, M.; Caloro, G.A.; Laino, L.; Alovisi, M.; Sovereto, D.; Crincoli, V.; Aiuto, R.; Coccia, E.; Troiano, G.; Lo Muzio, L. Circulating MiR-21 as a Potential Biomarker for the Diagnosis of Oral Cancer: A Systematic Review with Meta-Analysis. Cancers 2020, 12, 936. [Google Scholar] [CrossRef]
- Shreya Reddy, C.S.; Usman, P.P.A.S.; Ganapathy, D.M.; Ameya, K.P.; Sekar, D. MicroRNA-21 as a Biomarker in Terminal Stage Oral Squamous Cell Carcinoma (OSCC) in the South Indian Population. Oral Oncol. Rep. 2024, 9, 100139. [Google Scholar] [CrossRef]
- YANG, X.; WU, H.; LING, T. Suppressive Effect of MicroRNA-126 on Oral Squamous Cell Carcinoma in Vitro. Mol. Med. Rep. 2014, 10, 125–130. [Google Scholar] [CrossRef]
- Sasahira, T.; Kurihara, M.; Bhawal, U.K.; Ueda, N.; Shimomoto, T.; Yamamoto, K.; Kirita, T.; Kuniyasu, H. Downregulation of MiR-126 Induces Angiogenesis and Lymphangiogenesis by Activation of VEGF-A in Oral Cancer. Br. J. Cancer 2012, 107, 700–706. [Google Scholar] [CrossRef]
- Arunkumar, G.; Deva Magendhra Rao, A.K.; Manikandan, M.; Rao, H.P.S.; Subbiah, S.; Ilangovan, R.; Murugan, A.K.; Munirajan, A.K. Dysregulation of MiR-200 Family MicroRNAs and Epithelial-Mesenchymal Transition Markers in Oral Squamous Cell Carcinoma. Oncol. Lett. 2018, 15, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.-L.; Huang, C.-C.; Yu, C.-C. Emerging Role of MicroRNA-200 Family in Dentistry. Noncoding RNA 2021, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, L.; Wang, R.; Miao, L.; Jiang, H.; Yuan, H.; Ma, H.; Chen, N. Genetic Variants in Let-7/Lin28 Modulate the Risk of Oral Cavity Cancer in a Chinese Han Population. Sci. Rep. 2014, 4, 7434. [Google Scholar] [CrossRef]
- Peng, C.; Wang, T.; Lee, S.; Hsieh, P.; Liao, Y.; Tsai, L.; Fang, C.; Yu, C.; Hsieh, C. Let-7c Restores Radiosensitivity and Chemosensitivity and Impairs Stemness in Oral Cancer Cells through Inhibiting Interleukin-8. J. Oral Pathol. Med. 2018, 47, 590–597. [Google Scholar] [CrossRef]
- Hilly, O.; Pillar, N.; Stern, S.; Strenov, Y.; Bachar, G.; Shomron, N.; Shpitzer, T. Distinctive Pattern of Let-7 Family MicroRNAs in Aggressive Carcinoma of the Oral Tongue in Young Patients. Oncol. Lett. 2016, 12, 1729–1736. [Google Scholar] [CrossRef]
- Chang, C.J.; Hsu, C.C.; Chang, C.H.; Tsai, L.L.; Chang, Y.C.; Lu, S.W.; Yu, C.H.; Huang, H.S.; Wang, J.J.; Tsai, C.H.; et al. Let-7d Functions as Novel Regulator of Epithelial-Mesenchymal Transition and Chemoresistant Property in Oral Cancer. Oncol. Rep. 2011, 26, 1003–1010. [Google Scholar] [CrossRef]
- Frost, R.J.A.; Olson, E.N. Control of Glucose Homeostasis and Insulin Sensitivity by the Let-7 Family of MicroRNAs. Proc. Natl. Acad. Sci. USA 2011, 108, 21075–21080. [Google Scholar] [CrossRef]
- Su, Y.-F.; Lin, C.-S.; Shen, P.-C.; Chuang, S.-E.; Dai, Y.-H.; Huang, T.-W.; Lin, C.-Y.; Hung, Y.-J.; Shieh, Y.-S. MiR-34a Functions as a Tumor Suppressor in Oral Cancer through the Inhibition of the Axl/Akt/GSK-3β Pathway. J. Dent. Sci. 2024, 19, 428–437. [Google Scholar] [CrossRef]
- Kalfert, D.; Ludvikova, M.; Pesta, M.; Ludvik, J.; Dostalova, L.; Kholová, I. Multifunctional Roles of MiR-34a in Cancer: A Review with the Emphasis on Head and Neck Squamous Cell Carcinoma and Thyroid Cancer with Clinical Implications. Diagnostics 2020, 10, 563. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Cai, H.; Liu, W.; Pan, E.; Yu, D.; He, S. Upregulation of IGF2BP2 Promotes Oral Squamous Cell Carcinoma Progression That Is Related to Cell Proliferation, Metastasis and Tumor-Infiltrating Immune Cells. Front. Oncol. 2022, 12, 809589. [Google Scholar] [CrossRef]
- Olatunde, A.; Nigam, M.; Singh, R.K.; Panwar, A.S.; Lasisi, A.; Alhumaydhi, F.A.; Jyoti Kumar, V.; Mishra, A.P.; Sharifi-Rad, J. Cancer and Diabetes: The Interlinking Metabolic Pathways and Repurposing Actions of Antidiabetic Drugs. Cancer Cell Int. 2021, 21, 499. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yi, Z.; Chen, Y.; Huang, J.; Mao, X.; Zhang, L.; Zeng, Y.; Cheng, Q.; Ye, W.; Liu, Z.; et al. Efficacy of Metformin Therapy in Patients with Cancer: A Meta-Analysis of 22 Randomised Controlled Trials. BMC Med. 2022, 20, 402. [Google Scholar] [CrossRef]
- Chen, C.-H.; Tsai, H.-T.; Chuang, H.-C.; Shiu, L.-Y.; Su, L.-J.; Chiu, T.-J.; Luo, S.-D.; Fang, F.-M.; Huang, C.-C.; Chien, C.-Y. Metformin Disrupts Malignant Behavior of Oral Squamous Cell Carcinoma via a Novel Signaling Involving Late SV40 Factor/Aurora-A. Sci. Rep. 2017, 7, 1358. [Google Scholar] [CrossRef]
- Ji, M.; Lv, Y.; Chen, C.; Xing, D.; Zhou, C.; Zhao, J.; Qi, Y.; Zhang, J.; Wang, Y.; Ma, X.; et al. Metformin Inhibits Oral Squamous Cell Carcinoma Progression through Regulating RNA Alternative Splicing. Life Sci. 2023, 315, 121274. [Google Scholar] [CrossRef]
- Qi, X.; Xu, W.; Xie, J.; Wang, Y.; Han, S.; Wei, Z.; Ni, Y.; Dong, Y.; Han, W. Metformin Sensitizes the Response of Oral Squamous Cell Carcinoma to Cisplatin Treatment through Inhibition of NF-ΚB/HIF-1α Signal Axis. Sci. Rep. 2016, 6, 35788, Erratum in Sci. Rep. 2017, 7, 42934. [Google Scholar] [CrossRef]
- Bouland, C.; Vanden Eynden, X.; Lalmand, M.; Buset, T.; Yanni, A.; Javadian, R.; Rodriguez, A.; Loeb, I.; Lechien, J.R.; Journe, F.; et al. Preventive and Therapeutic Effect of Metformin in Head and Neck Cancer: A Concise Review. J. Clin. Med. 2023, 12, 6195. [Google Scholar] [CrossRef]
- Gutkind, J.S.; Molinolo, A.A.; Wu, X.; Wang, Z.; Nachmanson, D.; Harismendy, O.; Alexandrov, L.B.; Wuertz, B.R.; Ondrey, F.G.; Laronde, D.; et al. Inhibition of MTOR Signaling and Clinical Activity of Metformin in Oral Premalignant Lesions. J. Clin. Investig. 2021, 6, e147096. [Google Scholar] [CrossRef]
- Vitale-Cross, L.; Molinolo, A.A.; Martin, D.; Younis, R.H.; Maruyama, T.; Patel, V.; Chen, W.; Schneider, A.; Gutkind, J.S. Metformin Prevents the Development of Oral Squamous Cell Carcinomas from Carcinogen-Induced Premalignant Lesions. Cancer Prev. Res. 2012, 5, 562–573. [Google Scholar] [CrossRef]
- Madera, D.; Vitale-Cross, L.; Martin, D.; Schneider, A.; Molinolo, A.A.; Gangane, N.; Carey, T.E.; McHugh, J.B.; Komarck, C.M.; Walline, H.M.; et al. Prevention of Tumor Growth Driven by PIK3CA and HPV Oncogenes by Targeting MTOR Signaling with Metformin in Oral Squamous Carcinomas Expressing OCT3. Cancer Prev. Res. 2015, 8, 197–207. [Google Scholar] [CrossRef]
- Hu, X.; Xiong, H.; Chen, W.; Huang, L.; Mao, T.; Yang, L.; Wang, C.; Huang, D.; Wang, Z.; Yu, J.; et al. Metformin Reduces the Increased Risk of Oral Squamous Cell Carcinoma Recurrence in Patients with Type 2 Diabetes Mellitus: A Cohort Study with Propensity Score Analyses. Surg. Oncol. 2020, 35, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yeerna, H.; Goto, Y.; Ando, T.; Wu, V.H.; Zhang, X.; Wang, Z.; Amornphimoltham, P.; Murphy, A.N.; Tamayo, P.; et al. Metformin Inhibits Progression of Head and Neck Squamous Cell Carcinoma by Acting Directly on Carcinoma-Initiating Cells. Cancer Res. 2019, 79, 4360–4370. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.J.; Dhar, M.; Carlson, E.R. Metformin as a Potential Neoadjuvant Therapeutic Agent in Head and Neck Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2020, 78, e93. [Google Scholar] [CrossRef]
- Wilbon, S.S.; Kolonin, M.G. GLP1 Receptor Agonists—Effects beyond Obesity and Diabetes. Cells 2023, 13, 65. [Google Scholar] [CrossRef]
- Wolff Sagy, Y.; Ramot, N.; Battat, E.; Arbel, R.; Reges, O.; Dicker, D.; Lavie, G. Glucagon-like Peptide-1 Receptor Agonists Compared with Bariatric Metabolic Surgery and the Risk of Obesity-Related Cancer: An Observational, Retrospective Cohort Study. eClinicalMedicine 2025, 83, 103213. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Z.; Zhou, J.; Cheng, B.; Huang, Y. Semaglutide, a Glucagon-like Peptide-1 Receptor Agonist, Inhibits Oral Squamous Cell Carcinoma Growth through P38 MAPK Signaling Pathway. J. Cancer Res. Clin. Oncol. 2025, 151, 103. [Google Scholar] [CrossRef]
- Trakoonsenathong, R.; Kunprom, W.; Aphivatanasiri, C.; Yueangchantuek, P.; Pimkeeree, P.; Sorin, S.; Khawkhiaw, K.; Chiu, C.-F.; Okada, S.; Wongkham, S.; et al. Liraglutide Exhibits Potential Anti-Tumor Effects on the Progression of Intrahepatic Cholangiocarcinoma, in Vitro and in Vivo. Sci. Rep. 2024, 14, 13726. [Google Scholar] [CrossRef]
- Lu, X.; Xu, C.; Dong, J.; Zuo, S.; Zhang, H.; Jiang, C.; Wu, J.; Wei, J. Liraglutide Activates Nature Killer Cell-Mediated Antitumor Responses by Inhibiting IL-6/STAT3 Signaling in Hepatocellular Carcinoma. Transl. Oncol. 2021, 14, 100872. [Google Scholar] [CrossRef]
- Eftekhari, S.; Montazeri, H.; Tarighi, P. Synergistic Anti-Tumor Effects of Liraglutide, a Glucagon-like Peptide-1 Receptor Agonist, along with Docetaxel on LNCaP Prostate Cancer Cell Line. Eur. J. Pharmacol. 2020, 878, 173102. [Google Scholar] [CrossRef]
- Lu, R.; Yang, J.; Wei, R.; Ke, J.; Tian, Q.; Yu, F.; Liu, J.; Zhang, J.; Hong, T. Synergistic Anti-Tumor Effects of Liraglutide with Metformin on Pancreatic Cancer Cells. PLoS ONE 2018, 13, e0198938. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Wang, B.; Zhang, X.; Gu, L.; Guo, K.; Zhang, X.; Zhou, Z. GLP-1 Receptor Agonist Liraglutide Inhibits the Proliferation and Migration of Thyroid Cancer Cells. Cell Mol. Biol. 2023, 69, 221–225. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, X.; Zhou, Z.; Sun, B.; Gu, W.; Liu, J.; Zhang, H. Liraglutide Inhibits the Proliferation and Promotes the Apoptosis of MCF-7 Human Breast Cancer Cells through Downregulation of MicroRNA-27a Expression. Mol. Med. Rep. 2018, 17, 5202–5212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Duan, X.X.; Yuan, M.-C.; Yu, J.; Hu, X.; Han, X.; Lan, L.; Liu, B.-W.; Wang, Y.; Qin, J.-F. Glucagon-like Peptide-1 Receptor Activation by Liraglutide Promotes Breast Cancer through NOX4/ROS/VEGF Pathway. Life Sci. 2022, 294, 120370. [Google Scholar] [CrossRef]
- Pu, Z.; Yang, Y.; Qin, S.; Li, X.; Cui, C.; Chen, W. The Effect of Liraglutide on Lung Cancer and Its Potential Protective Effect on High Glucose-Induced Lung Senescence and Oxidative Damage. Front. Biosci. Landmark 2023, 28, 259. [Google Scholar] [CrossRef]
- Chequin, A.; Costa, L.E.; de Campos, F.F.; Moncada, A.D.B.; de Lima, L.T.F.; Sledz, L.R.; Picheth, G.F.; Adami, E.R.; Acco, A.; Gonçalves, M.B.; et al. Antitumoral Activity of Liraglutide, a New DNMT Inhibitor in Breast Cancer Cells in Vitro and in Vivo. Chem. Biol. Interact. 2021, 349, 109641. [Google Scholar] [CrossRef]
- Alanteet, A.; Attia, H.; Alfayez, M.; Mahmood, A.; Alsaleh, K.; Alsanea, S. Liraglutide Attenuates Obese-Associated Breast Cancer Cell Proliferation via Inhibiting PI3K/Akt/MTOR Signaling Pathway. Saudi Pharm. J. 2024, 32, 101923. [Google Scholar] [CrossRef]
- Kanda, R.; Hiraike, H.; Wada-Hiraike, O.; Ichinose, T.; Nagasaka, K.; Sasajima, Y.; Ryo, E.; Fujii, T.; Osuga, Y.; Ayabe, T. Expression of the Glucagon-like Peptide-1 Receptor and Its Role in Regulating Autophagy in Endometrial Cancer. BMC Cancer 2018, 18, 657. [Google Scholar] [CrossRef]
- Tong, G.; Peng, T.; Chen, Y.; Sha, L.; Dai, H.; Xiang, Y.; Zou, Z.; He, H.; Wang, S. Effects of GLP-1 Receptor Agonists on Biological Behavior of Colorectal Cancer Cells by Regulating PI3K/AKT/MTOR Signaling Pathway. Front. Pharmacol. 2022, 13, 901559. [Google Scholar] [CrossRef]
- Alanteet, A.A.; Attia, H.A.; Shaheen, S.; Alfayez, M.; Alshanawani, B. Anti-Proliferative Activity of Glucagon-Like Peptide-1 Receptor Agonist on Obesity-Associated Breast Cancer: The Impact on Modulating Adipokines’ Expression in Adipocytes and Cancer Cells. Dose-Response 2021, 19, 1559325821995651. [Google Scholar] [CrossRef]
- Dutka, M.; Bobiński, R.; Francuz, T.; Garczorz, W.; Zimmer, K.; Ilczak, T.; Ćwiertnia, M.; Hajduga, M.B. SGLT-2 Inhibitors in Cancer Treatment—Mechanisms of Action and Emerging New Perspectives. Cancers 2022, 14, 5811. [Google Scholar] [CrossRef]
- Mohite, P.; Lokwani, D.K.; Sakle, N.S. Exploring the Therapeutic Potential of SGLT2 Inhibitors in Cancer Treatment: Integrating in Silico and in Vitro Investigations. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 6107–6119. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, G. Empagliflozin Inhibits OSCC Proliferation and Migration by Suppressing SLC2A3 and NLRP3. Namık Kemal Tıp Derg. 2025, 13, 302–310. [Google Scholar] [CrossRef]
- Hanabata, Y.; Nakajima, Y.; Morita, K.; Kayamori, K.; Omura, K. Coexpression of SGLT1 and EGFR Is Associated with Tumor Differentiation in Oral Squamous Cell Carcinoma. Odontology 2012, 100, 156–163. [Google Scholar] [CrossRef]
- Doss, D.M.; Nirmal, R.M.; Veeran, V.; Saravanan, R.; Sridevi, J.; Rachel, J.B. Immunoevaluation of GLUT-1 in Oral Squamous Cell Carcinoma. J. Oral Maxillofac. Pathol. 2025, 29, 179–185. [Google Scholar] [CrossRef]
- Mostafa, R.G.; Hashim, M.I.; Bawahab, A.A.; Baloush, R.A.A.; Abdelwahed, M.S.; Hasan, A.; Ismail, K.A.; Abd-Elhameed, N.R.; Embaby, A.; Sharfeldeen, A.E.R.M. Immunohistochemical Expression of Glucose Transporter-1 in Oral Epithelial Dysplasia and Different Grades of Oral Squamous Cell Carcinoma. Medicina 2025, 61, 557. [Google Scholar] [CrossRef]
- Rafik, R.; Andrew, R.; Mark, R. NF-ΚB Inhibitors in Clinical Management for Cancer and Diabetes Treatment: The IL-6 Potential. Am. Heart J. 2024, 267, 143–144. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, N.K.; Mishra, N.; Mahajan, A.; Krishnan, A.; Rajpoot, R.; Kumar, J.A.; Pandey, A. Effects of Curcumin on Oral Cancer at Molecular Level: A Systematic Review. Natl. J. Maxillofac. Surg. 2023, 14, 9–15. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, M.; Gao, S.-H.; Liu, J.-L. Curcumin and Diabetes: A Systematic Review. Evid. Based Complement. Altern. Med. 2013, 2013, 636053. [Google Scholar] [CrossRef]
- Mikolaskova, I.; Crnogorac-Jurcevic, T.; Smolkova, B.; Hunakova, L. Nutraceuticals as Supportive Therapeutic Agents in Diabetes and Pancreatic Ductal Adenocarcinoma: A Systematic Review. Biology 2023, 12, 158. [Google Scholar] [CrossRef]
- Wang, C.-R.; Tsai, H.-W. Anti- and Non-Tumor Necrosis Factor-α-Targeted Therapies Effects on Insulin Resistance in Rheumatoid Arthritis, Psoriatic Arthritis and Ankylosing Spondylitis. World J. Diabetes 2021, 12, 238–260. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.; Elizalde, P.V.; Schillaci, R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers 2021, 13, 564. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, Y.; Liu, Y.; Vadgama, J. Diabetes-Associated Dysregulated Cytokines and Cancer. Integr. Cancer Sci. Ther. 2016, 3, 370–378. [Google Scholar] [CrossRef][Green Version]
- Kim, N.-H.; Kim, S.-K.; Kim, D.-S.; Zhang, D.; Park, J.-A.; Yi, H.; Kim, J.-S.; Shin, H.-C. Anti-Proliferative Action of IL-6R-Targeted Antibody Tocilizumab for Non-Small Cell Lung Cancer Cells. Oncol. Lett. 2015, 9, 2283–2288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 Signaling by Natural Products-Can It Alleviate Diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An Update of Nrf2 Activators and Inhibitors in Cancer Prevention/Promotion. Cell Commun. Signal. 2022, 20, 100. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Luo, G.; Zhou, J.; Wang, N.; Wang, S.; Zhao, R.; Cao, X.; Ma, Y.; Liu, G.; et al. Nox4 as a Novel Therapeutic Target for Diabetic Vascular Complications. Redox Biol. 2023, 64, 102781. [Google Scholar] [CrossRef]
- Konaté, M.M.; Antony, S.; Doroshow, J.H. Inhibiting the Activity of NADPH Oxidase in Cancer. Antioxid. Redox Signal. 2020, 33, 435–454. [Google Scholar] [CrossRef]
- Stalin, J.; Garrido-Urbani, S.; Heitz, F.; Szyndralewiez, C.; Jemelin, S.; Coquoz, O.; Ruegg, C.; Imhof, B.A. Inhibition of Host NOX1 Blocks Tumor Growth and Enhances Checkpoint Inhibitor–Based Immunotherapy. Life Sci. Alliance 2019, 2, e201800265. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef]
- Shafras, M.; Sabaragamuwa, R.; Suwair, M. Role of Dietary Antioxidants in Diabetes: An Overview. Food Chem. Adv. 2024, 4, 100666. [Google Scholar] [CrossRef]
- Ali, E.S.; Mitra, K.; Akter, S.; Ramproshad, S.; Mondal, B.; Khan, I.N.; Islam, M.T.; Sharifi-Rad, J.; Calina, D.; Cho, W.C. Recent Advances and Limitations of MTOR Inhibitors in the Treatment of Cancer. Cancer Cell Int. 2022, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B.; Walter, T.; Cariou, B. Endocrine Side Effects of Anti-Cancer Drugs: Effects of Anti-Cancer Targeted Therapies on Lipid and Glucose Metabolism. Eur. J. Endocrinol. 2014, 170, R43–R55. [Google Scholar] [CrossRef]
- Umezawa, S.; Higurashi, T.; Nakajima, A. AMPK: Therapeutic Target for Diabetes and Cancer Prevention. Curr. Pharm. Des. 2017, 23, 3629–3644. [Google Scholar] [CrossRef]
- Li, J.; Dong, R.; Yu, J.; Yi, S.; Da, J.; Yu, F.; Zha, Y. Inhibitor of IGF1 Receptor Alleviates the Inflammation Process in the Diabetic Kidney Mouse Model without Activating SOCS2. Drug Des. Dev. Ther. 2018, 12, 2887–2896. [Google Scholar] [CrossRef]
- Guven, D.C.; Ahmed, J.; Stephen, B.; Naing, A. IGF-1R Inhibitors in Cancer: A Review of Available Evidence and Future Outlook. Crit. Rev. Oncol. Hematol. 2025, 214, 104809. [Google Scholar] [CrossRef]
- Elebiyo, T.C.; Rotimi, D.; Evbuomwan, I.O.; Maimako, R.F.; Iyobhebhe, M.; Ojo, O.A.; Oluba, O.M.; Adeyemi, O.S. Reassessing Vascular Endothelial Growth Factor (VEGF) in Anti-Angiogenic Cancer Therapy. Cancer Treat. Res. Commun. 2022, 32, 100620. [Google Scholar] [CrossRef]
- Carmeliet, P.; Wong, B.W.; De Bock, K. Treating Diabetes by Blocking a Vascular Growth Factor. Cell Metab. 2012, 16, 553–555. [Google Scholar] [CrossRef][Green Version]
- Semenza, G.L. Development of Small Molecule Inhibitors of Hypoxia-Inducible Factors for Cancer Therapy. Pharmacol. Rev. 2025, 77, 100075. [Google Scholar] [CrossRef]
- Kao, T.-W.; Bai, G.-H.; Wang, T.-L.; Shih, I.-M.; Chuang, C.-M.; Lo, C.-L.; Tsai, M.-C.; Chiu, L.-Y.; Lin, C.-C.; Shen, Y.-A. Novel Cancer Treatment Paradigm Targeting Hypoxia-Induced Factor in Conjunction with Current Therapies to Overcome Resistance. J. Exp. Clin. Cancer Res. 2023, 42, 171. [Google Scholar] [CrossRef]
- Ilegems, E.; Bryzgalova, G.; Correia, J.; Yesildag, B.; Berra, E.; Ruas, J.L.; Pereira, T.S.; Berggren, P.-O. HIF-1α Inhibitor PX-478 Preserves Pancreatic β Cell Function in Diabetes. Sci. Transl. Med. 2022, 14, eaba9112. [Google Scholar] [CrossRef]
- Nekoua, M.P.; Fachinan, R.; Atchamou, A.K.; Nouatin, O.; Amoussou-Guenou, D.; Amoussou-Guenou, M.K.; Moutairou, K.; Yessoufou, A. Modulation of Immune Cells and Th1/Th2 Cytokines in Insulin-Treated Type 2 Diabetes Mellitus. Afr. Health Sci. 2016, 16, 712. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Yu, X.; Sun, Q.; Li, H.; Sun, C.; Liu, L. Polysaccharides Regulate Th1/Th2 Balance: A New Strategy for Tumor Immunotherapy. Biomed. Pharmacother. 2024, 170, 115976. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Watt, W.C.; Cecil, D.L. Th1 Epitope Selection for Clinically Effective Cancer Vaccines. Oncoimmunology 2014, 3, e954971. [Google Scholar] [CrossRef]
- Kang, Y.E.; Kim, H.J.; Shong, M. Regulation of Systemic Glucose Homeostasis by T Helper Type 2 Cytokines. Diabetes Metab. J. 2019, 43, 549. [Google Scholar] [CrossRef]
- Cho, Y.K.; Jung, C.H. Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice. Diabetes Metab. J. 2023, 47, 757–766. [Google Scholar] [CrossRef]
- Aden, D.; Zaheer, S.; Sureka, N.; Trisal, M.; Chaurasia, J.K.; Zaheer, S. Exploring Immune Checkpoint Inhibitors: Focus on PD-1/PD-L1 Axis and Beyond. Pathol. Res. Pract. 2025, 269, 155864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).