Diabetes Mellitus and Cardiopulmonary Bypass (CPB): Pathophysiological Mechanisms Related to Inflammation and Cardiovascular Disease
Abstract
1. Introduction
2. Diabetes Mellitus: Classification, Risk Factors, and Cardiovascular Associations
3. Metabolism and Inflammation: The Inflammatory State Characterizing Type 2 Diabetes Mellitus with Cardiovascular Pathophysiology Associations
3.1. Metabolism and Inflammation: The Generalized Inflammatory State of Type 2 Diabetes Mellitus with a Focus on Macrophages and T-Lymphocyte Populations
3.2. Metabolism and Inflammation: The Generalized Inflammatory State of Type 2 Diabetes Mellitus with Cardiovascular Pathophysiology Associations
4. Cardiopulmonary Bypass (CPB) and Inflammation: General Overview of Cardiopulmonary Bypass with a Focus on Macrophage, T-Lymphocyte Populations
4.1. Cardiopulmonary Bypass: Overview of the Circuit, Effects of Cardioplegic Arrest on Tissue Structure and Function
4.2. Cardiopulmonary Bypass: General Overview of the Inflammatory Response During Cardiopulmonary Bypass
4.3. Cardiopulmonary Bypass: Contributions of Macrophage and T-Lymphocyte Populations to the Inflammatory Response and Immune Dysfunction with Cardiopulmonary Bypass
5. Cardiopulmonary Bypass (CPB) and Its Effects on the Inflammatory State in Type 2 Diabetes Mellitus
| Study (Reference) | Description | Model | Immune Effects of Cardiac Surgery with CPB |
|---|---|---|---|
| Antunes et al., 1997 [198] | Increased risk for postoperative wound infections, mediastinitis; risk augmented with bilateral ITA harvesting | Human | Mediastinitis (Increase) |
| Matata and Galiñanes, 2000 [199] | Increase in pro-inflammatory factors and cytokines; increase in complement factors (C3a) greater and more immediate, activation of immune cells (neutrophils) and secretion of elastase persists for longer periods after CPB | Human | Qualitative differences in inflammatory reaction in DM versus non-DM cohorts |
| Groom et al., 2004 [200] | DM, temperature-interacting variables in infectious mediastinitis; rates of mediastinitis increase from 0.7% (lower than 37° C) up to 3.3% (greater than 38 °C); no such effect observed in non-DM groups | Human | Mediastinitis (Increase) |
| Voisine et al., 2004 [201] | Upregulation of transcriptional activators related to inflammation (MYC, IL-8, IL-1β, VEGF, amphiregulin, and IRS-1) | Human | Pro-inflammatory gene expression (Increase) |
| Kremen et al., 2006 [171] | Increase in pro-inflammatory cytokine secretion from subcutaneous (IL-6, TNF-α, CD45, resistin, and MCP-1), epicardial adipose tissue (IL-6, resistin, and MCP-1); association of pro-inflammatory factor secretion with insulin resistance up to 24 h after CPB | Human | Pro-inflammatory factors (Increase) |
| de Lange et al., 2007 [172] | Higher levels of IL-6 in animals with DM compared to non-DM groups | Rat | Pro-inflammatory factors (Increase) |
| Emani et al., 2009 [176] | Higher levels and larger increase in circulating pro-inflammatory cytokines (VEGF, HGF) with increased expression of pro-inflammatory factors HIF-1α, CREB, and EP300 in DM compared to non-DM groups | Human | Pro-inflammatory cytokines (Increase), pro-inflammatory gene expression (Increase) |
| Zakrzewski et al., 2010 [202] | No association of the postoperative inflammatory response with renal failure | Human | N/A |
| Le Guillou et al., 2012 [203] | Higher levels and larger increase in TNF-α levels DM groups | Rat | Pro-inflammatory cytokines (Increase) |
| Zhou et al., 2024 [168] | Higher levels and larger increase in IL-6, TNF-α, inflammation, and caspase-1-mediated pyroptosis in T2DM groups (cardiac muscle) | Rat | Pro-inflammatory cytokines (Increase) |
| Study (Reference) | Description | Location | Model | Vascular Response to Cardiac Surgery with CPB |
|---|---|---|---|---|
| Feng et al., 2012 [187] | Arteriolar response to vasodilatory substances (ADP, substance P) impaired in poorly controlled DM; Increased levels of PKC-α, PKC-β | Skeletal muscle microvessels | Human | Response to vasodilation (Decrease) |
| Le Guillou et al., 2012 [203] | Arteriolar response to vasoconstrictor substances (phenylephrine) is enhanced, while response to vasodilatory substances (acetylcholine) is impaired | Mesentery microvessels | Rat | Response to vasodilation (Decrease), response to vasoconstriction (Increase) |
| Feng et al., 2013 [186] | Arteriolar response to vasoconstrictor substances (TXA2) impaired in all patients, effect augmented in patients with poorly controlled DM | Cardiac muscle, coronary microvessels | Human | Response to vasoconstriction (Decrease) |
| Changes in protein kinase expression, including p38-MAPK (decrease), ERK1/2 (decrease), and JNK (increase); MKP-1 levels higher in poorly controlled DM with no significant changes due to CPB | Cardiac muscle | Alterations in levels of MAPK, ERK, and JNK kinase activity | ||
| Feng et al., 2016 [180] | Arteriolar response to vasodilatory substances (ADP) impaired in poorly controlled DM | Cardiac muscle, coronary microvessels | Human | Response to vasodilation (Decrease) |
| Higher levels of phosphorylated endothelial VE-Cadherin in poorly controlled DM; higher rates of VE-Cadherin, β-, γ-catenin degradation with endothelial cell-to-cell junction disruption in poorly controlled DM | Adherens-junction activation/localization in coronary endothelial cells (Decrease) | |||
| Feng et al., 2017 [189] | Arteriolar response to vasodilatory substances (bradykinin) impaired in poorly controlled DM; higher levels and larger increase in COX-2 in poorly controlled DM; no effects on COX-1 expression | Skeletal muscle microvessels | Human | Response to vasodilation (Decrease) |
| Study (Reference) | Description | Model | Effects of Cardiac Surgery with CPB on Oxidative Stress |
|---|---|---|---|
| Matata and Galiñanes, 2000 [199] | Higher levels and larger increase in lipid hydroperoxides, protein carbonyls, and NOX4 proteins in DM groups | Human | Increase |
| Doenst et al., 2005 [204] | Hyperglycemia is an independent risk factor for mortality regardless of DM due to mechanisms involving immune cells (monocyte, neutrophil), endothelial function, and the pro-inflammatory state; association with insulin resistance and other comorbidities (CAD, diabetic cardiomyopathy) | Human | N/A |
| de Lange et al., 2007 [172] | No differences in neurocognitive performance tests (MWM) in DM versus non-DM groups | Rat | N/A |
| Marty et al., 2008 [205] | Increase in ascorbyl radical/vitamin C ratios, decrease in ORAC values in DM; reduced capacity for the neutralization of oxidative stress | Human | Increase |
| Feng et al., 2012 [187] | Increased levels of protein oxidation in skeletal muscle, contribution to levels of protein oxidation by cardiac surgery with CPB not significant | Human | No significant effect |
| Cao et al., 2013 [170] | Reduction in adiponectin due to inflammation contributes to perioperative insulin resistance up to 48 h after CPB | Human | N/A |
| Mahmood et al., 2019 [206] | Baseline levels of PGC-1α reduced in DM preoperatively, decrease is further augmented postoperatively; reduced levels of antioxidant (NOX4, GPX4), angiogenic (TGF-β, NT3, Ang1), and anti-apoptotic (BCM-xL) factors in DM postoperatively; downregulation of proteins involved in mitochondrial energy production (CREB5, SLC25A40) and angiogenesis | Human | Impaired mitochondrial function and beta-oxidation |
| Zhang et al., 2021 [181] | Absence of the physiological postoperative FOXO3α response, alterations in levels of effectors involved in mitochondrial FA β-oxidation (PGC-1α) and autophagy (SIRT1, FOXO3α), and downregulation of autophagy in cardiac tissue | Human | Paradoxical decrease in FOXO3α, absence of physiological upregulation in SIRT1 and PGC-1α |
| No explicit association between upregulation of autophagy/mitochondrial biogenesis and improved clinical outcomes in this study | |||
| Snel et al., 2024 [207] | Increase in ketone bodies throughout cardiac surgery, peak observed at the end of aortic cross-clamp; no differences in ketone body concentrations based on T2DM status | Human | No significant effect |
| Zhou et al., 2024 [168] | Levels of lipid deposition, disruption of myocardial fiber architecture, and cardiomyocyte apoptosis increased in T2DM groups | Rat | Increase |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AdipoR1 | Adiponectin receptor-1 |
| ADP | Adenosine Diphosphate |
| AGE | Advanced glycation end-products |
| AKI | Acute kidney injury |
| AKT | Protein kinase B |
| AMP | Adenosine Monophosphate |
| AMPK | AMP-activated protein kinase |
| AP-1 | Activator Protein-1 |
| Arg-1 | Arginase-1 |
| ATP | Adenosine triphosphate |
| C3aR | Complement C3a receptor |
| C5aR1 | Complement C5a receptor 1 |
| Ca2+ | Calcium |
| cAMP | Cyclic adenosine monophosphate |
| CCR2 | C-C Motif Chemokine Receptor 2 |
| CHOP | C/EBP homologous protein or growth arrest and DNA damage-inducible protein 153 (GADD153) |
| CI | Cardiac index |
| CK-MB | Creatine kinase-MB |
| CPB | Cardiopulmonary bypass |
| CR3 | Complement receptor 3 |
| CRP | C-Reactive protein |
| CTLA-4 | Cytotoxic T-lymphocyte–associated protein 4 |
| CXCL1/2/6/8A/9/10/12 | C-X-C motif chemokine ligand 12 |
| CXCR2/3/4/7 | C-X-C motif chemokine receptor 2/3/4/7 |
| DAMP | Damage-associated molecular patterns |
| DM | Diabetes mellitus |
| DNA | Deoxyribonucleic acid |
| ECM | Extracellular matrix |
| eGFR | estimated Glomerular filtration rate |
| eNOS | Endothelial nitric oxide synthase |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-related kinase |
| FFA | free fatty acid |
| FOX | Forkhead box |
| FOXO | Forkhead box O |
| FOXO1 | Forkhead box O1 |
| FOXP3 | Forkhead box P3 |
| G6Pase | Glucose-6-phosphatase |
| GCK | Glucokinase |
| GDM | Gestational diabetes mellitus |
| GLUT1/2/4 | Glucose transporter 1/2/4 |
| GpIIb/IIIa/IV | Glycoprotein IIb/IIIa/IV |
| GSK3/3β | Glycogen synthase kinase 3/3 beta |
| HbA1c | Glycated hemoglobin A |
| HCV | Hepatitis C virus |
| HDL | High density lipoprotein |
| HDL-C | High density lipoprotein-C |
| HIF-1α | Hypoxia inducible factor 1 alpha |
| HIT | Heparin induced thrombocytopenia |
| HLA-DR | Human leukocyte antigen-DR isotype |
| HMGB1 | High Mobility Group Box 1 |
| HMWK | High-molecular-weight kininogen |
| HTK | Histidine, tryptophan and ketoglutarate |
| I/R | Ischemia/Reperfusion injury |
| IABP | Intra-aortic balloon pump |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IFN-γ | Interferon gamma |
| IGF1-R | Insulin growth factor 1-receptor |
| IL-1β/4/5/6/8/9/10/12/13/17A/18/21/22/23/36 | Interleukin-1β/4/5/6/8/9/10/12/13/17A/18/21/22/23/36 |
| IL-6/9R | Interleukin-6/9 receptor |
| iNOS | Inducible nitric oxide synthase |
| IRS-1 | Insulin receptor substrate-1 |
| JAK1/3 | Janus kinase 1/3 |
| JNK | C-Jun N-terminal kinase 1 |
| K+ | Potassium |
| LC3B | Microtubule associated protein 1 light chain 3 beta |
| LCOS | Low cardiac output syndrome |
| LDL | Low-density lipoprotein |
| LFA-1 | Lymphocyte function-associated antigen 1 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-associated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MD-2 | Myeloid differentiation-2 |
| Mef2d | Myocyte enhancer factor 2d |
| Mg2+ | Magnesium |
| MHC | Major histocompatibility complex |
| MIF | Macrophage migration inhibitory factor |
| MLCK | Myosin light chain kinase |
| MMP-1/9/13 | Matrix metalloproteinase-1/9/13 |
| mTOR | Mammalian target of rapamycin |
| MPTP | Mitochondrial permeability transition pore |
| MYC | Myelocytomatosis oncogene |
| MYH6 | Myosin heavy chain 6 |
| Na+ | Sodium |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NAFLD | Non-alcoholic fatty liver disease |
| NET | Neutrophil extracellular traps |
| NF-κB | Nuclear factor kappa B subunit 1 |
| NK cells | Natural killer cells |
| NLR | Nod-like receptors |
| NLRP3 | NLR family pyrin domain containing 3 |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NOX | Nicotinamide adenine dinucleotide phosphate (NADPH) Oxidase |
| p53 | Tumor protein p53 |
| PAF | Platelet activating factor |
| PAI-1 | Plasminogen activator inhibitor 1 |
| PAMP | Pathogen-associated molecular structures |
| PAR | Protease-activated receptors |
| PD-1 | Programmed cell death protein 1 |
| PDK1 | Pyruvate dehydrogenase kinase 1 |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PFK-2 | Phosphofructokinase-2 |
| PI3K | Phosphoinositide-3-kinase |
| PKC/Cδ/ε | Protein kinase C/C delta/C epsilon |
| PKM2 | Pyruvate kinase M2 |
| POAF | Postoperative atrial fibrillation |
| PPARG | Peroxisome proliferator activated receptor gamma (gene) |
| PPARγ | Peroxisome proliferator activated receptor gamma (protein) |
| PSGL-1 | P-Selectin glycoprotein ligand-1 |
| PTCA | Platelet-T-lymphocyte aggregates |
| PTDM | Post-transplantation diabetes mellitus |
| RAAS | Renin angiotensin system |
| RAGE | Receptor for advanced glycation end-products |
| RBC | Red blood cell |
| RNAi | Ribonucleic acid (RNA) interference |
| ROR-γt | Retinoic acid receptor-related orphan receptor gamma-t |
| ROS | Reactive oxygen species |
| RYR2 | Ryanodine receptor 2 |
| SERCA2a | ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 |
| SGK1 | Serum-glucocorticoid regulated kinase 1 |
| SGLT2 | Na+-Glucose cotransporter 2 |
| sIL-6R | Soluble IL-6 receptor |
| SIRS | Systemic inflammatory response syndrome |
| SIRT1 | Sirtuin 1 |
| STAT1/3/5/6 | Signal transducer and activator of transcription 1/3/5/6 |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| T3DM | Type 3c diabetes mellitus |
| TAC | Transverse aortic constriction |
| TAG | Triacylglycerol |
| TCA | Tricarboxylic acid cycle |
| TCR | T-cell receptor |
| TGF-β | Transforming growth factor beta |
| TIMP-1/2 | Tissue inhibitor of metalloproteinase-1/2 |
| TLR/2/4 | Toll-like receptor/-2/4 |
| TNF-α | Tumor necrosis factor alpha |
| tPA | Tissue plasminogen activator |
| TRALI | Transfusion-related acute lung injury |
| UPR | Unfolded protein response |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
| VEGFA | Vascular endothelial growth factor A |
| VSMC | Vascular smooth muscle cells |
| WHO | World Health Organization |
References
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 29 August 2025).
- Siam, N.H.; Snigdha, N.N.; Tabasumma, N.; Parvin, I. Diabetes Mellitus and Cardiovascular Disease: Exploring Epidemiology, Pathophysiology, and Treatment Strategies. Rev. Cardiovasc. Med. 2024, 25, 436. [Google Scholar] [CrossRef]
- Hattar, L.; Mumtaz, T.; El Mouhayyar, C.; Matevossian, A.; Johnstone, M. The Effects and Treatment of Inflammation on Diabetes Mellitus and Cardiovascular Disease. In Diabetes and Cardiovascular Disease; Johnstone, M., Veves, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 307–329. ISBN 978-3-031-13177-6. [Google Scholar]
- Squiccimarro, E.; Stasi, A.; Lorusso, R.; Paparella, D. Narrative Review of the Systemic Inflammatory Reaction to Cardiac Surgery and Cardiopulmonary Bypass. Artif. Organs 2022, 46, 568–577. [Google Scholar] [CrossRef]
- Knapik, P.; Nadziakiewicz, P.; Urbanska, E.; Saucha, W.; Herdynska, M.; Zembala, M. Cardiopulmonary Bypass Increases Postoperative Glycemia and Insulin Consumption After Coronary Surgery. Ann. Thorac. Surg. 2009, 87, 1859–1865. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes Mellitus: Classification, Mediators, and Complications; A Gate to Identify Potential Targets for the Development of New Effective Treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Martagon, A.J.; Zubirán, R.; González-Arellanes, R.; Praget-Bracamontes, S.; Rivera-Alcántara, J.A.; Aguilar-Salinas, C.A. HDL Abnormalities in Type 2 Diabetes: Clinical Implications. Atherosclerosis 2024, 394, 117213. [Google Scholar] [CrossRef]
- Yamada, T.; Kimura-Koyanagi, M.; Sakaguchi, K.; Ogawa, W.; Tamori, Y. Obesity and Risk for Its Comorbidities Diabetes, Hypertension, and Dyslipidemia in Japanese Individuals Aged 65 Years. Sci. Rep. 2023, 13, 2346. [Google Scholar] [CrossRef]
- Mitrovic, B.; Gluvic, Z.M.; Obradovic, M.; Radunovic, M.; Rizzo, M.; Banach, M.; Isenovic, E.R. Non-Alcoholic Fatty Liver Disease, Metabolic Syndrome, and Type 2 Diabetes Mellitus: Where Do We Stand Today? Arch. Med. Sci. 2022, 19, 884–894. [Google Scholar] [CrossRef]
- Sprenger, L.; Maechler, M.; Vonbank, A.; Larcher, B.; Mader, A.; Plattner, T.; Leiherer, A.; Muendlein, A.; Drexel, H.; Saely, C.H. Type 2 Diabetes, Chronic Kidney Disease and Major Cardiovascular Events in Patients with Established Cardiovascular Disease. JACC 2023, 81, 1698. [Google Scholar] [CrossRef]
- Borén, J.; Öörni, K.; Catapano, A.L. The Link between Diabetes and Cardiovascular Disease. Atherosclerosis 2024, 394, 117607. [Google Scholar] [CrossRef]
- Popoviciu, M.S.; Kaka, N.; Sethi, Y.; Patel, N.; Chopra, H.; Cavalu, S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J. Pers. Med. 2023, 13, 422. [Google Scholar] [CrossRef]
- Vergès, B. Cardiovascular Disease in Type 1 Diabetes, an Underestimated Danger: Epidemiological and Pathophysiological Data. Atherosclerosis 2024, 394, 117158. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.-M.; Eliasson, B.; Gudbjörnsdottir, S. Excess Mortality and Cardiovascular Disease in Young Adults with Type 1 Diabetes in Relation to Age at Onset: A Nationwide, Register-Based Cohort Study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Gottumukkala, R.V.S.R.K.; Lv, H.; Cornivelli, L.; Wagers, A.J.; Kwong, R.Y.; Bronson, R.; Stewart, G.C.; Schulze, P.C.; Chutkow, W.; Wolpert, H.A.; et al. Myocardial Infarction Triggers Chronic Cardiac Autoimmunity in Type 1 Diabetes. Sci. Transl. Med. 2012, 4, 138ra80. [Google Scholar] [CrossRef]
- Dong, B.; Qi, D.; Yang, L.; Huang, Y.; Xiao, X.; Tai, N.; Wen, L.; Wong, F.S. TLR4 Regulates Cardiac Lipid Accumulation and Diabetic Heart Disease in the Nonobese Diabetic Mouse Model of Type 1 Diabetes. Am. J. Physiol.-Heart Circ. Physiol. 2012, 303, H732–H742. [Google Scholar] [CrossRef]
- Tannus, L.R.M.; Drummond, K.R.G.; Clemente, E.L.d.S.; da Matta, M.d.F.B.; Gomes, M.B.; on behalf of the Brazilian Type 1 Diabetes Study Group (BrazDiab1SG). Predictors of Cardiovascular Autonomic Neuropathy in Patients with Type 1 Diabetes. Front. Endocrinol. 2014, 5, 191. [Google Scholar] [CrossRef]
- de Ferranti, S.D.; de Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 Diabetes Mellitus and Cardiovascular Disease. Circulation 2014, 130, 1110–1130. [Google Scholar] [CrossRef]
- Ferrannini, G.; Manca, M.L.; Magnoni, M.; Andreotti, F.; Andreini, D.; Latini, R.; Maseri, A.; Maggioni, A.P.; Ostroff, R.M.; Williams, S.A.; et al. Coronary Artery Disease and Type 2 Diabetes: A Proteomic Study. Diabetes Care 2020, 43, 843–851, Erratum in Diabetes Care 2021, 44, 1071. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Patel, N.T.; Villarreal-Gonzalez, M.; Taub, P.R. Prevalence of Diabetic Cardiomyopathy in Patients with Type 2 Diabetes in a Large Academic Medical Center. BMC Med. 2024, 22, 195. [Google Scholar] [CrossRef]
- Ma, C.-X.; Ma, X.-N.; Guan, C.-H.; Li, Y.-D.; Mauricio, D.; Fu, S.-B. Cardiovascular Disease in Type 2 Diabetes Mellitus: Progress toward Personalized Management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Leutner, M.; Harreiter, J. Sex Differences in Type 2 Diabetes. Diabetologia 2023, 66, 986–1002. [Google Scholar] [CrossRef]
- Yoo, D.; Kang, M.; Jung, J. Risk of Ischemic Heart Disease in Patients With Postpancreatectomy Diabetes and Pancreatic Cancer: A Population-Based Study. J. Am. Heart Assoc. 2023, 12, e031321. [Google Scholar] [CrossRef]
- Wayne, C.D.; Benbetka, C.; Besner, G.E.; Narayanan, S. Challenges of Managing Type 3c Diabetes in the Context of Pancreatic Resection, Cancer and Trauma. J. Clin. Med. 2024, 13, 2993. [Google Scholar] [CrossRef]
- Hjelmesæth, J.; Hartmann, A.; Leivestad, T.; Holdaas, H.; Sagedal, S.; Olstad, M.; Jenssen, T. The Impact of Early-Diagnosed New-Onset Post-Transplantation Diabetes Mellitus on Survival and Major Cardiac Events. Kidney Int. 2006, 69, 588–595. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Radek, M.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. Diabetes and Cardiovascular Risk in Renal Transplant Patients. Int. J. Mol. Sci. 2021, 22, 3422. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.-U.; Schold, J.D.; Srinivas, T.R.; Reed, A.; Kaplan, B. Kidney Transplantation Halts Cardiovascular Disease Progression in Patients with End-Stage Renal Diseas. Am. J. Transplant. 2004, 4, 1662–1668. [Google Scholar] [CrossRef]
- Wu, H.-X.; Chu, T.-Y.; Iqbal, J.; Jiang, H.-L.; Li, L.; Wu, Y.-X.; Zhou, H.-D. Cardio-Cerebrovascular Outcomes in MODY, Type 1 Diabetes, and Type 2 Diabetes: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2023, 108, 2970–2980. [Google Scholar] [CrossRef]
- Mao, Y.; Hu, W.; Xia, B.; Liu, L.; Han, X.; Liu, Q. Association Between Gestational Diabetes Mellitus and the Risks of Type-Specific Cardiovascular Diseases. Front. Public Health 2022, 10, 940335. [Google Scholar] [CrossRef]
- Chen, A.; Tan, B.; Du, R.; Chong, Y.S.; Zhang, C.; Koh, A.S.; Li, L.-J. Gestational Diabetes Mellitus and Development of Intergenerational Overall and Subtypes of Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Cardiovasc. Diabetol. 2024, 23, 320. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Chawla, A. Connecting Type 1 and Type 2 Diabetes through Innate Immunity. Cold Spring Harb. Perspect. Med. 2012, 2, a007724. [Google Scholar] [CrossRef]
- Toyoshima, Y.; Nakamura, K.; Taguchi, Y.; Tokita, R.; Takeuchi, S.; Osawa, H.; Teramoto, N.; Sugihara, H.; Yoshizawa, F.; Yamanouchi, K.; et al. Deletion of IRS-1 Leads to Growth Failure and Insulin Resistance with Downregulation of Liver and Muscle Insulin Signaling in Rats. Sci. Rep. 2025, 15, 649. [Google Scholar] [CrossRef]
- Jaldin-Fincati, J.R.; Pavarotti, M.; Frendo-Cumbo, S.; Bilan, P.J.; Klip, A. Update on GLUT4 Vesicle Traffic: A Cornerstone of Insulin Action. Trends Endocrinol. Metab. 2017, 28, 597–611. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Y.; Zhang, Z.; Chen, X.; Hu, Z.; Tong, Q.; Chang, J.; Feng, X.-H.; Lin, X. SCP4 Promotes Gluconeogenesis Through FoxO1/3a Dephosphorylation. Diabetes 2018, 67, 46–57. [Google Scholar] [CrossRef]
- Markuns, J.F.; Wojtaszewski, J.F.P.; Goodyear, L.J. Insulin and Exercise Decrease Glycogen Synthase Kinase-3 Activity by Different Mechanisms in Rat Skeletal Muscle. J. Biol. Chem. 1999, 274, 24896–24900. [Google Scholar] [CrossRef]
- Takano, A.; Usui, I.; Haruta, T.; Kawahara, J.; Uno, T.; Iwata, M.; Kobayashi, M. Mammalian Target of Rapamycin Pathway Regulates Insulin Signaling via Subcellular Redistribution of Insulin Receptor Substrate 1 and Integrates Nutritional Signals and Metabolic Signals of Insulin. Mol. Cell Biol. 2001, 21, 5050–5062. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 Diabetes Mellitus in Adults: Pathogenesis, Prevention and Therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef]
- Małodobra-Mazur, M.; Cierzniak, A.; Myszczyszyn, A.; Kaliszewski, K.; Dobosz, T. Histone Modifications Influence the Insulin-Signaling Genes and Are Related to Insulin Resistance in Human Adipocytes. Int. J. Biochem. Cell Biol. 2021, 137, 106031. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Li, H.; Xu, P.; Liu, Q.; Sun, Y.; Zhang, Z.; Wu, T.; Tang, Q.; Jia, Q.; et al. B3galt5 Functions as a PXR Target Gene and Regulates Obesity and Insulin Resistance by Maintaining Intestinal Integrity. Nat. Commun. 2024, 15, 5919. [Google Scholar] [CrossRef]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2019, 9, 1–58. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted Disruption of AdipoR1 and AdipoR2 Causes Abrogation of Adiponectin Binding and Metabolic Actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef]
- Suzuki, T.; Gao, J.; Ishigaki, Y.; Kondo, K.; Sawada, S.; Izumi, T.; Uno, K.; Kaneko, K.; Tsukita, S.; Takahashi, K.; et al. ER Stress Protein CHOP Mediates Insulin Resistance by Modulating Adipose Tissue Macrophage Polarity. Cell Rep. 2017, 18, 2045–2057. [Google Scholar] [CrossRef]
- Kang, K.; Reilly, S.M.; Karabacak, V.; Gangl, M.R.; Fitzgerald, K.; Hatano, B.; Lee, C.-H. Adipocyte-Derived Th2 Cytokines and Myeloid PPARδ Regulate Macrophage Polarization and Insulin Sensitivity. Cell Metab. 2008, 7, 485–495. [Google Scholar] [CrossRef]
- Li, X.; Xiao, G.-Y.; Guo, T.; Song, Y.-J.; Li, Q.-M. Potential Therapeutic Role of Pyroptosis Mediated by the NLRP3 Inflammasome in Type 2 Diabetes and Its Complications. Front. Endocrinol. 2022, 13, 986565. [Google Scholar] [CrossRef]
- Cucak, H.; Grunnet, L.G.; Rosendahl, A. Accumulation of M1-like Macrophages in Type 2 Diabetic Islets Is Followed by a Systemic Shift in Macrophage Polarization. J. Leukoc. Biol. 2014, 95, 149–160. [Google Scholar] [CrossRef]
- León-Pedroza, J.I.; González-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodríguez, D.; Escobedo, G.; González-Chávez, A. Low-Grade Systemic Inflammation and the Development of Metabolic Diseases: From the Molecular Evidence to the Clinical Practice. Cirugía Y Cir. 2015, 83, 543–551. [Google Scholar] [CrossRef]
- Kunz, H.E.; Hart, C.R.; Gries, K.J.; Parvizi, M.; Laurenti, M.; Man, C.D.; Moore, N.; Zhang, X.; Ryan, Z.; Polley, E.C.; et al. Adipose Tissue Macrophage Populations and Inflammation Are Associated with Systemic Inflammation and Insulin Resistance in Obesity. Am. J. Physiol.-Endocrinol. Metab. 2021, 321, E105–E121. [Google Scholar] [CrossRef]
- Shirakawa, K.; Yan, X.; Shinmura, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yamamoto, T.; Anzai, A.; Isobe, S.; Yoshida, N.; et al. Obesity Accelerates T Cell Senescence in Murine Visceral Adipose Tissue. J. Clin. Investig. 2016, 126, 4626–4639. [Google Scholar] [CrossRef]
- McLaughlin, T.; Liu, L.-F.; Lamendola, C.; Shen, L.; Morton, J.; Rivas, H.; Winer, D.; Tolentino, L.; Choi, O.; Zhang, H.; et al. T-Cell Profile in Adipose Tissue Is Associated With Insulin Resistance and Systemic Inflammation in Humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2637–2643. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Folco, E.J.; Sukhova, G.; Shimizu, K.; Gotsman, I.; Vernon, A.H.; Libby, P. Interferon-Gamma, a Th1 Cytokine, Regulates Fat Inflammation: A Role for Adaptive Immunity in Obesity. Circ. Res. 2008, 103, 467–476. [Google Scholar] [CrossRef]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of Obesity-Associated Insulin Resistance through Immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic Reticulum Stress: Molecular Mechanism and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Thakkar, H.; Vincent, V.; Chaurasia, B. Ceramide Signaling in Immunity: A Molecular Perspective. Lipids Health Dis. 2025, 24, 225. [Google Scholar] [CrossRef]
- Delcheva, G.; Stefanova, K.; Stankova, T. Ceramides—Emerging Biomarkers of Lipotoxicity in Obesity, Diabetes, Cardiovascular Diseases, and Inflammation. Diseases 2024, 12, 195. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Liu, J.; Ma, Y.; Qiu, C.; Liu, C.; Gong, Y.; Yuwen, Y.; Guan, G.; Zhang, Y.; et al. Palmitic Acid in Type 2 Diabetes Mellitus Promotes Atherosclerotic Plaque Vulnerability via Macrophage Dll4 Signaling. Nat. Commun. 2024, 15, 1281. [Google Scholar] [CrossRef]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin Promotes Macrophage Polarization toward an Anti-Inflammatory Phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef]
- Xia, C.; Rao, X.; Zhong, J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J. Diabetes Res. 2017, 2017, 6494795. [Google Scholar] [CrossRef]
- Meshkani, R.; Vakili, S. Tissue Resident Macrophages: Key Players in the Pathogenesis of Type 2 Diabetes and Its Complications. Clin. Chim. Acta 2016, 462, 77–89. [Google Scholar] [CrossRef]
- Pavlou, S.; Lindsay, J.; Ingram, R.; Xu, H.; Chen, M. Sustained High Glucose Exposure Sensitizes Macrophage Responses to Cytokine Stimuli but Reduces Their Phagocytic Activity. BMC Immunol. 2018, 19, 24. [Google Scholar] [CrossRef]
- Witcoski Junior, L.; de Lima, J.D.; Somensi, A.G.; de Souza Santos, L.B.; Paschoal, G.L.; Uada, T.S.; Bastos, T.S.B.; de Paula, A.G.P.; Dos Santos Luz, R.B.; Czaikovski, A.P.; et al. Metabolic Reprogramming of Macrophages in the Context of Type 2 Diabetes. Eur. J. Med. Res. 2024, 29, 497. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced Glycation End Products (AGE) and Diabetes: Cause, Effect, or Both? Curr. Diabetes Rep. 2013, 14, 453. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.-S.; Ko, S.-H. Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus. Nutrients 2022, 14, 3086. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Rajbhandari, J.; Fernandez, C.J.; Agarwal, M.; Yeap, B.X.Y.; Pappachan, J.M. Diabetic Heart Disease: A Clinical Update. World J. Diabetes 2021, 12, 383–406. [Google Scholar] [CrossRef]
- Lei, X.; Qiu, S.; Yang, G.; Wu, Q. Adiponectin and Metabolic Cardiovascular Diseases: Therapeutic Opportunities and Challenges. Genes. Dis. 2023, 10, 1525–1536. [Google Scholar] [CrossRef]
- Avagimyan, A.; Fogacci, F.; Pogosova, N.; Kakrurskiy, L.; Kogan, E.; Urazova, O.; Kobalava, Z.; Mikhaleva, L.; Vandysheva, R.; Zarina, G.; et al. Diabetic Cardiomyopathy: 2023 Update by the International Multidisciplinary Board of Experts. Curr. Probl. Cardiol. 2024, 49, 102052. [Google Scholar] [CrossRef]
- Ussher, J.R. The Role of Cardiac Lipotoxicity in the Pathogenesis of Diabetic Cardiomyopathy. Expert. Rev. Cardiovasc. Ther. 2014, 12, 345–358. [Google Scholar] [CrossRef]
- Guo, W.; Yang, C.; Zou, J.; Yu, T.; Li, M.; He, R.; Chen, K.; Hell, R.C.R.; Gross, E.R.; Zou, X.; et al. Interleukin-1β Polarization in M1 Macrophage Mediates Myocardial Fibrosis in Diabetes. Int. Immunopharmacol. 2024, 131, 111858. [Google Scholar] [CrossRef]
- Zi, C.; He, L.; Yao, H.; Ren, Y.; He, T.; Gao, Y. Changes of Th17 Cells, Regulatory T Cells, Treg/Th17, IL-17 and IL-10 in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocrine 2022, 76, 263–272. [Google Scholar] [CrossRef]
- Nekoua, M.P.; Fachinan, R.; Atchamou, A.K.; Nouatin, O.; Amoussou-Guenou, D.; Amoussou-Guenou, M.K.; Moutairou, K.; Yessoufou, A. Modulation of Immune Cells and Th1/Th2 Cytokines in Insulin-Treated Type 2 Diabetes Mellitus. Afr. Health Sci. 2016, 16, 712–724. [Google Scholar] [CrossRef]
- Olson, N.C.; Doyle, M.F.; de Boer, I.H.; Huber, S.A.; Jenny, N.S.; Kronmal, R.A.; Psaty, B.M.; Tracy, R.P. Associations of Circulating Lymphocyte Subpopulations with Type 2 Diabetes: Cross-Sectional Results from the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS ONE 2015, 10, e0139962. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Yin, R.; Xu, Y.; Zhang, L.; Zhang, Y.; Yang, L.; Zhao, D. Central Role of Cardiac Fibroblasts in Myocardial Fibrosis of Diabetic Cardiomyopathy. Front. Endocrinol. 2023, 14, 1162754. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Ge, X.; Meng, L.; Kong, J.; Meng, X. Regulatory T Cells Protect against Diabetic Cardiomyopathy in Db/Db Mice. J. Diabetes Investig. 2024, 15, 1191–1201. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Zhao, M.; Zhang, B.; Chi, J.; Liu, W.; Chen, W.; Fu, Y.; Liu, Y.; Yin, X. H2 and H3 Relaxin Inhibit High Glucose-Induced Apoptosis in Neonatal Rat Ventricular Myocytes. Biochimie 2015, 108, 59–67. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, J.; Miao, Y.; Wang, Y.; Cui, W.; Guo, J.; Qiu, S.; Han, Y.; Jia, L.; Li, H.; et al. Serum-Glucocorticoid Regulated Kinase 1 Regulates Alternatively Activated Macrophage Polarization Contributing to Angiotensin II–Induced Inflammation and Cardiac Fibrosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1675–1686. [Google Scholar] [CrossRef]
- Li, J.-M.; Gall, N.P.; Grieve, D.J.; Chen, M.; Shah, A.M. Activation of NADPH Oxidase during Progression of Cardiac Hypertrophy to Failure. Hypertension 2002, 40, 477–484. [Google Scholar] [CrossRef]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin Resistance and Hyperinsulinaemia in Diabetic Cardiomyopathy. Nat. Rev. Endocrinol. 2016, 12, 144–153. [Google Scholar] [CrossRef]
- Zlobine, I.; Gopal, K.; Ussher, J.R. Lipotoxicity in Obesity and Diabetes-Related Cardiac Dysfunction. Biochim. Biophys. Acta 2016, 1861, 1555–1568. [Google Scholar] [CrossRef]
- Luo, S.; Xu, R.; Xie, P.; Liu, X.; Ling, C.; Liu, Y.; Zhang, X.; Xia, Z.; Chen, Z.; Tang, J. EGFR of Platelet Regulates Macrophage Activation and Bacterial Phagocytosis Function. J. Inflamm. 2024, 21, 10. [Google Scholar] [CrossRef]
- Ferreira, I.A.; Eybrechts, K.L.; Mocking, A.I.M.; Kroner, C.; Akkerman, J.-W.N. IRS-1 Mediates Inhibition of Ca2+ Mobilization by Insulin via the Inhibitory G-Protein Gi. J. Biol. Chem. 2004, 279, 3254–3264. [Google Scholar] [CrossRef]
- Lee, W.J.; Tateya, S.; Cheng, A.M.; Rizzo-DeLeon, N.; Wang, N.F.; Handa, P.; Wilson, C.L.; Clowes, A.W.; Sweet, I.R.; Bomsztyk, K.; et al. M2 Macrophage Polarization Mediates Anti-Inflammatory Effects of Endothelial Nitric Oxide Signaling. Diabetes 2015, 64, 2836–2846. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mussa, S.; Gastaldi, D.; Sadowski, J.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Mechanisms of Increased Vascular Superoxide Production in Human Diabetes Mellitus: Role of NAD(P)H Oxidase and Endothelial Nitric Oxide Synthase. Circulation 2002, 105, 1656–1662. [Google Scholar] [CrossRef]
- Lemkes, B.A.; Hermanides, J.; Devries, J.H.; Holleman, F.; Meijers, J.C.M.; Hoekstra, J.B.L. Hyperglycemia: A Prothrombotic Factor? J. Thromb. Haemost. 2010, 8, 1663–1669. [Google Scholar] [CrossRef]
- Xia, Y.; Gao, D.; Wang, X.; Liu, B.; Shan, X.; Sun, Y.; Ma, D. Role of Treg Cell Subsets in Cardiovascular Disease Pathogenesis and Potential Therapeutic Targets. Front. Immunol. 2024, 15, 1331609. [Google Scholar] [CrossRef]
- Glöckner, A.; Ossmann, S.; Ginther, A.; Kang, J.; Borger, M.A.; Hoyer, A.; Dieterlen, M.-T. Relevance and Recommendations for the Application of Cardioplegic Solutions in Cardiopulmonary Bypass Surgery in Pigs. Biomedicines 2021, 9, 1279. [Google Scholar] [CrossRef]
- Nielsen, E.W.; Miller, Y.; Brekke, O.-L.; Grond, J.; Duong, A.H.; Fure, H.; Ludviksen, J.K.; Pettersen, K.; Reubsaet, L.; Solberg, R.; et al. A Novel Porcine Model of Ischemia-Reperfusion Injury After Cross-Clamping the Thoracic Aorta Revealed Substantial Cardiopulmonary, Thromboinflammatory and Biochemical Changes Without Effect of C1-Inhibitor Treatment. Front. Immunol. 2022, 13, 852119. [Google Scholar] [CrossRef]
- Pagehgiri, H.D.; Puruhito, I.; Aditiawarman, A. Ischemia Reperfusion Injury on Temporary Aortic Cross-Clamping. Ital. J. Vasc. Endovasc. Surg. 2023, 30, 57–63. [Google Scholar] [CrossRef]
- Ergene, S.; Hemsinli, D.; Karakisi, S.O.; Tümkaya, L.; Mercantepe, T.; Yilmaz, A.; Yel, I. Resveratrol Attenuates Degeneration and Apoptosis of Cardiomyocytes Induced by Aortic Clamping. Braz. J. Cardiovasc. Surg. 2023, 38, e20230224. [Google Scholar] [CrossRef]
- Prata, M.P.; Jaldin, R.G.; Lourenção, P.L.T.d.A.; Sobreira, M.L.; Yoshida, R.d.A.; Terra, S.A.; Viero, R.M.; Yoshida, W.B. Acute Aortic Wall Injury Caused by Aortic Cross-Clamping: Morphological and Biomechanical Study of the Aorta in a Swine Model of Three Aortic Surgery Approaches. J. Vasc. Bras. 2020, 19, e20190025. [Google Scholar] [CrossRef]
- Rylski, B.; Beyersdorf, F.; Czerny, M.; Siepe, M. Two Aortic Ruptures in Two Months—Role of Cross–Clamp-Associated Late Injury. Ann. Vasc. Surg. 2016, 32, 129.e17–129.e19. [Google Scholar] [CrossRef]
- Nguyen, Q.-S.; Banks, D. Strategies of Myocardial Protection During Cardiopulmonary Bypass. In Clinical Perfusion for Cardiac Surgery: A Step-by-Step Guide to the Fundamentals; Awad, A.S., DiNardo, J., Huang, J., Hancock, M.F., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 353–368. ISBN 978-3-031-91163-7. [Google Scholar]
- Aykut, G.; Ulugöl, H.; Aksu, U.; Akin, S.; Karabulut, H.; Alhan, C.; Toraman, F.; Ince, C. Microcirculatory Response to Blood vs. Crystalloid Cardioplegia During Coronary Artery Bypass Grafting With Cardiopulmonary Bypass. Front. Med. 2022, 8, 736214. [Google Scholar] [CrossRef]
- Brown, A.J.; Chambers, D.J. Physiology and Cardioplegia: Safety in Operating. Surgery 2021, 39, 126–131. [Google Scholar] [CrossRef]
- Francica, A.; Tonelli, F.; Rossetti, C.; Tropea, I.; Luciani, G.B.; Faggian, G.; Dobson, G.P.; Onorati, F. Cardioplegia between Evolution and Revolution: From Depolarized to Polarized Cardiac Arrest in Adult Cardiac Surgery. J. Clin. Med. 2021, 10, 4485. [Google Scholar] [CrossRef]
- Jonjev, Ź.S.; Schwertz, D.W.; Beck, J.M.; Ross, J.D.; Law, W.R. Subcellular Distribution of Protein Kinase C Isozymes during Cardioplegic Arrest. J. Thorac. Cardiovasc. Surg. 2003, 126, 1880–1885. [Google Scholar] [CrossRef][Green Version]
- Sodha, N.R.; Clements, R.T.; Bianchi, C.; Sellke, F.W. Cardiopulmonary Bypass with Cardioplegic Arrest Activates Protein Kinase C in the Human Myocardium. J. Am. Coll. Surg. 2008, 206, 33–41. [Google Scholar] [CrossRef]
- Khoynezhad, A.; Jalali, Z.; Tortolani, A.J. Apoptosis: Pathophysiology and Therapeutic Implications for the Cardiac Surgeon. Ann. Thorac. Surg. 2004, 78, 1109–1118. [Google Scholar] [CrossRef]
- Fischer, U.M.; Cox, C.S.; Laine, G.A.; Mehlhorn, U.; Bloch, W.; Allen, S.J. Induction of Cardioplegic Arrest Immediately Activates the Myocardial Apoptosis Signal Pathway. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H1630–H1633. [Google Scholar] [CrossRef]
- Shuja, F.; Tabbara, M.; Li, Y.; Liu, B.; Butt, M.U.; Velmahos, G.C.; DeMoya, M.; Alam, H.B. Profound Hypothermia Decreases Cardiac Apoptosis through Akt Survival Pathway. J. Am. Coll. Surg. 2009, 209, 89–99. [Google Scholar] [CrossRef]
- Ji, M.J.; Hong, J.H. A Cardioplegic Solution with an Understanding of a Cardiochannelopathy. Antioxidants 2021, 10, 1878. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Wang, Y.-C.; Wu, Y.-C.; Chu, J.-J.; Lin, P.J. Continuous Tepid Blood Cardioplegia Can Preserve Coronary Endothelium and Ameliorate the Occurrence of Cardiomyocyte Apoptosis. Chest 2003, 123, 1647–1654. [Google Scholar] [CrossRef]
- Panconesi, R.; Widmer, J.; Carvalho, M.F.; Eden, J.; Dondossola, D.; Dutkowski, P.; Schlegel, A. Mitochondria and Ischemia Reperfusion Injury. Curr. Opin. Organ Transplant. 2022, 27, 434–445. [Google Scholar] [CrossRef]
- De Hert, S.; Moerman, A. Myocardial Injury and Protection Related to Cardiopulmonary Bypass. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 137–149. [Google Scholar] [CrossRef]
- Muraki, S.; Morris, C.D.; Budde, J.M.; Zhao, Z.-Q.; Guyton, R.A.; Vinten-Johansen, J. Blood Cardioplegia Supplementation with the Sodium-Hydrogen Ion Exchange Inhibitor Cariporide to Attenuate Infarct Size and Coronary Artery Endothelial Dysfunction after Severe Regional Ischemia in a Canine Model. J. Thorac. Cardiovasc. Surg. 2003, 125, 155–164. [Google Scholar] [CrossRef][Green Version]
- de Oliveira, M.A.B.; Brandi, A.C.; dos Santos, C.A.; Botelho, P.H.H.; Cortez, J.L.L.; Goissis, G.; Braile, D.M. The Calcium Paradox—What Should We Have to Fear? Rev. Bras. Cir. Cardiovasc. 2014, 29, 249–254. [Google Scholar] [CrossRef]
- Saldanha, C.; Hearse, D.J. Cardioplegia and Vascular Injury: Dissociation of the Effects of Ischemia from Those of the Cardioplegic Solution. J. Thorac. Cardiovasc. Surg. 1994, 108, 279–290. [Google Scholar] [CrossRef]
- Keller, M.W.; Geddes, L.; Spotnitz, W.; Kaul, S.; Duling, B.R. Microcirculatory Dysfunction Following Perfusion with Hyperkalemic, Hypothermic, Cardioplegic Solutions and Blood Reperfusion. Effects of Adenosine. Circulation 1991, 84, 2485–2494. [Google Scholar] [CrossRef]
- Xue, H.-M.; Hou, H.-T.; Sun, W.-T.; Wang, S.-F.; Guo, S.; Yang, Q.; He, G.-W. Del Nido Cardioplegia Better Preserves Cardiac Diastolic Function but Histidine–Tryptophan–Ketoglutarate Is Better for Endothelial Function. Eur. J. Cardiothorac. Surg. 2022, 61, 1368–1378. [Google Scholar] [CrossRef]
- Ferrera, R.; Michel, P.; Ovize, M. Paradoxical Toxicity of Cardioplegic Compounds on Ischemic Cardiomyocyte Using Optimal Design Strategy. J. Heart Lung Transplant. 2005, 24, 904–911. [Google Scholar] [CrossRef]
- Shimoda, T.; Liu, C.; Mathis, B.J.; Goto, Y.; Ageyama, N.; Kato, H.; Matsubara, M.; Ohigashi, T.; Gosho, M.; Suzuki, Y.; et al. Effect of Cardiopulmonary Bypass on Coagulation Factors II, VII and X in a Primate Model: An Exploratory Pilot Study. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 37, ivad194. [Google Scholar] [CrossRef]
- Volk, L.E.; Mavroudis, C.D.; Ko, T.; Hallowell, T.; Delso, N.; Roberts, A.L.; Starr, J.; Landis, W.; Lin, Y.; Hefti, M.; et al. Increased Cerebral Mitochondrial Dysfunction and Reactive Oxygen Species with Cardiopulmonary Bypass. Eur. J. Cardiothorac. Surg. 2021, 59, 1256–1264. [Google Scholar] [CrossRef]
- Kramer, R.S.; Herron, C.R.; Groom, R.C.; Brown, J.R. Acute Kidney Injury Subsequent to Cardiac Surgery. J. Extra Corpor. Technol. 2015, 47, 16–28. [Google Scholar] [CrossRef]
- Woods, B.D.; Sladen, R.N. Perioperative Considerations for the Patient with Asthma and Bronchospasm. Br. J. Anaesth. 2009, 103, i57–i65. [Google Scholar] [CrossRef]
- Kim, L.; Nguyen, H.-Y.; Senawong, T.; Wei, C. Protamine-Related Non-Cardiogenic Pulmonary Edema during Routine Heparin Reversal for Cardiopulmonary Bypass. Med. Rep. 2025, 12, 100205. [Google Scholar] [CrossRef]
- Salomon, J.D.; Qiu, H.; Feng, D.; Owens, J.; Khailova, L.; Osorio Lujan, S.; Iguidbashian, J.; Chhonker, Y.S.; Murry, D.J.; Riethoven, J.-J.; et al. Piglet Cardiopulmonary Bypass Induces Intestinal Dysbiosis and Barrier Dysfunction Associated with Systemic Inflammation. Dis. Model. Mech. 2023, 16, dmm049742. [Google Scholar] [CrossRef]
- Rhaleb, N.-E.; Yang, X.-P.; Carretero, O.A. The Kallikrein-Kinin System as a Regulator of Cardiovascular and Renal Function. Compr. Physiol. 2011, 1, 971–993. [Google Scholar] [CrossRef]
- Chatterjee, K.; Thornton, J.L.; Bauer, J.W.; Vogler, E.A.; Siedlecki, C.A. Moderation of Prekallikrein-Factor XII Interactions in Surface Activation of Coagulation by Protein-Adsorption Competition. Biomaterials 2009, 30, 4915–4920. [Google Scholar] [CrossRef]
- Bekassy, Z.; Lopatko Fagerström, I.; Bader, M.; Karpman, D. Crosstalk between the Renin–Angiotensin, Complement and Kallikrein–Kinin Systems in Inflammation. Nat. Rev. Immunol. 2022, 22, 411–428. [Google Scholar] [CrossRef]
- Banerjee, D.; Feng, J.; Sellke, F.W. Strategies to Attenuate Maladaptive Inflammatory Response Associated with Cardiopulmonary Bypass. Front. Surg. 2024, 11, 1224068. [Google Scholar] [CrossRef]
- Somer, F.D.; Belleghem, Y.V.; Caes, F.; François, K.; Overbeke, H.V.; Arnout, J.; Taeymans, Y.; Nooten, G.V. Tissue Factor as the Main Activator of the Coagulation System during Cardiopulmonary Bypass. J. Thorac. Cardiovasc. Surg. 2002, 123, 951–958. [Google Scholar] [CrossRef]
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue Factor as a Link between Inflammation and Coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303. [Google Scholar] [CrossRef]
- Sang, Y.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between Platelets and Coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef]
- Kelchtermans, H.; Pelkmans, L.; Bouwhuis, A.; Schurgers, E.; Lindhout, T.; Huskens, D.; Miszta, A.; Hemker, H.C.; Lancé, M.D.; de Laat, B. Simultaneous Measurement of Thrombin Generation and Fibrin Formation in Whole Blood under Flow Conditions. Thromb. Haemost. 2016, 116, 134–145. [Google Scholar] [CrossRef]
- Parolari, A.; Colli, S.; Mussoni, L.; Eligini, S.; Naliato, M.; Wang, X.; Gandini, S.; Tremoli, E.; Biglioli, P.; Alamanni, F. Coagulation and Fibrinolytic Markers in a Two-Month Follow-up of Coronary Bypass Surgery. J. Thorac. Cardiovasc. Surg. 2003, 125, 336–343. [Google Scholar] [CrossRef][Green Version]
- Olson, S.A.; Osborn, B.K.; Cotton, M.E.; Krocker, J.D.; Koami, H.; White, N.; Cardenas, J.C. Fibrinogen Fragment X Mediates Endothelial Barrier Disruption via Suppression of VE-Cadherin. J. Surg. Res. 2024, 293, 639–646. [Google Scholar] [CrossRef]
- Amara, U.; Rittirsch, D.; Flierl, M.; Bruckner, U.; Klos, A.; Gebhard, F.; Lambris, J.D.; Huber-Lang, M. Interaction Between the Coagulation and Complement System. Adv. Exp. Med. Biol. 2008, 632, 71–79. [Google Scholar] [CrossRef]
- Kaur, J.; Woodman, R.C.; Ostrovsky, L.; Kubes, P. Selective Recruitment of Neutrophils and Lymphocytes by Thrombin: A Role for NF-κB. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H784–H795. [Google Scholar] [CrossRef]
- Gould, T.J.; Vu, T.T.; Swystun, L.L.; Dwivedi, D.J.; Mai, S.H.C.; Weitz, J.I.; Liaw, P.C. Neutrophil Extracellular Traps Promote Thrombin Generation Through Platelet-Dependent and Platelet-Independent Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1977–1984. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Neagoe, P.-E.; Gagnon, D.; Denault, A.Y.; Sirois, M.G. Increased Circulating Levels of Neutrophil Extracellular Traps During Cardiopulmonary Bypass. CJC Open 2019, 2, 39–48. [Google Scholar] [CrossRef]
- Jung, Y.; Choi, J.W.; Hwang, H.Y.; Gu, J.Y.; Kim, K.H.; Kim, H.K. Elevated Circulating Levels of Neutrophil Extracellular Traps after Cardiopulmonary Bypass Surgery as Risk Factors of Postoperative Atrial Fibrillation and Mortality. J. Thorac. Dis. 2024, 16, 4319–4328. [Google Scholar] [CrossRef]
- Chenoweth, D.E.; Cooper, S.W.; Hugli, T.E.; Stewart, R.W.; Blackstone, E.H.; Kirklin, J.W. Complement Activation during Cardiopulmonary Bypass: Evidence for Generation of C3a and C5a Anaphylatoxins. N. Engl. J. Med. 1981, 304, 497–503. [Google Scholar] [CrossRef]
- Fitch, J.C.K.; Rollins, S.; Matis, L.; Alford, B.; Aranki, S.; Collard, C.D.; Dewar, M.; Elefteriades, J.; Hines, R.; Kopf, G.; et al. Pharmacology and Biological Efficacy of a Recombinant, Humanized, Single-Chain Antibody C5 Complement Inhibitor in Patients Undergoing Coronary Artery Bypass Graft Surgery With Cardiopulmonary Bypass. Circulation 1999, 100, 2499–2506. [Google Scholar] [CrossRef]
- Kefalogianni, R.; Kamani, F.; Gaspar, M.; Aw, T.; Donovan, J.; Laffan, M.; Pickering, M.C.; Arachchillage, D.J. Complement Activation during Cardiopulmonary Bypass and Association with Clinical Outcomes. eJHaem 2022, 3, 86–96. [Google Scholar] [CrossRef]
- Denk, S.; Taylor, R.P.; Wiegner, R.; Cook, E.M.; Lindorfer, M.A.; Pfeiffer, K.; Paschke, S.; Eiseler, T.; Weiss, M.; Barth, E.; et al. Complement C5a-Induced Changes in Neutrophil Morphology during Inflammation. Scand. J. Immunol. 2017, 86, 143–155. [Google Scholar] [CrossRef]
- Mannes, M.; Pechtl, V.; Hafner, S.; Dopler, A.; Eriksson, O.; Manivel, V.A.; Wohlgemuth, L.; Messerer, D.A.C.; Schrezenmeier, H.; Ekdahl, K.N.; et al. Complement and Platelets: Prothrombotic Cell Activation Requires Membrane Attack Complex–Induced Release of Danger Signals. Blood Adv. 2023, 7, 6367–6380. [Google Scholar] [CrossRef]
- Aydin, N.B.; Gercekoglu, H.; Aksu, B.; Ozkul, V.; Sener, T.; Kıygıl, İ.; Turkoglu, T.; Cimen, S.; Babacan, F.; Demirtas, M. Endotoxemia in Coronary Artery Bypass Surgery: A Comparison of the off-Pump Technique and Conventional Cardiopulmonary Bypass. J. Thorac. Cardiovasc. Surg. 2003, 125, 843–848. [Google Scholar] [CrossRef]
- Ciesielska, A.; Krawczyk, M.; Sas-Nowosielska, H.; Hromada-Judycka, A.; Kwiatkowska, K. CD14 Recycling Modulates LPS-Induced Inflammatory Responses of Murine Macrophages. Traffic 2022, 23, 310–330. [Google Scholar] [CrossRef]
- Dayang, E.-Z.; Plantinga, J.; Ter Ellen, B.; van Meurs, M.; Molema, G.; Moser, J. Identification of LPS-Activated Endothelial Subpopulations With Distinct Inflammatory Phenotypes and Regulatory Signaling Mechanisms. Front. Immunol. 2019, 10, 1169. [Google Scholar] [CrossRef]
- Siepe, M.; Goebel, U.; Mecklenburg, A.; Doenst, T.; Benk, C.; Stein, P.; Beyersdorf, F.; Loop, T.; Schlensak, C. Pulsatile Pulmonary Perfusion During Cardiopulmonary Bypass Reduces the Pulmonary Inflammatory Response. Ann. Thorac. Surg. 2008, 86, 115–122. [Google Scholar] [CrossRef]
- Luecht, J.; Pauli, C.; Seiler, R.; Herre, A.-L.; Brankova, L.; Berger, F.; Schmitt, K.R.L.; Tong, G. Prolonged Extracorporeal Circulation Leads to Inflammation and Higher Expression of Mediators of Vascular Permeability Through Activation of STAT3 Signaling Pathway in Macrophages. Int. J. Mol. Sci. 2024, 25, 12398. [Google Scholar] [CrossRef]
- Dreyer, W.J.; Phillips, S.C.; Lindsey, M.L.; Jackson, P.; Bowles, N.E.; Michael, L.H.; Entman, M.L. Interleukin 6 Induction in the Canine Myocardium after Cardiopulmonary Bypass. J. Thorac. Cardiovasc. Surg. 2000, 120, 256–263. [Google Scholar] [CrossRef][Green Version]
- Fontes, J.A.; Rose, N.R.; Čiháková, D. The Varying Faces of IL-6: From Cardiac Protection to Cardiac Failure. Cytokine 2015, 74, 62–68. [Google Scholar] [CrossRef]
- Puchinger, J.; Ryz, S.; Nixdorf, L.; Edlinger-Stanger, M.; Lassnigg, A.; Wiedemann, D.; Hiesmayr, M.; Spittler, A.; Bernardi, M.H. Characteristics of Interleukin-6 Signaling in Elective Cardiac Surgery—A Prospective Cohort Study. J. Clin. Med. 2022, 11, 590. [Google Scholar] [CrossRef]
- Luo, C.; Xie, X.; Feng, X.; Lei, B.; Fang, C.; Li, Y.; Cai, X.; Ling, G.; Zheng, B. Deficiency of Interleukin-36 Receptor Protected Cardiomyocytes from Ischemia-Reperfusion Injury in Cardiopulmonary Bypass. Med. Sci. Monit. 2020, 26, e918933-1–e918933-11. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxid. Med. Cell Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef]
- Lou, X.; Duan, S.; Li, M.; Yuan, Y.; Chen, S.; Wang, Z.; Wang, Z.; Sun, L. IL-36α Inhibits Melanoma by Inducing pro-Inflammatory Polarization of Macrophages. Cancer Immunol. Immunother. 2023, 72, 3045–3061. [Google Scholar] [CrossRef]
- Flier, S.; Concepcion, A.N.; Versteeg, D.; Kappen, T.H.; Hoefer, I.E.; de Lange, D.W.; Pasterkamp, G.; Buhre, W.F. Monocyte Hyporesponsiveness and Toll-like Receptor Expression Profiles in Coronary Artery Bypass Grafting and Its Clinical Implications for Postoperative Inflammatory Response and Pneumonia: An Observational Cohort Study. Eur. J. Anaesthesiol. 2015, 32, 177–188. [Google Scholar] [CrossRef]
- Gaudriot, B.; Uhel, F.; Gregoire, M.; Gacouin, A.; Biedermann, S.; Roisne, A.; Flecher, E.; Le Tulzo, Y.; Tarte, K.; Tadié, J.-M. Immune Dysfunction After Cardiac Surgery with Cardiopulmonary Bypass: Beneficial Effects of Maintaining Mechanical Ventilation. Shock 2015, 44, 228–233. [Google Scholar] [CrossRef]
- Lesouhaitier, M.; Belicard, F.; Tadié, J.-M. Cardiopulmonary Bypass and VA-ECMO Induced Immune Dysfunction: Common Features and Differences, a Narrative Review. Crit. Care 2024, 28, 300. [Google Scholar] [CrossRef]
- Hou, L.; Yang, Z.; Wang, Z.; Zhang, X.; Zhao, Y.; Yang, H.; Zheng, B.; Tian, W.; Wang, S.; He, Z.; et al. NLRP3/ASC-Mediated Alveolar Macrophage Pyroptosis Enhances HMGB1 Secretion in Acute Lung Injury Induced by Cardiopulmonary Bypass. Lab. Investig. 2018, 98, 1052–1064. [Google Scholar] [CrossRef]
- Kotani, N.; Hashimoto, H.; Sessler, D.I.; Muraoka, M.; Wang, J.-S.; O’Connor, M.F.; Matsuki, A. Cardiopulmonary Bypass Produces Greater Pulmonary than Systemic Proinflammatory Cytokines. Anesth. Analg. 2000, 90, 1039–1045. [Google Scholar] [CrossRef]
- Sumaiya, K.; Langford, D.; Natarajaseenivasan, K.; Shanmughapriya, S. Macrophage Migration Inhibitory Factor (MIF): A Multifaceted Cytokine Regulated by Genetic and Physiological Strategies. Pharmacol. Ther. 2022, 233, 108024. [Google Scholar] [CrossRef]
- Toso, C.; Emamaullee, J.A.; Merani, S.; Shapiro, A.M.J. The Role of Macrophage Migration Inhibitory Factor on Glucose Metabolism and Diabetes. Diabetologia 2008, 51, 1937–1946. [Google Scholar] [CrossRef]
- Bernhagen, J.; Krohn, R.; Lue, H.; Gregory, J.L.; Zernecke, A.; Koenen, R.R.; Dewor, M.; Georgiev, I.; Schober, A.; Leng, L.; et al. MIF Is a Noncognate Ligand of CXC Chemokine Receptors in Inflammatory and Atherogenic Cell Recruitment. Nat. Med. 2007, 13, 587–596. [Google Scholar] [CrossRef]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage Migration Inhibitory Factor Stimulates AMP-Activated Protein Kinase in the Ischaemic Heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef]
- Atsumi, T.; Cho, Y.-R.; Leng, L.; McDonald, C.; Yu, T.; Danton, C.; Hong, E.-G.; Mitchell, R.A.; Metz, C.; Niwa, H.; et al. The Proinflammatory Cytokine Macrophage Migration Inhibitory Factor Regulates Glucose Metabolism during Systemic Inflammation. J. Immunol. 2007, 179, 5399–5406. [Google Scholar] [CrossRef]
- Stoppe, C.; Werker, T.; Rossaint, R.; Dollo, F.; Lue, H.; Wonisch, W.; Menon, A.; Goetzenich, A.; Bruells, C.S.; Coburn, M.; et al. What Is the Significance of Perioperative Release of Macrophage Migration Inhibitory Factor in Cardiac Surgery? Antioxid. Redox Signal. 2013, 19, 231–239. [Google Scholar] [CrossRef]
- Stoppe, C.; Grieb, G.; Rossaint, R.; Simons, D.; Coburn, M.; Götzenich, A.; Strüssmann, T.; Pallua, N.; Bernhagen, J.; Rex, S. High Postoperative Blood Levels of Macrophage Migration Inhibitory Factor Are Associated with Less Organ Dysfunction in Patients after Cardiac Surgery. Mol. Med. 2012, 18, 843–850. [Google Scholar] [CrossRef]
- Furtado de Mendonça-Filho, H.T.; Gomes, R.V.; Campos, L.A.d.A.; Tura, B.; Nunes, E.M.; Gomes, R.; Bozza, F.; Bozza, P.T.; Castro-Faria-Neto, H.C. Circulating Levels of Macrophage Migration Inhibitory Factor Are Associated with Mild Pulmonary Dysfunction After Cardiopulmonary Bypass. Shock 2004, 22, 533–537. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Rodríguez-López, J.M.; Iglesias-González, J.L.; Lozano-Sánchez, F.S.; Palomero-Rodríguez, M.Á.; Sánchez-Conde, P. Inflammatory Response, Immunosuppression and Arginase Activity after Cardiac Surgery Using Cardiopulmonary Bypass. J. Clin. Med. 2022, 11, 4187. [Google Scholar] [CrossRef]
- Li, W.-J.; Peng, Y.-X.; Zhao, L.-Q.; Wang, H.-Y.; Liu, W.; Bai, K.; Chen, S.; Lu, Y.; Huang, J. T-Cell Lymphopenia Is Associated with an Increased Infecting Risk in Children after Cardiopulmonary Bypass. Pediatr. Res. 2024, 95, 227–232. [Google Scholar] [CrossRef]
- Farhid, F.; Hosseini, E.; Kargar, F.; Ghasemzadeh, M. Interplay between Platelet and T Lymphocyte after Coronary Artery Bypass Grafting (CABG): Evidence for Platelet Mediated Post-CABG Immunomodulation. Microvasc. Res. 2025, 160, 104805. [Google Scholar] [CrossRef]
- Hosseini, E.; Ahmadi, J.; Kargar, F.; Ghasemzadeh, M. Coronary Artery Bypass Grafting (CABG) Induces pro-Inflammatory and Immunomodulatory Phenotype of Platelets in the Absence of a pro-Aggregatory State. Microvasc. Res. 2024, 153, 104669. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, Y.; Feng, Z.; Zhang, Y.; Chen, Y.; Yu, T.; Wang, H. Inhibition of Caspase-1-Dependent Pyroptosis Alleviates Myocardial Ischemia/Reperfusion Injury during Cardiopulmonary Bypass (CPB) in Type 2 Diabetic Rats. Sci. Rep. 2024, 14, 19420. [Google Scholar] [CrossRef]
- Schweizer, T.; Nossen, C.M.; Galova, B.; Schild, C.; Huber, M.; Bally, L.; Vogt, A.; Siepe, M.; Nagler, M.; Fischer, K.; et al. In Vitro Investigation of Insulin Dynamics During 4 Hours of Simulated Cardiopulmonary Bypass. Anesth. Analg. 2025, 141, 267–272. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, T.; Yu, S.; Sun, G.; Gu, C.; Yi, D. Relationships of Adiponectin with Markers of Systemic Inflammation and Insulin Resistance in Infants Undergoing Open Cardiac Surgery. Mediat. Inflamm. 2013, 2013, 187940. [Google Scholar] [CrossRef]
- Kremen, J.; Dolinkova, M.; Krajickova, J.; Blaha, J.; Anderlova, K.; Lacinova, Z.; Haluzikova, D.; Bosanska, L.; Vokurka, M.; Svacina, S.; et al. Increased Subcutaneous and Epicardial Adipose Tissue Production of Proinflammatory Cytokines in Cardiac Surgery Patients: Possible Role in Postoperative Insulin Resistance. J. Clin. Endocrinol. Metab. 2006, 91, 4620–4627. [Google Scholar] [CrossRef]
- de Lange, F.; Dieleman, J.M.; Jungwirth, B.; Kalkman, C.J. Effects of Cardiopulmonary Bypass on Neurocognitive Performance and Cytokine Release in Old and Diabetic Rats. Br. J. Anaesth. 2007, 99, 177–183. [Google Scholar] [CrossRef][Green Version]
- Hoedemaekers, C.W.; Pickkers, P.; Netea, M.G.; van Deuren, M.; Van der Hoeven, J.G. Intensive Insulin Therapy Does Not Alter the Inflammatory Response in Patients Undergoing Coronary Artery Bypass Grafting: A Randomized Controlled Trial [ISRCTN95608630]. Crit. Care 2005, 9, R790–R797. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory Cytokine Concentrations Are Acutely Increased by Hyperglycemia in Humans: Role of Oxidative Stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- Wasmuth, H.E.; Kunz, D.; Graf, J.; Stanzel, S.; Purucker, E.A.; Koch, A.; Gartung, C.; Heintz, B.; Gressner, A.M.; Matern, S.; et al. Hyperglycemia at Admission to the Intensive Care Unit Is Associated with Elevated Serum Concentrations of Interleukin-6 and Reduced Ex Vivo Secretion of Tumor Necrosis Factor-Alpha. Crit. Care Med. 2004, 32, 1109–1114. [Google Scholar] [CrossRef]
- Emani, S.; Ramlawi, B.; Sodha, N.R.; Li, J.; Bianchi, C.; Sellke, F.W. Increased Vascular Permeability after Cardiopulmonary Bypass in Patients with Diabetes Is Associated with Increased Expression of Vascular Endothelial Growth Factor and Hepatocyte Growth Factor. J. Thorac. Cardiovasc. Surg. 2009, 138, 185–191. [Google Scholar] [CrossRef]
- Serraf, A.; Aznag, H.; Baudet, B.; Détruit, H.; Séccatore, F.; Mazmanian, M.G.; Planché, C. Pulmonary Vascular Endothelial Growth Factor and Nitric Oxide Interaction during Total Cardiopulmonary Bypass in Neonatal Pigs. J. Thorac. Cardiovasc. Surg. 2003, 125, 1050–1057. [Google Scholar] [CrossRef]
- Gavard, J.; Gutkind, J.S. VEGF Controls Endothelial-Cell Permeability by Promoting the β-Arrestin-Dependent Endocytosis of VE-Cadherin. Nat. Cell Biol. 2006, 8, 1223–1234. [Google Scholar] [CrossRef]
- Lambeng, N.; Wallez, Y.; Rampon, C.; Cand, F.; Christé, G.; Gulino-Debrac, D.; Vilgrain, I.; Huber, P. Vascular Endothelial-Cadherin Tyrosine Phosphorylation in Angiogenic and Quiescent Adult Tissues. Circ. Res. 2005, 96, 384–391. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.; Sabe, A.A.; Sadek, A.A.; Singh, A.K.; Sodha, N.R.; Sellke, F.W. Differential Impairment of Adherens-Junction Expression/Phosphorylation after Cardioplegia in Diabetic versus Non-Diabetic Patients. Eur. J. Cardio-Thorac. Surg. 2016, 49, 937–943. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, R.; Chaudhary, O.; Mahmood, E.; Baribeau, Y.; Rashid, R.; Khabbaz, K.R.; Chu, L.M.; Liu, D.C.; Senthilnathan, V.; et al. Cardiopulmonary Bypass Suppresses Forkhead Box O3 and Downstream Autophagy in the Diabetic Human Heart. Ann. Thorac. Surg. 2021, 111, 937–944. [Google Scholar] [CrossRef]
- Yu, W.; Gao, B.; Li, N.; Wang, J.; Qiu, C.; Zhang, G.; Liu, M.; Zhang, R.; Li, C.; Ji, G.; et al. Sirt3 Deficiency Exacerbates Diabetic Cardiac Dysfunction: Role of Foxo3A-Parkin-Mediated Mitophagy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1973–1983. [Google Scholar] [CrossRef]
- Xin, Z.; Ma, Z.; Jiang, S.; Wang, D.; Fan, C.; Di, S.; Hu, W.; Li, T.; She, J.; Yang, Y. FOXOs in the Impaired Heart: New Therapeutic Targets for Cardiac Diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 486–498. [Google Scholar] [CrossRef]
- Sun, D.-M.; Yuan, X.; Wei, H.; Zhu, S.-J.; Zhang, P.; Zhang, S.-J.; Fan, H.-G.; Li, Y.; Zheng, Z.; Liu, X.-C. Impaired Myocardium Energetics Associated with the Risk for New-Onset Atrial Fibrillation after Isolated Coronary Artery Bypass Graft Surgery. Coron. Artery Dis. 2014, 25, 224–229. [Google Scholar] [CrossRef]
- Garcia, L.; Verdejo, H.E.; Kuzmicic, J.; Zalaquett, R.; Gonzalez, S.; Lavandero, S.; Corbalan, R. Impaired Cardiac Autophagy in Patients Developing Postoperative Atrial Fibrillation. J. Thorac. Cardiovasc. Surg. 2012, 143, 451–459.e1. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.; Dobrilovic, N.; Singh, A.K.; Sabe, A.A.; Guan, Y.; Bianchi, C.; Sellke, F.W. Altered Expression and Activation of Mitogen-Activated Protein Kinases in Diabetic Heart during Cardioplegic Arrest and Cardiopulmonary Bypass. Surgery 2013, 154, 436–443. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.; Chu, L.M.; Singh, A.K.; Dobrilovic, N.; Fingleton, J.G.; Clements, R.T.; Bianchi, C.; Sellke, F.W. Changes in Microvascular Reactivity after Cardiopulmonary Bypass in Patients with Poorly Controlled versus Controlled Diabetes. Circulation 2012, 126 (Suppl. S1), S73–S80. [Google Scholar] [CrossRef]
- Kizub, I.V.; Klymenko, K.I.; Soloviev, A.I. Protein Kinase C in Enhanced Vascular Tone in Diabetes Mellitus. Int. J. Cardiol. 2014, 174, 230–242. [Google Scholar] [CrossRef]
- Feng, J.; Anderson, K.; Liu, Y.; Singh, A.K.; Ehsan, A.; Sellke, F.W. Cyclooxygenase 2 Contributes to Bradykinin-Induced Microvascular Responses in Peripheral Arterioles after Cardiopulmonary Bypass. J. Surg. Res. 2017, 218, 246–252. [Google Scholar] [CrossRef]
- Schoonen, A.; van Klei, W.A.; van Wolfswinkel, L.; van Loon, K. Definitions of Low Cardiac Output Syndrome after Cardiac Surgery and Their Effect on the Incidence of Intraoperative LCOS: A Literature Review and Cohort Study. Front. Cardiovasc. Med. 2022, 9, 926957. [Google Scholar] [CrossRef]
- Chen, A.L.; Kindzelski, B.A.; Robinson, J.P.; Altshuler, J.M.; Schwann, T.A.; Vivacqua, A. Deciphering Low Cardiac Output Syndrome: Insights and Management in Post-Cardiac Surgery. HSF 2024, 27, 1237–1244. [Google Scholar] [CrossRef]
- Álvarez, J. Levosimendan and Low Cardiac Output Syndrome. Does Mortality Really Decrease? Rev. Española Cardiol. (Engl. Ed.) 2008, 61, 454–457. [Google Scholar] [CrossRef]
- Bielawska, M.; Warszyńska, M.; Stefańska, M.; Błyszczuk, P. Autophagy in Heart Failure: Insights into Mechanisms and Therapeutic Implications. J. Cardiovasc. Dev. Dis. 2023, 10, 352. [Google Scholar] [CrossRef]
- Khan, T.A.; Voisine, P.; Sellke, F.W. Cardiac Surgery and Diabetes Mellitus. In Diabetes and Cardiovascular Disease; Johnstone, M.T., Veves, A., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 543–553. ISBN 978-1-59259-908-0. [Google Scholar]
- Awad, A.K.; Elbahloul, M.A.; Al-omoush, O.; Abdelnasser, O.; Hajali, M.; Abdelnasser, A.; Saleh, O.; Altiti, A.; Elgharably, H.; El Diasty, M. Impact of Postoperative Atrial Fibrillation (POAF) on Outcomes after Coronary Artery Bypass Grafting: A Meta-Analysis of Unique 247,270 Patients from 50 Studies. Am. Heart J. Plus Cardiol. Res. Pract. 2025, 59, 100621. [Google Scholar] [CrossRef]
- Duncan, A.E.; Kartashov, A.; Robinson, S.B.; Randall, D.; Zhang, K.; Luber, J.; James, R.A.; Halvorson, S.; Bokesch, P. Risk Factors, Resource Use, and Cost of Postoperative Low Cardiac Output Syndrome. J. Thorac. Cardiovasc. Surg. 2022, 163, 1890–1898.e10. [Google Scholar] [CrossRef]
- Lomivorotov, V.V.; Efremov, S.M.; Kirov, M.Y.; Fominskiy, E.V.; Karaskov, A.M. Low-Cardiac-Output Syndrome After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 291–308. [Google Scholar] [CrossRef]
- Antunes, P.E.; Bernardo, J.E.; Eugénio, L.; de Oliveira, J.F.; Antunes, M.J. Mediastinitis after Aorto-Coronary Bypass Surgery. Eur. J. Cardiothorac. Surg. 1997, 12, 443–449. [Google Scholar] [CrossRef][Green Version]
- Matata, B.M.; Galiñanes, M. Cardiopulmonary Bypass Exacerbates Oxidative Stress but Does Not Increase Proinflammatory Cytokine Release in Patients with Diabetes Compared with Patients without Diabetes: Regulatory Effects of Exogenous Nitric Oxide. J. Thorac. Cardiovasc. Surg. 2000, 120, 1–11. [Google Scholar] [CrossRef]
- Groom, R.C.; Rassias, A.J.; Cormack, J.E.; DeFoe, G.R.; DioDato, C.; Krumholz, C.K.; Forest, R.J.; Pieroni, J.W.; O’Connor, B.; Warren, C.S.; et al. Highest Core Temperature during Cardiopulmonary Bypass and Rate of Mediastinitis. Perfusion 2004, 19, 119–125. [Google Scholar] [CrossRef]
- Voisine, P.; Ruel, M.; Khan, T.A.; Bianchi, C.; Xu, S.-H.; Kohane, I.; Libermann, T.A.; Otu, H.; Saltiel, A.R.; Sellke, F.W. Differences in Gene Expression Profiles of Diabetic and Nondiabetic Patients Undergoing Cardiopulmonary Bypass and Cardioplegic Arrest. Circulation 2004, 110 (Suppl. S1), II-280. [Google Scholar] [CrossRef]
- Zakrzewski, D.; Janas, J.; Heretyk, H.; Stepińska, J. Inflammatory Response and Postoperative Kidney Failure in Patients with Diabetes Type 2 or Impaired Glucose Tolerance Undergoing Heart Valve Surgery. Kardiol. Pol. 2010, 68, 530–536. [Google Scholar]
- Le Guillou, V.; Tamion, F.; Jouet, I.; Richard, V.; Mulder, P.; Bessou, J.P.; Doguet, F. Mesenteric Endothelial Dysfunction in a Cardiopulmonary Bypass Rat Model: The Effect of Diabetes. Diabetes Vasc. Dis. Res. 2012, 9, 270–279. [Google Scholar] [CrossRef]
- Doenst, T.; Wijeysundera, D.; Karkouti, K.; Zechner, C.; Maganti, M.; Rao, V.; Borger, M.A. Hyperglycemia during Cardiopulmonary Bypass Is an Independent Risk Factor for Mortality in Patients Undergoing Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2005, 130, 1144.e1–1144.e8. [Google Scholar] [CrossRef]
- Marty, J.C.; Bendhadra, S.; Amoureux, S.; Guilland, J.-C.; Vergely, C.; Rochette, L.; Girard, C. Oxidative stress is exacerbated in diabetic patients during cardiopulmonary bypass. Ann. Cardiol. Angeiol. 2008, 57, 155–160. [Google Scholar] [CrossRef]
- Mahmood, E.; Jeganathan, J.; Feng, R.; Saraf, M.; Khabbaz, K.; Mahmood, F.; Venkatachalam, S.; Liu, D.; Chu, L.; Parikh, S.M.; et al. Decreased PGC-1α Post-Cardiopulmonary Bypass Leads to Impaired Oxidative Stress in Diabetic Patients. Ann. Thorac. Surg. 2019, 107, 467–476. [Google Scholar] [CrossRef]
- Snel, L.I.P.; Li, X.; Weber, N.C.; Zuurbier, C.J.; Preckel, B.; van Raalte, D.H.; Hermanides, J.; Hulst, A.H. Ketonaemia during Cardiopulmonary Bypass Surgery: A Prospective Observational Study. Br. J. Anaesth. 2024, 133, 689–691. [Google Scholar] [CrossRef]
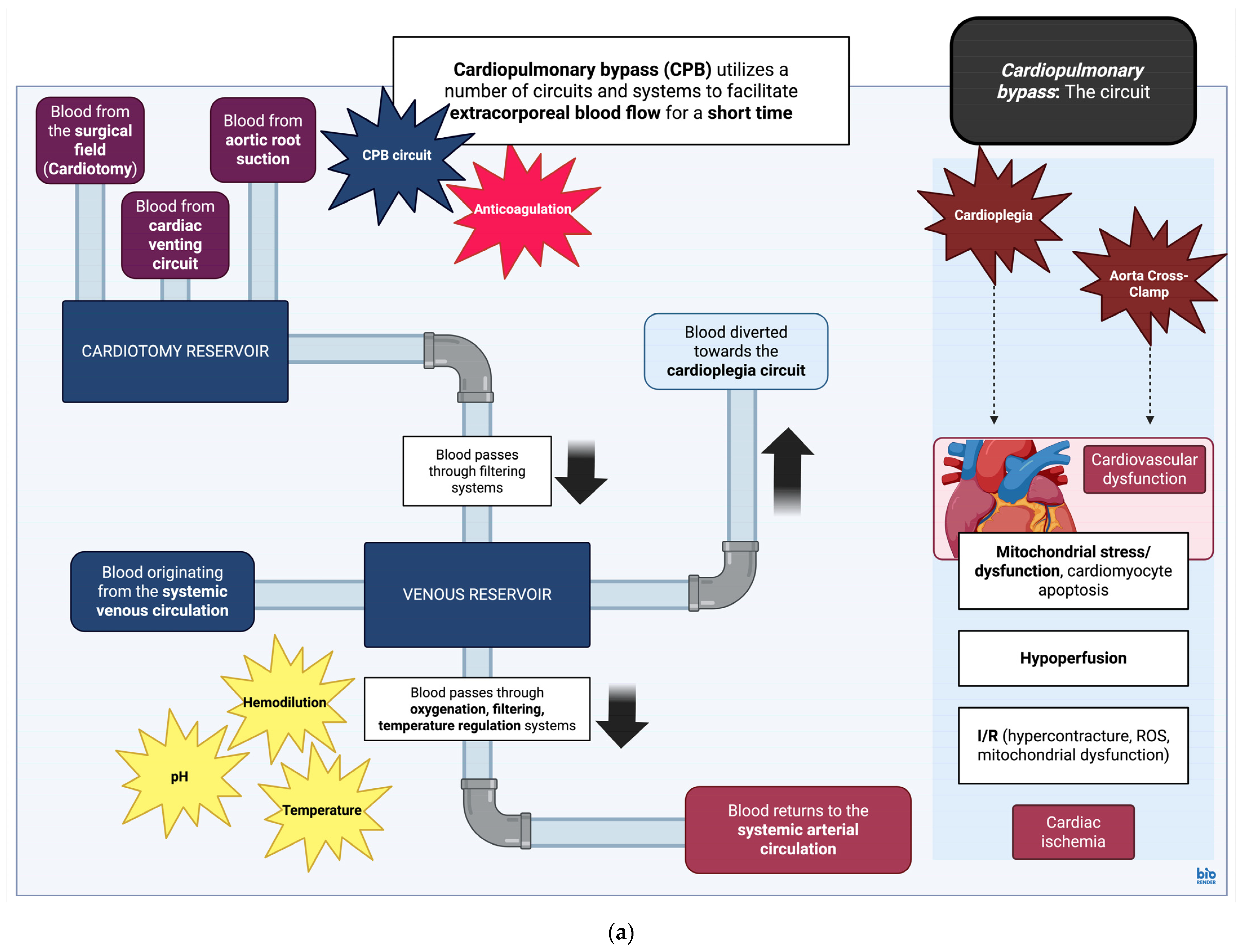
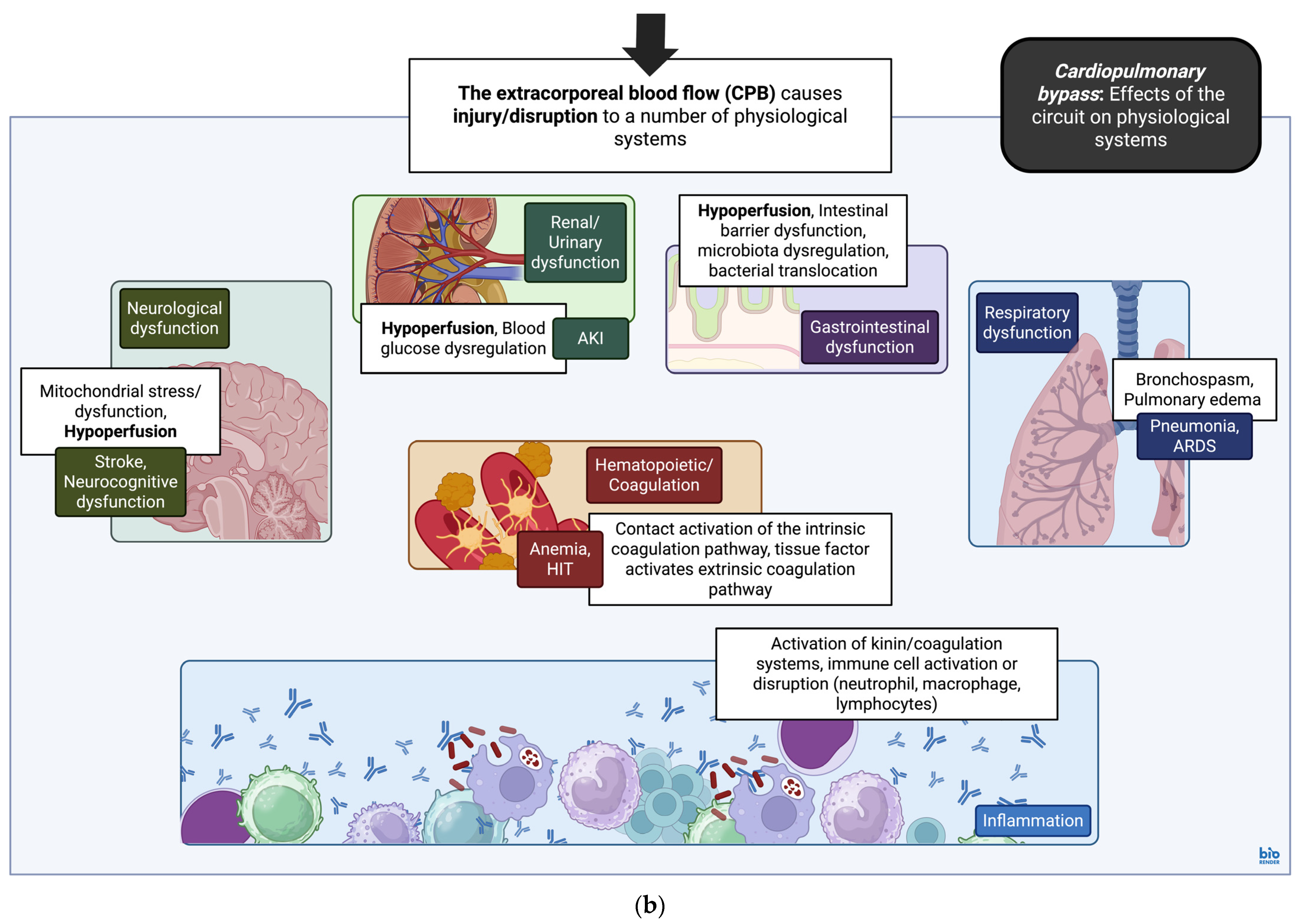
| Classification | Cardiovascular Associations | References |
|---|---|---|
| T1DM | CAD (disease is more diffuse, concentric compared to T2DM), autoimmune myocarditis post MI; diabetic cardiomyopathy; CAN (cardiac arrythmia, sudden death, and no strong direct associations with increased cardiovascular risk); cerebrovascular disease; PAD; risk factors affect men, women equally | [13,15,16,17,18] |
| T2DM | CAD; diabetic cardiomyopathy; cerebrovascular disease; PAD; risk factor burden for T2DM diagnosis higher in women, men diagnosed at lower BMI and age, compared to women; NAFLD in association with metabolic syndrome increases risk for both T2DM and cardiovascular disease; T2DM and CKD both independent risk factors for major adverse cardiovascular events in patients with known cardiovascular disease | [9,10,18,19,20,21,22] |
| T3DM | CAD | [23,24] |
| PTDM | CAD (MI risk higher in PTDM [relative risk of 1.6 compared to risk in pre-existing DM of 1.1]); cardiac arrhythmia; cerebrovascular disease | [25,26,27] |
| Monogenic DM | CAD; heart failure; cerebrovascular disease; all adverse cardiovascular events (MODY) show lower incidence compared to T2DM and higher incidence compared to T1DM in a prospective cohort study of 26,198 patients in the UK | [28] |
| GDM | CAD; heart failure; cerebrovascular disease; association with CAD and heart failure stronger compared to cerebrovascular disease in one national cohort study of 12,025 patients (NHNES), partly attributable to T2DM; increased risk of cardiovascular complications later in life (increased risk of ~ 23%) in embryos that develop within a hyperglycemic environment | [29,30] |
| Mediator | Description | Immune Cell Response | References |
|---|---|---|---|
| FFA | Accumulation of TAG in adipose tissue causing accumulation of FFA in the ER; maladaptive UPR response and ER stress trigger NF-κB signaling/NLRP3 inflammasome assembly (IL-1β, IL-18) | Upregulation of M1 macrophage polarization | [45,46,55] |
| Lipid metabolites | Ceramides (derived from sphingolipid metabolism, TLR4 receptor activation, TNF-α, IL-1β, and IL-6 signaling) accumulate (liver, skeletal muscle); ROS production and chronic oxidative stress contribute to inflammation via NF-κB signaling/NLRP3 inflammasome assembly | Upregulation of M1 macrophage polarization | [56,57] |
| Downregulation of M2 macrophage polarization | |||
| Saturated FA | Palmitic acid leads to activation of TLR4/ERK/FOXC2 signaling, immune cell activation | Upregulation of M1 macrophage polarization | [58] |
| CHOP | Chaperone protein activation by ER stress, downregulation of anti-inflammatory Th2-lymphocyte cytokine secretion (IL-4, IL-13) | Downregulation of M2 macrophage polarization | [45] |
| Adipocyte stress | Release of MCP-1, prevention of adipocyte differentiation due to IL-13 downregulation | Upregulation of pro-inflammatory circulating monocyte and T-lymphocyte accumulation (MCP-1) | [45,46] |
| M1 macrophage polarization | |||
| Adipose tissue | Pro-inflammatory mediators (TNF-α, IL-6, ROS) reduce adiponectin secretion with disruption of FA oxidation, glucose uptake (muscle), and physiological gluconeogenesis suppression (liver) processes | Downregulation of M2 macrophage polarization | [59] |
| M1 macrophage | Secretion of pro-inflammatory mediators (IL-1β, IL-6, TNF-α, MCP-1, and PAI-1) | N/A | [45,46] |
| T-lymphocyte | General accumulation of Th1-, γδT-, Th17, and CD8+ T-lymphocyte types; downregulation of groups that attenuate/regulate immune responses (NK cells, Th2-, and Treg-lymphocyte) | N/A | [54,60] |
| Th1-lymphocyte | Secretion of pro-inflammatory mediators (IFN-γ), induce expression of pro-inflammatory mediators in adipose tissue (TNF-α, MCP-1) | Upregulation of pro-inflammatory circulating monocyte accumulation (MCP-1) | [53] |
| Mediator | Mechanism | Description with Cardiovascular Disease Association | Immune Cell Response | References |
|---|---|---|---|---|
| Cardiomyocyte | A | ER stress, hyperglycemia-mediated oxidative stress both lead to caspase-8 and caspase-9 activation triggering caspase-3 activation, cell death with release of DAMP; ROS stimulate NLRP3 inflammasome, release of TNF-α, IL-1β, and MCP-1 | Upregulation of pro-inflammatory circulating monocyte accumulation (MCP-1) | [77,78] |
| Diabetic cardiomyopathy | ||||
| G | Inflammation (TNF-α, IL-6, TNF-α, IFN-γ, and LPS) and oxidative stress (NOX2, NOX4, and ROS) contribute to cardiomyocyte hypertrophy | Upregulation of M1 macrophage polarization | [71,79] | |
| Diabetic cardiomyopathy | ||||
| CF | E | RAAS axis activation and SGK1/STAT3 signaling, RAAS-mediated activation of NF-κB signaling with secretion of TNF-α, MCP-1, IL-6, and IL-8 | Upregulation of M1 macrophage polarization | [78,80] |
| Diabetic cardiomyopathy | ||||
| LDL | B | Modified LDL (glycation, oxidation) interacts with subendothelial proteoglycan, leads to macrophage-mediated phagocytosis of LDL with upregulation of pro-inflammatory IL-1β, IL-6, TNF-α, and MMP | Upregulation of M1 macrophage polarization | [68] |
| Atherosclerosis, diabetic macrovascular disease | ||||
| M1 macrophage | B | Increased atherosclerotic plaque instability due to increased M1 macrophage polarization, VSMC senescence | N/A | [58] |
| Atherosclerosis, diabetic macrovascular disease | ||||
| M1 macrophage | E | IL-1β produced by M1 macrophages stimulates cardiac fibroblasts | N/A | [71] |
| Diabetic cardiomyopathy | ||||
| Mitochondria | C | Increased FA oxidation with cardiomyocyte hypoxia, increased production of lipid intermediaries (DAG, acylcarnitine, and ceramide) triggering ROS production, ER stress | Upregulation of M1 macrophage polarization | [56,57,81] |
| Diabetic cardiomyopathy, cardiac lipotoxicity | ||||
| Platelets | F | Lipid peroxidation generates F2-isoprostanes (8-iso-PGF2α) due to low-grade inflammation, enhances platelet aggregation | Upregulation of M1 macrophage polarization | [82,83] |
| Atherosclerosis, diabetic macrovascular disease | ||||
| ROS | D | Increased NOX activity, absence of compensatory low-level NO production (dysfunctional eNOS) | Upregulation of M1 macrophage polarization, downregulation of M2 macrophage polarization | [84,85] |
| Diabetic macrovascular disease | ||||
| Saturated FA | B | High levels of palmitic acid in T2DM trigger TLR4/ERK/FOXC2 signaling | Upregulation of M1 macrophage polarization | [58] |
| Atherosclerosis, diabetic macrovascular disease | ||||
| TF | F | TF upregulation in endothelial cells and circulating monocytes due to low-grade inflammation (TNF-α, IL-6) | N/A | [86] |
| Atherosclerosis, diabetic macrovascular disease | ||||
| Th1-lymphocyte | E | Integrin-α4 cell-to-cell interactions with cardiac fibroblasts, transdifferentiation into myofibroblasts | N/A | [75] |
| Diabetic cardiomyopathy | ||||
| Treg-lymphocyte | E | Reduced levels of Treg-lymphocytes in T2DM augment cardiac fibrosis/hypertrophy | Upregulation of M1 macrophage polarization, downregulation of M2 macrophage polarization | [76,87] |
| Diabetic cardiomyopathy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stougiannou, T.M.; Koufakis, T.; Papanas, N.; Karangelis, D. Diabetes Mellitus and Cardiopulmonary Bypass (CPB): Pathophysiological Mechanisms Related to Inflammation and Cardiovascular Disease. Curr. Issues Mol. Biol. 2025, 47, 911. https://doi.org/10.3390/cimb47110911
Stougiannou TM, Koufakis T, Papanas N, Karangelis D. Diabetes Mellitus and Cardiopulmonary Bypass (CPB): Pathophysiological Mechanisms Related to Inflammation and Cardiovascular Disease. Current Issues in Molecular Biology. 2025; 47(11):911. https://doi.org/10.3390/cimb47110911
Chicago/Turabian StyleStougiannou, Theodora M., Theocharis Koufakis, Nikolaos Papanas, and Dimos Karangelis. 2025. "Diabetes Mellitus and Cardiopulmonary Bypass (CPB): Pathophysiological Mechanisms Related to Inflammation and Cardiovascular Disease" Current Issues in Molecular Biology 47, no. 11: 911. https://doi.org/10.3390/cimb47110911
APA StyleStougiannou, T. M., Koufakis, T., Papanas, N., & Karangelis, D. (2025). Diabetes Mellitus and Cardiopulmonary Bypass (CPB): Pathophysiological Mechanisms Related to Inflammation and Cardiovascular Disease. Current Issues in Molecular Biology, 47(11), 911. https://doi.org/10.3390/cimb47110911









