Genome-Wide Identification and Functional Evolution of NLR Gene Family in Capsicum annuum
Abstract
1. Introduction
2. Materials and Methods
2.1. NLR Gene Identification
2.2. Phylogenetic Analysis
2.3. Gene Duplication and Synteny Analysis
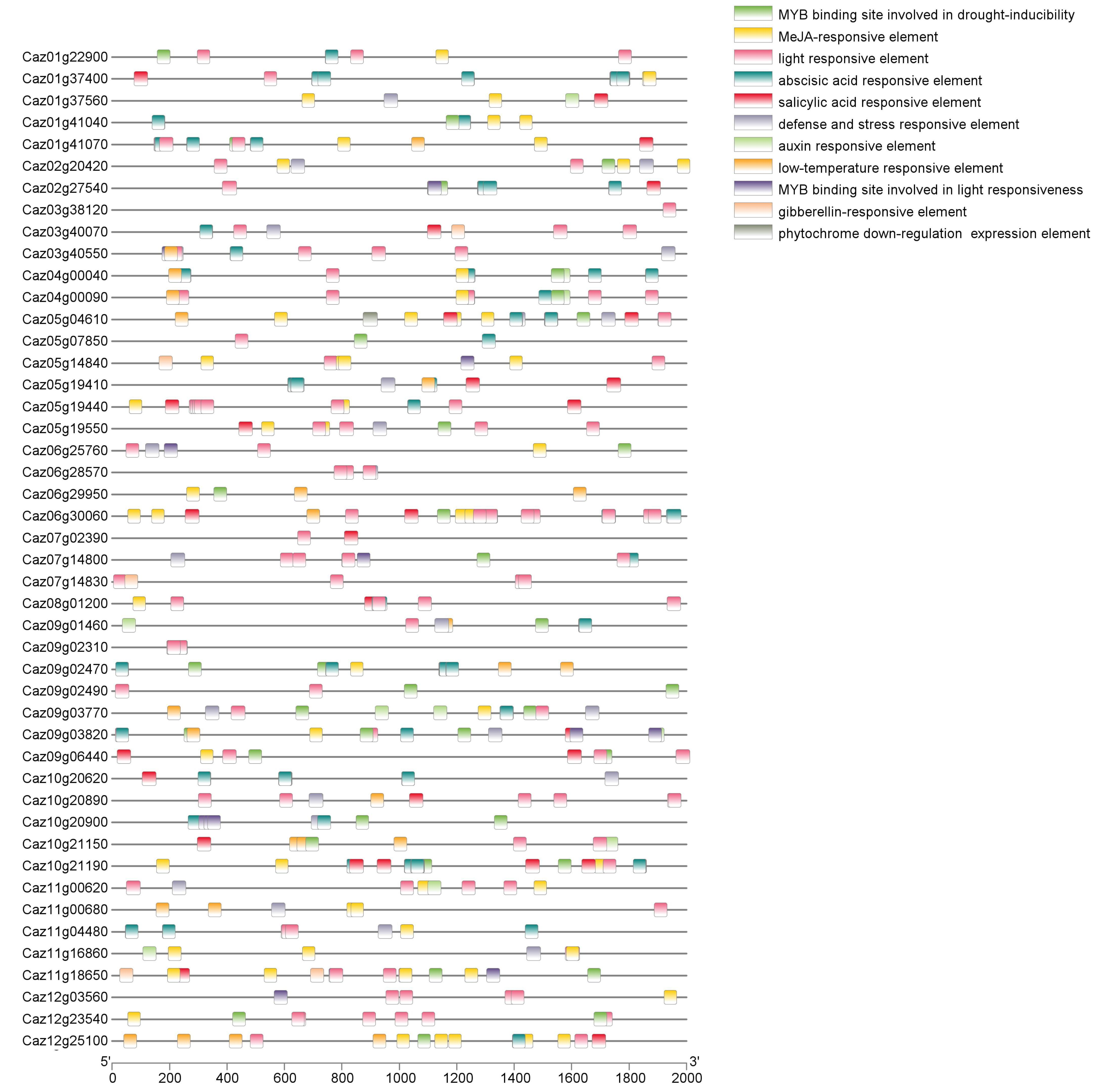
2.4. Cis-Regulatory Element (CRE) Prediction
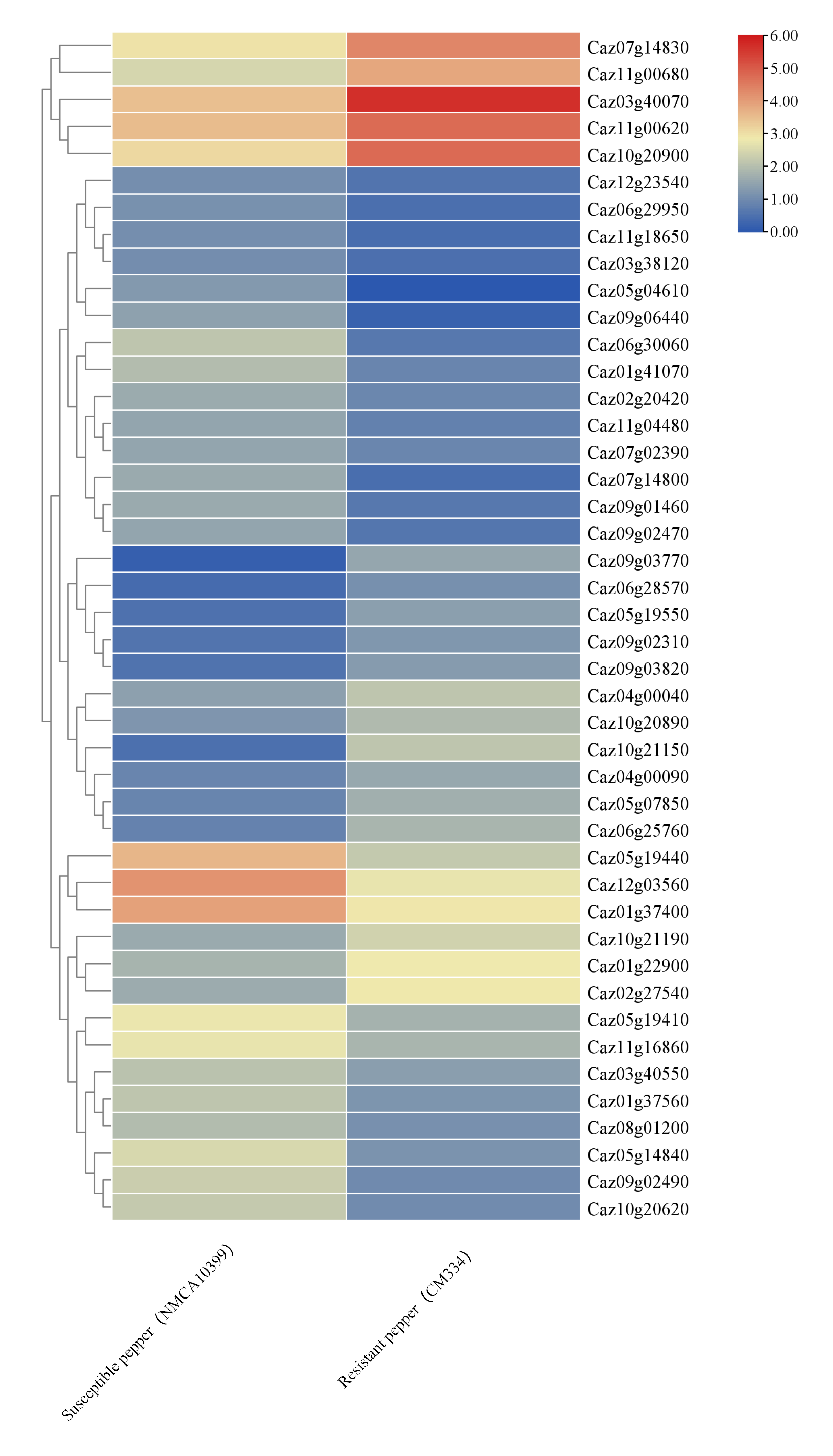
2.5. RNA-Seq Analysis
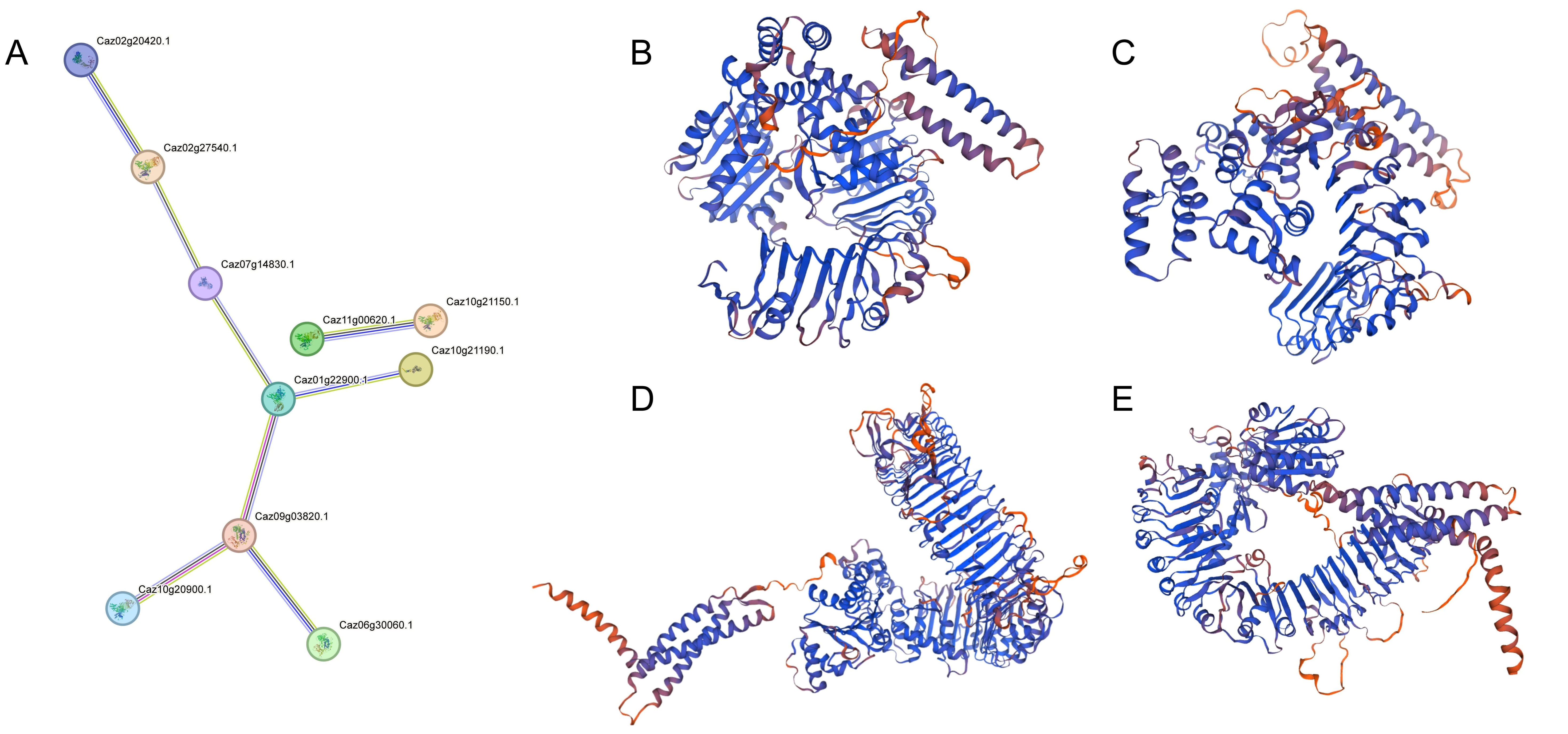
2.6. Protein–Protein Interaction (PPI) Network and Protein Structure Prediction
2.7. RT-qPCR Analysis
3. Results
3.1. Genome-Wide Identification and Classification of NLR Genes in Pepper
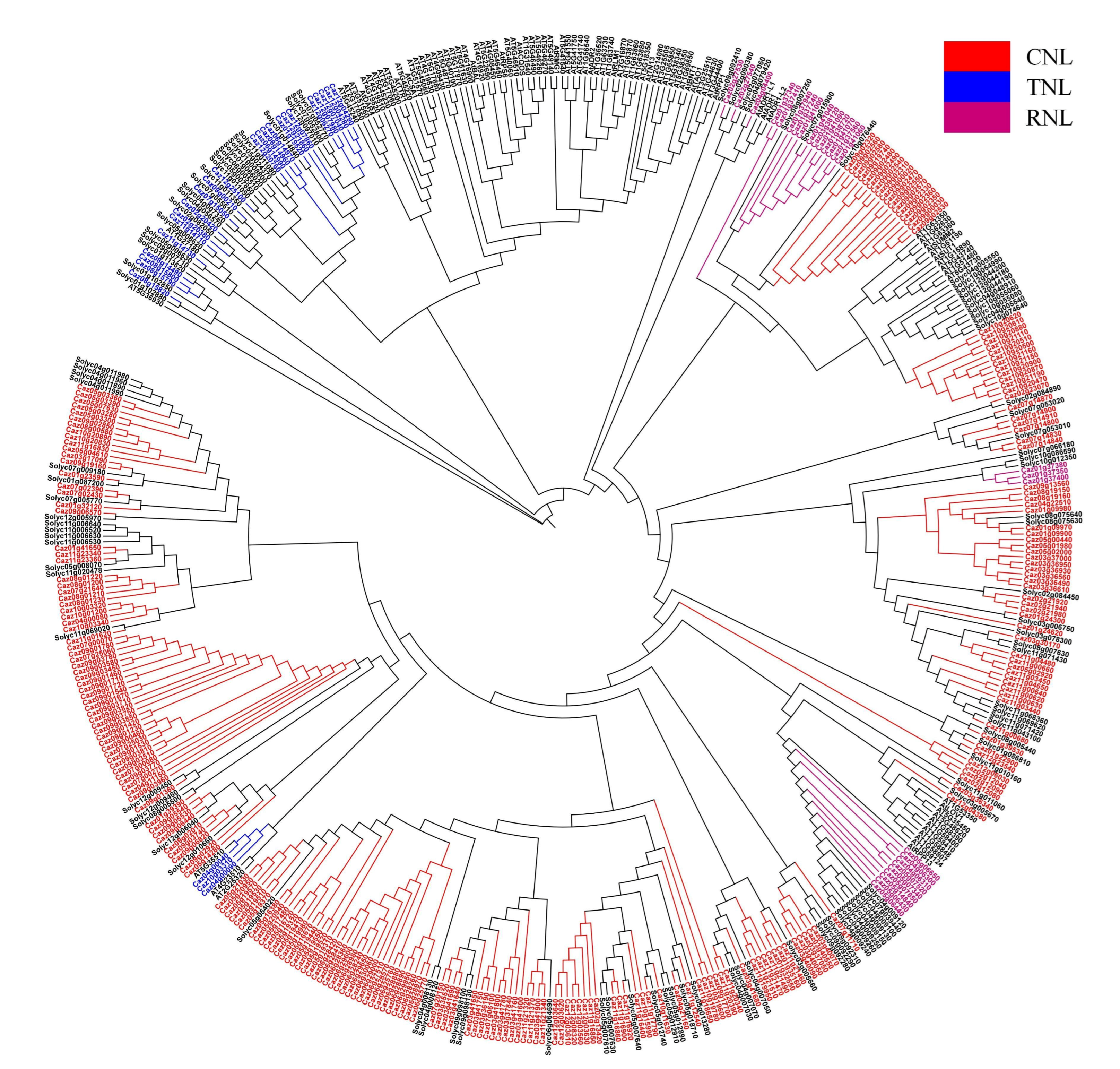
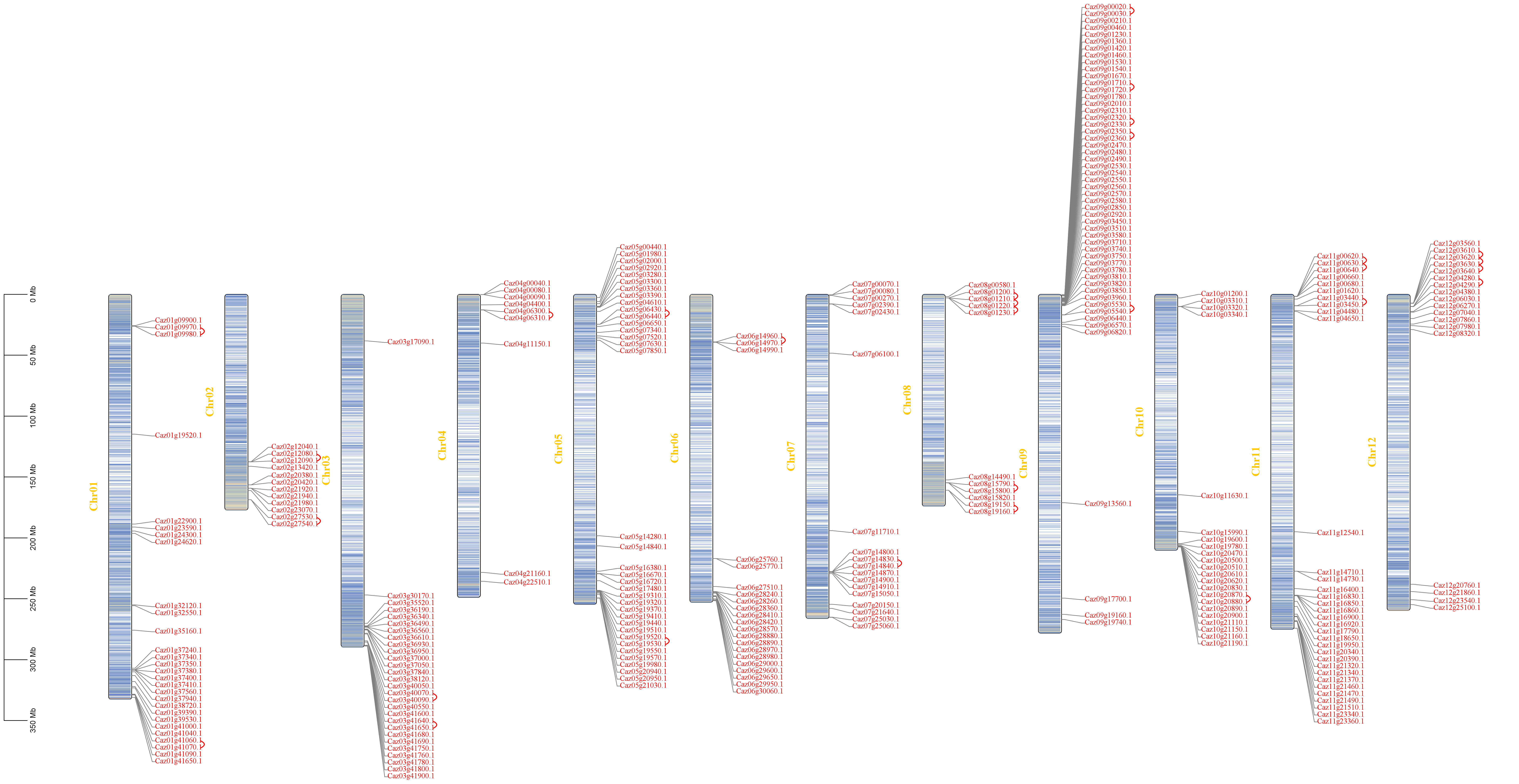
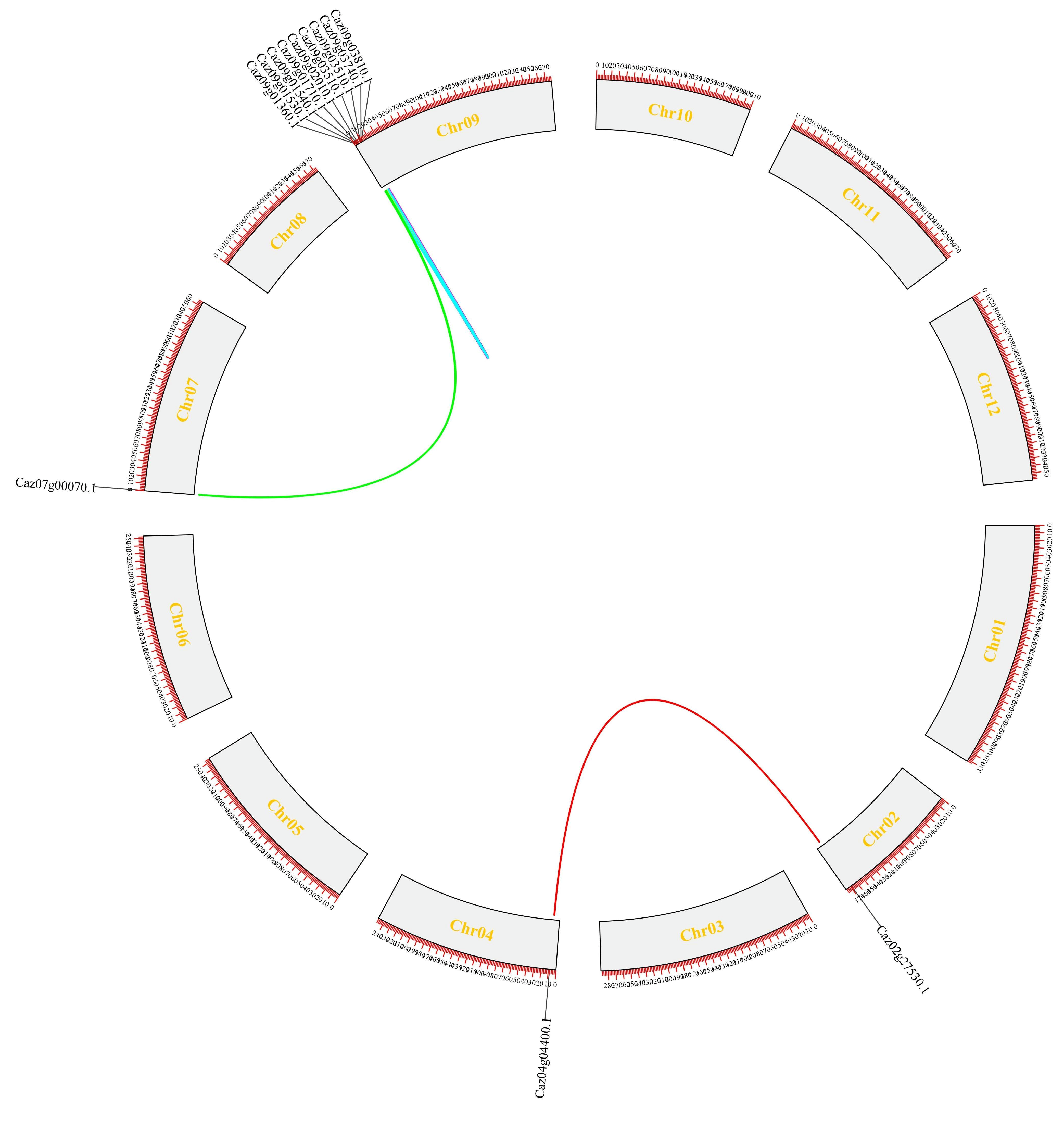
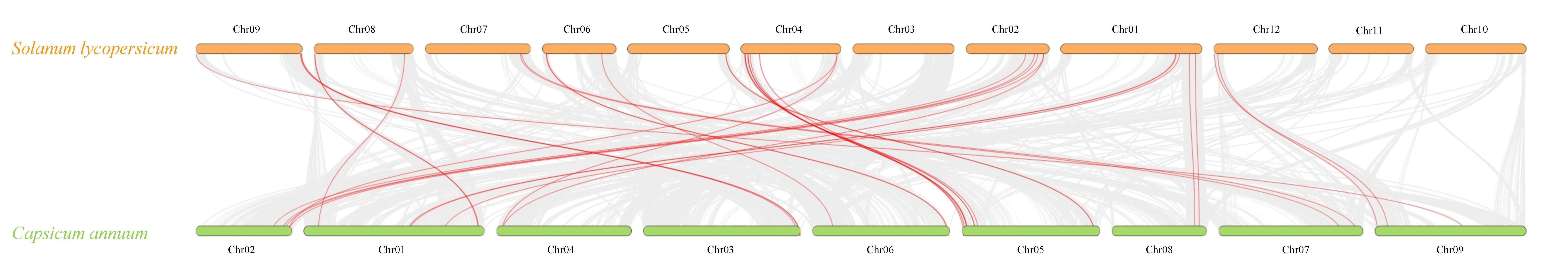
3.2. Tandem Duplication Drives NLR Family Expansion
3.3. NLR Expression Dynamics and Regulatory Element Analysis Reveal Immune-Related Genes
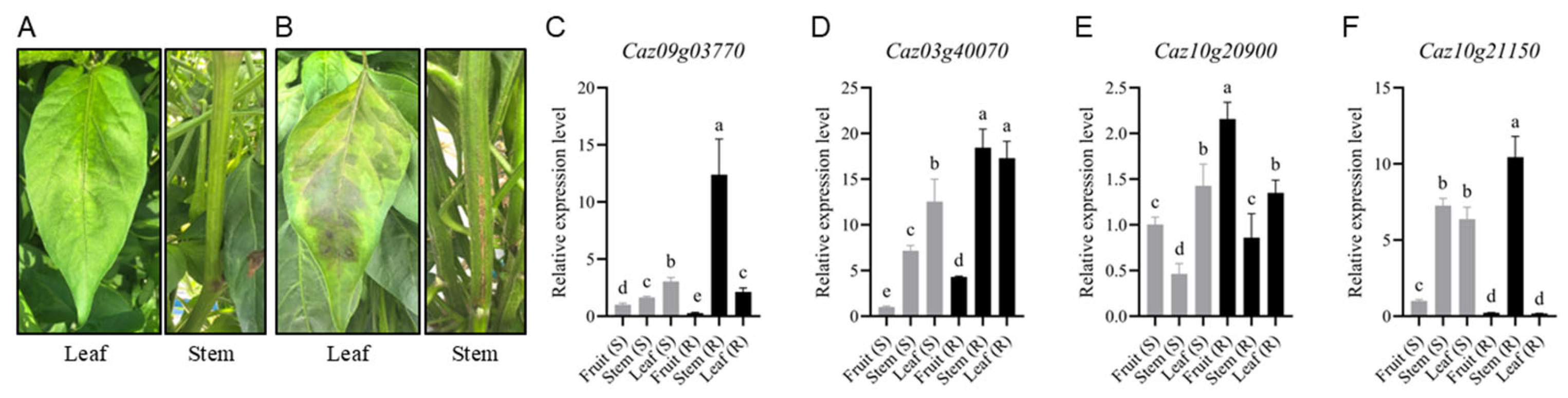
3.4. Disease Symptom Phenotyping in Resistant and Susceptible Cultivars and Organ-Specific Expression of NLR Candidates
3.5. Protein–Protein Interaction (PPI) Network and Protein Structure Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-Recognition Receptors Are Required for NLR-Mediated Plant Immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Yu, X.Q.; Niu, H.Q.; Liu, C.; Wang, H.L.; Yin, W.; Xia, X. PTI-ETI Synergistic Signal Mechanisms in Plant Immunity. Plant Biotechnol. J. 2024, 22, 2113–2128. [Google Scholar] [CrossRef]
- Locci, F.; Parker, J.E. Plant NLR Immunity Activation and Execution: A Biochemical Perspective. Open Biol. 2024, 14, 230387. [Google Scholar] [CrossRef]
- Ma, S.; An, C.; Lawson, A.W.; Cao, Y.; Sun, Y.; Tan, E.Y.J.; Pan, J.; Jirschitzka, J.; Kümmel, F.; Mukhi, N.; et al. Oligomerization-Mediated Autoinhibition and Cofactor Binding of a Plant NLR. Nature 2024, 632, 869–876. [Google Scholar] [CrossRef]
- Wang, J.; Song, W.; Chai, J. Structure, Biochemical Function, and Signaling Mechanism of Plant NLRs. Mol. Plant 2023, 16, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Liang, D.; Li, H.; Jiao, F.; Yan, B.; Liu, J.; Lei, Z.; Huang, L.; Gong, X.; Wang, X.; et al. NLRs Guard Metabolism to Coordinate Pattern- and Effector-Triggered Immunity. Nature 2022, 601, 245–251. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, L.; Zhang, X.; Zhang, Q.; Jia, Y.; Wang, G.; Li, S.; Tian, D.; Li, W.H.; Yang, S. Large-Scale Identification and Functional Analysis of NLR Genes in Blast Resistance in the Tetep Rice Genome Sequence. Proc. Natl. Acad. Sci. USA 2019, 116, 18479–18487. [Google Scholar] [CrossRef]
- Prigozhin, D.M.; Krasileva, K.V. Analysis of Intraspecies Diversity Reveals a Subset of Highly Variable Plant Immune Receptors and Predicts Their Binding Sites. Plant Cell 2021, 33, 998–1015. [Google Scholar] [CrossRef] [PubMed]
- Aguileta, G.; Refrégier, G.; Yockteng, R.; Fournier, E.; Giraud, T. Rapidly Evolving Genes in Pathogens: Methods for Detecting Positive Selection and Examples Among Fungi, Bacteria, Viruses and Protists. Infect. Genet. Evol. 2009, 9, 656–670. [Google Scholar] [CrossRef]
- Singh, P.K.; Ray, S.; Thakur, S.; Rathour, R.; Sharma, V.; Sharma, T.R. Co-Evolutionary Interactions Between Host Resistance and Pathogen Avirulence Genes in Rice-Magnaporthe Oryzae Pathosystem. Fungal Genet. Biol. 2018, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pál, C.; Papp, B. How Selection Shapes the Short- and Long-Term Dynamics of Molecular Evolution. Proc. Natl. Acad. Sci. USA 2023, 120, e2311012120. [Google Scholar] [CrossRef]
- Good, B.H.; McDonald, M.J.; Barrick, J.E.; Lenski, R.E.; Desai, M.M. The Dynamics of Molecular Evolution over 60,000 Generations. Nature 2017, 551, 45–50. [Google Scholar] [CrossRef]
- Bashir, S.; Rehman, N.; Fakhar Zaman, F.; Naeem, M.K.; Jamal, A.; Tellier, A.; Ilyas, M.; Silva Arias, G.A.; Khan, M.R. Genome-Wide Characterization of the NLR Gene Family in Tomato (Solanum lycopersicum) and Their Relatedness to Disease Resistance. Front. Genet. 2022, 13, 931580. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, F.; Chen, X.; Zhang, C.; Zhang, H.; Wang, T.; Zhang, J.; He, C.; Wang, S.; Zhang, X.; et al. Stacking Potato NLR Genes Activates a Calcium-Dependent Protein Kinase and Confers Broad-Spectrum Disease Resistance to Late Blight. J. Integr. Plant Biol. 2025, 1910-1927, 1910–1927. [Google Scholar] [CrossRef]
- Forero, D.A.; Chand, V. Methods in Molecular Biology and Genetics: Looking to the Future. BMC Res. Notes 2023, 16, 26. [Google Scholar] [CrossRef]
- Yang, X.; Pan, Y.; Singh, P.; He, X.; Ren, Y.; Zhao, L.; Zhang, N.; Cheng, S.; Chen, F. Investigation and Genome-Wide Association Study for Fusarium Crown Rot Resistance in Chinese common wheat. BMC Plant. Biol. 2019, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Dakouri, A.; Zhang, X.; Peng, G.; Falk, K.; Gossen, B.; Strelkov, S.; Yu, F. Analysis of Genome-Wide Variants Through Bulked Segregant RNA Sequencing Reveals a Major Gene For Resistance to Plasmodiophora Brassicae in Brassica oleracea. Sci. Rep. 2018, 8, 17657. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, J.; Sun, H.; Xiong, C.; Sun, X.; Wang, X.; Wang, Z.; Jarret, R.; Wang, J.; Tang, B.; et al. Genomes of Cultivated and Wild Capsicum Species Provide Insights into Pepper Domestication and Population Differentiation. Nat. Commun. 2023, 14, 5487. [Google Scholar] [CrossRef] [PubMed]
- Thakur, H.; Jindal, S.K.; Sharma, A.; Dhaliwal, M.S. Chilli Leaf Curl Virus Disease: A Serious Threat for Chilli Cultivation. J. Plant Dis. Prot. 2018, 125, 239–249. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Z.; Zhang, Y.M.; Li, Q.; Jiang, X.M.; Jiang, Z.; Tang, J.H.; Chen, D.; Wang, Q.; Chen, J.Q.; et al. An Angiosperm NLR Atlas Reveals That NLR Gene Reduction Is Associated with Ecological Specialization and Signal Transduction Component Deletion. Mol. Plant 2021, 14, 2015–2031. [Google Scholar] [CrossRef]
- Reams, A.B.; Roth, J.R. Mechanisms of Gene Duplication and Amplification. Cold Spring Harb. Perspect. Biol. 2015, 7, a016592. [Google Scholar] [CrossRef]
- Shepherd, S.; Yuen, E.L.H.; Carella, P.; Bozkurt, T.O. The Wheels of Destruction: Plant NLR Immune Receptors Are Mobile and Structurally Dynamic Disease Resistance Proteins. Curr. Opin. Plant Biol. 2023, 74, 102372. [Google Scholar] [CrossRef]
- Castel, B.; Ngou, P.M.; Cevik, V.; Redkar, A.; Kim, D.S.; Yang, Y.; Ding, P.; Jones, J.D.G. Diverse NLR Immune Receptors Activate Defence via the RPW8-NLR NRG1. New Phytol. 2019, 222, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Zhang, G.; Pan, B.; Diao, W.; Liu, J.; Ge, W.; Gao, C.; Zhang, Y.; Jiang, C.; Wang, S. Development and Application of InDel Markers for Capsicum spp. Based on Whole-Genome Re-Sequencing. Sci. Rep. 2019, 9, 3691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, S.; Fang, X. Emerging Roles of Phase Separation in Plant Transcription and Chromatin Organization. Curr. Opin. Plant Biol. 2023, 75, 102387. [Google Scholar] [CrossRef]
- Fang, X.; May, A.I.; Sporbeck, K.; Hauer, L.; Knorr, R.L. Wet Scissors: How Biomolecular Condensates Cut Cellular Membranes. Curr. Opin. Plant Biol. 2025, 86, 102740. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, W.; Niu, Y.; Wang, T.; Dong, J. Liquid-Liquid Phase Separation in Plants: Advances and Perspectives from Model Species to Crops. Plant Commun. 2024, 5, 100663. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Dong, K.; Wen, D.; Cui, Z.; Cao, J. A review of m6A modification in plant development and potential quality improvement. Int. J. Biol. Macromol. 2025, 308, 142597. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Chen, Q.; Liu, W.; Li, T.; Ji, T. Genome-Wide Identification and Functional Evolution of NLR Gene Family in Capsicum annuum. Curr. Issues Mol. Biol. 2025, 47, 867. https://doi.org/10.3390/cimb47100867
Feng C, Chen Q, Liu W, Li T, Ji T. Genome-Wide Identification and Functional Evolution of NLR Gene Family in Capsicum annuum. Current Issues in Molecular Biology. 2025; 47(10):867. https://doi.org/10.3390/cimb47100867
Chicago/Turabian StyleFeng, Chong, Qi Chen, Wenhao Liu, Tengfei Li, and Tuo Ji. 2025. "Genome-Wide Identification and Functional Evolution of NLR Gene Family in Capsicum annuum" Current Issues in Molecular Biology 47, no. 10: 867. https://doi.org/10.3390/cimb47100867
APA StyleFeng, C., Chen, Q., Liu, W., Li, T., & Ji, T. (2025). Genome-Wide Identification and Functional Evolution of NLR Gene Family in Capsicum annuum. Current Issues in Molecular Biology, 47(10), 867. https://doi.org/10.3390/cimb47100867




