Clinical Trial of Autologous Dendritic Cell Administration Effect on Water Molecule Diffusion and Anti-Inflammatory Biomarkers in Diabetic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
Study Design
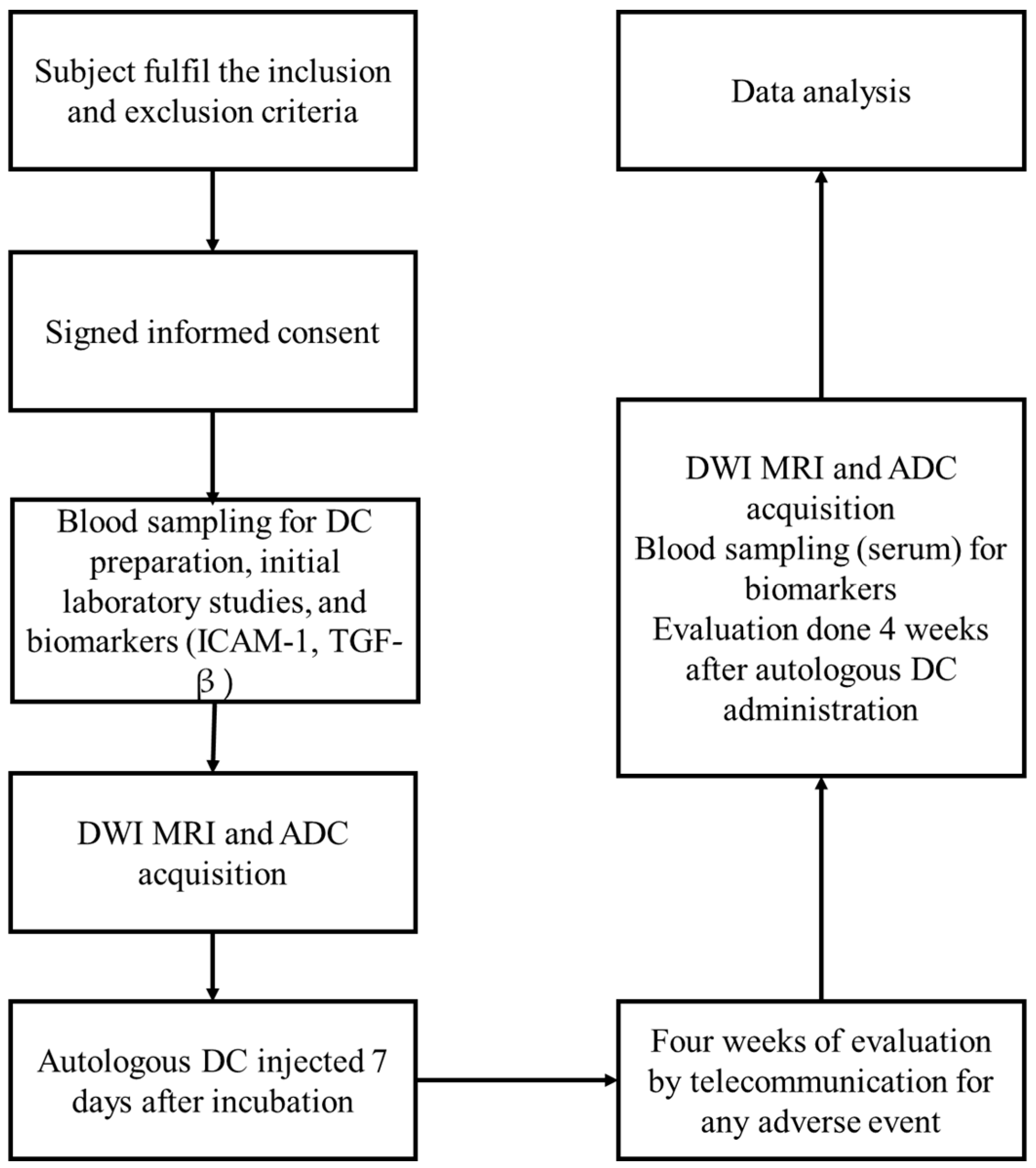
3. Results
3.1. Subject Characteristics
3.2. Correlation of ADC and eGFR
| ADC Values (×103 mm2/s) | Lef Kidney | Right Kidney | ||||
|---|---|---|---|---|---|---|
| ROI 1 | ROI 2 | ROI 3 | ROI 4 | ROI 5 | ROI 6 | |
| Pre | 1.734 | 1.740 | 1.779 | 1.839 | 1.951 | 1.818 |
| Post | 1.529 | 1.728 | 1.736 | 1.675 | 1.824 | 1.773 |
| p-value | 0.017 | 0.910 | 0.615 | 0.277 | 0.101 | 0.709 |

| Correlation of ADC and eGFR | p-Value * | |
|---|---|---|
| eGFR > 60 mL/min/1.73 m2 | −0.267 | 0.457 |
| eGFR < 60 mL/min/1.73 m2 | 0.079 | 0.806 |
3.3. Changes in ADC
| Variables | Median (Q1–Q3) | p-Value Hypothesis Test |
|---|---|---|
| ADC Pre | 1.75 mm2/s (1.51–1.87) | 0.223 a |
| ADC Post | 1.64 mm2/s (1.46–1.75) | |
| ICAM-1 Pre | 325.6 ng/mL (256–355.3) | 0.359 b |
| ICAM-1 Post | 336.7 (283.8–375.3) | |
| TGF-β Pre | 39.55 ng/mL (30.9–49) | 0.506 a |
| TGF-β Post | 41.35 ng/mL (32.4–53.7) |

3.4. Changes in ICAM-1 and TGF-β Levels
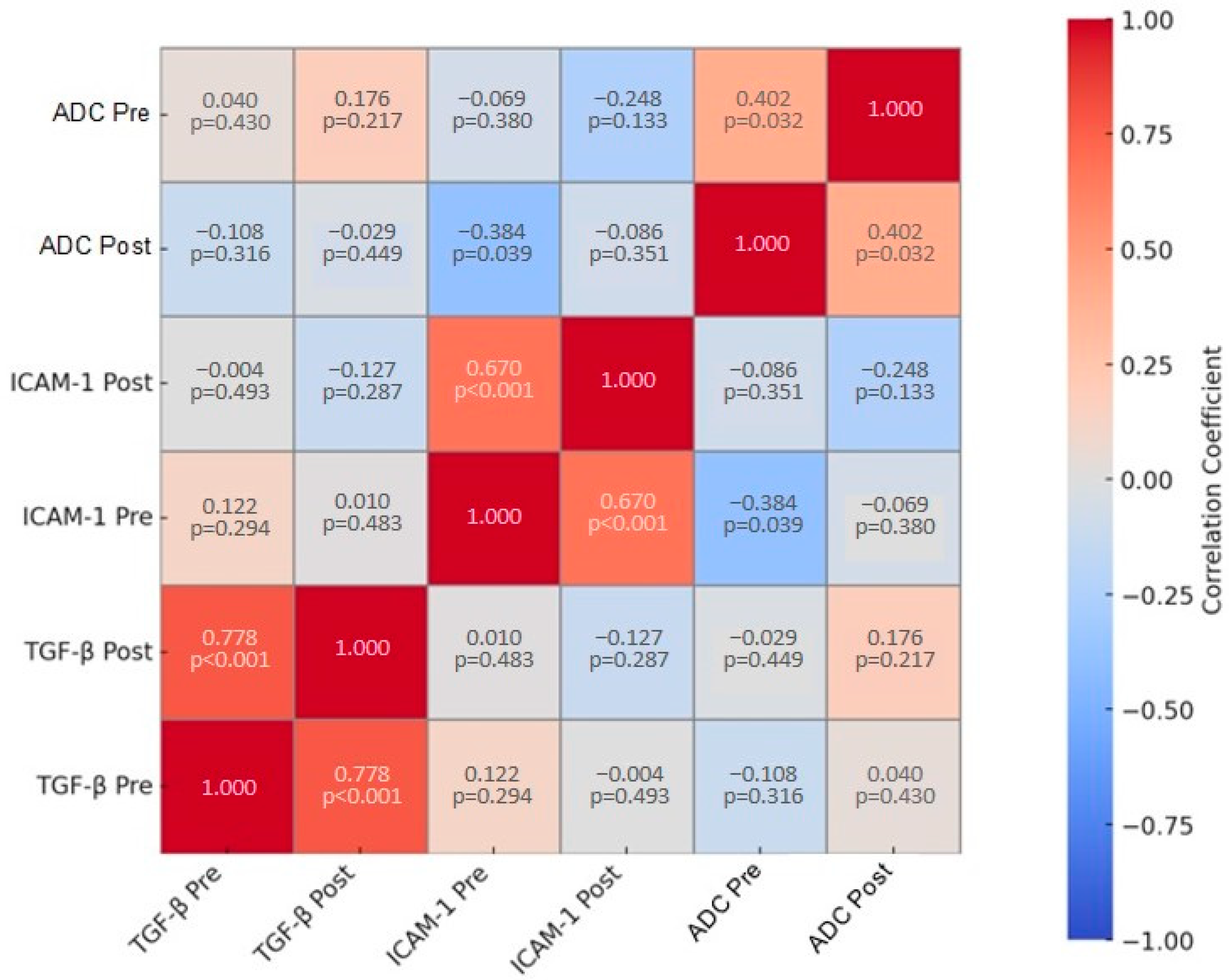
3.5. Correlation of ADC, TGF-β, and VCAM
| TGF-β Pre | TGF-β Post | ICAM-1 Pre | ICAM-1 Post | Mean ADC Pre | Mean ADC Post | |
|---|---|---|---|---|---|---|
| TGF-β Pre | 1.000 | 0.778 | 0.122 | −0.004 | −0.108 | 0.040 |
| p-value | 0.000 | 0.294 | 0.493 | 0.316 | 0.430 | |
| TGF-β Post | 0.778 | 1.000 | 0.010 | −0.127 | −0.029 | 0.176 |
| p-value | 0.000 | 0.483 | 0.287 | 0.449 | 0.217 | |
| ICAM-1 Pre | 0.122 | 0.010 | 1.000 | 0.670 | −0.384 | −0.069 |
| p-value | 0.294 | 0.483 | 0.000 | 0.039 | 0.380 | |
| ICAM-1 Post | −0.004 | −0.127 | 0.670 | 1.000 | −0.086 | −0.248 |
| p-value | 0.493 | 0.287 | 0.000 | 0.351 | 0.133 | |
| Mean ADC Pre | −0.108 | −0.029 | −0.384 | −0.086 | 1.000 | 0.402 |
| p-value | 0.316 | 0.449 | 0.039 | 0.351 | 0.032 | |
| Mean ADC Post | 0.040 | 0.176 | −0.069 | −0.248 | 0.402 | 1.000 |
| p-value | 0.430 | 0.217 | 0.380 | 0.133 | 0.032 |
| ADC Ratio | ||
|---|---|---|
| Correlation | p-Value * | |
| ICAM-1 Ratio | −0.490 | 0.010 |
| TGF-β ratio | −0.12 | 0.479 |
3.6. Changes in ADC Values, Serum Creatinine Levels, ICAM-1, and TGF-β in Gender Subgroups
| Paired t-Test | Wilcoxon Signed Rank Test | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ICAM-1 (Post–Pre) | p-Value | Z TGF-β | p-Value | Z ADC | p-Value | Z Creatinine | p-Value | |
| Men | −18.23 ± 55.1 | 0.35 | −1.244 | 0.214 | −0.533 | 0.594 | −0.59 | 0.953 |
| Women | 31.77 ± 49.8 | 0.04 | −0.105 | 0.917 | −0.943 | 0.345 | −2.030 | 0.042 |
3.7. Changes in ADC Values, Serum Creatinine Levels, ICAM-1, and TGF-β Based on Cardiovascular Comorbidities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Pernefri 11th Report of Indonesian Renal Registry 2018. 2018. Available online: https://www.indonesianrenalregistry.org/ (accessed on 15 May 2024).
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney Disease and Increased Mortality Risk in Type 2 Diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Nurtandhee, M. Estimasi Biaya Pelayanan Kesehatan sebagai Upaya Pencegahan Defisit Dana Jaminan Sosial untuk Penyakit Gagal Ginjal. J. Jaminan Kesehat. Nas. 2023, 3, 84–101. [Google Scholar] [CrossRef]
- Umanath, K.; Lewis, J.B. Update on Diabetic Nephropathy: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 71, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Nicholas, S.B. Pathomechanisms of Diabetic Kidney Disease. J. Clin. Med. 2023, 12, 7349. [Google Scholar] [CrossRef]
- Karimi, Z.; Kahe, F.; Jamil, A.; Marszalek, J.; Ghanbari, A.; Afarideh, M.; Khajeh, E.; Noshad, S.; Esteghamati, A.; Chi, G. Intercellular adhesion molecule-1 in diabetic patients with and without microalbuminuria. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 365–368. [Google Scholar] [CrossRef]
- Çakmak, P.; Yaǧci, A.B.; Dursun, B.; Herek, D.; Fenkçi, S.M. Renal diffusion-weighted imaging in diabetic nephropathy: Correlation with clinical stages of disease. Diagn. Interv. Radiol. 2014, 20, 374–378. [Google Scholar] [CrossRef]
- Dongwei, L.; Zhengyong, L.; Zhangsuo, L. Treatment of diabetic kidney disease: Research development, current hotspots and future directions. Natl. Med. J. China 2021, 101, 683–686. [Google Scholar] [CrossRef]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kawanami, D.; Utsunomiya, K.; Nishimura, R. Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. Int. J. Mol. Sci. 2019, 20, 3393. [Google Scholar] [CrossRef]
- Bell, G.M.; Anderson, A.E.; Diboll, J.; Reece, R.; Eltherington, O.; Harry, R.A.; Fouweather, T.; MacDonald, C.; Chadwick, T.; McColl, E.; et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 2017, 76, 227–234. [Google Scholar] [CrossRef]
- Kassianos, A.J.; Sampangi, S.; Wang, X.; Roper, K.E.; Beagley, K.W.; Healy, H.; Wilkinson, R. Human proximal tubule epithelial cells modulate autologous dendritic cell function. Nephrol. Dial. Transplant. 2013, 28, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Sampangi, S.; Kassianos, A.J.; Wang, X.; Beagley, K.W.; Klein, T.J.; Afrin, S.; Healy, H.; Wilkinson, R. The Mechanisms of Human Renal Epithelial Cell Modulation of Autologous Dendritic Cell Phenotype and Function. PLoS ONE 2015, 10, e0134688. [Google Scholar] [CrossRef] [PubMed]
- Jonny; Sitepu, E.C.; Nidom, C.A.; Wirjopranoto, S.; Sudiana, I.K.; Ansori, A.N.M.; Putranto, T.A. Ex Vivo-Generated Tolerogenic Dendritic Cells: Hope for a Definitive Therapy of Autoimmune Diseases. Curr. Issues Mol. Biol. 2024, 46, 4035–4048. [Google Scholar] [CrossRef] [PubMed]
- Jonny, J.; Putranto, T.A.; Sitepu, E.C.; Irfon, R. Dendritic cell vaccine as a potential strategy to end the COVID-19 pandemic. Why should it be Ex Vivo? Expert Rev. Vaccines 2022, 21, 1111–1120. [Google Scholar] [CrossRef]
- Jonny; Putranto, T.A.; Purnama, Y.; Djatmiko, R.; Yana, M.L.; Sitepu, E.C.; Irfon, R. Significant improvement of systemic lupus erythematosus manifestation in children after autologous dendritic cell transfer: A case report and review of literature. Ther. Adv. Vaccines Immunother. 2023, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jonny, J.; Sitepu, E.C.; Lister, I.N.E.; Chiuman, L.; Putranto, T.A. The Potential of Anti-Inflammatory DC Immunotherapy in Improving Proteinuria in Type 2 Diabetes Mellitus. Vaccines 2024, 12, 972. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Z.; Li, X.; Li, C.; Wang, R.; Zhang, Y.; Niu, G. Diffusion-weighted imaging for staging chronic kidney disease: A meta-analysis. Br. J. Radiol. 2018, 91, 20170952. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; He, J.; Li, Y. Immune responses in diabetic nephropathy: Pathogenic mechanisms and therapeutic target. Front. Immunol. 2022, 13, 958790. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Diverse Role of TGF-β in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, Y.; Liu, F. Transforming Growth Factor-Beta1 in Diabetic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 187. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Ballantyne, C.M.; Sharrett, A.R.; Smith, L.C.; Davis, C.E.; Gotto, A.M.; Boerwinkle, E. Circulating Adhesion Molecules VCAM-1, ICAM-1, and E-selectin in Carotid Atherosclerosis and Incident Coronary Heart Disease Cases. Circulation 1997, 96, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Hay, M.; Johnson, A.K. The Protective Role of Estrogen and Brain Estrogen Receptors in the Pathogenesis of Hypertension. In Sex Differences in Cardiovascular Physiology and Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–39. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128131978000038 (accessed on 19 November 2024).
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chimal, J.; Jaisser, F. Pathophysiologic mechanisms in diabetic kidney disease: A focus on current and future therapeutic targets. Diabetes Obes. Metab. 2020, 22, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, R.; Koya, D.; Haneda, M. Progression of diabetic nephropathy. Am. J. Kidney Dis. 2003, 41, S19–S21. [Google Scholar] [CrossRef]
| Baseline Characteristics | ||
|---|---|---|
| Number of subjects | 22 | |
| Gender, n (%) | Men | 9 (41) |
| Women | 13 (59) | |
| Age, mean ± SD | 64 ± 7.8 | |
| Comorbidities, n (%) | Hypertension | 20 (90.9) |
| Heart Disease | 10 (45.5) | |
| Stroke | 3 (13.6) | |
| Neuropathy | 14 (63.6) | |
| BMI categories, n (%) | Underweight (≤17.5 kg/m2) | 1 (4.5) |
| Normal weight (17.50–22.99 kg/m2) | 6 (27.3) | |
| Overweight (23.00–27.99 kg/m2) | 9 (40.9) | |
| Obesity (≥28 kg/m2) | 6 (27.3) | |
| Types of anti-diabetics consumed, n (%) | Sulphonylurea | 6 (27.3) |
| Biguanide | 5 (22.7) | |
| Dipeptidyl peptidase-4 inhibitors | 4 (18.2) | |
| Sodium-glucose cotransporter-2 inhibitors | 8 (36.4) | |
| Insulin | 17 (77.3) | |
| Anti-hypertensive types consumed, n (%) | Angiotensin receptor blocker | 15 (68.2) |
| Angiotensin-converting enzyme inhibitors | 3 (13.6) | |
| Calcium channel blockers | 16 (72.7) | |
| Beta-blockers | 9 (40.9) | |
| Variables | Time Point | Group | |||||
|---|---|---|---|---|---|---|---|
| Heart Disease Comorbidity | Resistant Hypertension | ||||||
| Absent (n = 12) | Present (n = 10) | Absent (n = 14) | Present (n = 8) | ||||
| Mean ± SD | p-Value 1 | Mean ± SD | p-Value 1 | ||||
| ADC a (mm2/s) | Pre | 1.680 (0.229) | 1.683 (0.891) | 0.895 | 1.668 (0.381) | 1.806 (0.611) | 0.539 |
| Post | 1.757 (0.288) | 1.1517 (0.308) | 1.637 (0.287) | 1.664 (0.767) | |||
| p-value 2 | 0.480 | 0.445 | 0.510 | 0.208 | |||
| ICAM-1 b (ng/mL) | Pre | 329.97 ± 27.87 | 312.33 ± 19.44 | 0.578 | 338.25 ± 21.68 | 293.42 ± 27.53 | 0.300 |
| Post | 334.94 ± 16.97 | 331.26 ± 16.23 | 339.89 ± 14.41 | 321.67 ± 19.96 | |||
| p-value 2 | 0.767 | 0.333 | 0.893 | 0.311 | |||
| TGF b (ng/mL) | Pre | 44.67 ± 47.14 | 36.422 ± 28.41 | 0.465 | 45.47 ± 39.70 | 3.29 ± 2.56 | 0.502 |
| Post | 45.51 ± 40.78 | 40.54 ± 43.75 | 46.67 ± 38.98 | 3.73 ± 3.87 | |||
| p-value 2 | 0.796 | 0.193 | 0.667 | 0.282 | |||
| Creatinine b (mg/dL) | Pre | 1.27 ± 0.15 | 1.59 ± 0.17 | 0.544 | 1.56 ± 0.15 | 1.16 ± 0.17 | 0.468 |
| Post | 1.24 ± 0.17 | 1.50 ± 0.14 | 1.52 ± 0.13 | 1.06 ± 0.19 | |||
| p-value 2 | 0.636 | 0.200 | 0.595 | 0.199 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimu, P.S.; Icksan, A.G.; Farhat; Jonny; Hernowo, B.A.; Putranto, T.A. Clinical Trial of Autologous Dendritic Cell Administration Effect on Water Molecule Diffusion and Anti-Inflammatory Biomarkers in Diabetic Kidney Disease. Curr. Issues Mol. Biol. 2024, 46, 13767-13779. https://doi.org/10.3390/cimb46120822
Dimu PS, Icksan AG, Farhat, Jonny, Hernowo BA, Putranto TA. Clinical Trial of Autologous Dendritic Cell Administration Effect on Water Molecule Diffusion and Anti-Inflammatory Biomarkers in Diabetic Kidney Disease. Current Issues in Molecular Biology. 2024; 46(12):13767-13779. https://doi.org/10.3390/cimb46120822
Chicago/Turabian StyleDimu, Paulus Stefanus, Aziza Ghanie Icksan, Farhat, Jonny, Bhimo Aji Hernowo, and Terawan Agus Putranto. 2024. "Clinical Trial of Autologous Dendritic Cell Administration Effect on Water Molecule Diffusion and Anti-Inflammatory Biomarkers in Diabetic Kidney Disease" Current Issues in Molecular Biology 46, no. 12: 13767-13779. https://doi.org/10.3390/cimb46120822
APA StyleDimu, P. S., Icksan, A. G., Farhat, Jonny, Hernowo, B. A., & Putranto, T. A. (2024). Clinical Trial of Autologous Dendritic Cell Administration Effect on Water Molecule Diffusion and Anti-Inflammatory Biomarkers in Diabetic Kidney Disease. Current Issues in Molecular Biology, 46(12), 13767-13779. https://doi.org/10.3390/cimb46120822







