Abstract
Earliness in crop plants has a vital role in prevention of heat-induced drought stress and in combating global warming, which is predicted to exacerbate in the near future. Furthermore, earliness may expand production into northern areas or higher altitudes, having relatively shorter growing season and may also expand arable lands to meet global food demands. The primary objective of the present study was to investigate quantitative trait loci (QTLs) for super-earliness and important agro-morphological traits in a recombinant inbred line (RIL) population derived from an interspecific cross. A population of 114 RILs developed through single-seed descent from an interspecific cross involving Pisum sativum L. and P. fulvum Sibth. et Sm. was evaluated to identify QTLs for super-earliness and important agro-morphological traits. A genetic map was constructed with 44 SSRs markers representing seven chromosomes with a total length of 262.6 cM. Of the 14 QTLs identified, two were for super-earliness on LG2, one for plant height on LG3, six for number of pods per plant on LG2, LG4, LG5 and LG6, one for number of seeds per pod on LG6, one for pod length on LG4 and three for harvest index on LG3, LG5, and LG6. AA205 and AA372-1 flanking markers for super-earliness QTLs were suggested for marker-assisted selection (MAS) in pea breeding programs due to high heritability of the trait. This is the first study to map QTLs originating from P. sativum and P. fulvum recently identified species with super-earliness character and the markers (AA205 and AA372-1) linked to QTLs were valuable molecular tools for pea breeding.
1. Introduction
The Pisum L. genus is classified in the Fabaceae (Legumes) family, Fabaoideae (Papilionoideae) sub-family and Fabeae Rchb. tribe. The genus Pisum consists of three cultivated species including P. sativum L. (garden pea), P. arvense (L.) Poir. (field pea) and P. abyssinicum A.Br. (Dekoko or Abyssinian/Ethiopian pea) and there are seven taxa in the genus. These taxa were classified as follows: P. sativum L subsp. sativum var. sativum, P. sativum subsp. sativum var. arvense and P. sativum subsp. abyssinicum as cultivated species, while P. elatius (M.Bieb.) Asch. and Graebn. complex contains three varieties including P. sativum subsp. elatius var. elatius, P. sativum subsp. elatius var. pumilio Meikle and P. sativum subsp. elatius var. brevipedinculatum Davis and Meikle [1]. P. fulvum Sibth. et Sm. with a small distribution in Middle East and Turkey is the most distinct relative of the garden pea [2,3].
Pea has a central place in the history of genetics as an experimental plant since Mendel studied the famous laws of heredity [4]. Garden pea is among the most important food legumes, fodders and vegetable crops. It is grown in 99 countries worldwide. World annual production quantity of garden pea was reported to be 14.6 million tons for dry pea and 19.9 million tons for vegetable pea in 2020 [5]. It is used for various purposes, including food (leaves, green pods, unripe fresh seeds and dry mature seeds) and feed (direct grazing and silage). Garden pea is quite rich in protein (21–33%), starch (37–49%), soluble sugars (5%), fiber (2–9%), minerals and vitamins [2,6,7].
Garden pea, like faba bean (Vicia faba L.), is a cool season food legume and more susceptible to droughts than chickpea (Cicer arietinum L.), lentil (Lens culinaris Medik.) and grass pea (Lathyrus sativus L.) [8]. Like other crop plants, earliness provides many advantages in garden pea cultivation, such as prevention from drought-induced heat stress. Three resistance mechanisms have been reported for heat and drought: (i) escape, (ii) avoidance and (iii) tolerance. Escape is provided by early phenology including earliness [9,10]. Drought-induced heat stress has already increased due to climate change and is predicted to worsen due to a rise in temperature up to 1.5–4 °C in the near future [11]. Breeding for heat-tolerant garden pea has crucial importance with early flowered and matured cultivars [12]. Success in the selection for heat-tolerant garden pea depends on accuracy of selection with few efforts and short times by marker-assisted selection (MAS) [13,14]. New genetic sources for earliness, yield and yield-related traits should therefore be studied and mapped in garden pea.
Pea has a quite large genome of about 4.45 Gb [15]. Thanks to high-resolution genetic maps, it is possible to identify genes or QTLs controlling important agro-morphological and desirable traits. QTL mapping studies were performed in pea for many characteristics using maps constructed with molecular markers [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Dirlewanger et al. [16] identified a QTL for earliness on chromosome 6 (LG2) in the F2 population of pea. Prioul et al. [19] discovered three QTLs for days to flowering on LGs 2, 3 and 6 in RILs, derived from intraspecific crosses. Timmerman-Vaughan et al. [20] identified four QTLs for days to flowering on LGs 1, 2b, 3 and 5b in A26 × Rovar population and three QTLs on LGs 1, 3 and 5b in A88 × Rovar population. Fondevilla et al. [23] detected four QTLs for earliness on LG2, LG3, and LG6 in RILs, derived from the cross between P. sativum subsp. syriacum and P. sativum. Furthermore, four QTLs were determined for days to flowering on LGs 3, 4 and 5 in interspecific crosses between P. sativum and P. fulvum by Jha et al. [28]. Huang et al. [30] identified three QTLs for days to flowering on LGs 2, 3 and 6b in intraspecific crosses.
There are also important QTL studies on pea seed quality. QTLs were determined in Va and Vb of the linkage map for seed protein content [31]. In addition to important minerals such as Ca, Fe, K, Mg, Mn, Mo, and P [32], QTLs of starch, fiber, and phytate contents [33] in pea were also defined. Various QTL mapping studies have been performed for agro-morphological traits of pea. The majority of QTLs for plant height were determined on LG3 [18,19,28,33,34,35,36,37]. Furthermore, several QTLs were identified for morphological traits, like number of pods per plant, number of seeds per pod, pod size, biological yield, seed yield and harvest index on all seven linkage groups [31,38,39,40,41]. However, because quantitative traits are polygenic and influenced by environmental conditions, it may be scattered in linkage groups. The QTL studies using plant populations derived from interspecific crosses in pea are very limited [28,42].
Promising interspecific crosses of pea were previously reported to have a potential for further improvement of super-earliness [43]. Thus, the objectives of this study were to: (i) generate a genetic linkage map of a pea recombinant inbred line (RIL) population derived from a cross between P. sativum (♀) and P. fulvum (♂) × (ii) discover novel QTLs associated with super-earliness and important agro-morphological traits.
2. Materials and Methods
2.1. Plant Material
A total of 114 F4 recombinant inbred lines (RILs) derived from the P. sativum × P. fulvum interspecific crosses were used as the plant material of the present study. The parents were selected based on contrasting or significant differences in morphological and phenological characteristics. The female parent, ACP 20 (P. sativum), is a large, wrinkled and cream color-seeded and early-flowered genotype, while the male parent, AWP 600 (P. fulvum), is a small, smooth and black color-seeded and late-flowered genotype [43].
ACP 20 is a landrace from Antalya, Turkey, whereas AWP 600 originated in Turkey and was obtained from USDA GRIN in the United States. Each recombinant inbred line was advanced as five seeds after the F2 population. That is, from F3 to F4, each line was advanced as a family consisting of five individuals. Both parents and RILs were evaluated in the years 2019 and 2020 under glasshouse conditions.
2.2. Phenotyping
Phenotyping for QTL was recorded on 12 characteristics, namely as flower color (FC), days to flowering (DF; days), days to pod setting (DP; days), plant height (PH; cm), first pod height (FH; cm), internode length (IN; cm), number of pods per plant (PP), number of seeds per pod (SP), pod length (PL; cm), biological yield per plant (BY; g), seed yield per plant (SY; g) and harvest index per plant (HI; %). Phenotyping was recorded in parents and F4 lines derived from interspecific crosses P. sativum × P. fulvum. Phenotyping was evaluated by averaging the five families grown on F4 lines for each characteristic. DF was recorded as the number of days after germination until the first flowering. DP was recorded as the number of days after germination until the first pod setting. PH and FH were recorded in cm as the height of a plant from the ground to the top of the plant and as the height from ground to the first pod, respectively. Internode length (IN) was measured at the distance between two stipules with a ruler. PP and SP were recorded as the total number of pods per plant and seeds per pod, respectively. PL was recorded in cm as the length of a pod. BY was recorded in grams (g) as the total weight of a plant after harvest, while SY was recorded in g as the weight of seeds per plant after harvest. HI was calculated in percentage (%), as the ratio of SY to BY multiplied by 100. For PL, three randomly selected pods of each plant were used and SP of the same pods was recorded.
2.3. Genotyping
DNA isolation was carried out according to the CTAB method developed by Doyle and Doyle [44] using young leaves. In order to create the genetic map and determine the QTLs, a total of 70 SSR markers, 10 from each linkage group (LG), were selected from the SSR markers mapped by Loridon et al. [21]. Forty-five SSRs markers showing polymorphism in both the female and the male parent were used in this study. Some information about polymorphic markers is presented in Table 1.

Table 1.
List of SSRs primers used for this study with primer sequences.
The polymerase chain reaction (PCR) mix was prepared in a total volume of 15.47 µL, 1.5 µL dNTP, 1.25 µL MgCl2, 1.5 µL PCR buffer, 0.1 µL Taq DNA polymerase, 7.62 µL ultrapure water, 1 µL F primer, 1 µL R primer and 1.5 µL DNA for each sample. In PCR amplification, initial denaturation at 95 °C for 3 min, then at 94 °C for 50 s, at 45–55 °C for 40 s, at 72 °C for 50 s, final extension after 35 cycles at 72 °C for 5 min were completed. After the PCR amplification was completed, 3% agarose gel electrophoresis was used to visually examine and score the separation of the formed bands based on the size differences of the obtained PCR products. A 1 kB plus marker (Thermo Scientific GeneRuler 1kB plus DNA Ladder) was used to determine product sizes (molecular weights). The electrophoresis tank, which is connected to the power source, was run at 75 volts for about 100 min and the bands were separated from each other.
Of the F4 RILs, those with the same band size as the female parent were scored as “A”, those with the same band size as the male parent as “B” and those with both parent bands were scored as “H”. Lines that did not show bands were scored as “-”.
2.4. Genetic Mapping
The genetic linkage map was created using the Join-Map 4.1 software [45]. Markers were assigned to linkage groups (LGs) with a LOD greater than 3 using the Kosambi map method of Join-Map 4.1. The linkage groups identified in this study were aligned to seven pea chromosomes based on common markers in the pea genetic map previously reported [21].
2.5. QTL Analyses
The QGene software was used for QTL analysis of days to flowering, days to pod setting, plant height, first pod height, internodes, number of pods per plant, number of seeds per pod, pod length, biological yield, seed yield and harvest index. Using the Composite Interval Mapping (CIM) method, the QTL was determined for each quantitative trait with LOD > 3. MapChart 2.0 [46] software was used to mark the determined QTLs on the created genetic map.
Gene action was calculated by dividing the absolute value of the estimated dominance effect (|d|) by the absolute value of the estimated additive effect (|a|) [47]. |d|/|a| = 0–0.20 additive (A); partial dominance (PD) between 0.21–0.80; dominance (D) between 0.81–1.20; and >1.20 have been classified as over-dominance (OD).
3. Results
3.1. Phenotypic Characteristics
Days to flowering was recorded as 53 days for P. sativum and 117 days for P. fulvum, while the earliest lines flowered 24 days after germination in the F4 population. Days to pod setting was 62 days for P. sativum and 128 days for P. fulvum. On the other hand, the earliest lines formed pods in 30 days in the F4 population (Table 2). Plant height for P. sativum and P. fulvum was 90 and 41 cm, respectively, whereas it varied between 15 to 266.7 cm for the F4 population. The number of pods per plant was 11 for P. sativum and 23 for P. fulvum, ranging between 2 to 197 in the F4 population. Pod length was 10 cm for P. sativum and 4 cm for P. fulvum, while it was between 3 to 9 cm in F4 lines. The number of seeds per pod in P. sativum and P. fulvum was seven and three, respectively, and it was between one to seven in F4 lines. The harvest index was 47% for P. sativum and 15% for P. fulvum, whereas it ranged from 1% to 58% in F4 lines (Table 2). Distributions of parents and lines for each characteristic are presented in Figure 1.

Table 2.
Minimum (Min) and maximum (Max) values, means ( ) and ± standard errors ( ) for phenological and morphological traits in parents and F4 population originated from interspecific crosses P. sativum × P. fulvum.
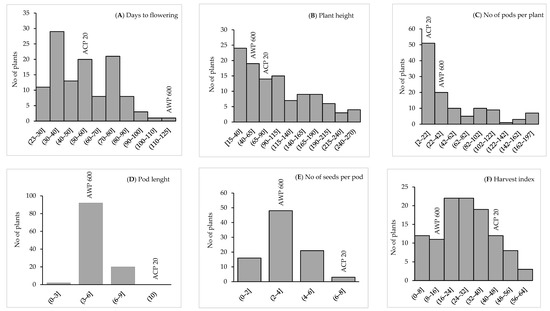
Figure 1.
Frequency distribution of (A) days to flowering, (B) plant height, (C) no of pods per plant, (D) pod length, (E) no of seeds per pod, and (F) harvest index in the ACP 20 (P. sativum), AWP 600 (P. fulvum) and F4 population derived from interspecific crosses between P. sativum × P. fulvum.
3.2. Genetic Mapping
A population of 114 F4 RILs obtained from P. sativum × P. fulvum interspecific crosses was screened with 70 codominant SSR markers. Of these 70 SSRs, 45 of them showed polymorphism between parents (Figure 2). After each marker was scored on the population, the markers with a LOD greater than three were selected and a genetic linkage map was generated, resulting seven linkage groups. The 44 SSR markers were mapped on LGs. The total length of the map is 262.6 cM, with an average marker resolution of 5.9 cM (Table 3). The number, names and resolution of the SSR markers for each LG are given in Table 3.
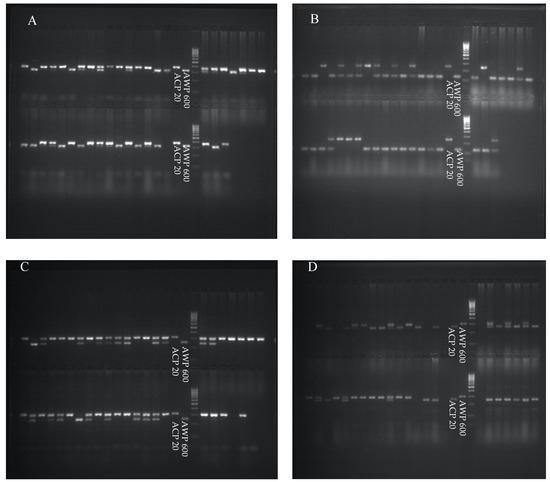
Figure 2.
Agarose gel images of markers (A) AA67, (B) AD148, (C) AD270, and (D) AA285 between parents and F4 lines derived from interspecific crosses involving P. sativum × P. fulvum.

Table 3.
Linkage group (LG), number of SSR markers, length (cM) and average marker distance (cM) of the linkage groups developed with 44 SSRs using F4 lines involving P. sativum × P. fulvum.
3.3. QTLs Analyses
A total of 14 QTLs on five different linkage groups were determined for earliness and important agro-morphological traits. Two QTLs were determined on LG2 for flowering time. The first QTL FLO2.1 had a LOD value of 3.6. The AA205 marker, which explained 14% of the phenotypic variance, was the closest to the QTL (Table 4). The second QTL FLO2.2 explained 14% of the phenotypic variance with a LOD of 3.2. The marker AA372.1 was the closest to the QTL (Figure 3). FLO2.1 and FLO2.2 showed a dominance/additive (d/a) ratio of 1.06 and 0.71, indicating a dominant and partially dominant gene action, respectively (Table 4). The QTLs for flowering time explained 28% of the total phenotypic variance.

Table 4.
QTLs detected for days to flowering, plant height, no. of pods per plant, no. of seeds per pod, pod length and harvest index under glasshouse conditions.
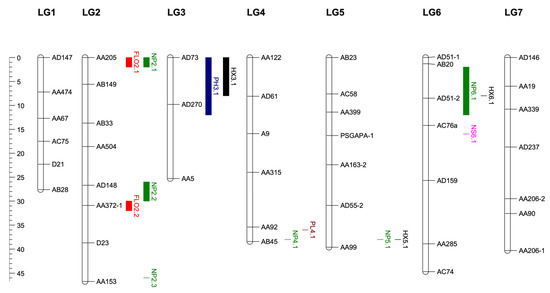
Figure 3.
QTLs mapping for FLO: days to flowering, PH: plant height, NP: number of pods per plant, NS: number of seeds per pod, PL: pod length, HX: harvest index in 114 RILs developed from interspecific crosses between P. sativum and P. fulvum.
One QTL for plant height was identified on LG3 with a LOD value of 4.35. Two flanking markers were determined for PH3.1 QTL (Figure 3). The QTL flanked by markers AD73 and AD270 explained 16% of phenotypic variation. PH3.1 showed d/a ratio of 1.65, indicating an over-dominance gene action (Table 4).
Six QTLs were determined for the number of pods per plant, one of the most important yield components. Three QTLs were mapped on LG2, one on LG4, one on LG5 and one on LG6. The NP2.1, NP2.2 and NP2.3 on LG2 were mapped with LOD values of 4.3, 3.5 and 3.6, respectively (Figure 3, Table 4). The NP2.1 and NP2.2 explained 16% and 13% of the phenotypic variation, respectively.
The NP2.3, NP4.1 and NP5.1 explained 13%, 14% and 14% of the variation (Table 4, Figure 3). The NP6.1 QTL associated with the number of pods per plant explained 17% of the phenotypic variation (Table 4). The six QTL determined for the number of pods per plant within the scope of this study explained a total of 87% of the variation. NP2.1, NP2.2, NP5.1 and NP 6.1 had over dominance gene action. The HX3.1 QTL associated with the harvest index explained 13% of the phenotypic variation (Table 4). The second QTL (HX5.1) explained 12% of the phenotypic variation for the same trait. The third QTL (HX6.1) on LG6 explained 12% of the phenotypic variation. The three QTLs for harvest index explained 37% of the total phenotypic variation. The PL4.1 QTL associated with the pod length explained 12% of the phenotypic variation (Figure 3, Table 4). The NS6.1 associated with the number of seeds per pod explained 13% of the phenotypic variation (Table 4).
4. Discussion
A total of 114 F4 RILs derived from P. sativum × P. fulvum interspecific crosses were used for phenotyping and genotyping. The 70 SSR markers were selected from the genetic map created by Loridon et al. [21]. The 45 SSRs showed parental polymorphisms. The linkage map with seven linkage groups was created using 44 SSRs with a LOD greater than 3.0 using the Kosambi function. Each LG represents a pea chromosome and the total map length was 262.6 cM with an average marker resolution of 5.9 cM (Table 3). The pea genetic map with the highest number of SSR markers was reported by Loridon et al. [21] with 239 polymorphic markers. In this study, a genetic linkage map was created by using of the RIL population developed from interspecific crossing. Common markers used in both studies are indicative of cross-population transferability.
Days to flowering, days to pod setting, plant height, first pod height, internode, number of pods per plant, number of seeds per pod, pod length, biological yield, seed yield and harvest index were evaluated to determine QTLs in this study. The evaluated characteristics are important targets for pea breeding. Of these characteristics, 14 QTLs were determined for a total of six traits: days to flowering, plant height, number of pods per plant, number of seeds per pod, pod length and harvest index. In the QTL analyses, composite interval mapping (CIM) was used. The F4 RIL population of 114 individuals derived from P. sativum × P. fulvum interspecific crosses was used to determine the QTLs. In a study comparing RIL populations for QTL detection, it was concluded that the F4 RIL population may be as effective as the F6-7 populations [48].
Flowering time is one of the main determinants of adaptation to different ecological and geographical regions. Early-flowering genotypes in pea play an important role in minimizing bottlenecks such as abiotic and biotic stresses. There are growing global concerns about the impact of climate change on food production, livelihoods and food security [49,50]. Global warming is thought to harm agricultural production and is one of the most serious threats to food supply. The second threat is the increasing world population, estimated at 8 billion by 2030, which will require a 60% increase in current food production [51,52]. The majority of the world’s population lives in cities, and considering the reasons for migration from rural areas to cities, it is inevitable that the consumption rate will create even more food deficits [50]. According to the data of the International Panel on Climate Change (IPCC), global warming will exceed 1.5 °C by 2030, causing permanent loss of the most sensitive ecosystems. It is thought to cause a crisis for societies in underdeveloped and developing countries. Super-early individuals from the previous study could escape high temperature stress, while late-maturing individuals were exposed to heat stress during the flowering and pod setting periods [43]. The earliest lines in the F4 population flowered 24 days after germination, while P. sativum flowered in 53 days and P. fulvum flowered in 117 days (Table 2). P. sativum required 62 days to reach pod setting, while P. fulvum required 128 days. In the F4 population, the earliest lines developed pods in 30 days (Figure 1). In a previous study, the earliest days to flowering in the F2 and F3 generations of the same population were 17 and 13 days under short-days, respectively [43].
More than 20 loci related to flowering time and flowering development had been identified in pea and the interactions of these loci determined flowering time. Late-flowering (Lf) [53], high-response (Hr), sterile nodes (Sn), early (E), photoperiod (Ppd) [54] and die Neutralis (Dne) loci are the most important ones [55,56,57]. The Ppd and Lf loci were mapped on LG2 [54,56], while the Hr and Dne loci were mapped on LG3 [58,59]. In this study, two QTLs associated with the markers AA205 and AA372.1 were found for flowering time, which are in the same linkage group (on LG2) as the Ppd and Lf loci. Guindon et al. [40] reported that seed diameter and seed weight characteristics were associated with the AA205 marker in peas. Three genomic regions controlling flowering time were identified on LG2, LG3, and LG6 by Prioul et al. [19]. QTL flo1 was mapped on LG2, the same linkage group as the QTL found in this study, contributing most of the variation [19]. QTL determined on LG2 was associated with the AB33 marker. The marker flanking the AB33 marker was AA372.1 and it was linked with the FLO2.2 determined in this study (Table 3). In addition, QTL flo2 was mapped on LG3 and QTL mpIII-3 was reported to be in the same region with the pea blight resistance QTL. Resistance alleles in the blight resistance-related QTLs had been associated with alleles that delay flowering time [19]. Burstin et al. [60] mapped one QTL in 49 cM of LGV where the Det gene is located for flowering time. Foucher et al. [61] reported that the Det gene played a role in the regulation of flowering time. Fondevilla et al. [62] determined two QTLs on LG3 for earliness in pea. In addition, it was reported that the QTLs were close to the AB64 and AA175 markers. QTL was mapped for earliness in pea on LG2 by Dirlewanger et al. [16]. Jha et al. [28] identified four QTLs for flowering time at LGs 3, 4 and 5. In a recent study, three QTLs, two on LG1 and one on LG2, were mapped for flowering time in F2 and F3 populations obtained from DDR14 and Explorer intraspecific crosses [40]. Fondevilla et al. [23] defined QTLs for flowering time on LG6 and LG3. QTL on LG3 determined by Fondevilla et al. [23] was reported to be related to earliness in the study by Timmerman-Vaughan et al. [20]. Although QTLs determined for flowering time in the previous studies were close to AA205 and AA372.1 markers, it was not directly related. QTL studies on flowering time in peas are limited and two more new QTLs were found on LG2 with this study.
Major and minor QTLs have been identified for plant height in peas in previous studies. Tar’an et al. [18] determined three main QTLs with a total variation of 64.6% and Hamon et al. [34] identified three minor QTLs on LG3. Three QTLs were determined for plant height in LG2, LG3, and LG7 by Prioul et al. [19] and it was emphasized that the QTL on LG3 explained 63% of the variation. Gali et al. [36] identified a major QTL for plant height on LG3, explaining 33–65% of the phenotypic variance in the three RIL populations. Also, Ferrari et al. [35] mapped QTL for plant height on LG3. Gali et al. [33] identified four loci on LG3 associated with plant height using the GWAS (genome-wide association) method. Guindon et al. [40] found QTL on LG2 for plant height. In one study, two QTLs were found, one QTL on LG3 and one QTL on LG5 [37]. In Jha et al. [28], in which quantitative loci of blight disease in pea were studied, five QTLs associated with plant height were identified. Three of the QTLs were positioned on LG3, LG4 and LG7. Although QTLs were found in different linkage groups related to plant height in previous studies, the majority of QTLs that explain the phenotypic variation were identified on LG3, as reported in this study.
Previous studies using RIL populations have identified multiple QTLs associated with the number of pods per plant in more than one linkage group. For the number of pods, a total of five QTLs were determined on LG1, LG2, LG3, LG5 and LG6 [38]. Guindon et al. [40] detected a QTL in the LG1 for the same trait. Sadras et al. [41] determined the QTL in LG2 for the number of pods per m2. QTL was determined for the number of seeds per pod on LG2 in an RIL population [31]. Two QTLs were identified for the number of seeds per pod on LG1 by Guindon et al. [40]. Timmerman-Vaughan et al. [39] determined a total of seven QTLs on LG1, LG2, LG3, LG4 and LG7 linkage groups for the number of seeds per m2. Sadras et al. [41] found QTL on LG2 and LG3 for the number of seeds per pod. In addition, it was reported that the QTLs of flowering time and yield components were mostly on LG2 [41]. Four QTLs were identified, explaining 40% of the total phenotypic variation for number of seeds per plant by Timmerman-Vaughan et al. [39]. Two of these QTLs were mapped on LG3, one on LG1 and one on LG2 [39]. A QTL (PL4.1) associated with the pod length was detected in LG4 with a LOD value of 3.08 (Table 1). It explained 12% of the phenotypic variation. The closest marker to the PL4.1 QTL was AA92 and its position on the map was 36 cM (Figure 3). One QTL for pod size was mapped on LG2 [40].
Another QTL mapped in this study was the harvest index. The HX3.1 QTL explained 13%, and HX5.1 and HX6.1 QTL each explained 12% of the phenotypic variation. The three QTLs explained a total of 37% of the phenotypic variation (Table 4). In a similar study, four QTLs were identified that explained a total of 40% of phenotypic variation. Two of these were mapped on LG3, one on LG1 and one on LG2 [39]. Yield components can be included in many linkage groups due to the characteristics that are easily affected by the environment and the populations.
QTL studies were carried out in pea for various characteristics. In addition to physiological traits, yield and yield components, QTL studies were also conducted against biotic and abiotic stress factors. In a study investigating the relationship between lodging resistance and plant height in pea, lodging resistance was mapped on LG3 [18], and in pea blight disease resistance on LG2, LG3, LG5 and LG6 [19,28], seed color, grain weight, grain yield, biological yield, protein content, broomrape resistance and powdery mildew resistance QTLs were reported in all seven linkage groups for many characteristics [22,63,64,65,66]. Studying QTLs mapped in the same linkage group in future studies may strengthen the functionality of the markers used.
The super-earliness character has a high heritability in this interspecific population [43] and thus MAS can be employed to transfer it into cultivated types.
In conclusion, a 262.6 cM long genetic map was constructed with 44 SSRs markers. A total of 14 QTLs were mapped, two QTLs for super-earliness on LG2, one for plant height on LG3, six QTLs for number of pods per plant on LG2, LG4, LG5 and LG6, one for number of seeds per pod on LG6, one for pod length on LG4 and three for harvest index on LG3, LG5, and LG6. The SSR markers AA205 and AA372-1 flanking super-earliness QTLs can potentially contribute significantly to future marker-assisted pea breeding programs.
Author Contributions
Conceptualization, H.S. and C.T.; methodology, H.S., N.M. and I.C.; software, H.S. and I.C.; validation, T.E. and H.S.T.; formal analysis, H.S., C.T. and N.M.; investigation, H.S., T.E. and H.S.T.; writing—original draft preparation, H.S. and C.T.; writing—review and editing, H.S., C.T., N.M. and I.C.; visualization, H.S. and T.E.; supervision, C.T.; project administration, C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Akdeniz University, grant number FDK-2020-5257.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davis, P.H. Flora of Turkey and the East Aegean Islands.; Davis, P.H., Ed.; University Press: Edinburgh, Scotland, 1970; Volume 3. [Google Scholar]
- Ladizinsky, G.; Abbo, S. The Search for Wild Relatives of Cool Season Legumes; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Smỳkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N. Legume Crops Phylogeny and Genetic Diversity for Science and Breeding. Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar] [CrossRef]
- Mendel, G. Experiments in Plant Hybridization (1865). In Verhandlungen Naturforschenden Ver. Brünn Available Online; 1996; Available online: http://old.esp.org/foundations/genetics/classical/gm-65-a.pdf (accessed on 20 December 2022).
- FAOSTAT. Food and Agriculture Organisation Statistics Database. Available online: https://search.library.wisc.edu/database/UWI12320 (accessed on 20 December 2022).
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the Health Benefits of Peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Smỳkal, P.; Aubert, G.; Burstin, J.; Coyne, C.J.; Ellis, N.T.; Flavell, A.J.; Ford, R.; Hỳbl, M.; Macas, J.; Neumann, P. Pea (Pisum sativum L.) in the Genomic Era. Agronomy 2012, 2, 74–115. [Google Scholar] [CrossRef]
- Toker, C.; Yadav, S.S. Legumes Cultivars for Stress Environments. In Climate change and management of cool season grain legume crops; Springer: Berlin/Heidelberg, Germany, 2010; pp. 351–376. [Google Scholar]
- Blum, A. Plant Breeding for Stress Environments; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Toker, C.; Lluch, C.; Tejera, N.A.; Serraj, R.; Siddique, K.H.M. 23 Abiotic Stresses. Chickpea Breed. Manag. 2007, 474. Available online: https://www.researchgate.net/profile/Cengiz-Toker/publication/285964620_Conventional_breeding_methods/links/5b19399a45851587f2987c0c/Conventional-breeding-methods.pdf#page=500 (accessed on 20 December 2022).
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.C.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R. Global Warming of 1.5 °C: Summary for Policy Makers; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Jiang, Y.; Lindsay, D.L.; Davis, A.R.; Wang, Z.; MacLean, D.E.; Warkentin, T.D.; Bueckert, R.A. Impact of Heat Stress on Pod-Based Yield Components in Field Pea (Pisum sativum L.). J. Agron. Crop Sci. 2020, 206, 76–89. [Google Scholar] [CrossRef]
- Parihar, A.K.; Dixit, G.P.; Bohra, A.; Sen Gupta, D.; Singh, A.K.; Kumar, N.; Singh, D.; Singh, N.P. Genetic Advancement in Dry Pea (Pisum sativum L.): Retrospect and Prospect. In Accelerated Plant Breeding, Volume 3; Springer: Berlin/Heidelberg, Germany, 2020; pp. 283–341. [Google Scholar]
- Tafesse, E.G.; Gali, K.K.; Lachagari, V.R.; Bueckert, R.; Warkentin, T.D. Genome-Wide Association Mapping for Heat Stress Responsive Traits in Field Pea. Int. J. Mol. Sci. 2020, 21, 2043. [Google Scholar] [CrossRef]
- Kreplak, J.; Madoui, M.-A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A Reference Genome for Pea Provides Insight into Legume Genome Evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Dirlewanger, E.; Isaac, P.G.; Ranade, S.; Belajouza, M.; Cousin, R.; De Vienne, D. Restriction Fragment Length Polymorphism Analysis of Loci Associated with Disease Resistance Genes and Developmental Traits in Pisum sativum L. Theor. Appl. Genet. 1994, 88, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Irzykowska, L.; Wolko, B.; Swiecicki, W.K. Interval Mapping of QTLs Controlling Some Morphological Traits in Pea. Cell. Mol. Biol. Lett. 2002, 7, 417–422. [Google Scholar]
- Tar’an, B.; Warkentin, T.; Somers, D.J.; Miranda, D.; Vandenberg, A.; Blade, S.; Woods, S.; Bing, D.; Xue, A.; DeKoeyer, D. Quantitative Trait Loci for Lodging Resistance, Plant Height and Partial Resistance to Mycosphaerella Blight in Field Pea (Pisum sativum L.). Theor. Appl. Genet. 2003, 107, 1482–1491. [Google Scholar] [CrossRef]
- Prioul, S.; Frankewitz, A.; Deniot, G.; Morin, G.; Baranger, A. Mapping of Quantitative Trait Loci for Partial Resistance to Mycosphaerella Pinodes in Pea (Pisum sativum L.), at the Seedling and Adult Plant Stages. Theor. Appl. Genet. 2004, 108, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Timmerman-Vaughan, G.M.; Frew, T.J.; Butler, R.; Murray, S.; Gilpin, M.; Falloon, K.; Johnston, P.; Lakeman, M.B.; Russell, A.; Khan, T. Validation of Quantitative Trait Loci for Ascochyta Blight Resistance in Pea (Pisum sativum L.), Using Populations from Two Crosses. Theor. Appl. Genet. 2004, 109, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Loridon, K.; McPhee, K.; Morin, J.; Dubreuil, P.; Pilet-Nayel, M.-L.; Aubert, G.; Rameau, C.; Baranger, A.; Coyne, C.; Lejeune-Henaut, I. Microsatellite Marker Polymorphism and Mapping in Pea (Pisum sativum L.). Theor. Appl. Genet. 2005, 111, 1022–1031. [Google Scholar] [CrossRef]
- Pilet-Nayel, M.-L.; Muehlbauer, F.J.; McGee, R.J.; Kraft, J.M.; Baranger, A.; Coyne, C.J. Consistent Quantitative Trait Loci in Pea for Partial Resistance to Aphanomyces Euteiches Isolates from the United States and France. Phytopathology 2005, 95, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Fondevilla, S.; Satovic, Z.; Rubiales, D.; Moreno, M.T.; Torres, A.M. Mapping of Quantitative Trait Loci for Resistance to Mycosphaerella Pinodes in Pisum sativum subsp. syriacum. Mol. Breed. 2008, 21, 439–454. [Google Scholar] [CrossRef]
- Dumont, E.; Fontaine, V.; Vuylsteker, C.; Sellier, H.; Bodèle, S.; Voedts, N.; Devaux, R.; Frise, M.; Avia, K.; Hilbert, J.-L. Association of Sugar Content QTL and PQL with Physiological Traits Relevant to Frost Damage Resistance in Pea under Field and Controlled Conditions. Theor. Appl. Genet. 2009, 118, 1561–1571. [Google Scholar] [CrossRef]
- Fondevilla, S.; Fernández-Aparicio, M.; Satovic, Z.; Emeran, A.A.; Torres, A.M.; Moreno, M.T.; Rubiales, D. Identification of Quantitative Trait Loci for Specific Mechanisms of Resistance to Orobanche Crenata Forsk. in Pea (Pisum sativum L.). Mol. Breed. 2010, 25, 259–272. [Google Scholar] [CrossRef]
- Ubayasena, L.; Bett, K.; Tar’an, B.; Vijayan, P.; Warkentin, T. Genetic Control and QTL Analysis of Cotyledon Bleaching Resistance in Green Field Pea (Pisum sativum L.). Genome 2010, 53, 346–359. [Google Scholar] [CrossRef]
- Feng, J.; Hwang, R.; Chang, K.F.; Conner, R.L.; Hwang, S.F.; Strelkov, S.E.; Gossen, B.D.; McLaren, D.L.; Xue, A.G. Identification of Microsatellite Markers Linked to Quantitative Trait Loci Controlling Resistance to Fusarium Root Rot in Field Pea. Can. J. Plant Sci. 2011, 91, 199–204. [Google Scholar] [CrossRef]
- Jha, A.B.; Tar’an, B.; Stonehouse, R.; Warkentin, T.D. Identification of QTLs Associated with Improved Resistance to Ascochyta Blight in an Interspecific Pea Recombinant Inbred Line Population. Crop Sci. 2016, 56, 2926–2939. [Google Scholar] [CrossRef]
- Timmerman-Vaughan, G.M.; Moya, L.; Frew, T.J.; Murray, S.R.; Crowhurst, R. Ascochyta Blight Disease of Pea (Pisum sativum L.): Defence-Related Candidate Genes Associated with QTL Regions and Identification of Epistatic QTL. Theor. Appl. Genet. 2016, 129, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gali, K.K.; Tar’an, B.; Warkentin, T.D.; Bueckert, R.A. Pea Phenology: Crop Potential in a Warming Environment. Crop Sci. 2017, 57, 1540–1551. [Google Scholar] [CrossRef]
- Krajewski, P.; Bocianowski, J.; Gaw\lowska, M.; Kaczmarek, Z.; Pniewski, T.; Święcicki, W.; Wolko, B. QTL for Yield Components and Protein Content: A Multienvironment Study of Two Pea (Pisum sativum L.) Populations. Euphytica 2012, 183, 323–336. [Google Scholar] [CrossRef]
- Ma, Y.; Coyne, C.J.; Grusak, M.A.; Mazourek, M.; Cheng, P.; Main, D.; McGee, R.J. Genome-Wide SNP Identification, Linkage Map Construction and QTL Mapping for Seed Mineral Concentrations and Contents in Pea (Pisum sativum L.). BMC Plant Biol. 2017, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Gali, K.K.; Sackville, A.; Tafesse, E.G.; Lachagari, V.R.; McPhee, K.; Hybl, M.; Mikić, A.; Smỳkal, P.; McGee, R.; Burstin, J. Genome-Wide Association Mapping for Agronomic and Seed Quality Traits of Field Pea (Pisum sativum L.). Front. Plant Sci. 2019, 10, 1538. [Google Scholar] [CrossRef]
- Hamon, C.; Coyne, C.J.; McGee, R.J.; Lesné, A.; Esnault, R.; Mangin, P.; Hervé, M.; Le Goff, I.; Deniot, G.; Roux-Duparque, M. QTL Meta-Analysis Provides a Comprehensive View of Loci Controlling Partial Resistance to Aphanomyces Euteichesin Four Sources of Resistance in Pea. BMC Plant Biol. 2013, 13, 1–19. [Google Scholar] [CrossRef]
- Ferrari, B.; Romani, M.; Aubert, G.; Boucherot, K.; Burstin, J.; Pecetti, L.; Huart, M.; Klein, A.; Annicchiarico, P. Association of SNP Markers with Agronomic and Quality Traits of Field Pea in Italy. Czech J. Genet. Plant Breed. 2016, 52, 83–93. [Google Scholar] [CrossRef]
- Gali, K.K.; Liu, Y.; Sindhu, A.; Diapari, M.; Shunmugam, A.S.; Arganosa, G.; Daba, K.; Caron, C.; Lachagari, R.V.; Tar’an, B. Construction of High-Density Linkage Maps for Mapping Quantitative Trait Loci for Multiple Traits in Field Pea (Pisum sativum L.). BMC Plant Biol. 2018, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Gawlowska, M.; Knopkiewicz, M.; Święcicki, W.; Boros, L.; Wawer, A. Quantitative Trait Loci for Stem Strength Properties and Lodging in Two Pea Biparental Mapping Populations. Crop Sci. 2021, 61, 1682–1697. [Google Scholar] [CrossRef]
- Irzykowska, L.; Wolko, B. Interval Mapping of QTLs Controlling Yield-Related Traits and Seed Protein Content in Pisum sativum. J. Appl. Genet. 2004, 45, 297–306. [Google Scholar]
- Timmerman-Vaughan, G.M.; Mills, A.; Whitfield, C.; Frew, T.; Butler, R.; Murray, S.; Michael, L.; John, M.; Adrian, R.; Derek, W. Linkage Mapping of QTL for Seed Yield, Yield Components, and Developmental Traits in Pea. Crop Sci. 2005, 45, 1336–1344. [Google Scholar] [CrossRef]
- Guindon, M.F.; Martin, E.; Cravero, V.; Gali, K.K.; Warkentin, T.D.; Cointry, E. Linkage Map Development by GBS, SSR, and SRAP Techniques and Yield-Related QTLs in Pea. Mol. Breed. 2019, 39, 1–16. [Google Scholar] [CrossRef]
- Sadras, V.O.; Lake, L.; Kaur, S.; Rosewarne, G. Phenotypic and Genetic Analysis of Pod Wall Ratio, Phenology and Yield Components in Field Pea. Field Crop Res. 2019, 241, 107551. [Google Scholar] [CrossRef]
- Aryamanesh, N.; Zeng, Y.; Byrne, O.; Hardie, D.C.; Al-Subhi, A.M.; Khan, T.; Siddique, K.H.M.; Yan, G. Identification of Genome Regions Controlling Cotyledon, Pod Wall/Seed Coat and Pod Wall Resistance to Pea Weevil through QTL Mapping. Theor. Appl. Genet. 2014, 127, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Sari, H.; Sari, D.; Eker, T.; Toker, C. De Novo Super-Early Progeny in Interspecific Crosses Pisum sativum L. × P. fulvum Sibth. et Sm. Sci. Rep. 2021, 11, 19706. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Van Ooijen, J.W. Multipoint Maximum Likelihood Mapping in a Full-Sib Family of an Outbreeding Species. Genet. Res. 2011, 93, 343–349. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the Graphical Presentation of Linkage Maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Stuber, C.W.; Edwards, M.D.; Wendel, J.F. Molecular Marker-Facilitated Investigations of Quantitative Trait Loci in Maize. II. Factors Influencing Yield and Its Component Traits1. Crop Sci. 1987, 27, 639–648. [Google Scholar] [CrossRef]
- Takuno, S.; Terauchi, R.; Innan, H. The Power of QTL Mapping with RILs. PLoS ONE 2012, 7, e46545. [Google Scholar] [CrossRef]
- Onyekachi, O.G.; Bonifeca, O.O.; Gemlack, N.F.; Nicholas, N. Abiotic and Biotic Stress in Plants; BoD—Books on Demand: Norderstedt, Germany, 2019; ISBN 978-1-78923-811-2. [Google Scholar]
- Schiermeier, Q. Eat Less Meat: UN Climate-Change Report Calls for Change to Human Diet. Nature 2019, 572, 291–293. [Google Scholar] [CrossRef] [PubMed]
- FAO. The Future of Food and Agriculture–Trends and Challenges. Annual Report; Food Agriculture Organization: Rome, Italy, 2017; pp. 1–180. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition; Food & Agriculture Organization: Rome, Italy, 2018; ISBN 978-92-5-130571-3. [Google Scholar]
- White, O.E. Studies of Inheritance in Pisum. II. The Present State of Knowledge of Heredity and Variation in Peas. Proc. Am. Philos. Soc. 1917, 56, 487–588. [Google Scholar]
- Murfet, I.C. Flowering in Pisum. Three Distinct Phenotypic Classes Determined by the Interaction of a Dominant Early and a Dominant Late Gene. Heredity 1971, 26, 243–257. [Google Scholar] [CrossRef]
- King, W.M.; Murfet, I.C. Flowering in Pisum: A Sixth Locus, Dne. Ann. Bot. 1985, 56, 835–846. [Google Scholar] [CrossRef]
- Arumingtyas, E.L.; Murfet, I.C. Flowering in Pisum: A Further Gene Controlling Response to Photoperiod. J. Hered. 1994, 85, 12–17. [Google Scholar] [CrossRef]
- Weller, J.L.; Ortega, R. Genetic Control of Flowering Time in Legumes. Front. Plant Sci. 2015, 6, 207. [Google Scholar] [CrossRef]
- Lejeune-Hénaut, I.; Hanocq, E.; Béthencourt, L.; Fontaine, V.; Delbreil, B.; Morin, J.; Petit, A.; Devaux, R.; Boilleau, M.; Stempniak, J.-J.; et al. The Flowering Locus Hr Colocalizes with a Major QTL Affecting Winter Frost Tolerance in Pisum sativum L. Theor. Appl. Genet. 2008, 116, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.C.; Hecht, V.; Laurie, R.E.; Knowles, C.L.; Vander Schoor, J.K.; Macknight, R.C.; Weller, J.L. DIE NEUTRALIS and LATE BLOOMER 1 Contribute to Regulation of the Pea Circadian Clock. Plant Cell 2009, 21, 3198–3211. [Google Scholar] [CrossRef]
- Burstin, J.; Marget, P.; Huart, M.; Moessner, A.; Mangin, B.; Duchene, C.; Desprez, B.; Munier-Jolain, N.; Duc, G. Developmental Genes Have Pleiotropic Effects on Plant Morphology and Source Capacity, Eventually Impacting on Seed Protein Content and Productivity in Pea. Plant Physiol. 2007, 144, 768–781. [Google Scholar] [CrossRef]
- Foucher, F.; Morin, J.; Courtiade, J.; Cadioux, S.; Ellis, N.; Banfield, M.J.; Rameau, C. DETERMINATE and LATE FLOWERING Are Two TERMINAL FLOWER1/CENTRORADIALIS Homologs That Control Two Distinct Phases of Flowering Initiation and Development in Pea. Plant Cell 2003, 15, 2742–2754. [Google Scholar] [CrossRef]
- Fondevilla, S.; Almeida, N.F.; Satovic, Z.; Rubiales, D.; Vaz Patto, M.C.; Cubero, J.I.; Torres, A.M. Identification of Common Genomic Regions Controlling Resistance to Mycosphaerella pinodes, Earliness and Architectural Traits in Different Pea Genetic Backgrounds. Euphytica 2011, 182, 43–52. [Google Scholar] [CrossRef]
- Timmerman-Vaughan, G.M.; McCallum, J.A.; Frew, T.J.; Weeden, N.F.; Russell, A.C. Linkage Mapping of Quantitative Trait Loci Controlling Seed Weight in Pea (Pisum sativum L.). Theor. Appl. Genet. 1996, 93, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tar’an, B.; Warkentin, T.; Somers, D.J.; Miranda, D.; Vandenberg, A.; Blade, S.; Bing, D. Identification of Quantitative Trait Loci for Grain Yield, Seed Protein Concentration and Maturity in Field Pea (Pisum sativum L.). Euphytica 2004, 136, 297–306. [Google Scholar] [CrossRef]
- Valderrama, M.R.; Román, B.; Satovic, Z.; Rubiales, D.; Cubero, J.I.; Torres, A.M. Locating Quantitative Trait Loci Associated with Orobanche Crenata Resistance in Pea. Weed Res. 2004, 44, 323–328. [Google Scholar] [CrossRef]
- Fondevilla, S.; Torres, A.M.; Moreno, M.T.; Rubiales, D. Identification of a New Gene for Resistance to Powdery Mildew in Pisum fulvum, a Wild Relative of Pea. Breed. Sci. 2007, 57, 181–184. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).