Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus spp. Buds
Abstract
:1. Introduction
2. Results and Discussion
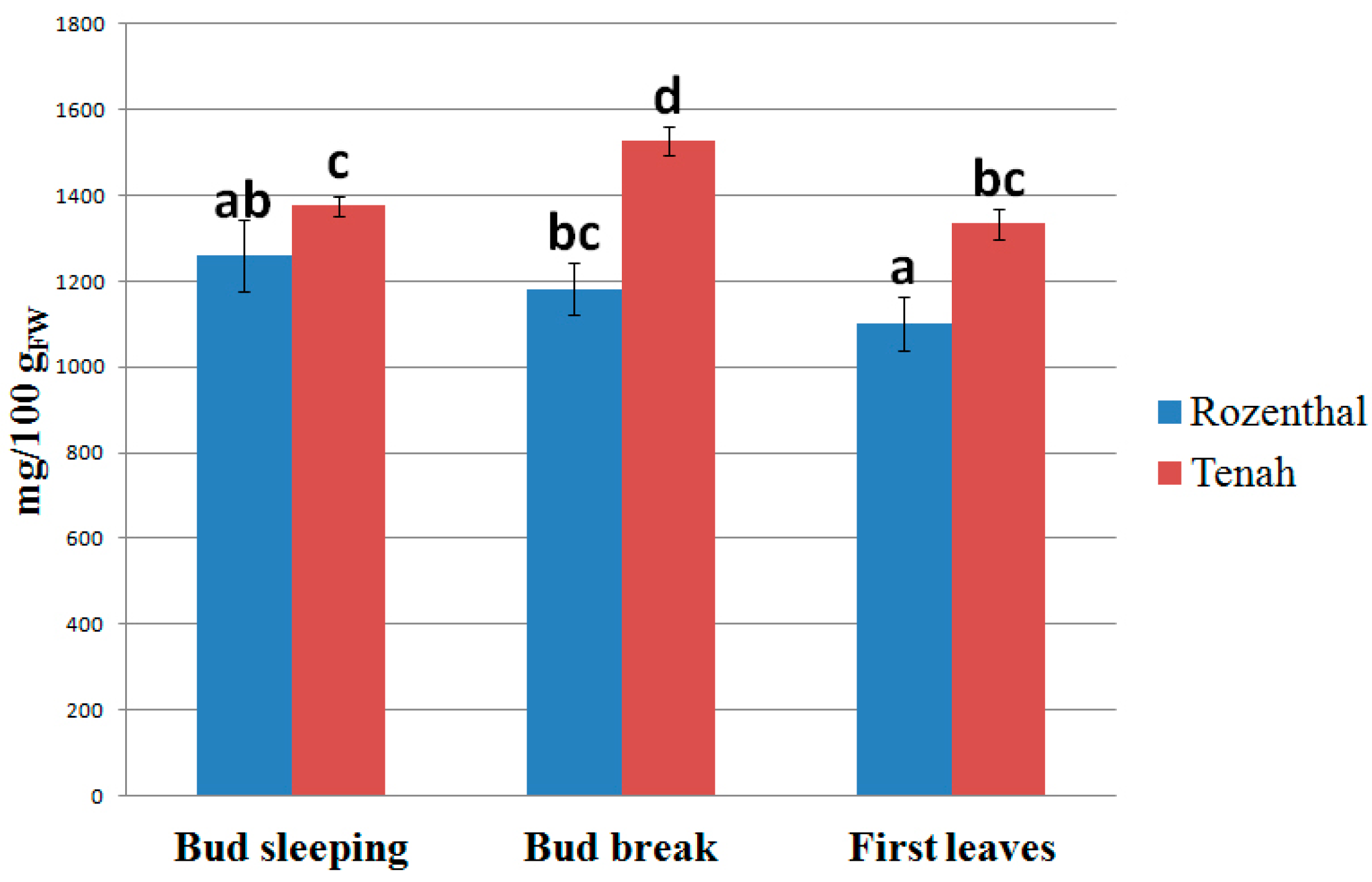
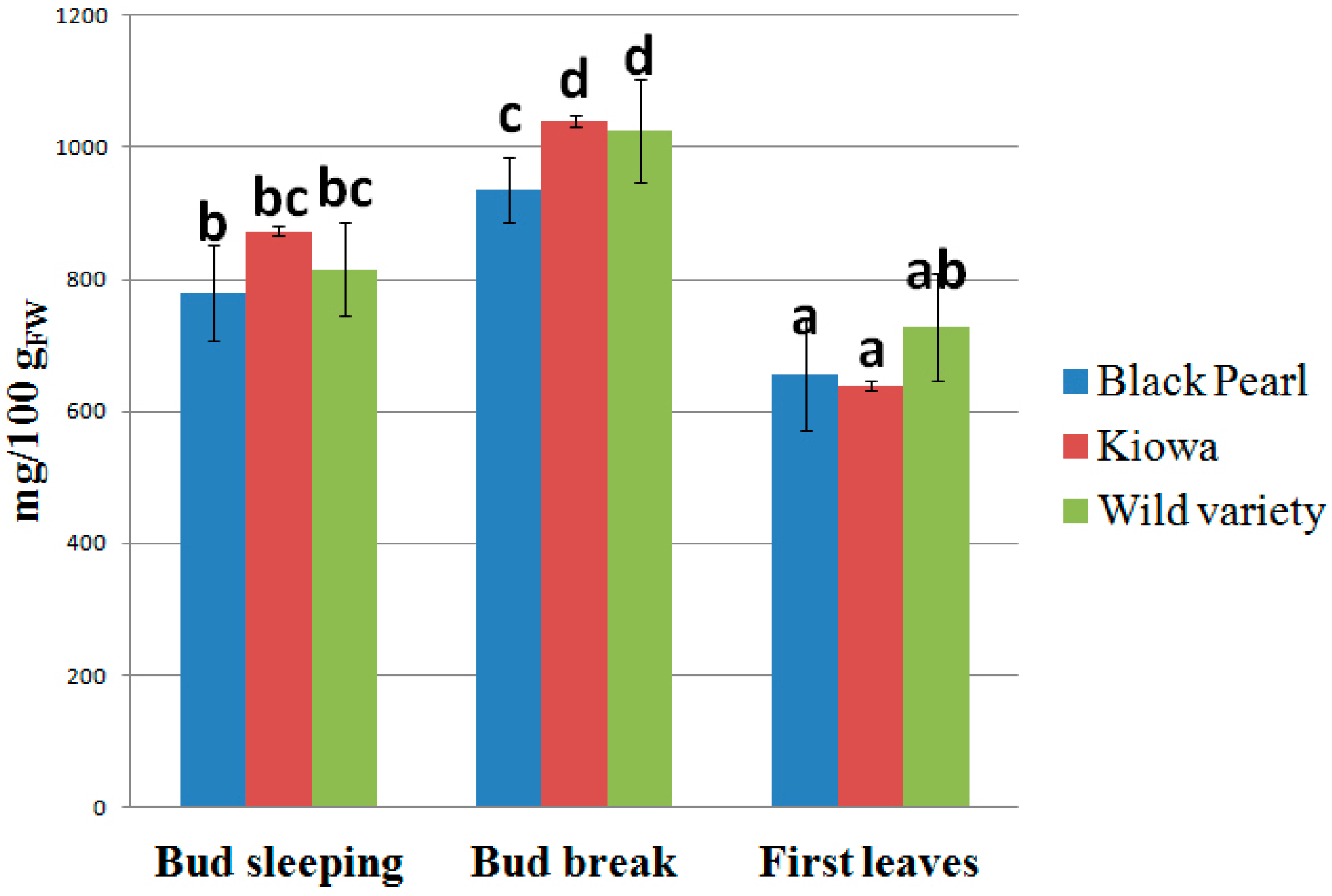
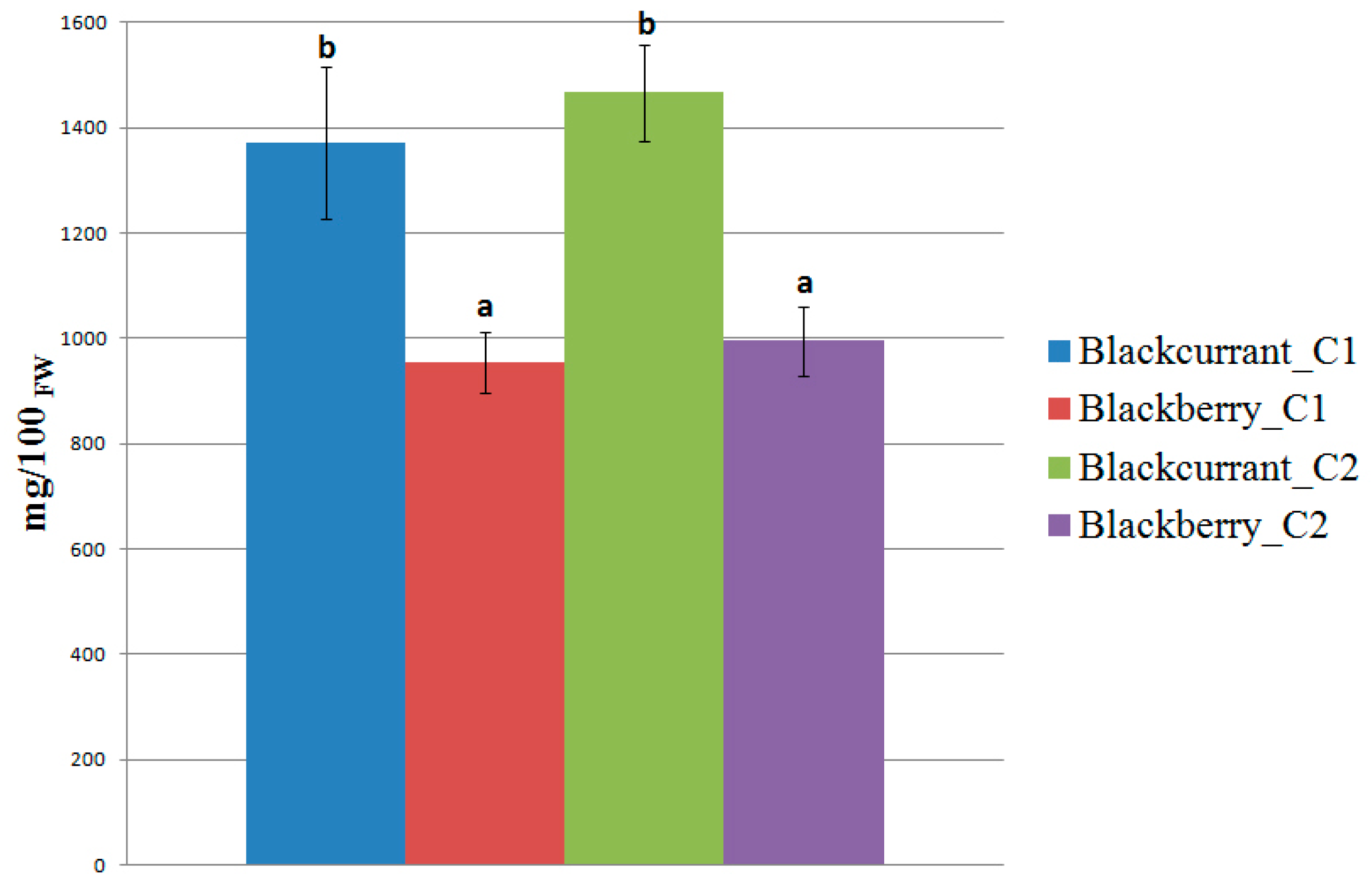
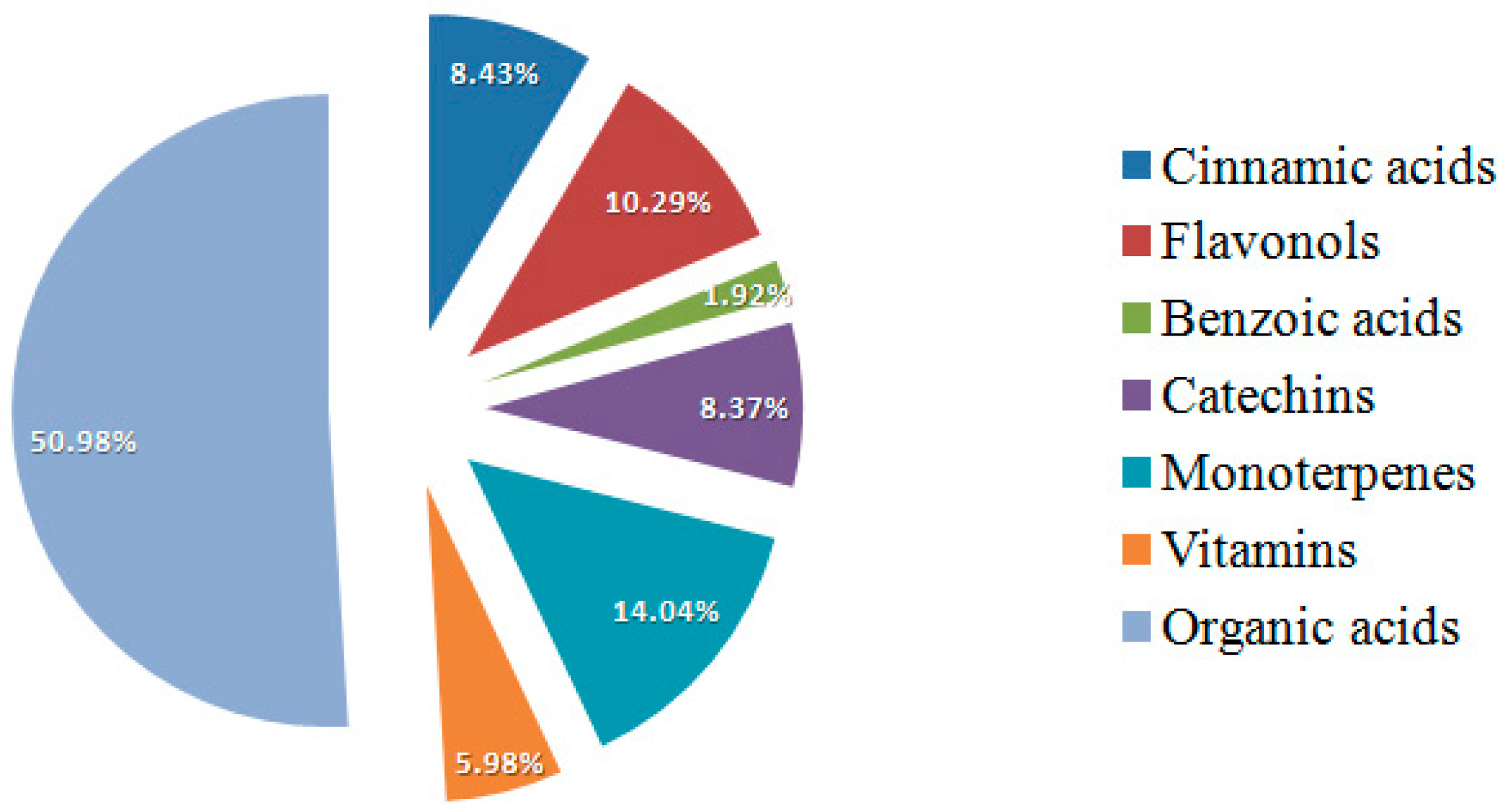
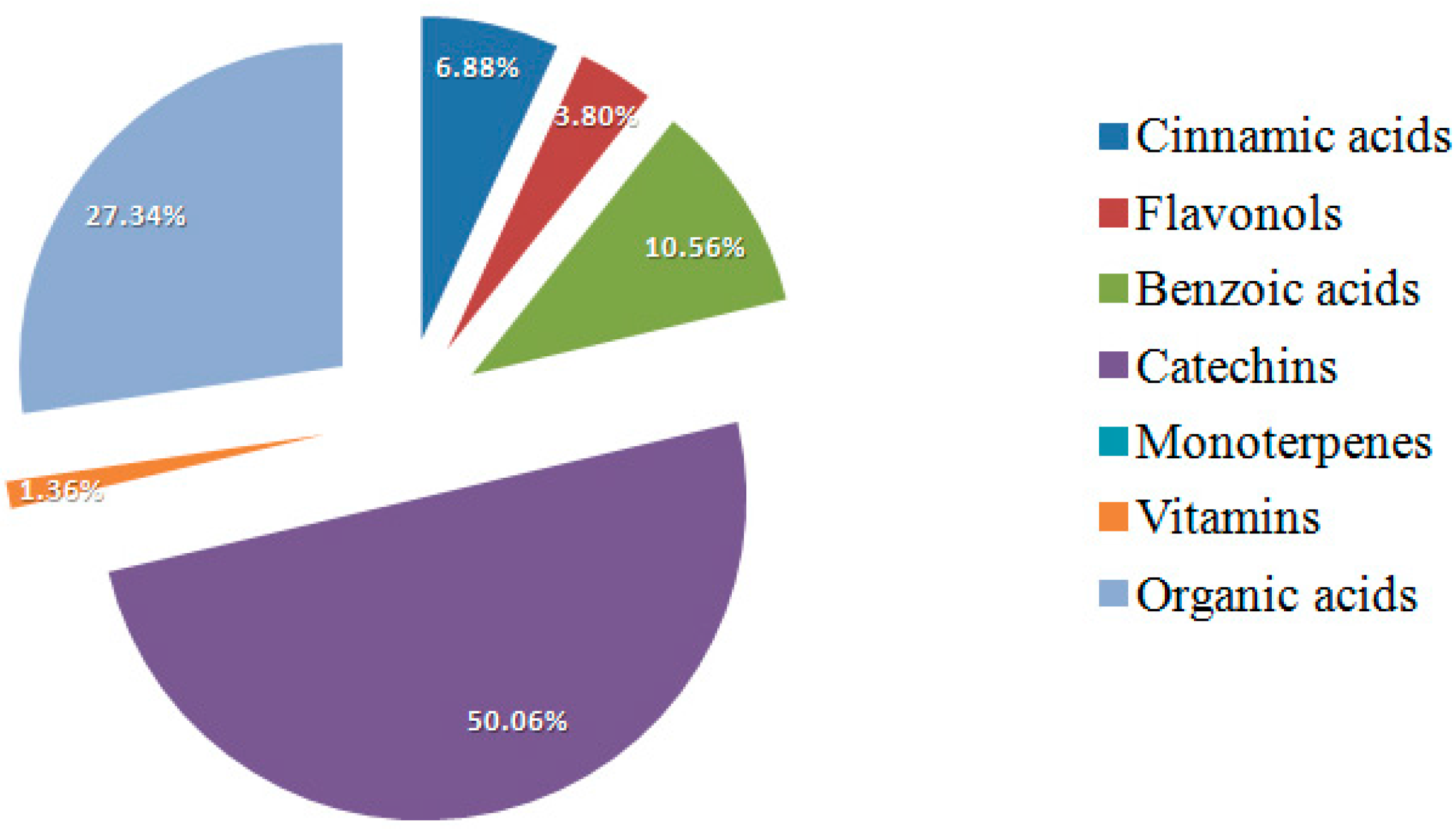
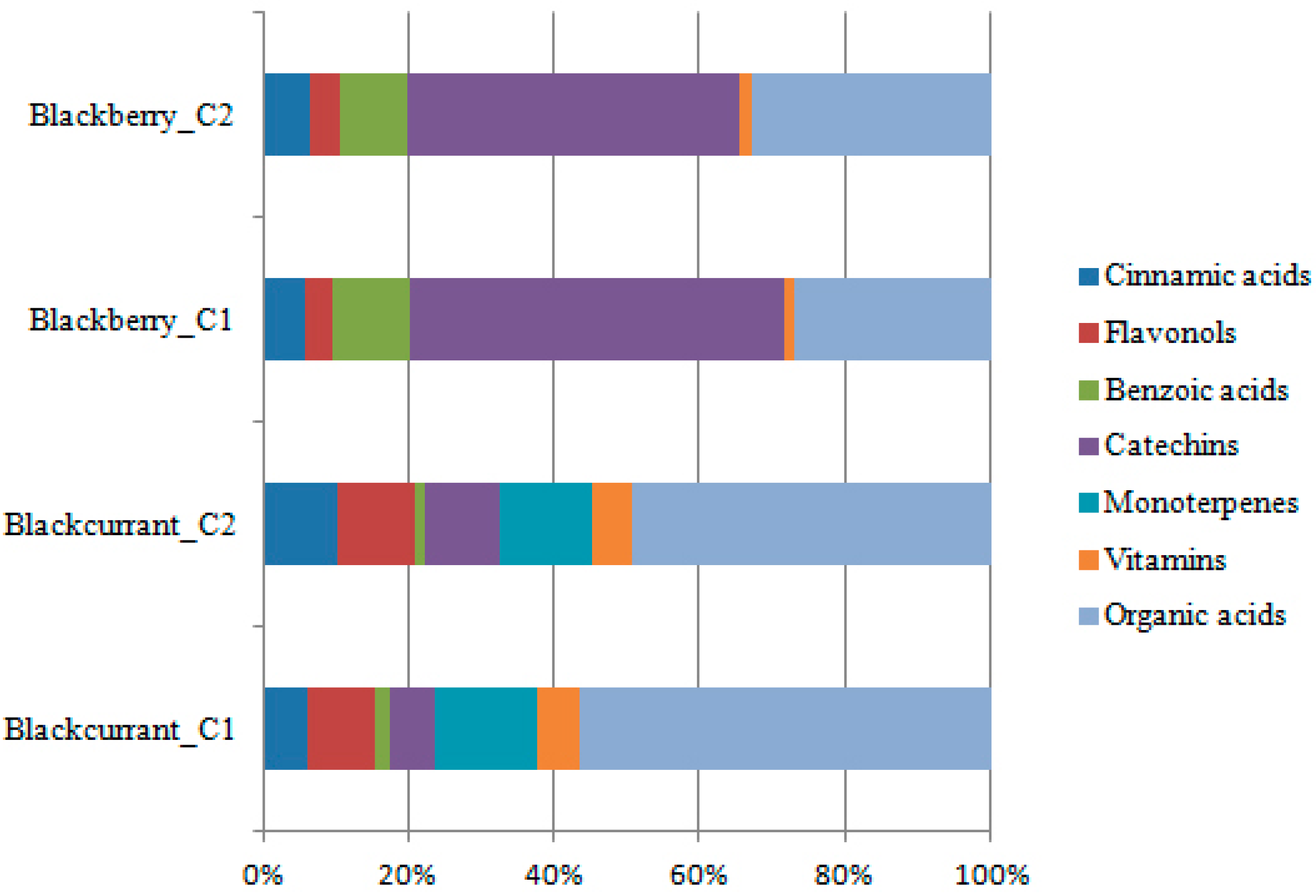
3. Materials and Methods
3.1. Plant Material
| University bud-preparations | ||||
|---|---|---|---|---|
| Species | Genotype | Year | Germplasm repository | Identification code |
| Ribes nigrum L. | Rozenthal | 2014 | San Secondo di Pinerolo, Torino, Italy | RR |
| Tenah | RT | |||
| Rubus ulmifolius Schott | Black Pearl | 2014 | Grugliasco, Torino, Italy | RRBP |
| Kiowa | RRK | |||
| Wild variety | RRW | |||
| Commercial bud-preparations | ||||
| Species | Company | Year | Germplasm repository | Identification code |
| Ribes nigrum L. | Company 1 | 2013 | San Gregorio di Catania, Catania, Italy | RC1 |
| Company 2 | Predappio, Forlì-Cesena, Italy | RC2 | ||
| Rubus ulmifolius Schott | Company 1 | 2013 | San Gregorio di Catania, Catania, Italy | RRC1 |
| Company 2 | Predappio, Forlì-Cesena, Italy | RRC2 | ||
3.2. Solvents and Chemicals
3.3. Sample Preparation Protocols
3.4. Apparatus and Chromatographic Conditions
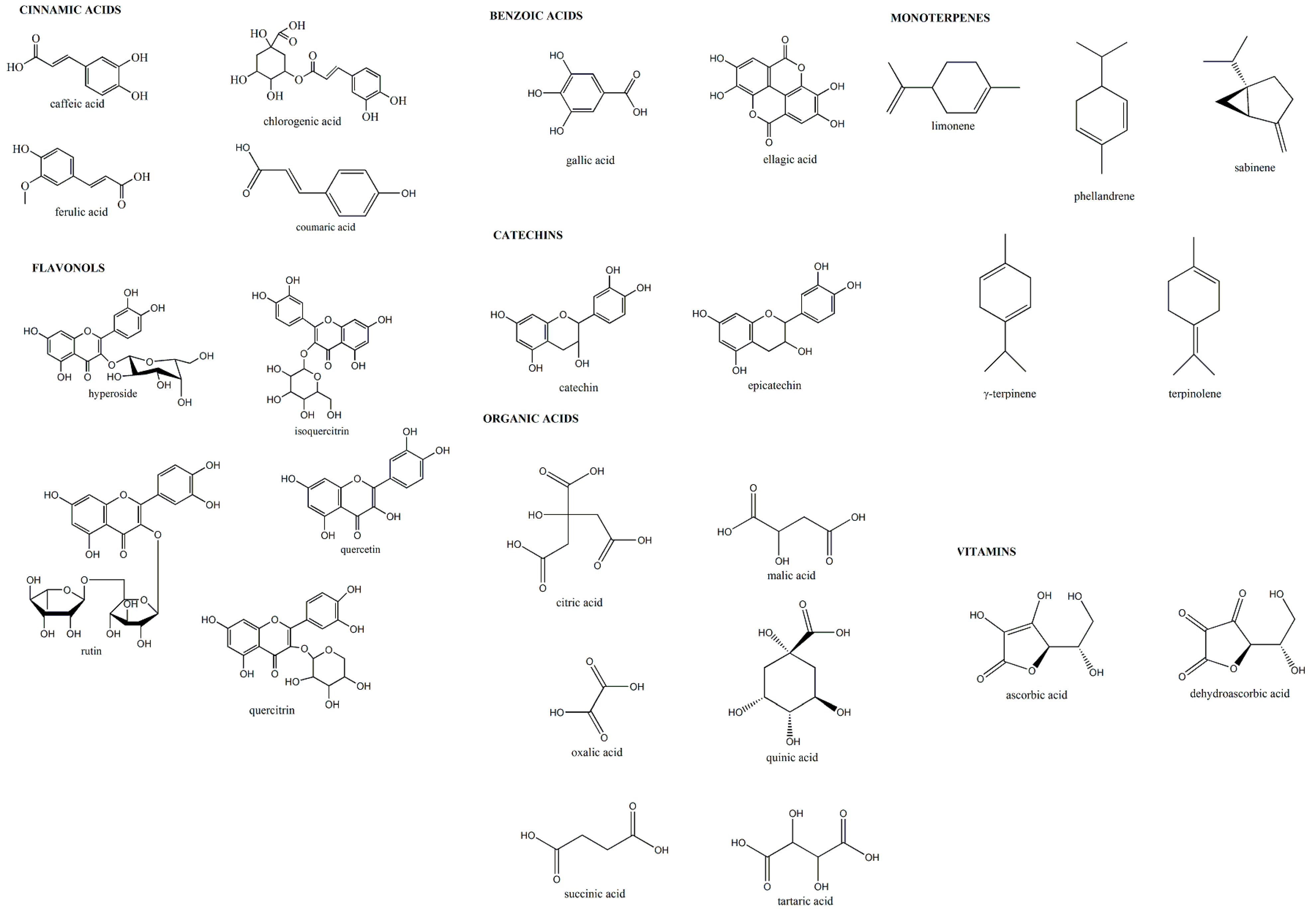
3.5. Identification and Quantification of Bioactive Compounds
| Method | Compounds of interest | Stationary phase | Mobile phase | Flow (mL min−1) | Time of analysis (min) | Gradient | Wavelenght (nm) |
|---|---|---|---|---|---|---|---|
| A | cinnamic acids, flavonols | KINETEX–C18 column (4.6×150 mm, 5 μm) | A: 10 mM KH2PO4/H3PO4, pH=2.8; | 1.5 | 20+2 (CT) | Yes | 330 |
| B: CH3CN | |||||||
| B | benzoic acids, catechins | KINETEX–C18 column (4.6×150 mm, 5 μm) | A: H2O/CH3OH/HCOOH (5:95:0.1 v/v/v), pH=2.5; | 0.6 | 23+2 (CT) | Yes | 280 |
| B: CH3OH/HCOOH (100:0.1 v/v) | |||||||
| C | monoterpenes | KINETEX–C18 column (4.6×150 mm, 5 μm) | A: H2O; | 1.0 | 17+3 (CT) | Yes | 210, 220, |
| B: CH3CN | 235, 250 | ||||||
| D | organic acids | KINETEX–C18 column (4.6×150 mm, 5 μm) | A: 10 mM KH2PO4/H3PO4, pH=2.8; | 0.6 | 13+2 (CT) | No | 214 |
| B: CH3CN | |||||||
| E | vitamins | KINETEX–C18 column (4.6×150 mm, 5 μm) | A: 5 mM C16H33N(CH3)3Br/50 mM KH2PO4, pH=2.5; | 0.9 | 10+5 (CT) | No | 261, 348 |
| B: CH3OH |
| Class | Standard | Identification code | Retention time (tR) (min) | Wavelenght (nm) | Method | Calibration curve equation | R2 | Calibration curve range (mg L−1) | LOD (mg L−1) | LOQ (mg L−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cinnamic acids | caffeic acid | 1 | 4.54 | 330 | A | y = 59.046x + 200.6 | 0.996 | 111–500 | 0.305 | 1.016 |
| chlorogenic acid | 2 | 3.89 | 330 | A | y = 13.583x + 760.05 | 0.984 | 111–500 | 0.940 | 3.134 | |
| coumaric acid | 3 | 6.74 | 330 | A | y = 8.9342x + 217.4 | 0.997 | 111–500 | 2.907 | 9.690 | |
| ferulic acid | 4 | 7.99 | 330 | A | y = 3.3963x − 4.9524 | 1.000 | 111–500 | 1.245 | 4.150 | |
| Flavonols | hyperoside | 5 | 10.89 | 330 | A | y = 7.1322x − 4.583 | 0.999 | 111–500 | 3.372 | 11.241 |
| isoquercitrin | 6 | 11.24 | 330 | A | y = 8.3078x + 26.621 | 0.999 | 111–500 | 0.252 | 0.840 | |
| quercetin | 7 | 17.67 | 330 | A | y = 3.4095x − 98.307 | 0.998 | 111–500 | 4.055 | 13.518 | |
| quercitrin | 8 | 13.28 | 330 | A | y = 2.7413x + 5.6367 | 0.998 | 111–500 | 5.456 | 18.187 | |
| rutin | 9 | 12.95 | 330 | A | y = 6.5808x + 30.831 | 0.999 | 111–500 | 2.937 | 9.790 | |
| Benzoic acids | ellagic acid | 10 | 18.65 | 280 | B | y = 29.954x + 184.52 | 0.998 | 62.5–250 | 0.611 | 2.035 |
| gallic acid | 11 | 4.26 | 280 | B | y = 44.996x + 261.86 | 0.999 | 62.5–250 | 0.435 | 1.451 | |
| Catechins | catechin | 12 | 10.31 | 280 | B | y = 8.9197x + 66.952 | 1.000 | 62.5–250 | 2.343 | 7.809 |
| epicatechin | 13 | 14.30 | 280 | B | y = 12.88x − 43.816 | 0.999 | 62.5–250 | 0.763 | 2.543 | |
| Monoterpenes | limonene | 14 | 3.35 | 250 | C | y = 0.1894x − 5.420 | 0.999 | 125–1000 | 8.654 | 28.847 |
| phellandrene | 15 | 3.57 | 210 | C | y = 8.783x − 145.3 | 0.998 | 125–1000 | 0.562 | 1.874 | |
| sabinene | 16 | 3.45 | 220 | C | y = 18.14x − 1004 | 0.998 | 125–1000 | 0.094 | 0.314 | |
| γ-terpinene | 17 | 3.28 | 235 | C | y = 0.4886x − 23.02 | 0.999 | 125–1000 | 17.577 | 58.590 | |
| terpinolene | 18 | 4.83 | 220 | C | y = 26.52x + 876.8 | 0.999 | 125–1000 | 0.241 | 0.804 | |
| Organic acids | citric acid | 19 | 5.30 | 214 | D | y = 1.0603x − 22.092 | 1.000 | 167–1000 | 18.805 | 62.682 |
| malic acid | 20 | 4.03 | 214 | D | y = 1.415x − 80.254 | 0.996 | 167–1000 | 15.721 | 52.404 | |
| oxalic acid | 21 | 7.85 | 214 | D | y = 6.4502x + 6.1503 | 0.998 | 167–1000 | 0.550 | 1.835 | |
| quinic acid | 22 | 3.21 | 214 | D | y = 0.8087x − 38.021 | 0.998 | 167–1000 | 26.106 | 87.021 | |
| succinic acid | 23 | 3.46 | 214 | D | y = 0.9236x − 8.0823 | 0.995 | 167–1000 | 7.135 | 23.783 | |
| tartaric acid | 24 | 5.69 | 214 | D | y = 1.8427x + 15.796 | 1.000 | 167–1000 | 8.520 | 28.401 | |
| Vitamins | ascorbic acid | 25 | 4.14 | 261 | E | y = 42.71x + 27.969 | 0.999 | 100–1000 | 0.836 | 2.786 |
| dehydroascorbic acid | 26 | 3.41 | 348 | E | y = 4.1628x + 140.01 | 0.999 | 30–300 | 1.095 | 3.649 |
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ramawat, K.; Dass, S.; Mathur, M. The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants. In Herbal Drugs: Ethnomedicine to Modern Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 7–32. [Google Scholar]
- Verpoorte, R. Medicinal plants: A renewable resource for novel leads and drugs. In Herbal Drugs: Ethnomedicine to Modern Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–5. [Google Scholar]
- Pal, S.K.; Shukla, Y. Herbal medicine: Current status and the future. Asian Pacific J. Cancer Prev. 2003, 4, 281–288. [Google Scholar]
- Chen, L.; Xin, X.L.; Yuan, Q.P.; Su, D.H.; Liu, W. Phytochemical properties and antioxidant capacities of various colored berries. J. Sci. Food Agric. 2014, 94, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Medicinal plants, chemical composition and quality: May blackcurrant buds and blackberry sprouts be a new polyphenol source for herbal preparations? J. Appl. Bot. Food Qual. 2013, 86, 79–89. [Google Scholar]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.-O.; Dommes, J. Antioxidant and anti-inflammatory activities of ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Cervellati, R.; Loi, M.C.; Innocenti, G. Evaluation of in vitro antioxidant properties of some traditional sardinian medicinal plants: Investigation of the high antioxidant capacity of rubus ulmifolius. Food Chem. 2008, 106, 745–749. [Google Scholar] [CrossRef]
- Panizzi, L.; Caponi, C.; Catalano, S.; Cioni, P.L.; Morelli, I. In vitro antimicrobial activity of extracts and isolated constituents of rubus ulmifolius. J. Ethnopharmacol. 2002, 79, 165–168. [Google Scholar] [CrossRef]
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. J. Food Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Konik, E.A.; Jungling, R.C.; Bauer, B.A. Herbs and dietary supplements in the european union: A review of the regulations with special focus on germany and poland. J. Diet. Suppl. 2011, 8, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Silano, V.; Coppens, P.; Larranaga-Guetaria, A.; Minghetti, P.; Roth-Ehrang, R. Regulations applicable to plant food supplements and related products in the european union. Food Funct. 2011, 2, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Fürst, R.; Zündorf, I. Evidence-based phytotherapy in europe: Where do we stand? Planta Med. 2015, 81, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M.; Casu, L. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Bud extracts as new phytochemical source for herbal preparations: Quality control and standardization by analytical fingerprint. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015; Volume 1, pp. 187–218. [Google Scholar]
- Fong, H.H.S. Integration of herbal medicine into modern medical practices: Issues and prospects. Integr. Cancer Ther. 2002, 1, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Gulati, O.P.; Berry Ottaway, P. Legislation relating to nutraceuticals in the european union with a particular focus on botanical-sourced products. Toxicology 2006, 221, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Knoss, W.; Chinou, I. Regulation of medicinal plants for public health—european community monographs on herbal substances. Planta Med. 2012, 78, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Bonvegna, L.; Bounous, G. Castanea spp. Buds as a phytochemical source for herbal preparations: Botanical fingerprint for nutraceutical identification and functional food standardisation. J. Sci. Food Agric. 2014, 94, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz. J. Med. Biol. Res. 2000, 33, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, G.M.; Cerutti, A.K.; Canterino, S.; Bounous, G. Effect of agronomic and environmental conditions on chemical composition of tree-species buds used for herbal preparations. Vegetos Int. J Plant Res. 2012, 25, 21–29. [Google Scholar]
- Vagiri, M.; Ekholm, A.; Öberg, E.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Phenols and ascorbic acid in black currants (ribes nigrum l.): Variation due to genotype, location, and year. J. Agric. Food Chem. 2013, 61, 9298–9306. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-Z.; Xie, P.; Chan, K. Quality control of herbal medicines. J. Chromatogr. B 2004, 812, 53–70. [Google Scholar] [CrossRef]
- Júnior, J.O.C.S.; Costa, R.M.R.; Teixeira, F.M.; Barbosa, W.L.R. Processing and quality control of herbal drugs and their derivatives. In Quality Control of Herbal Medicines and Related Areas; Intech: Rijeka, Croatia, 2011; pp. 195–222. [Google Scholar]
- Rossi Forim, M.; Perlatti, B.; Soares Costa, E.; Facchini Magnani, B.; Donizetti de Souza, G. Concerns and considerations about the quality control of natural products using chromatographic methods. Curr. Chromatogr. 2015, 2, 20–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.; Dai, J.; Wang, W.; Cao, H.; Wu, J.; Gou, X. Quality control method for herbal medicine-chemical fingerprint analysis. In Quality Control Herbal Medicines and Related Areas; InTech: Rijeka, Croatia, 2011; pp. 171–194. [Google Scholar]
- He, X.G. On-line identification of phytochemical constituents in botanical extracts by combined high-performance liquid chromatographic-diode array detection-mass spectrometric techniques. J. Chromatogr. A 2000, 880, 203–232. [Google Scholar] [CrossRef]
- Shi, Z.-Q.; Song, D.-F.; Li, R.-Q.; Yang, H.; Qi, L.-W.; Xin, G.-Z.; Wang, D.-Q.; Song, H.-P.; Chen, J.; Hao, H.; et al. Identification of effective combinatorial markers for quality standardization of herbal medicines. J. Chromatogr. A 2014, 1345, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B 2004, 812, 23–33. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Compos. Anal. 2010, 23, 561–568. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [PubMed]
- Hodges, D.M.; Kalt, W. Health Functionality of Small Fruit. Available online: http://www.actahort.org/books/626/626_1.htm (accessed on 3 February 2016).
- Shukitt-Hale, B.; Lau, F.C.; Joseph, J.A. Berry fruit supplementation and the aging brain. J. Agric. Food Chem. 2008, 56, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Dvaranauskaitė, A.; Venskutonis, P.R.; Raynaud, C.; Talou, T.; Viškelis, P.; Sasnauskas, A. Variations in the essential oil composition in buds of six blackcurrant (Ribes nigrum L.) cultivars at various development phases. Food Chem. 2009, 114, 671–679. [Google Scholar]
- Kerslake, M.F.; Menary, R.C. Varietal differences of extracts from blackcurrant buds (Ribes nigrum L.). J. Sci. Food Agric. 1985, 36, 343–351. [Google Scholar] [CrossRef]
- Dabbou, S.; Sifi, S.; Rjiba, I.; Esposto, S.; Taticchi, A.; Servili, M.; Montedoro, G.F.; Hammami, M. Effect of pedoclimatic conditions on the chemical composition of the sigoise olive cultivar. Chem. Biodivers. 2010, 7, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Marconi, V.; Bounous, G. Botanicals in ribes nigrum bud-preparations: An analytical fingerprinting to evaluate the bioactive contribution to total phytocomplex. Pharm. Biol. 2013, 51, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Wang, B.-T.; Liang, Y.-Z.; Chau, F.-T.; Fung, Y.-S. Variable selection for discriminating herbal medicines with chromatographic fingerprints. Anal. Chim. Acta 2006, 572, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Yang, H.; Chan, C.O.; Jin, D.; Mok, D.K.; Chen, S. Fingerprint analysis and simultaneous determination of phenolic compounds in extracts of curculiginis rhizoma by hplc-diode array detector. Chem. Pharm. Bull. 2013, 61, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Kong, W.; Wei, J.; Ou-Yang, Z.; Yang, M. Hplc fingerprint analysis combined with chemometrics for pattern recognition of ginger. Pharm. Biol. 2014, 52, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Lugasi, A.; Hóvári, J.; Kádár, G.; Dénes, F. Phenolics in raspberry, blackberry and currant cultivars grown in hungary. Acta Aliment. 2011, 40, 52–64. [Google Scholar] [CrossRef]
- Dugo, P.; Cacciola, F.; Donato, P.; Jacques, R.A.; Caramao, E.B.; Mondello, L. High efficiency liquid chromatography techniques coupled to mass spectrometry for the characterization of mate extracts. J. Chromatogr. A 2009, 1216, 7213–7221. [Google Scholar] [CrossRef] [PubMed]
- Kesting, J.R.; Huang, J.; Sorensen, D. Identification of adulterants in a chinese herbal medicine by lc-hrms and lc-ms-spe/nmr and comparative in vivo study with standards in a hypertensive rat model. J. Pharm. Biomed. Anal. 2010, 51, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.L.; Qu, J.; Luo, G.A.; Wang, Y.M. Rapid and reliable determination of illegal adulterant in herbal medicines and dietary supplements by lc/ms/ms. J. Pharm. Biomed. Anal. 2006, 40, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Ordre_National_des_Pharmaciens. Pharmacopée française, codex medicamentarius gallicus, codex français: Monographie, préparations homéopathiques, 8th ed.; Ministère de la santé publique et de la population: Paris, France, 1965; Available online: http://ansm.sante.fr/Mediatheque/Publications/Pharmacopee-francaise-Plan-Preambule-index (accessed on 4 February 2016).
- Mok, D.K.W.; Chau, F.T. Chemical information of chinese medicines: A challenge to chemist. Chemomet. Intell. Lab. Syst. 2006, 82, 210–217. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus spp. Buds. Pharmaceuticals 2016, 9, 7. https://doi.org/10.3390/ph9010007
Donno D, Mellano MG, Cerutti AK, Beccaro GL. Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus spp. Buds. Pharmaceuticals. 2016; 9(1):7. https://doi.org/10.3390/ph9010007
Chicago/Turabian StyleDonno, Dario, Maria Gabriella Mellano, Alessandro Kim Cerutti, and Gabriele Loris Beccaro. 2016. "Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus spp. Buds" Pharmaceuticals 9, no. 1: 7. https://doi.org/10.3390/ph9010007
APA StyleDonno, D., Mellano, M. G., Cerutti, A. K., & Beccaro, G. L. (2016). Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus spp. Buds. Pharmaceuticals, 9(1), 7. https://doi.org/10.3390/ph9010007









