Polyoxypregnane Aryl Esters Prepared from Metaplexis japonica (Thunb.) Makino and Their Role in Reversing Multidrug Resistance in HepG2/Dox Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Identification of Compounds 1 and 1a
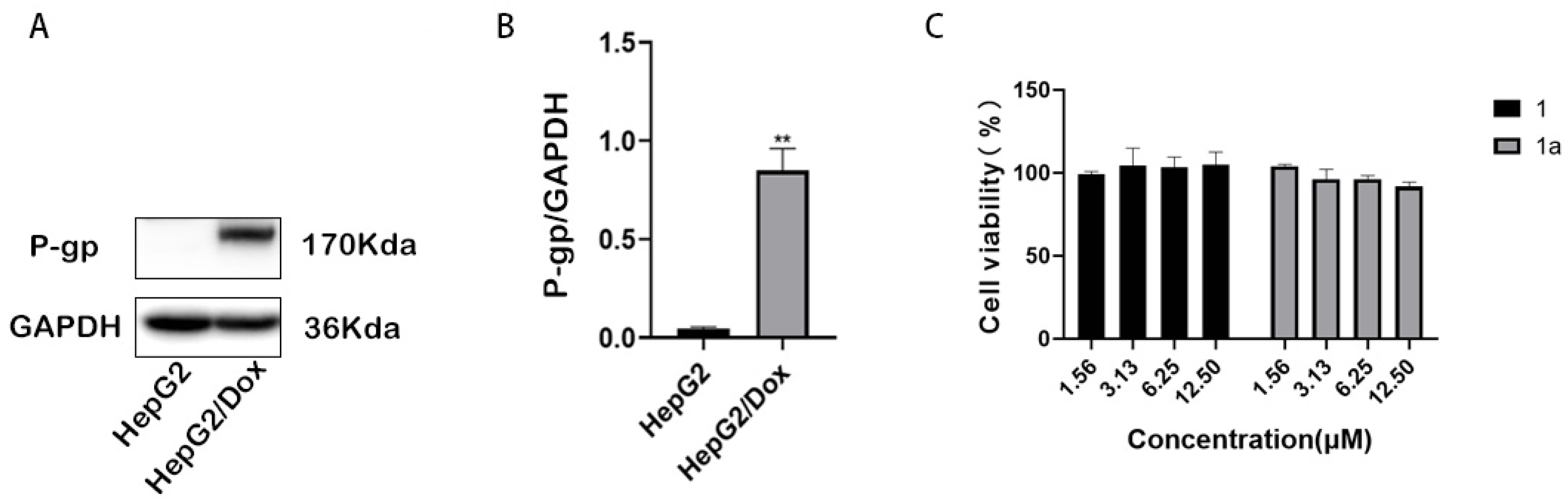
2.2. Compounds 1 and 1a Significantly Reversed P-gp-Mediated MDR in HepG2/Dox Cells
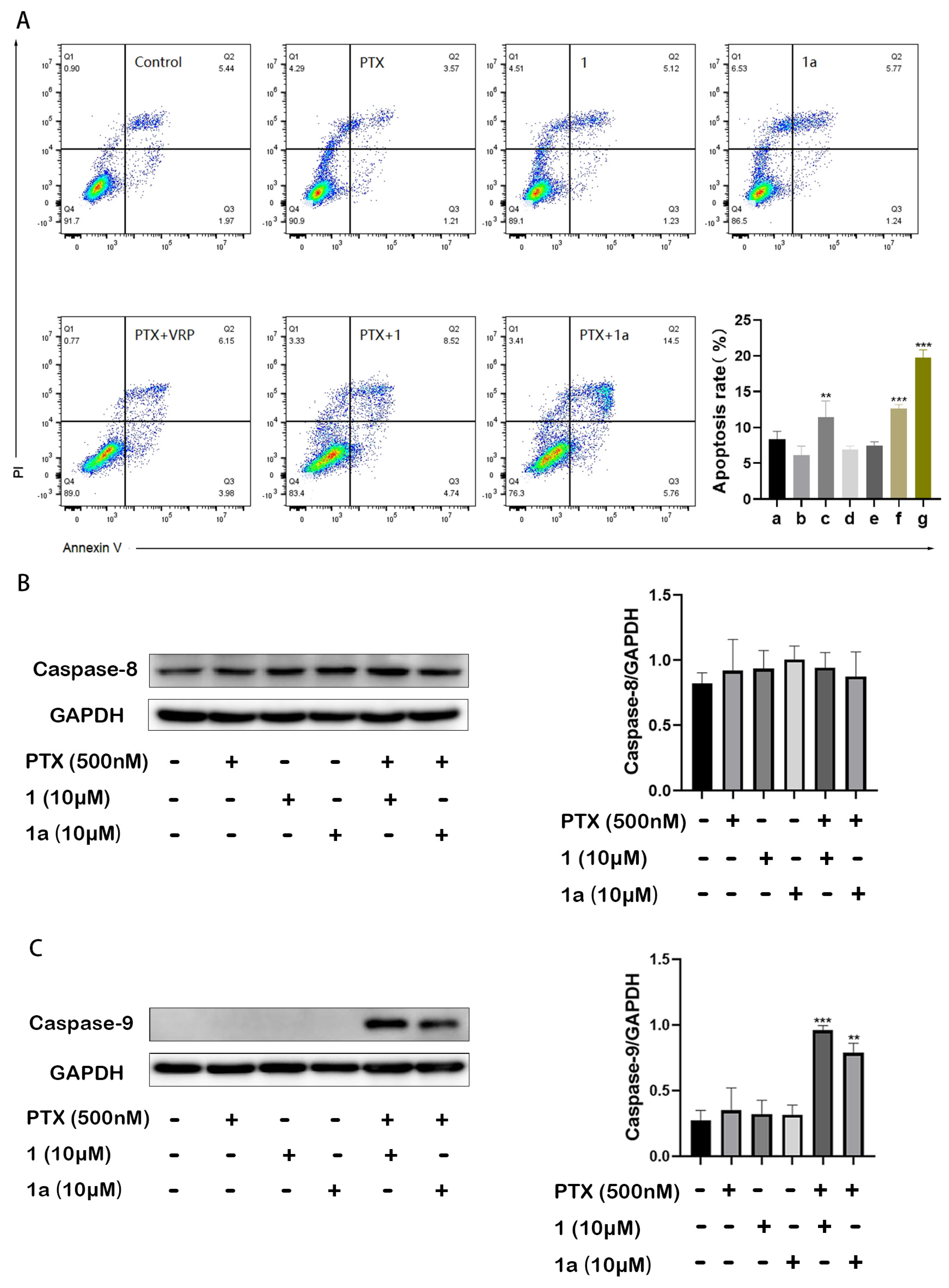
2.3. Compounds 1 and 1a Significantly Enhanced Paclitaxel-Induced Intrinsic Apoptosis in HepG2/Dox Cells
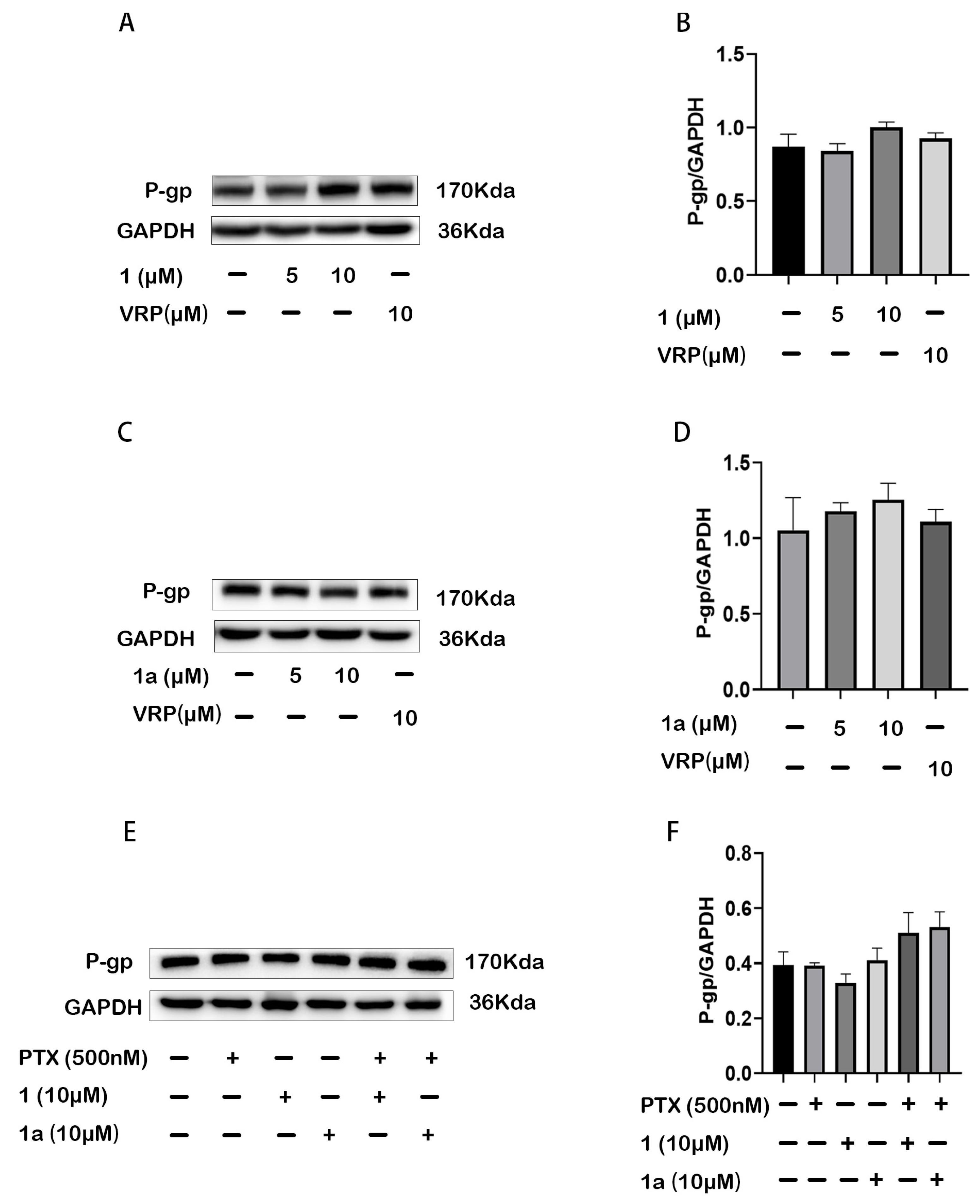
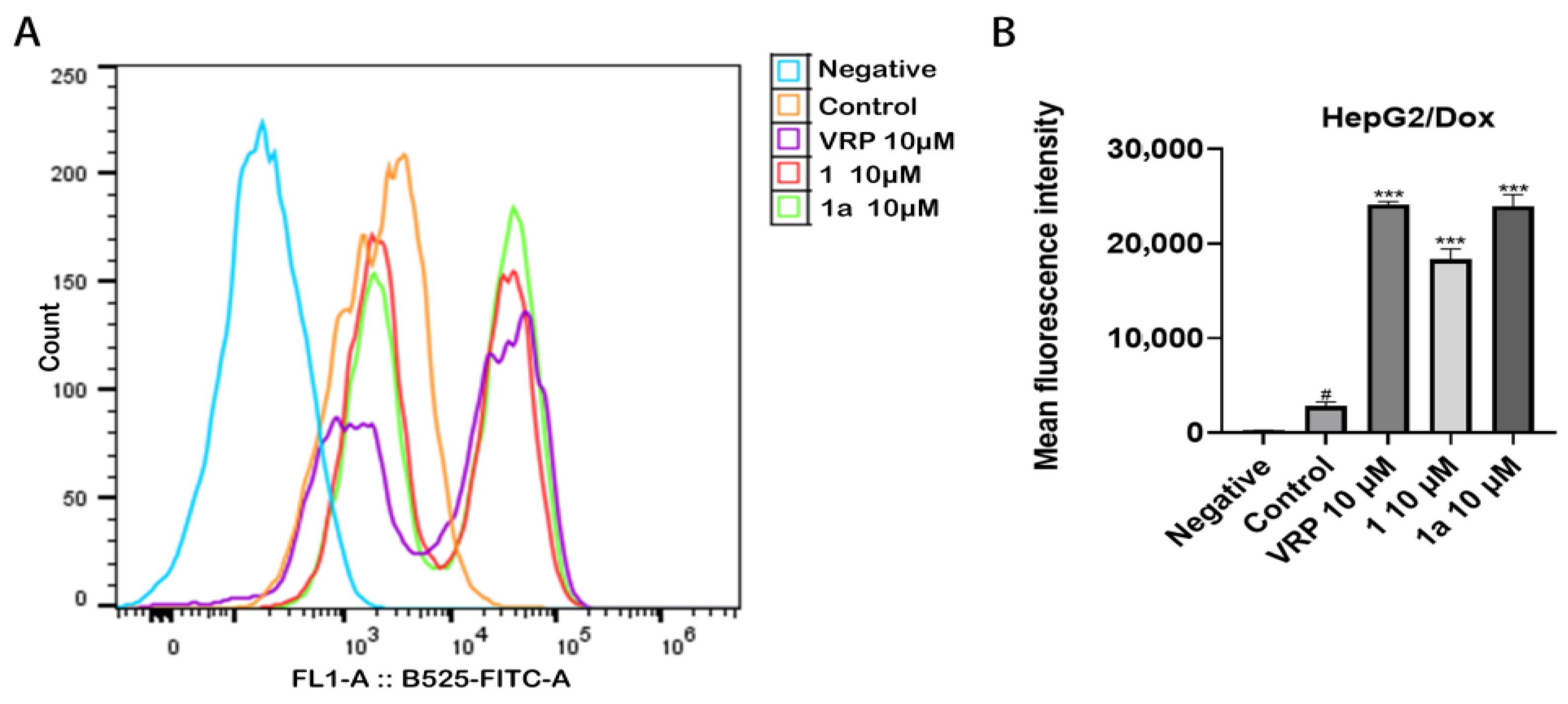
2.4. Compounds 1 and 1a Did Not Affect the Expression but Inhibited the Function of P-gp in HepG2/Dox Cells
2.5. Compounds 1 and 1a Acted as Non-Substrates of P-gp in Caco-2 Cell Monolayer
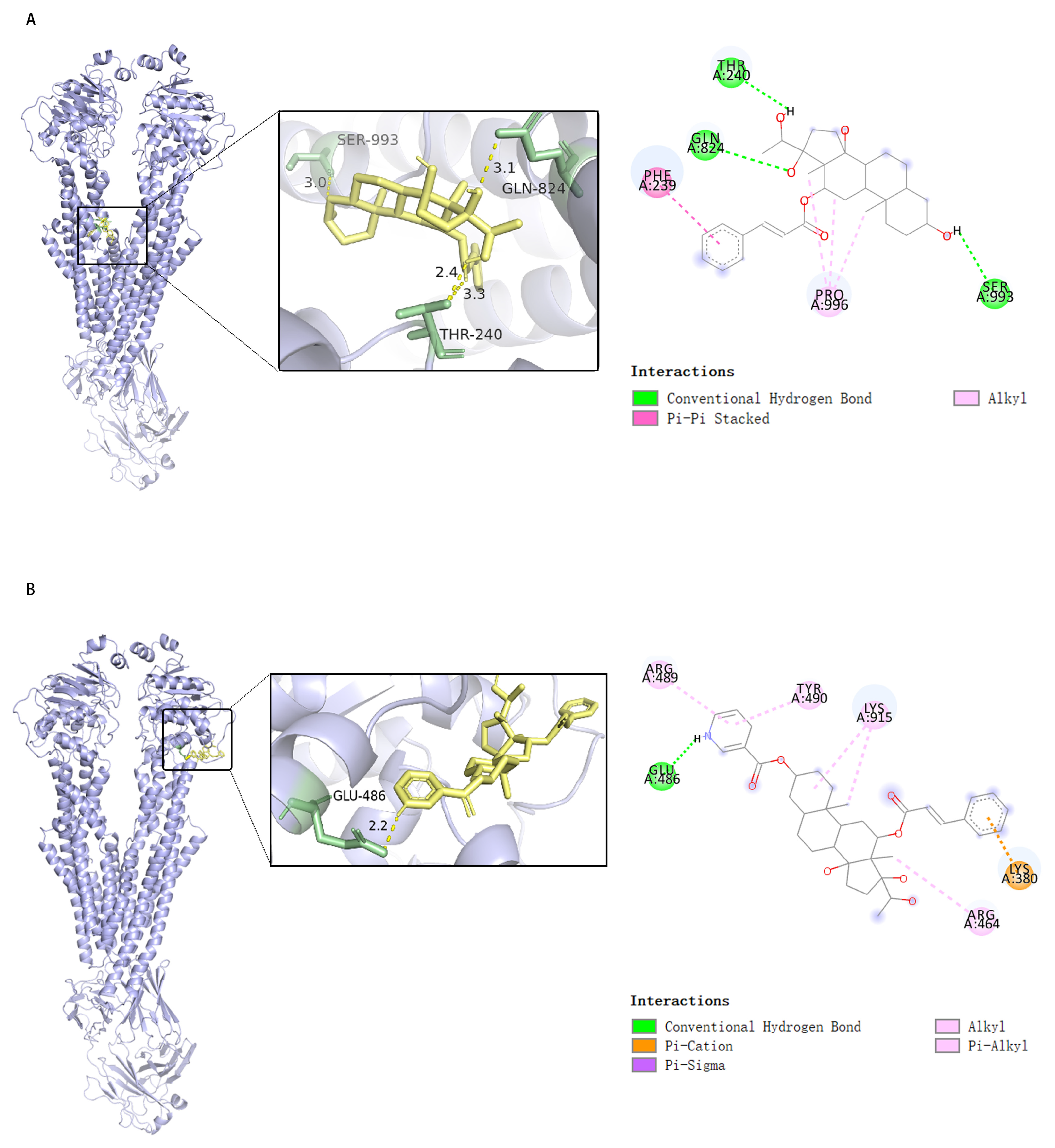
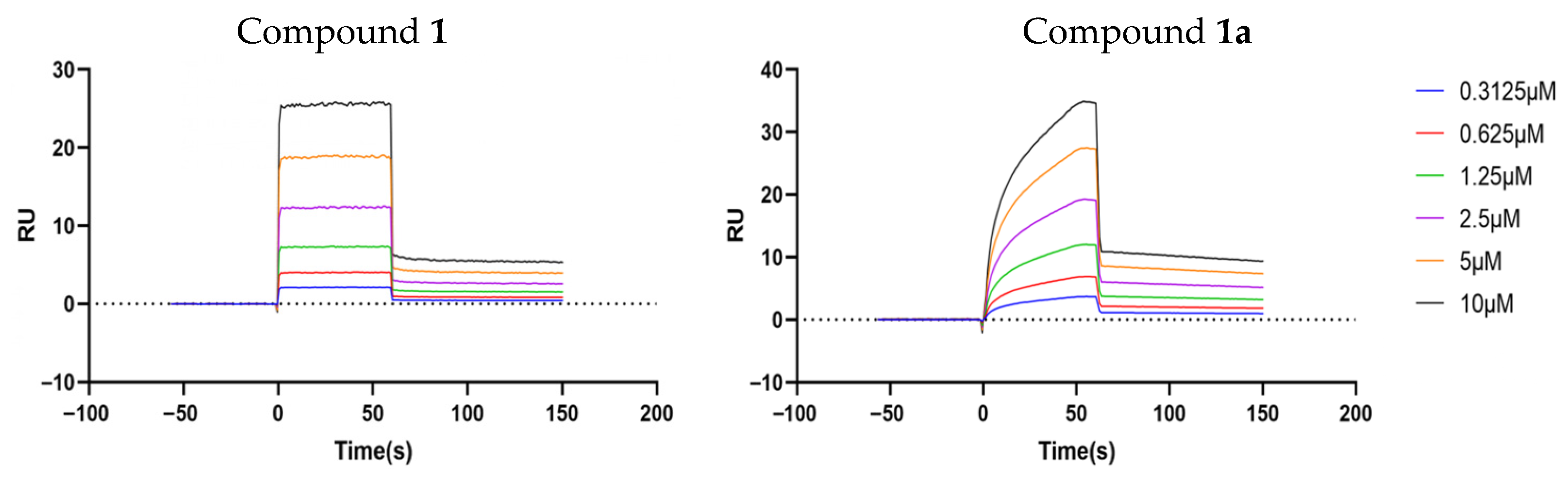
2.6. Compounds 1 and 1a Interact with P-gp with High Affinity but at Different Sites
3. Materials and Methods
3.1. Materials and Reagents
3.2. Equipment
3.3. Synthesis of Compounds 1 and 1a
3.4. Evaluation of MDR Reversal Effects of Compound 1 and 1a
3.4.1. Cell Line and Cell Culture
3.4.2. Detection of Cell Viability via Cell Counting Kit 8
3.4.3. Observation of Apoptosis via Annexin V/PI Staining
3.5. Study on the MDR-Reversing Mechanism of Test Compounds
3.5.1. Detection of Cellular Protein Expression via Western Blotting
3.5.2. Rhodamine 123 Efflux Assay for Detection of P-gp Function
3.5.3. Bidirectional Transport Assay on Caco-2 Cell Monolayers
3.6. Molecular Docking
3.7. Surface Plasmon Resonance
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCK-8 | Cell counting kit-8 |
| Dox | Doxorubicin |
| ER | Efflux Ratio |
| HBSS | Hank’s balanced salt solution |
| HMBC | Heteronuclear multiple bond correlation |
| 1H-1H COSY | 1H-1H Correlated spectroscopy |
| 1H-NMR | 1H nuclear magnetic resonance |
| IC50 | Half inhibitory concentration |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| MDR | Multidrug resistance |
| P-gp | P-glycoprotein |
| PI | Propidium Iodide |
| PTX | Paclitaxel |
| Rh-123 | Rhodamine-123 |
| SDS | Sodium dodecyl sulfate |
| SPR | Surface plasmon resonance |
| TLC | Thin-Layer Chromatography |
| VRP | Verapamil |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Kukal, S.; Guin, D.; Rawat, C.; Bora, S.; Mishra, M.K.; Sharma, P.; Paul, P.R.; Kanojia, N.; Grewal, G.K.; Kukreti, S.; et al. Multidrug efflux transporter ABCG2: Expression and regulation. Cell. Mol. Life Sci. 2021, 78, 6887–6939. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.F.; Chang, P.M. Clinical perspective of FDA approved drugs with P-glycoprotein inhibition activities for potential cancer therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Shen, X.L.; Hu, Y.J.; Yu, Z.L.; Fong, W.F. Chinese herbal medicines as reversal agents for P-glycoprotein-mediated multidrug resistance in tumors. Chin. J. Nat. Med. 2009, 7, 465–475. [Google Scholar] [CrossRef]
- Hu, Y.J.; Shen, X.L.; Lu, H.L.; Zhang, Y.H.; Huang, X.A.; Fu, L.C.; Fong, W.F. Tenacigenin B derivatives reverse P-glycoprotein-mediated multidrug resistance in HepG2/Dox cells. J. Nat. Prod. 2008, 71, 1049–1051. [Google Scholar] [CrossRef]
- Dai, L.L.; Tian, R.H.; Zhang, Y.P.; Zhou, J.; Zhu, R.J.; Hu, Y.J.; Shen, X.L. Study of C21 steroidal constituents from Marsdenia tenacissima for enhancing antitumor activity of paclitexel. Trad. Chin. Drug Res. Clinl Pharmacol. 2015, 26, 269–274. [Google Scholar]
- Wu, Z.L.; Chen, Y.; Qu, Z.; Wu, G.Y.; He, X.F.; Huang, J.W.; Meng, Q.Q.; Hu, Y.H.; Shen, X.L.; Yang, R.Y.; et al. An ester derivative of tenacigenin B from Marsdenia tenacissima (Roxb.) Wight et Arn reversed paclitaxel-induced MDR in vitro and in vivo by inhibiting both P-gp and MRP2. J. Ethnopharmacol. 2022, 294, 115353. [Google Scholar] [CrossRef]
- Nomura, T.; Fukai, T.; Kuramochi, T. Components of Metaplexis japonica Makino. Planta Med. 1981, 41, 206–207. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Q.Y.; Liu, C.; Yang, J.; Lv, Q.T.; Tian, Z.H.; Jiang, H.Q.; Rong, R. Three new C21 steroidal glycosides isolated from Metaplexis japonica and their potential inhibitory effects on tyrosine protein kinases. Nat. Prod. Res. 2022, 36, 1988–1995. [Google Scholar] [CrossRef]
- Seto, H.; Hayashi, K.; Mitsuhashi, H. Studies on the constituents of Asclepiadaceae plants. XXXVI. Component of Marsdenia tomentosa Decne: Structure of tomentonin, tomentodin, and dehydrotomentosin and difference in the reactivity between utendin and tomentogenin diesters on mild alkaline hydrolysis. Chem. Pharm. Bull. 1976, 24, 443–449. [Google Scholar] [CrossRef]
- Fong, W.F.; Shen, X.L.; Globisch, C.; Wiese, M.; Chen, G.Y.; Zhu, G.Y.; Yu, Z.L.; Tse, A.K.; Hu, Y.J. Methoxylation of 3′,4′-aromatic side chains improve P-glycoprotein inhibitory and multidrug resistance reversal activities of 7,8-pyranocoumarin against cancer cells. Bioorg. Med. Chem. 2008, 16, 3694–3703. [Google Scholar] [CrossRef]
- Xiong, J.M.; Liu, H.; Chen, J.; Zou, Q.Q.; Wang, Y.Y.; Bi, G.S. Curcumin nicotinate suppresses abdominal aortic aneurysm pyroptosis via lncRNA PVT1/miR-26a/KLF4 axis through regulating the PI3K/AKT signaling pathway. Toxicol. Res. 2021, 10, 651–661. [Google Scholar] [CrossRef]
- Yang, D.M.; Yang, W.; Sun, F.; Sun, S.Y.; Chen, J.X.; Zhang, C.P.; Xiong, G.Z.; Tuo, Q.H.; Liao, R.F. Effects of CurTn on proliferation of VSMC. Chin. Pharmacol. Bull. 2016, 32, 1526–1530. [Google Scholar]
- Lo, Y.L.; Wang, W. Formononetin potentiates epirubicin-induced apoptosis via ROS production in HeLa cells in vitro. Chem. Biol. Interact. 2013, 205, 188–197. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, M.; Qu, Z.; Zhou, J.; Zhang, Q. Resveratrol enhances anticancer effects of paclitaxel in HepG2 human liver cancer cells. BMC Complement. Altern. Med. 2017, 17, 477. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Yalcin-Ozkat, G. Molecular modeling strategies of cancer multidrug resistance. Drug Resist. Updat. 2021, 59, 100789. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Lu, L.; Fang, Q.; Liu, C.; Lin, Z. Identification of small-molecule inhibitors of human MUS81-EME1/2 by FRET-based high-throughput screening. Bioorg Med. Chem. 2023, 90, 117383. [Google Scholar] [CrossRef]
- Tsuruo, T.; Iida, H.; Tsukagoshi, S.; Sakurai, Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981, 41, 1967–1972. [Google Scholar]
- Yang, C.P.; DePinho, S.G.; Greenberger, L.M.; Arceci, R.J.; Horwitz, S.B. Progesterone interats with P-glycoprotein in multidrug-resistant cells and in the endometrium of gravid uterus. J. Biol. Chem. 1989, 264, 782–788. [Google Scholar] [CrossRef]
- Mutoh, K.; Tsukahara, S.; Mitsuhashi, J.; Katayama, K.; Sugimoto, Y. Estrogen-mediated post transcriptional down-regulated of P-glycoprotein in MDR1-transduced human breast cancer cells. Cancer Sci. 2006, 97, 1198–1204. [Google Scholar] [CrossRef]
- Yuan, W.Q.; Zhang, R.R.; Wang, J.; Ma, Y.; Li, W.X.; Jiang, R.W.; Cai, S.H. Asclepiasterol, a novel C21 steroidal glycoside derived from Asclepias curassavica, reverses tumor multidrug resistance by down-regulating P-glycoprotein expression. Oncotarget. 2016, 7, 31466–31483. [Google Scholar] [CrossRef]
- To, K.K.W.; Wu, X.; Yin, C.; Chai, S.; Yao, S.; Kadioglu, O.; Efferth, T.; Ye, Y.; Lin, G. Reversal of multidrug resistance by Marsdenia tenacissima and its main active ingredients polyoxypregnanes. J. Ethnopharmacol. 2017, 203, 110–119. [Google Scholar] [CrossRef]
- Xie, B.; Lu, Y.Y.; Luo, Z.H.; Qu, Z.; Zheng, C.G.; Huang, X.A.; Zhou, H.Y.; Hu, Y.J.; Shen, X.L. Tenacigenin B ester derivatives from Marsdenia tenacissima actively inhibited CYP3A4 and enhanced in vivo antitumor activity of paclitaxel. J. Ethnopharmacol. 2019, 235, 309–319. [Google Scholar] [CrossRef]
- Zhu, R.J.; Shen, X.L.; Dai, L.L.; Ai, X.Y.; Tian, R.H.; Tang, R.; Hu, Y.J. Total aglycones from Marsdenia tenacissima increases antitumor efficacy of paclitaxel in nude mice. Molecules 2014, 19, 13965–13975. [Google Scholar] [CrossRef]
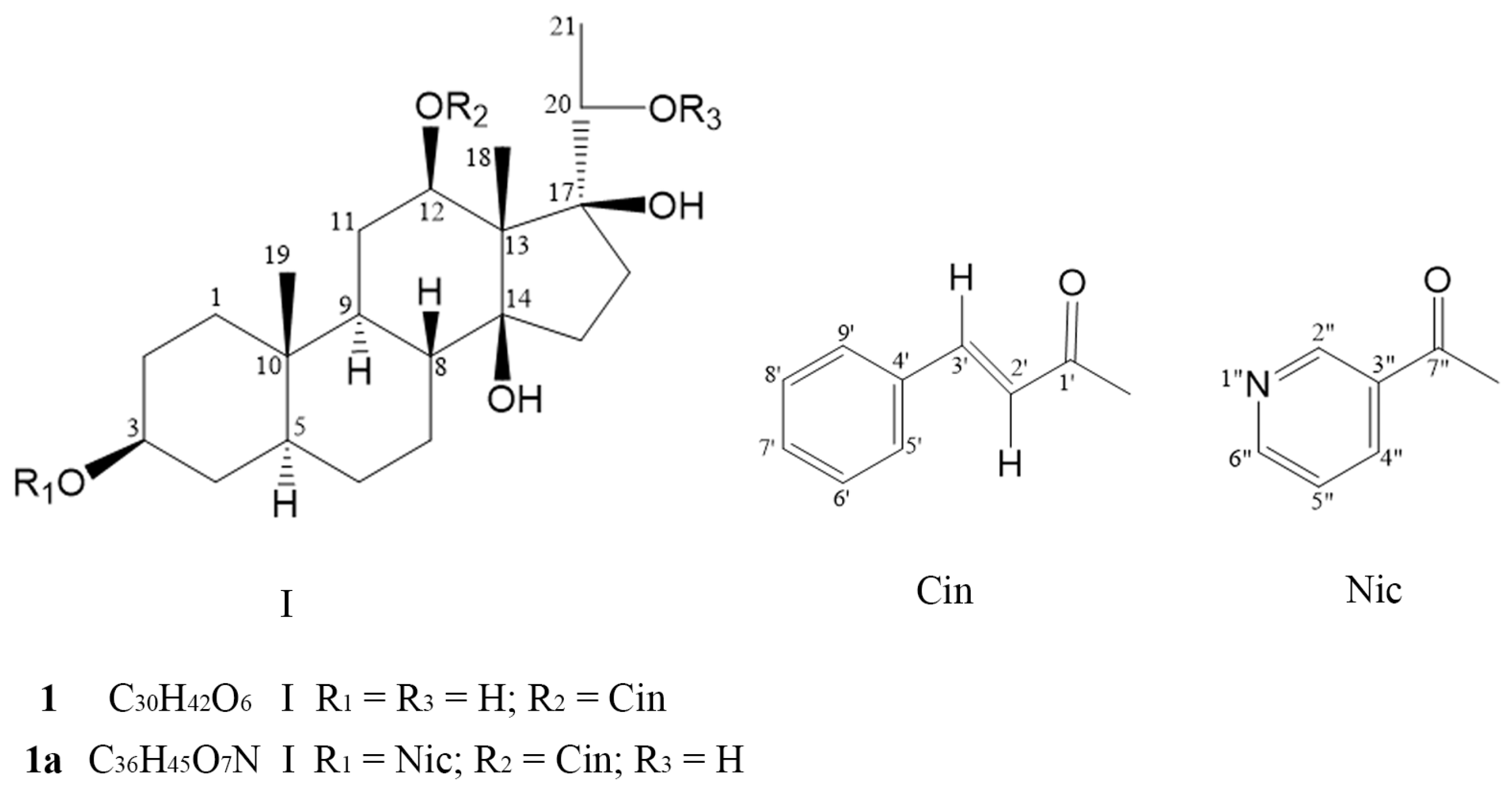
| C | 1 | 1a | |||
|---|---|---|---|---|---|
| δH, (J in Hz) | δC | δH, (J in Hz) | δC | Correlation | |
| 1 | 37.8, CH2 | 36.7, CH2 | |||
| 2 | 31.7, CH2 | 1.50, 1.53 | 27.3, CH2 | ||
| 3 | 3.607 (hept, 3α) | 71.0, CH | 4.993 (hept, 4.8, 3α) | 74.5, CH | COSY: 1.341 (18-H), 1.40, 1.53 (2-H), 1.95 (9-H) |
| 4 | 36.9, CH2 | 1.57 | 33.8, CH2 | ||
| 5 | 44.5, CH | 44.4, CH | |||
| 6 | 28.2, CH2 | 28.2, CH2 | |||
| 7 | 26.6, CH2 | 26.6, CH2 | |||
| 8 | 39.3, CH | 39.3, CH | |||
| 9 | 45.4, CH | 1.95 | 45.3, CH | ||
| 10 | / | 35.5, C | / | 35.6, C | |
| 11 | 27.0, CH2 | 1.38, 1.40, 1.57 | 27.0, CH2 | ||
| 12 | 4.665 (dd, 11.4, 4.2, 12α) | 74.6, CH | 4.692 (dd, 11.2, 4.8, 12α) | 74.7, CH | COSY: 1.50, 1.53, 1.57, 1.95 |
| 13 | / | 55.1, C | 55.1, C | HMBC: 166.1 (C-1′) | |
| 14 | / | 87.3, C | 87.2, C | ||
| 15 | 30.9, CH2 | 30.9, CH2 | |||
| 16 | 31.2, CH2 | 31.8, CH2 | |||
| 17 | / | 88.1, C | 88.1, C | ||
| 18 | 1.320 (s) | 12.2, CH3 | 1.341 (s) | 12.2, CH3 | HMBC: 74.7 (C-12), 55.1 (C-13), 87.2 (C-14) |
| 19 | 0.825 (s) | 9.7, CH3 | 0.908 (s) | 9.7, CH3 | HMBC: 35.6 (C-10), δC 44.4 (C-5) |
| 20 | 3.623 (q, 7.2) | 71.4, CH | 3.647 (q, 6.0) | 71.4, CH | |
| 21 | 1.111 (d, 7.2) | 17.2, CH3 | 1.122 (d, 6.4) | 17.2, CH3 | COSY: 3.647 (20-H); HMBC: 71.4 (C-20), 88.1 (C-17) |
| 1′ | / | 166.1, C | 166.1, C | ||
| 2′ | 6.445 (d, 17.6) | 117.2, CH | 6.444 (dd, 16.2, 2.0) | 117.2, CH | HMBC: 134.0 (C-4′), 166.1 (C-1′) |
| 3′ | 7.745 (d, 17.6) | 146.3, CH | 7.755 (dd, 16.2, 2.0) | 146.3, CH | HMBC: 128.3 (C-5′, C-9′), 134.0 (C-4′), 134.0 (C-4′), 117.2 (C-2′) |
| 4′ | / | 133.9, C | 134.0, C | ||
| 5′ | 7.53~7.56 (2H, m, 5′, 9′) | 128.4, CH | 7.54~7.56 (2H, m, 5′, 9′) | 128.3, CH | |
| 6′ | 7.39~7.44 (3H, m) | 129.0, CH | 7.41~7.37 (4H, m, 4″-H, 6′-H, 7′-H, 8′-H) | 129.0, CH | HMBC: 128.3 (C-5′, C-9′) |
| 7′ | 7.39~7.44 (3H, m) | 130.8, CH | 7.41~7.37 (4H, m, 4″-H, 6′-H, 7′-H, 8′-H) | 130.8, CH | |
| 8′ | 7.39~7.44 (3H, m) | 129.0, CH | 7.37~7.41 (4H, m, 4″-H, 6′-H, 7′-H, 8′-H) | 129.0, CH | |
| 9′ | 7.53~7.56 (2H, m, 5′-H, 9′-H) | 128.4, CH | 7.54~7.56 (2H, m, 5′-H, 9′-H) | 128.3, CH | |
| 2″ | / | / | 9.202 (s) | 150.8, CH | COSY: 8.293 (5″-H) |
| 3″ | / | / | 126.7, C | ||
| 4″ | / | / | 7.390 (m) in 7.37~7.41 (4H, m) | 123.3, CH | HMBC: 153.2 (C-6″) |
| 5″ | / | / | 8.293 (dd, 8.0, 4.8) | 137.1, CH | HMBC: 153.2 (C-6″), |
| 6″ | / | / | 8.756 (d, 4.8) | 153.2, CH | COSY: 7.390, 4″-H (in 7.37~7.41, m), HMBC: 137.1 (C-5″), 150.8 (C-2″), 123.3 (C-4″) |
| 7″ | / | / | / | 164.7, C | |
| OH/H2O | 3.722 | 4.491, 3.442 | |||
| Treatment | IC50 ± SD a (µM) | Fres b | Fsen c | |
|---|---|---|---|---|
| HepG2 | HepG2/Dox | |||
| Doxorubicin | 0.17 ± 0.024 | 6.03 ± 1.15 | 35.5 | 1 |
| + compound 1 (5 µM) | / | 2.33 ± 1.37 *** | 13.7 | 2.6 |
| + compound 1 (10 µM) | / | 0.85 ± 0.16 **** | 5.0 | 7.1 |
| + compound 1a (5 µM) | / | 0.32 ± 0.11 **** | 1.9 | 18.8 |
| + compound 1a (10 µM) | / | 0.70 ± 0.38 **** | 4.1 | 8.6 |
| + VRP (10 µM) | / | 0.19 ± 0.06 **** | 1.1 | 31.7 |
| Paclitaxel | 0.031 ± 0.002 | 4.03 ± 0.06 | 130.0 | 1 |
| + compound 1 (5 µM) | / | 0.12 ± 0.04 **** | 3.9 | 33.6 |
| + compound 1 (10 µM) | / | 0.034 ± 0.002 **** | 1.1 | 118.5 |
| + compound 1a (5 µM) | / | 0.026 ± 0.004 **** | 0.8 | 155.0 |
| + compound 1a (10 µM) | / | 0.012 ± 0.006 **** | 0.4 | 335.8 |
| + VRP (10 µM) | / | 0.039 ± 0.005 **** | 1.3 | 103.3 |
| Vinblastine | 0.017 ± 0.004 | 2.38 ± 0.62 | 140.0 | 1 |
| + compound 1 (5 µM) | / | 0.026 ± 0.001 **** | 1.5 | 91.5 |
| + compound 1 (10 µM) | / | 0.012 ± 0.002 **** | 0.7 | 198.3 |
| + compound 1a (5 µM) | / | 0.030 ± 0.0055 **** | 1.8 | 79.3 |
| + compound 1a (10 µM) | / | 0.017 ± 0.001 **** | 1.0 | 140.0 |
| + VRP (10 µM) | / | 0.036 ± 0.006 **** | 2.1 | 66.1 |
| Compound | Efflux Ratio | A→B Transport | B→A Transport | ||
|---|---|---|---|---|---|
| Papp | Solution Recovery % | Papp | Solution Recovery % | ||
| Compound 1 | 0.83 | 9.28 ± 0.88 | 44.6 ± 2.3 | 7.71 ± 0.61 | 50.4 ± 1.7 |
| Compound 1a | 0.89 | 0.27 ± 0.03 | 5.3 ± 0.5 | 0.24 ± 0.02 | 12.2 ± 0.7 |
| Digoxin | 181.00 | 0.12 ± 0.02 | 97.9 ± 8.1 | 22.3 ± 1.02 | 94.4 ± 1.9 |
| Receptor | Analyte | KD (M) | Ka (M−1s−1) | Kd (s−1) |
|---|---|---|---|---|
| Human ABCB1 | 1 | 5.53 × 10−6 | 6.58 × 104 | 3.64 × 10−1 |
| 1a | 3.72 × 10−6 | 4.16 × 104 | 1.55 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wu, H.; Wu, T.; Shen, X.; Hu, Y. Polyoxypregnane Aryl Esters Prepared from Metaplexis japonica (Thunb.) Makino and Their Role in Reversing Multidrug Resistance in HepG2/Dox Cells. Pharmaceuticals 2025, 18, 1187. https://doi.org/10.3390/ph18081187
Guo Y, Wu H, Wu T, Shen X, Hu Y. Polyoxypregnane Aryl Esters Prepared from Metaplexis japonica (Thunb.) Makino and Their Role in Reversing Multidrug Resistance in HepG2/Dox Cells. Pharmaceuticals. 2025; 18(8):1187. https://doi.org/10.3390/ph18081187
Chicago/Turabian StyleGuo, Yujia, Huiwen Wu, Taorui Wu, Xiaoling Shen, and Yingjie Hu. 2025. "Polyoxypregnane Aryl Esters Prepared from Metaplexis japonica (Thunb.) Makino and Their Role in Reversing Multidrug Resistance in HepG2/Dox Cells" Pharmaceuticals 18, no. 8: 1187. https://doi.org/10.3390/ph18081187
APA StyleGuo, Y., Wu, H., Wu, T., Shen, X., & Hu, Y. (2025). Polyoxypregnane Aryl Esters Prepared from Metaplexis japonica (Thunb.) Makino and Their Role in Reversing Multidrug Resistance in HepG2/Dox Cells. Pharmaceuticals, 18(8), 1187. https://doi.org/10.3390/ph18081187








