Nuclear Magnetic Resonance Based Metabolomics—A Rising Star in Traditional Chinese Medicine Research
Abstract
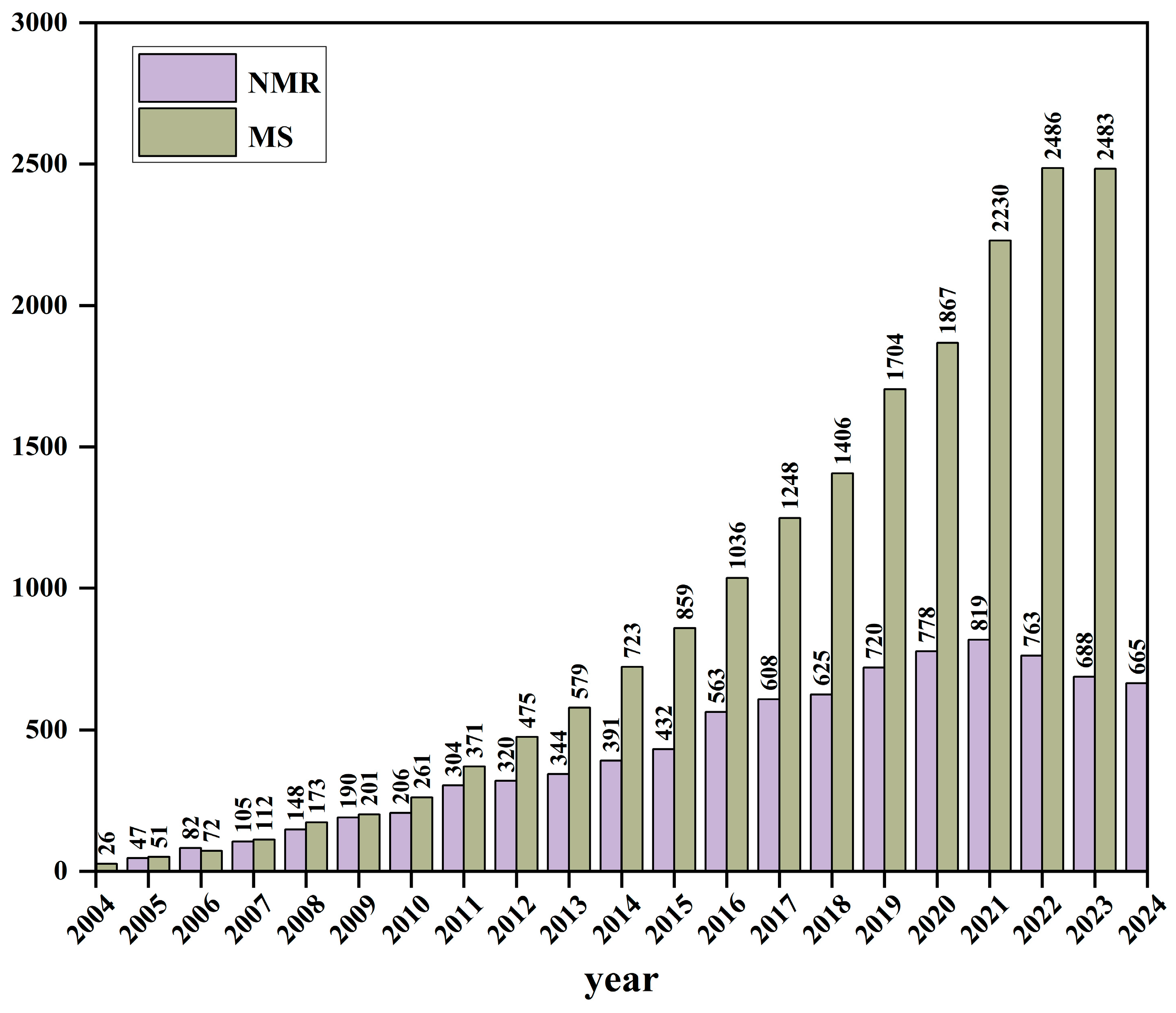
1. Introduction
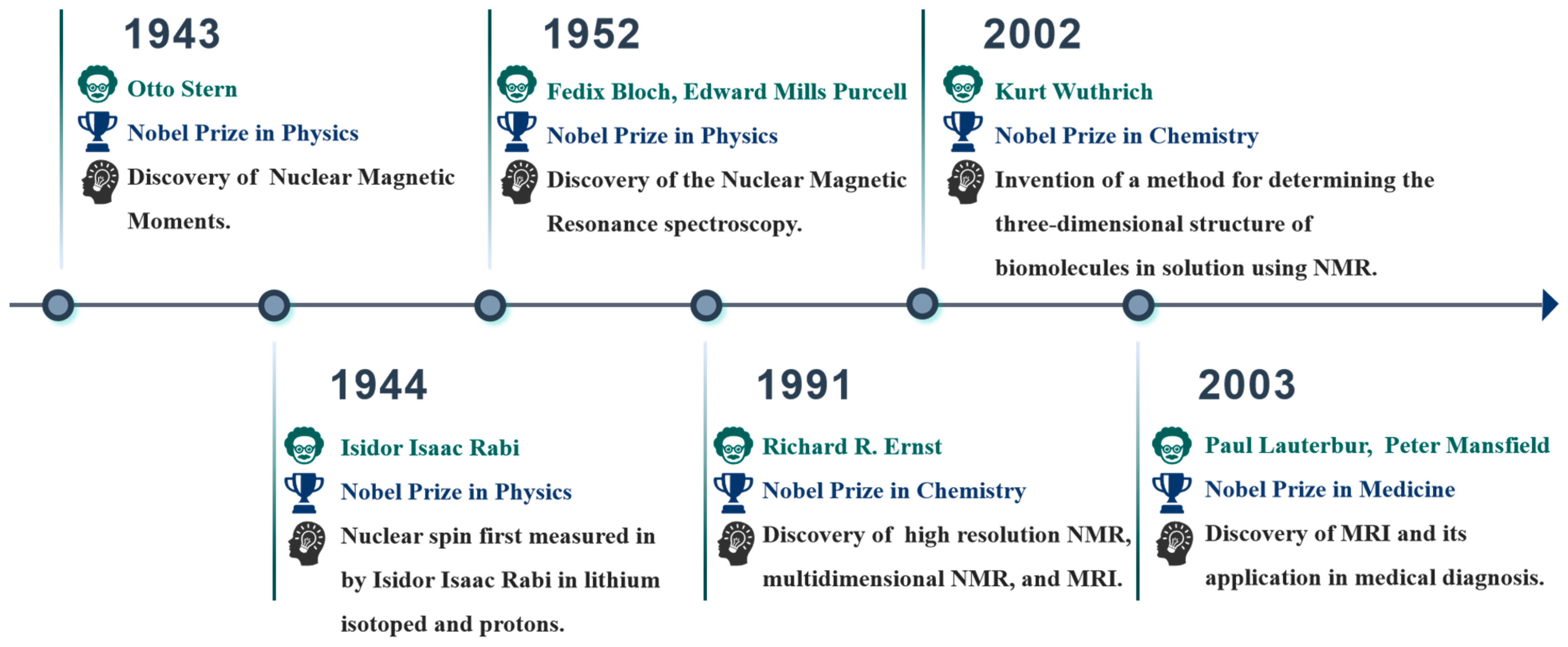
2. Introduction to NMR
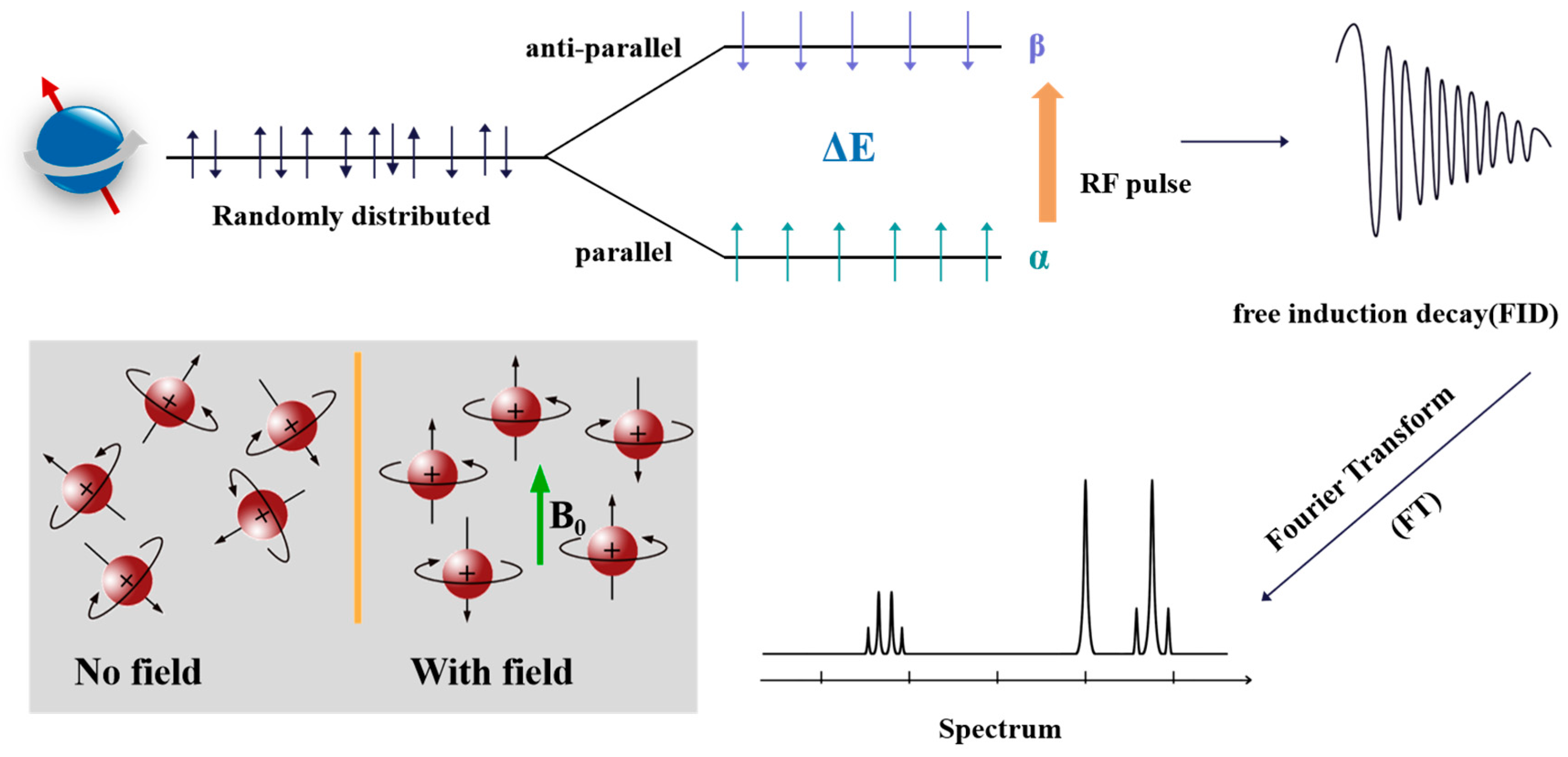
2.1. Basic Principles
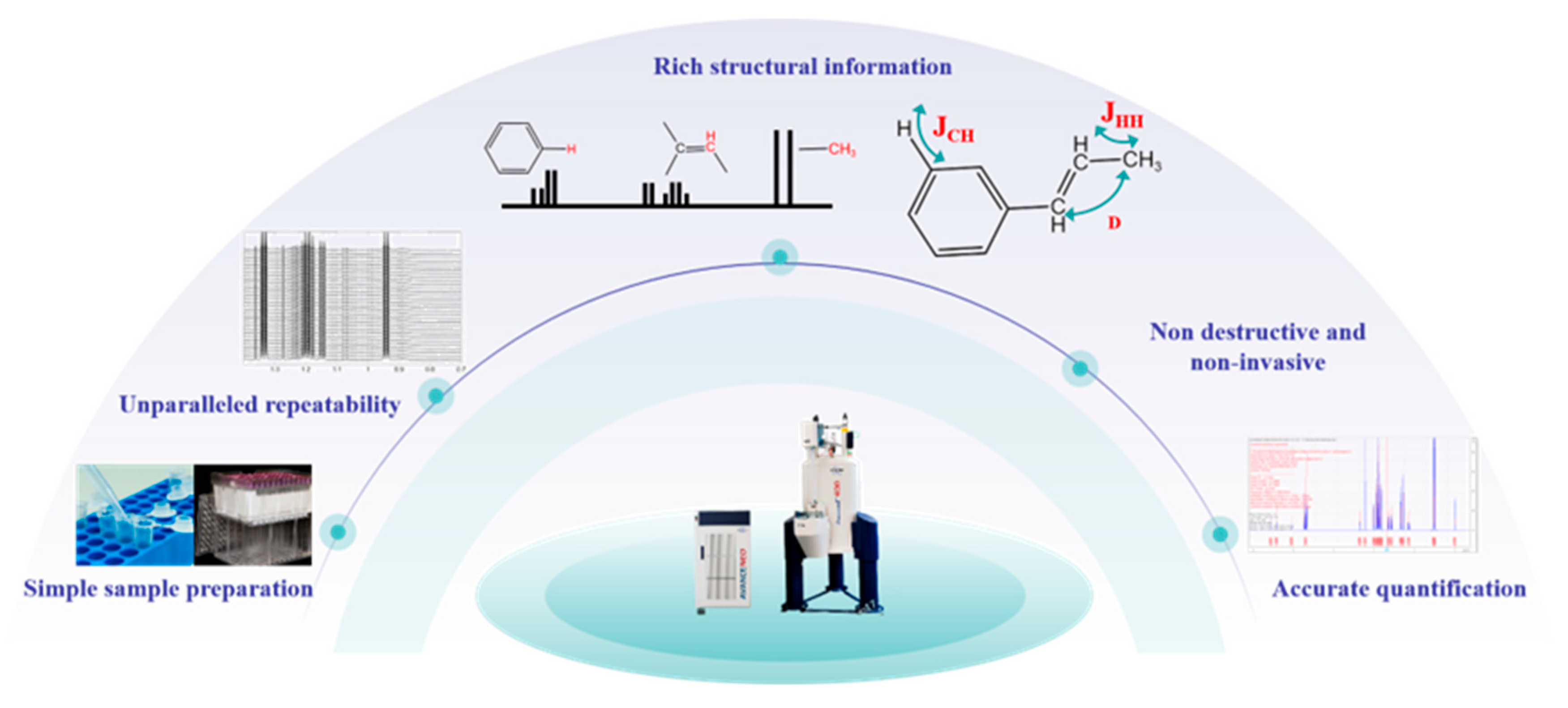
2.2. Characteristics of NMR
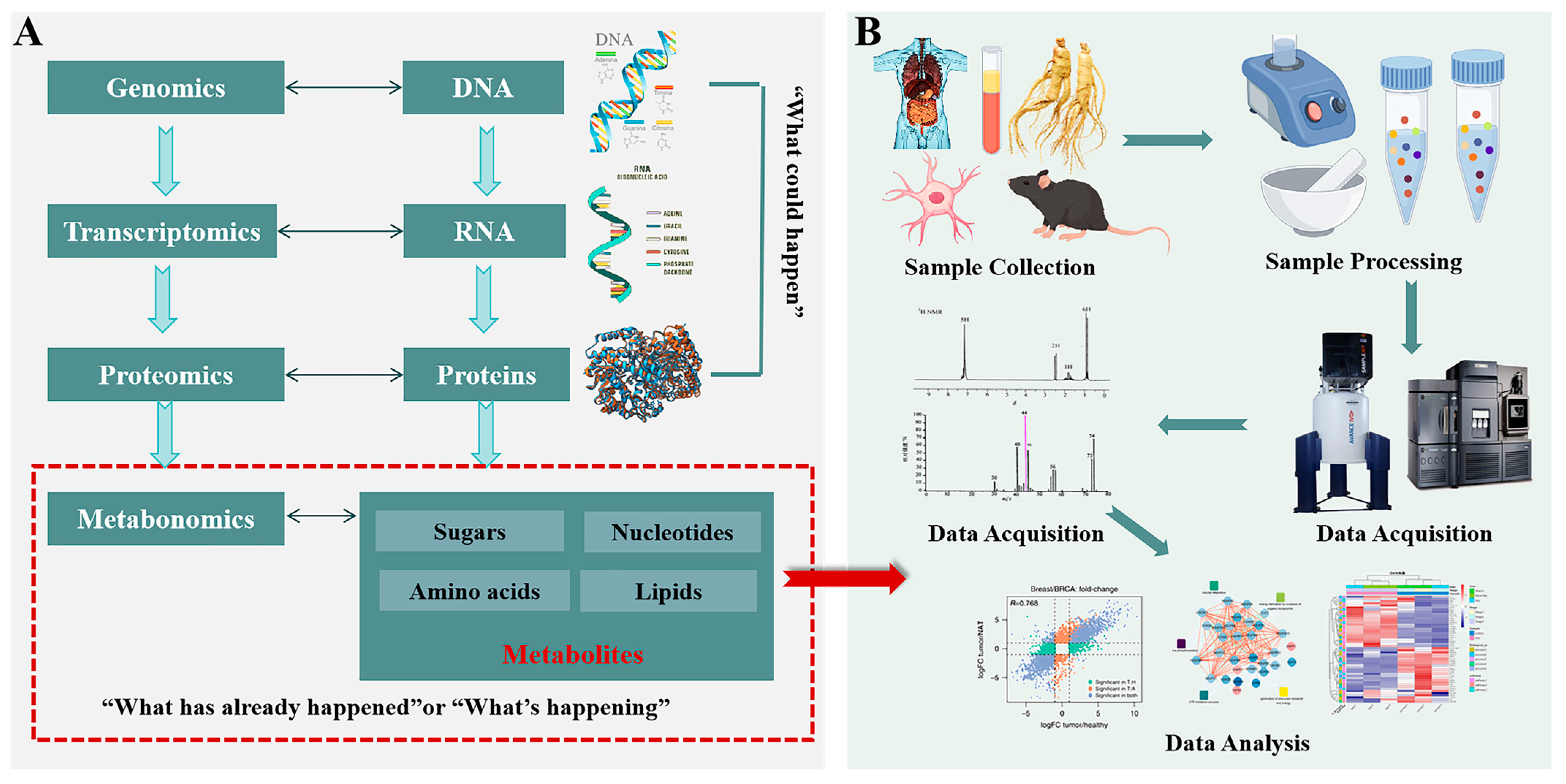
3. NMR-Based Metabolomics
3.1. Sample Preparation
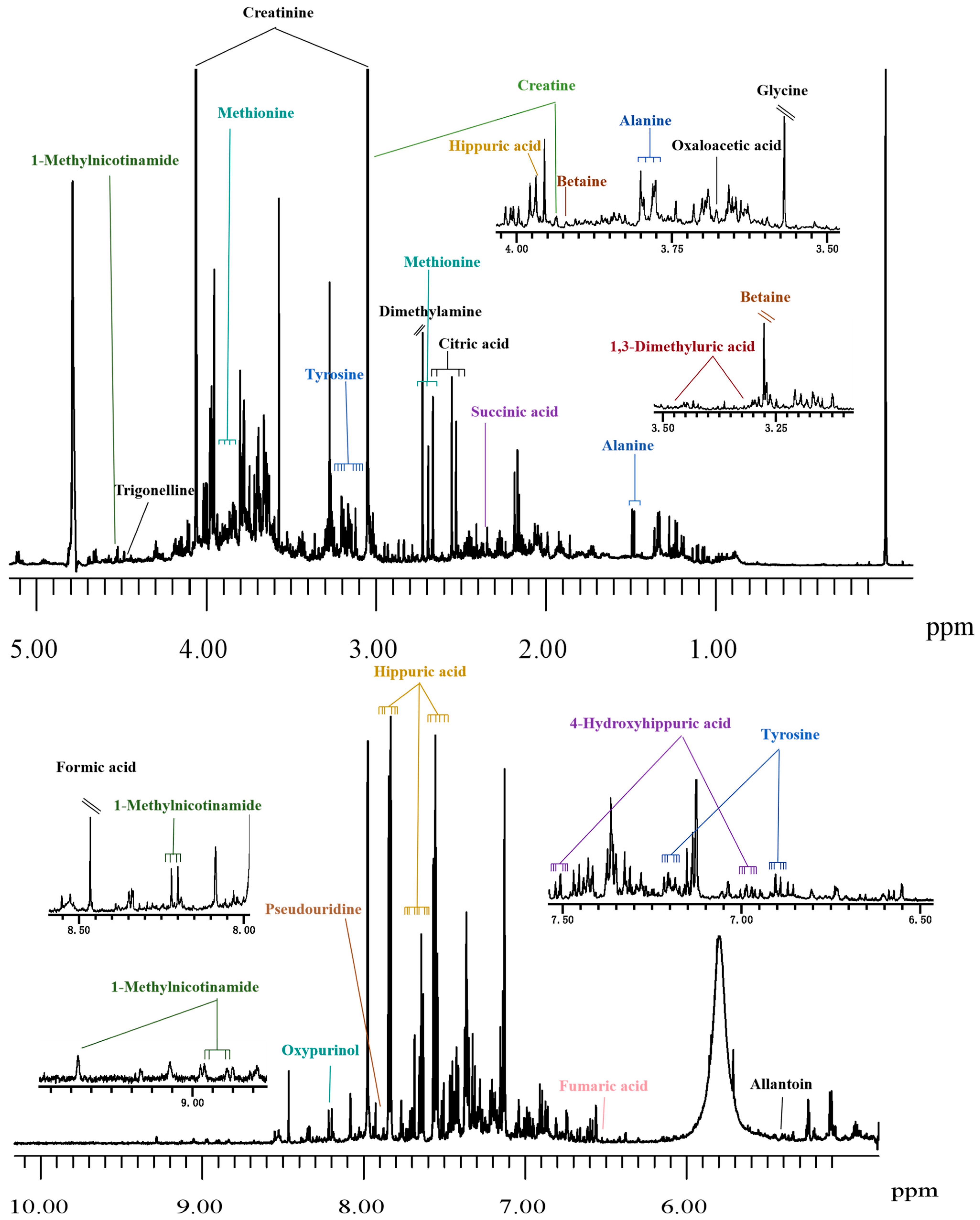
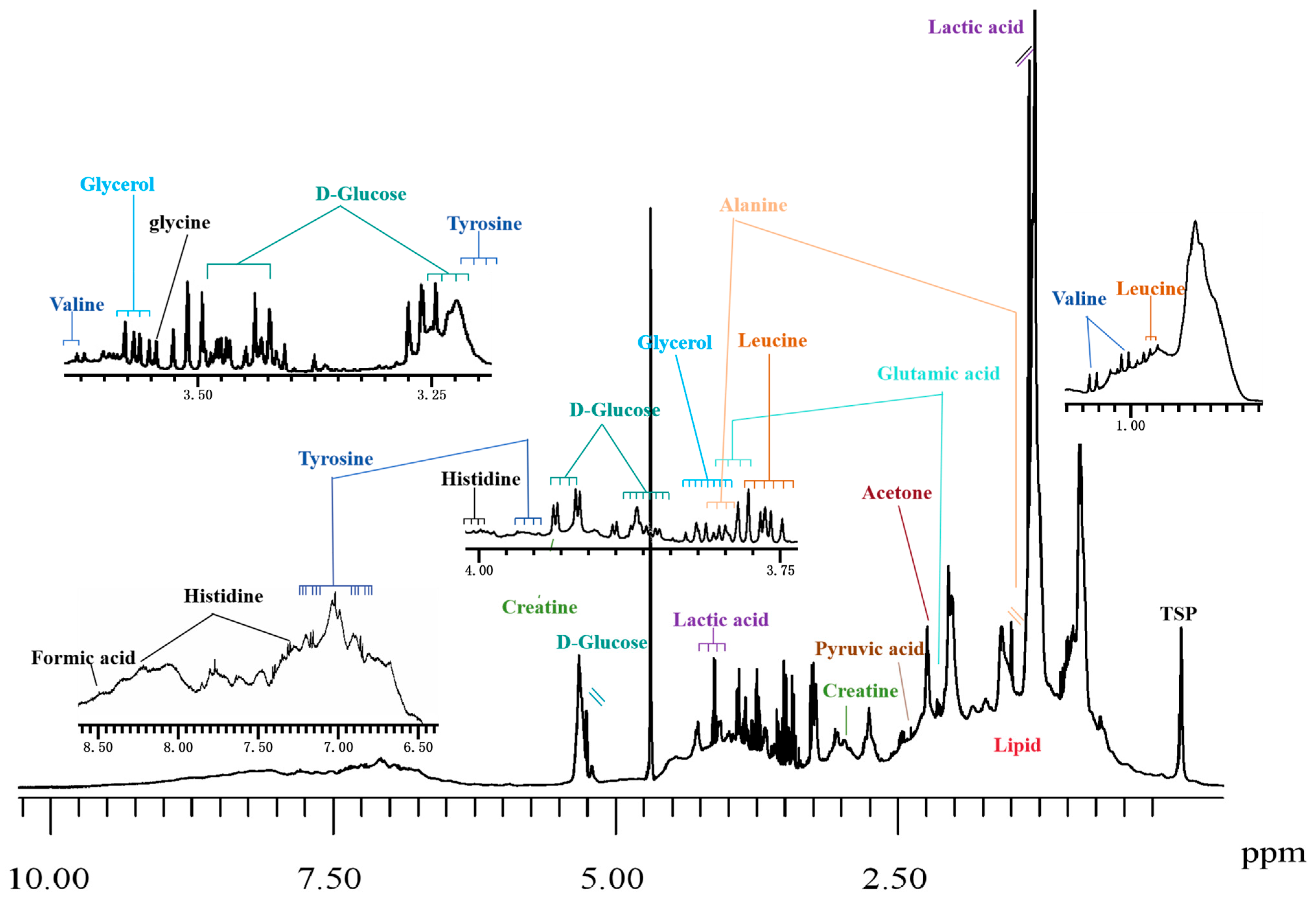
3.2. Data Acquisition
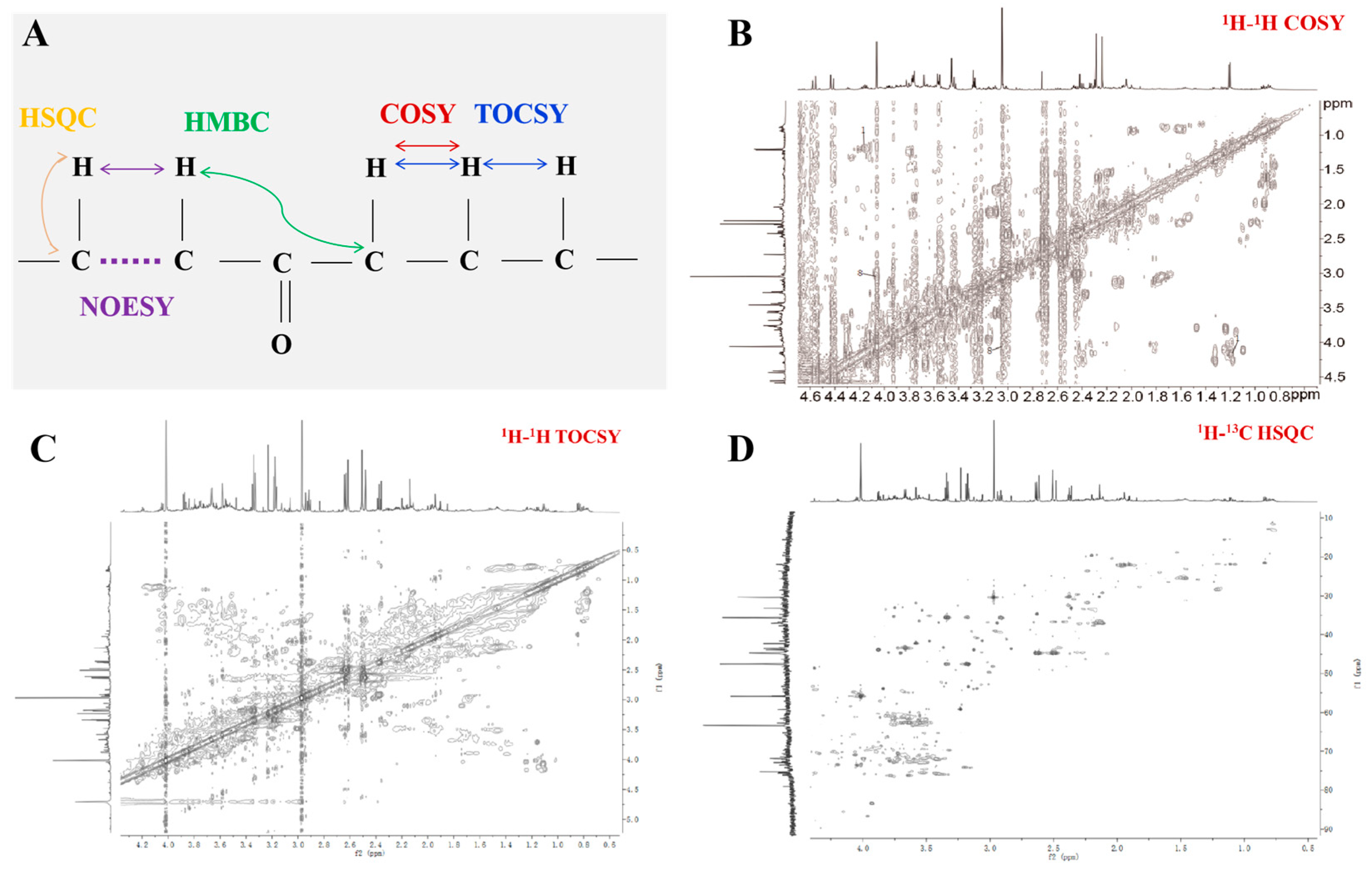
3.3. Data Processing
4. Application of NMR-Based Metabolomics in TCM Research
4.1. Discovery of Syndrome/Disease Biomarkers and Evaluation of the Efficacy of TCM
4.2. Exploration on the Toxicity and Detoxification Mechanisms of Toxic TCM
4.3. Decipher the Compatibility Rules of TCM Prescriptions
4.4. Quality Evaluation of Traditional Chinese Medicine
5. Challenges and Prospects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCM | Nuclear magnetic resonance |
| NMR | Mass spectrometry |
| MS | Radio frequency |
| RF | Cerebrospinal fluid |
| CSF | Carr-Purcell-Meiboom-Gill |
| CPMG | Signal-to-noise ratio |
| S/N | Nuclear Overhauser enhancement spectroscopy |
| NOESY | 1H-1H correlation spectroscopy |
| COSY | 1H-1H total correlation spectroscopy |
| TOCSY | Heteronuclear single quantum coherence spectroscopy |
| HSQC | Heteronuclear multiple quantum correlation spectroscopy |
| HMQC | Heteronuclear multiple bond correction spectroscopy |
| HMBC | Principal component analysis |
| PCA | Nasopharyngeal carcinoma |
| NPC | Induction chemotherapy |
| IC | Xiaoyao-San |
| XYS | Zhusha Anshen Wan |
| ZSASW | The Shugan group |
| SG | The Jianpi group |
| JP | Niuhuang Jiedu tablet |
| NJT | Radix Bupleuri |
| RB | Artificial intelligence |
| AI | Nuclear magnetic resonance |
References
- Chen, Y.Z.; Yuan, M.Y.; Chen, Y.L.; Zhang, X.; Xu, X.T.; Liu, S.L.; Liu, S.L.; Zou, X.; Tao, J.L.; Qiang, Y.H.; et al. The Gut Microbiota and Traditional Chinese Medicine: A New Clinical Frontier on Cancer. Curr. Drug Targets 2021, 22, 1222–1231. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, M.; Zeng, L.; Zhao, K.; Wang, C.; Sun, T.; Li, Z.; Liu, R. Role of traditional Chinese medicine in ameliorating mitochondrial dysfunction via non-coding RNA signaling: Implication in the treatment of neurodegenerative diseases. Front. Pharmacol. 2023, 14, 1123188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.W.; Liu, H.M.; Jia, X.H. The Role and Mechanisms of Traditional Chinese Medicine for Airway Inflammation and Remodeling in Asthma: Overview and Progress. Front. Pharmacol. 2022, 13, 917256. [Google Scholar] [CrossRef]
- Hu, T.; Li, L.; Ma, Q. Research Progress of Immunomodulation on Anti-COVID-19 and the Effective Components from Traditional Chinese Medicine. Am. J. Chin. Med. 2023, 51, 1337–1360. [Google Scholar] [CrossRef]
- Huang, M.; Liu, Y.Y.; Xiong, K.; Yang, F.W.; Jin, X.Y.; Wang, Z.Q.; Zhang, J.H.; Zhang, B.L. The role and advantage of traditional Chinese medicine in the prevention and treatment of COVID-19. J. Integr. Med. 2023, 21, 407–412. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Z.; Sun, X.; Zhong, N.; Liu, Z. Application of Traditional Chinese Medicine and Systems Pharmacology in Drug Prevention and Treatment against COVID-19. Am. J. Chin. Med. 2021, 49, 1045–1061. [Google Scholar] [CrossRef]
- Lin, A.X.; Chan, G.; Hu, Y.; Ouyang, D.; Ung, C.O.L.; Shi, L.; Hu, H. Internationalization of traditional Chinese medicine: Current international market, internationalization challenges and prospective suggestions. Chin. Med. 2018, 13, 9. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm. Sin. B 2022, 12, 4011–4039. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Muthubharathi, B.C.; Gowripriya, T.; Balamurugan, K. Metabolomics: Small molecules that matter more. Mol. Omics 2021, 17, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, M.J.; Powers, R. Multiplatform untargeted metabolomics. Magn. Reson. Chem. 2023, 61, 628–653. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics--the link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Li, X.; Li, X.; Wang, J.; He, J.; Wu, X.; Chen, S.; Zhang, Y.; Li, Y. Integrated Multi-Omics Techniques and Network Pharmacology Analysis to Explore the Material Basis and Mechanism of Simiao Pill in the Treatment of Rheumatoid Arthritis. ACS Omega 2023, 8, 11138–11150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ju, Y.; Qin, X. Studies on the compatibility mechanism and material basis of Danggui Buxue Decoction against anemia mice using metabonomics and network pharmacology. J. Pharm. Pharmacol. 2021, 73, 767–777. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Wang, S.; Qin, X. Gut microbiome and tissue metabolomics reveal the compatibility effects of Xiaoyaosan on depression based on “gut-liver-kidney” axis. Phytomedicine 2023, 111, 154628. [Google Scholar] [CrossRef]
- Ren, J.L.; Yang, L.; Qiu, S.; Zhang, A.H.; Wang, X.J. Efficacy evaluation, active ingredients, and multitarget exploration of herbal medicine. Trends Endocrinol. Metab. 2023, 34, 146–157. [Google Scholar] [CrossRef]
- Li, Q.; Ren, J.; Yang, L.; Sun, H.; Zhang, X.; Yan, G.; Han, Y.; Wang, X. Parsing the Q-Markers of Baoyin Jian to Treat Abnormal Uterine Bleeding by High-Throughput Chinmedomics Strategy. Pharmaceuticals 2023, 16, 719. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Zhang, X.; Sun, Y.; Sun, H.; Yan, G.; Zhao, Q.; Han, Y.; Wang, X. Quality marker discovery of Danggui Jianzhong decoction for treating primary dysmenorrhoea based on chinmedomics strategy. Phytomedicine 2023, 115, 154724. [Google Scholar] [CrossRef] [PubMed]
- Liebal, U.W.; Phan, A.N.T.; Sudhakar, M.; Raman, K.; Blank, L.M. Machine Learning Applications for Mass Spectrometry-Based Metabolomics. Metabolites 2020, 10, 243. [Google Scholar] [CrossRef]
- Lenz, E.M.; Wilson, I.D. Analytical strategies in metabonomics. J. Proteome Res. 2007, 6, 443–458. [Google Scholar] [CrossRef]
- Chen, C.J.; Lee, D.Y.; Yu, J.; Lin, Y.N.; Lin, T.M. Recent advances in LC-MS-based metabolomics for clinical biomarker discovery. Mass. Spectrom. Rev. 2023, 42, 2349–2378. [Google Scholar] [CrossRef]
- Marshall, D.D.; Powers, R. Beyond the paradigm: Combining mass spectrometry and nuclear magnetic resonance for metabolomics. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 100, 1–16. [Google Scholar] [CrossRef]
- Wishart, D.S.; Cheng, L.L.; Copié, V.; Edison, A.S.; Eghbalnia, H.R.; Hoch, J.C.; Gouveia, G.J.; Pathmasiri, W.; Powers, R.; Schock, T.B.; et al. NMR and Metabolomics-A Roadmap for the Future. Metabolites 2022, 12, 678. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef]
- Novak, R.F.; Koop, D.R.; Hollenberg, P.F. Liver microsomal metabolism of N-methylcarbazole: Structural identification of the four major metabolites of N-methylcarbazole using 1H Fourier transform NMR spectroscopy. Mol. Pharmacol. 1980, 17, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Menick, B.J.; Bobman, S.A.; Listerud, J.; Atlas, S.W. Thin-section, three-dimensional Fourier transform, steady-state free precession MR imaging of the brain. Radiology 1992, 183, 369–377. [Google Scholar] [CrossRef]
- Bhattacharya, A. Chemistry: Breaking the billion-hertz barrier. Nature 2010, 463, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, W.; Bacher, A. Advances of high-resolution NMR techniques in the structural and metabolic analysis of plant biochemistry. Phytochemistry 2007, 68, 2799–2815. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, K.; Wen, Y.; Wang, P.; Hu, Y.; Lai, Y.; Wang, Y.; Zhao, K.; Tang, S.; Zhang, A.; et al. Screening and diagnosis of cardiovascular disease using artificial intelligence-enabled cardiac magnetic resonance imaging. Nat. Med. 2024, 30, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Meng, X.; Wang, X.; Han, F.; Xing, H.; Lv, G.; Zhang, L.; Wu, S.; Jiang, X.; et al. A FAPα-activated MRI nanoprobe for precise grading diagnosis of clinical liver fibrosis. Nat. Commun. 2024, 15, 8036. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, T.Y.; Hong, J.H.; Cheng, T.J.; Lin, C.Y. NMR-based metabolomics to determine acute inhalation effects of nano- and fine-sized ZnO particles in the rat lung. Nanotoxicology 2016, 10, 924–934. [Google Scholar] [CrossRef]
- Stopyra, M.A.; Friederich, H.C.; Lavandier, N.; Mönning, E.; Bendszus, M.; Herzog, W.; Simon, J.J. Homeostasis and food craving in obesity: A functional MRI study. Int. J. Obes. 2021, 45, 2464–2470. [Google Scholar] [CrossRef]
- Liu, Y.; Basty, N.; Whitcher, B.; Bell, J.D.; Sorokin, E.P.; van Bruggen, N.; Thomas, E.L.; Cule, M. Genetic architecture of 11 organ traits derived from abdominal MRI using deep learning. Elife 2021, 10, e65554. [Google Scholar] [CrossRef]
- Bothwell, J.H.; Griffin, J.L. An introduction to biological nuclear magnetic resonance spectroscopy. Biol. Rev. Camb. Philos. Soc. 2011, 86, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Theillet, F.X.; Luchinat, E. In-cell NMR: Why and how? Prog. Nucl. Magn. Reson. Spectrosc. 2022, 132–133, 1–112. [Google Scholar] [CrossRef]
- Tynkkynen, T.; Wang, Q.; Ekholm, J.; Anufrieva, O.; Ohukainen, P.; Vepsäläinen, J.; Männikkö, M.; Keinänen-Kiukaanniemi, S.; Holmes, M.V.; Goodwin, M.; et al. Proof of concept for quantitative urine NMR metabolomics pipeline for large-scale epidemiology and genetics. Int. J. Epidemiol. 2019, 48, 978–993. [Google Scholar] [CrossRef]
- Bizzarri, D.; Reinders, M.J.T.; Kuiper, L.; Beekman, M.; Deelen, J.; van Meurs, J.B.J.; van Dongen, J.; Pool, R.; Boomsma, D.I.; Ghanbari, M.; et al. NMR metabolomics-guided DNA methylation mortality predictors. EBioMedicine 2024, 107, 105279. [Google Scholar] [CrossRef] [PubMed]
- Oexner, R.R.; Ahn, H.; Theofilatos, K.; Shah, R.A.; Schmitt, R.; Chowienczyk, P.; Zoccarato, A.; Shah, A.M. Serum metabolomics improves risk stratification for incident heart failure. Eur. J. Heart Fail. 2024, 26, 829–840. [Google Scholar] [CrossRef]
- Yoshii, A.; McMillen, T.S.; Wang, Y.; Zhou, B.; Chen, H.; Banerjee, D.; Herrero, M.; Wang, P.; Muraoka, N.; Wang, W.; et al. Blunted Cardiac Mitophagy in Response to Metabolic Stress Contributes to HFpEF. Circ. Res. 2024, 135, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Mevenkamp, J.; Bruls, Y.M.H.; Mancilla, R.; Grevendonk, L.; Wildberger, J.E.; Brouwers, K.; Hesselink, M.K.C.; Schrauwen, P.; Hoeks, J.; Houtkooper, R.H.; et al. Development of a (31)P magnetic resonance spectroscopy technique to quantify NADH and NAD(+) at 3 T. Nat. Commun. 2024, 15, 9159. [Google Scholar] [CrossRef]
- Robitaille, P.M.; Merkle, H.; Sako, E.; Lang, G.; Clack, R.M.; Bianco, R.; From, A.H.; Foker, J.; Uğurbil, K. Measurement of ATP synthesis rates by 31P-NMR spectroscopy in the intact myocardium in vivo. Magn. Reson. Med. 1990, 15, 8–24. [Google Scholar] [CrossRef]
- Hoult, D.I.; Busby, S.J.; Gadian, D.G.; Radda, G.K.; Richards, R.E.; Seeley, P.J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature 1974, 252, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Bittl, J.A.; Ingwall, J.S. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J. Biol. Chem. 1985, 260, 3512–3517. [Google Scholar] [CrossRef]
- Sehr, P.A.; Bore, P.J.; Papatheofanis, J.; Radda, G.K. Non-destructive measurement of metabolites and tissue pH in the kidney by 31P nuclear magnetic resonance. Br. J. Exp. Pathol. 1979, 60, 632–641. [Google Scholar]
- Nagana Gowda, G.A.; Raftery, D. NMR-Based Metabolomics. Adv. Exp. Med. Biol. 2021, 1280, 19–37. [Google Scholar] [CrossRef]
- Düz, S.A.; Mumcu, A.; Doğan, B.; Sarıdoğan, E.; Tuncay, G.; Onat, T.; Karaer, A. Metabolomics approach using HR-MAS NMR spectroscopy for the assessment of metabolic profiles of uterine fibroids. Anal. Biochem. 2025, 704, 115885. [Google Scholar] [CrossRef] [PubMed]
- Gogiashvili, M.; Nowacki, J.; Hergenröder, R.; Hengstler, J.G.; Lambert, J.; Edlund, K. HR-MAS NMR Based Quantitative Metabolomics in Breast Cancer. Metabolites 2019, 9, 19. [Google Scholar] [CrossRef]
- Arda Düz, S.; Mumcu, A.; Doğan, B.; Yılmaz, E.; İnci Çoşkun, E.; Sarıdogan, E.; Tuncay, G.; Karaer, A. Metabolomic analysis of endometrial cancer by high-resolution magic angle spinning NMR spectroscopy. Arch. Gynecol. Obstet. 2022, 306, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Cacciatore, S.; Zerbini, L.F. Metabolite Analyses Using Nuclear Magnetic Resonance (NMR) Spectroscopy in Plasma of Patients with Prostate Cancer. Methods Mol. Biol. 2023, 2675, 195–204. [Google Scholar] [CrossRef]
- Borges, R.M.; Ferreira, G.A.; Campos, M.M.; Teixeira, A.M.; Costa, F.D.N.; das Chagas, F.O.; Colonna, M. NMR as a tool for compound identification in mixtures. Phytochem. Anal. 2023, 34, 385–392. [Google Scholar] [CrossRef]
- van Beek, T.A. Low-field benchtop NMR spectroscopy: Status and prospects in natural product analysis(†). Phytochem. Anal. 2021, 32, 24–37. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. Quantitative NMR Methods in Metabolomics. Handb. Exp. Pharmacol. 2023, 277, 143–164. [Google Scholar] [CrossRef]
- Mo, H.; Raftery, D. Solvent signal as an NMR concentration reference. Anal. Chem. 2008, 80, 9835–9839. [Google Scholar] [CrossRef]
- Letertre, M.P.M.; Giraudeau, P.; de Tullio, P. Nuclear Magnetic Resonance Spectroscopy in Clinical Metabolomics and Personalized Medicine: Current Challenges and Perspectives. Front. Mol. Biosci. 2021, 8, 698337. [Google Scholar] [CrossRef]
- Styles, P.; Soffe, N.F.; Scott, C.A.; Cragg, D.A.; Row, F.; White, D.J.; White, P.C. A high-resolution NMR probe in which the coil and preamplifier are cooled with liquid helium. 1984. J. Magn. Reson. 2011, 213, 347–354. [Google Scholar] [CrossRef]
- Wang, J.H.; Byun, J.; Pennathur, S. Analytical approaches to metabolomics and applications to systems biology. Semin. Nephrol. 2010, 30, 500–511. [Google Scholar] [CrossRef]
- Tang, Y.P.; Xu, D.Q.; Yue, S.J.; Chen, Y.Y.; Fu, R.J.; Bai, X. Modern research thoughts and methods on bio-active components of TCM formulae. Chin. J. Nat. Med. 2022, 20, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A. Metabolomics- and systems-biology-guided discovery of metabolite lead compounds and druggable targets. Drug Discov. Today 2023, 28, 103460. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Deng, D.; Pan, C.; Ren, J.; Wei, T.; Wu, Z.; Zhang, B.; Li, S.; Yin, P.; Shang, D. Integration of transcriptomics, proteomics, and metabolomics data to reveal HER2-associated metabolic heterogeneity in gastric cancer with response to immunotherapy and neoadjuvant chemotherapy. Front. Immunol. 2022, 13, 951137. [Google Scholar] [CrossRef] [PubMed]
- Aerqin, Q.; Wang, Z.T.; Wu, K.M.; He, X.Y.; Dong, Q.; Yu, J.T. Omics-based biomarkers discovery for Alzheimer’s disease. Cell Mol. Life Sci. 2022, 79, 585. [Google Scholar] [CrossRef]
- Horgusluoglu, E.; Neff, R.; Song, W.M.; Wang, M.; Wang, Q.; Arnold, M.; Krumsiek, J.; Galindo-Prieto, B.; Ming, C.; Nho, K.; et al. Integrative metabolomics-genomics approach reveals key metabolic pathways and regulators of Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, 1260–1278. [Google Scholar] [CrossRef]
- Yan, L.; Su, P.; Sun, X. Role of multi-omics in advancing the understanding and treatment of prostate cancer (Review). Mol. Med. Rep. 2025, 31, 130. [Google Scholar] [CrossRef]
- Gu, C.; Hu, X.; Shan, B.; Wu, X.; Chen, J. Targeted and non-targeted metabolomics uncovering the effects of Er-Miao-Wan formula on rats with hyperuricemia. J. Pharm. Biomed. Anal. 2023, 226, 115246. [Google Scholar] [CrossRef]
- Ding, M.; Fan, J.L.; Huang, D.F.; Jiang, Y.; Li, M.N.; Zheng, Y.Q.; Yang, X.P.; Li, P.; Yang, H. From non-targeted to targeted GC-MS metabolomics strategy for identification of TCM preparations containing natural and artificial musk. Chin. Med. 2022, 17, 41. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Qin, X.; Du, G.; Zhou, Y. Integration of Non-Targeted Metabolomics and Targeted Quantitative Analysis to Elucidate the Synergistic Antidepressant Effect of Bupleurum Chinense DC-Paeonia Lactiflora Pall Herb Pair by Regulating Purine Metabolism. Front. Pharmacol. 2022, 13, 900459. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.; Haseeb, Y.B.; Haseeb, S. Metabolomics Work Flow and Analytics in Systems Biology. Curr. Mol. Med. 2022, 22, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Van Houcke, S.K.; Thienpont, L.M. “Good samples make good assays”—The problem of sourcing clinical samples for a standardization project. Clin. Chem. Lab. Med. 2013, 51, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Yuille, M.; Illig, T.; Hveem, K.; Schmitz, G.; Hansen, J.; Neumaier, M.; Tybring, G.; Wichmann, E.; Ollier, B. Laboratory management of samples in biobanks: European consensus expert group report. Biopreserv. Biobank. 2010, 8, 65–69. [Google Scholar] [CrossRef]
- Bernini, P.; Bertini, I.; Luchinat, C.; Nincheri, P.; Staderini, S.; Turano, P. Standard operating procedures for pre-analytical handling of blood and urine for metabolomic studies and biobanks. J. Biomol. NMR 2011, 49, 231–243. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Buckingham, M.J.; Sadler, P.J. High resolution 1H n.m.r. studies of vertebrate blood and plasma. Biochem. J. 1983, 211, 605–615. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. Whole Blood Metabolomics by (1)H NMR Spectroscopy Provides a New Opportunity To Evaluate Coenzymes and Antioxidants. Anal. Chem. 2017, 89, 4620–4627. [Google Scholar] [CrossRef]
- Cheng, L.L. High-resolution magic angle spinning NMR for intact biological specimen analysis: Initial discovery, recent developments, and future directions. NMR Biomed. 2023, 36, e4684. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Harris, N.G.; Williams, S.R.; Bhakoo, K.K. A comparison of cell and tissue extraction techniques using high-resolution 1H-NMR spectroscopy. NMR Biomed. 2002, 15, 37–44. [Google Scholar] [CrossRef]
- Bothwell, J.H.; Rae, C.; Dixon, R.M.; Styles, P.; Bhakoo, K.K. Hypo-osmotic swelling-activated release of organic osmolytes in brain slices: Implications for brain oedema in vivo. J. Neurochem. 2001, 77, 1632–1640. [Google Scholar] [CrossRef]
- Haslauer, K.E.; Hemmler, D.; Schmitt-Kopplin, P.; Heinzmann, S.S. Guidelines for the Use of Deuterium Oxide (D(2)O) in (1)H NMR Metabolomics. Anal. Chem. 2019, 91, 11063–11069. [Google Scholar] [CrossRef]
- Beteinakis, S.; Papachristodoulou, A.; Mikros, E.; Halabalaki, M. From sample preparation to NMR-based metabolic profiling in food commodities: The case of table olives. Phytochem. Anal. 2022, 33, 83–93. [Google Scholar] [CrossRef]
- Xiong, W.; Zirpel, F.; Cader, M.Z.; Anthony, D.C.; Probert, F. Extraction Methods for Brain Biopsy NMR Metabolomics: Balancing Metabolite Stability and Protein Precipitation. Metabolites 2024, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR Nomenclature: Nuclear Spin Properties and Conventions for Chemical Shifts. IUPAC Recommendations 2001. Solid State Nucl. Magn. Reson. 2002, 22, 458–483. [Google Scholar] [CrossRef] [PubMed]
- Nazarski, R.B. On the Use of Deuterated Organic Solvents without TMS to Report (1)H/(13)C NMR Spectral Data of Organic Compounds: Current State of the Method, Its Pitfalls and Benefits, and Related Issues. Molecules 2023, 28, 4369. [Google Scholar] [CrossRef]
- Guzman, A.L.; Hoye, T.R. TMS is Superior to Residual CHCl(3) for Use as the Internal Reference for Routine (1)H NMR Spectra Recorded in CDCl(3). J. Org. Chem. 2022, 87, 905–909. [Google Scholar] [CrossRef]
- Shimizu, A.; Ikeguchi, M.; Sugai, S. Appropriateness of DSS and TSP as internal references for (1)H NMR studies of molten globule proteins in aqueous media. J. Biomol. NMR 1994, 4, 859–862. [Google Scholar] [CrossRef]
- Ye, W.; Lin, Y.; Bezabeh, T.; Ma, C.; Liang, J.; Zhao, J.; Ouyang, T.; Tang, W.; Wu, R. (1) H NMR-based metabolomics of paired esophageal tumor tissues and serum samples identifies specific serum biomarkers for esophageal cancer. NMR Biomed. 2021, 34, e4505. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Foxall, P.J.; Spraul, M.; Farrant, R.D.; Lindon, J.C. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal. Chem. 1995, 67, 793–811. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Piotto, M.; Saudek, V.; Sklenár, V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR 1992, 2, 661–665. [Google Scholar] [CrossRef]
- Ogg, R.J.; Kingsley, P.B.; Taylor, J.S. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J. Magn. Reson. B 1994, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Brown, S.A. Purge NMR: Effective and easy solvent suppression. J. Magn. Reson. 2005, 175, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, A.; Tayyari, F.; Edison, A.S. Alternatives to Nuclear Overhauser Enhancement Spectroscopy Presat and Carr-Purcell-Meiboom-Gill Presat for NMR-Based Metabolomics. Anal. Chem. 2017, 89, 8582–8588. [Google Scholar] [CrossRef]
- Zambrello, M.A.; Craft, D.L.; Hoch, J.C.; Rovnyak, D.; Schuyler, A.D. The influence of the probability density function on spectral quality in nonuniformly sampled multidimensional NMR. J. Magn. Reson. 2020, 311, 106671. [Google Scholar] [CrossRef]
- Mora-Ortiz, M.; Nuñez Ramos, P.; Oregioni, A.; Claus, S.P. NMR metabolomics identifies over 60 biomarkers associated with Type II Diabetes impairment in db/db mice. Metabolomics 2019, 15, 89. [Google Scholar] [CrossRef]
- Humphries, T.L.R.; Gobe, G.C.; Urquhart, A.J.; Ellis, R.J.; Galloway, G.J.; Vesey, D.A.; Francis, R.S. Identifying biochemical changes in the kidney using proton nuclear magnetic resonance in an adenine diet chronic kidney disease mouse model. NMR Biomed. 2024, 37, e5257. [Google Scholar] [CrossRef] [PubMed]
- Rådjursöga, M.; Lindqvist, H.M.; Pedersen, A.; Karlsson, G.B.; Malmodin, D.; Brunius, C.; Ellegård, L.; Winkvist, A. The (1)H NMR serum metabolomics response to a two meal challenge: A cross-over dietary intervention study in healthy human volunteers. Nutr. J. 2019, 18, 25. [Google Scholar] [CrossRef]
- Da Silva, L.; Godejohann, M.; Martin, F.P.; Collino, S.; Bürkle, A.; Moreno-Villanueva, M.; Bernhardt, J.; Toussaint, O.; Grubeck-Loebenstein, B.; Gonos, E.S.; et al. High-resolution quantitative metabolome analysis of urine by automated flow injection NMR. Anal. Chem. 2013, 85, 5801–5809. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Kang, J.; He, Q.; Qi, Y.; Chen, X.; Wang, S.; Liang, S. A NMR-Based Metabonomics Approach to Determine Protective Effect of a Combination of Multiple Components Derived from Naodesheng on Ischemic Stroke Rats. Molecules 2019, 24, 1831. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wen, R.; Liu, Q.; Wang, J.; Lu, Y.; Zhao, M.; Guo, X.; Tu, P.; Jiang, Y. (1)H NMR-based dynamic metabolomics delineates the therapeutic effects of Baoyuan decoction on isoproterenol-induced cardiac hypertrophy. J. Pharm. Biomed. Anal. 2019, 163, 64–77. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Cui, Q.; Lewis, I.A.; Hegeman, A.D.; Anderson, M.E.; Li, J.; Schulte, C.F.; Westler, W.M.; Eghbalnia, H.R.; Sussman, M.R.; Markley, J.L. Metabolite identification via the Madison Metabolomics Consortium Database. Nat. Biotechnol. 2008, 26, 162–164. [Google Scholar] [CrossRef]
- Hoch, J.C.; Baskaran, K.; Burr, H.; Chin, J.; Eghbalnia, H.R.; Fujiwara, T.; Gryk, M.R.; Iwata, T.; Kojima, C.; Kurisu, G.; et al. Biological Magnetic Resonance Data Bank. Nucleic Acids Res. 2023, 51, D368–D376. [Google Scholar] [CrossRef]
- Lundberg, P.; Vogel, T.; Malusek, A.; Lundquist, P.O.; Cohen, L.; Dahlqvist Leinhard, O. MDL-The Magnetic Resonance Metabolomics Database (mdl.imv.liu.se). In Proceedings of the ESMRMB 22nd Annual Meeting, Basel, Switzerland, 15–18 September 2005. [Google Scholar]
- Padayachee, T.; Khamiakova, T.; Louis, E.; Adriaensens, P.; Burzykowski, T. The impact of the method of extracting metabolic signal from 1H-NMR data on the classification of samples: A case study of binning and BATMAN in lung cancer. PLoS ONE 2019, 14, e0211854. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, S.; Liu, P.; Bjorndahl, T.C.; Mandal, R.; Grant, J.R.; Wilson, M.; Eisner, R.; Sinelnikov, I.; Hu, X.; Luchinat, C.; et al. Accurate, fully-automated NMR spectral profiling for metabolomics. PLoS ONE 2015, 10, e0124219. [Google Scholar] [CrossRef]
- Röhnisch, H.E.; Eriksson, J.; Müllner, E.; Agback, P.; Sandström, C.; Moazzami, A.A. AQuA: An Automated Quantification Algorithm for High-Throughput NMR-Based Metabolomics and Its Application in Human Plasma. Anal. Chem. 2018, 90, 2095–2102. [Google Scholar] [CrossRef]
- Xia, J.; Bjorndahl, T.C.; Tang, P.; Wishart, D.S. MetaboMiner--semi-automated identification of metabolites from 2D NMR spectra of complex biofluids. BMC Bioinform. 2008, 9, 507. [Google Scholar] [CrossRef]
- Jung, Y.S.; Hyeon, J.S.; Hwang, G.S. Software-assisted serum metabolite quantification using NMR. Anal. Chim. Acta 2016, 934, 194–202. [Google Scholar] [CrossRef]
- Du, H.; Gu, X.; Chen, J.; Bai, C.; Duan, X.; Hu, K. GIPMA: Global Intensity-Guided Peak Matching and Alignment for 2D (1)H-(13)C HSQC-Based Metabolomics. Anal. Chem. 2023, 95, 3195–3203. [Google Scholar] [CrossRef]
- Domżał, B.; Nawrocka, E.K.; Gołowicz, D.; Ciach, M.A.; Miasojedow, B.; Kazimierczuk, K.; Gambin, A. Magnetstein: An Open-Source Tool for Quantitative NMR Mixture Analysis Robust to Low Resolution, Distorted Lineshapes, and Peak Shifts. Anal. Chem. 2024, 96, 188–196. [Google Scholar] [CrossRef]
- Jendrek, S.T.; Schmelter, F.; Schinke, S.; Hackel, A.; Graßhoff, H.; Lamprecht, P.; Humrich, J.Y.; Sina, C.; Müller, A.; Günther, U.; et al. Metabolomic signature identifies HDL and apolipoproteins as potential biomarker for systemic sclerosis with interstitial lung disease. Respir. Med. 2024, 234, 107825. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Burlingame, A.L. Deuterium and carbon-13 tracer studies of ethanol metabolism in the rat by 2H, 1H-decoupled 13C nuclear magnetic resonance. Biochem. Biophys. Res. Commun. 1974, 56, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.R.; Midgley, I. The use of 13C-nmr spectroscopy for the detection and identification of metabolites of carbon-13 labelled amitriptyline. J. Pharm. Pharmacol. 1978, 30, 547–553. [Google Scholar] [CrossRef]
- Williams, T.H.; Sasso, G.J.; Ryan, J.J.; Schwartz, M.A. Novel application of proton nuclear magnetic resonance spectroscopy in the identification of 2’-chloronordiazepam metabolites in the dog. J. Med. Chem. 1979, 22, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Ogawa, S.; Shulman, R.G. 13C NMR studies of gluconeogenesis in rat liver cells: Utilization of labeled glycerol by cells from euthyroid and hyperthyroid rats. Proc. Natl. Acad. Sci. USA 1979, 76, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- van der Greef, J.; Leegwater, D.C. Urine profile analysis by field desorption mass spectrometry, a technique for detecting metabolites of xenobiotics. Application to 3,5-dinitro-2-hydroxytoluene. Biomed. Mass. Spectrom. 1983, 10, 1–4. [Google Scholar] [CrossRef]
- Iles, R.A.; Chalmers, R.A.; Burns, S.P. High-resolution 1H-NMR spectroscopy of blood plasma for metabolic studies. Clin. Chem. 1995, 41, 1054–1056. [Google Scholar] [CrossRef]
- Foxall, P.J.; Parkinson, J.A.; Sadler, I.H.; Lindon, J.C.; Nicholson, J.K. Analysis of biological fluids using 600 MHz proton NMR spectroscopy: Application of homonuclear two-dimensional J-resolved spectroscopy to urine and blood plasma for spectral simplification and assignment. J. Pharm. Biomed. Anal. 1993, 11, 21–31. [Google Scholar] [CrossRef]
- Wang, Y.; Greenhalgh, T.; Wardle, J. Chinese herbal medicine (“3 medicines and 3 formulations”) for COVID-19: Rapid systematic review and meta-analysis. J. Eval. Clin. Pract. 2022, 28, 13–32. [Google Scholar] [CrossRef]
- Li, W.; Zhao, F.; Yang, J.; Pan, J.; Qu, H. Development of a comprehensive method based on quantitative 1H NMR for quality evaluation of Traditional Chinese Medicine injection: A case study of Danshen Injection. J. Pharm. Pharmacol. 2022, 74, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, M.; Wang, Y.; Zhao, D.; Zhao, S.; Li, S.; Qin, X. Deciphering the compatibility rules of traditional Chinese medicine prescriptions based on NMR metabolomics: A case study of Xiaoyaosan. J. Ethnopharmacol. 2020, 254, 112726. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Ghebrezadik, H.; Hylands, P.J. Metabolomic quality control of commercial Asian ginseng, and cultivated and wild American ginseng using (1)H NMR and multi-step PCA. J. Pharm. Biomed. Anal. 2015, 114, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.Y.; Li, M.H.; Xing, Y.X.; Hong, W.; Chen, C.; Chen, J.F.; Xu, H.; Zhao, W.L.; Wang, J.S. Hepatotoxicity and hepatoprotection of Polygonum multiflorum Thund. as two sides of the same biological coin. J. Ethnopharmacol. 2019, 230, 81–94. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.Z.; Lei, Z.H.; Qin, X.M.; Li, Z.Y. NMR based metabolomic comparison of the antitussive and expectorant effect of Farfarae Flos collected at different stages. J. Pharm. Biomed. Anal. 2018, 150, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Sun, H.M.; Jia, J.P.; Qin, X.M.; Li, Z.Y. Integrative hepatoprotective efficacy comparison of raw and vinegar-baked Radix Bupleuri using nuclear magnetic resonance-based metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 215–222. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, Z.; Qin, X.; Zheng, Q. Urinary metabolomics research for Huangqi Jianzhong Tang against chronic atrophic gastritis rats based on (1) H NMR and UPLC-Q/TOF MS. J. Pharm. Pharmacol. 2020, 72, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Duan, Y.; Liu, Y.; Liu, H.; Wei, C.; Wang, J.; Qin, X.; Wang, X.; Li, Z. Metabolomics coupled with SystemsDock reveal the protective effect and the potential active components of Naozhenning granule against traumatic brain injury. J. Ethnopharmacol. 2020, 246, 112247. [Google Scholar] [CrossRef]
- Su, Z.; Ling, X.; Ji, K.; Huang, H.; Liu, X.; Yin, C.; Zhu, H.; Guo, Y.; Mo, Y.; Lu, Y.; et al. (1)H NMR-based urinary metabonomic study of the antidiabetic effects of Rubus Suavissimus, S. Lee in STZ-induced T1DM rats. J. Chromatogr. B 2020, 1158, 122347. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Q.; Li, A.; Li, K.; Qin, X. Mechanisms exploration of herbal pair of HuangQi-DanShen on cerebral ischemia based on metabonomics and network pharmacology. J. Ethnopharmacol. 2020, 253, 112688. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, W.; Zhang, X.; Lv, C.; Lu, J. Icariin, the main prenylflavonoid of Epimedii Folium, ameliorated chronic kidney disease by modulating energy metabolism via AMPK activation. J. Ethnopharmacol. 2023, 312, 116543. [Google Scholar] [CrossRef]
- Cheng, P.; Xue, X.; Su, J.; Lu, M.; Wang, S.; Meng, J. (1)H NMR-based metabonomic revealed protective effect of Moutan Cortex charcoal on blood-heat and hemorrhage rats. J. Pharm. Biomed. Anal. 2019, 169, 151–158. [Google Scholar] [CrossRef]
- Hsu, W.H.; Shen, Y.C.; Shiao, Y.J.; Kuo, C.H.; Lu, C.K.; Lin, T.Y.; Ku, W.C.; Lin, Y.L. Combined proteomic and metabolomic analyses of cerebrospinal fluid from mice with ischemic stroke reveals the effects of a Buyang Huanwu decoction in neurodegenerative disease. PLoS ONE 2019, 14, e0209184. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Zhang, J.; Xie, D.; Pu, Y. Exploration of TCM syndrome types of the material basis and risk prediction of Wilson disease liver fibrosis based on (1)H NMR metabolomics. J. Pharm. Biomed. Anal. 2024, 245, 116167. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Su, F.; Tao, Y.; Wang, P.; Gu, J. NMR-based metabolomics approach to study the effect and related molecular mechanisms of Saffron essential oil against depression. J. Pharm. Biomed. Anal. 2024, 247, 116244. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Wang, H.; Gao, Y.; Chen, G.; Pei, Y.; Bai, J. 1H-NMR-Based Metabonomics of the Protective Effect of Coptis chinensis and Berberine on Cinnabar-Induced Hepatotoxicity and Nephrotoxicity in Rats. Molecules 2017, 22, 1855. [Google Scholar] [CrossRef]
- Zhao, S.J.; Tian, J.S.; Tai, G.; Gao, X.X.; Liu, H.L.; Du, G.H.; Liu, X.J.; Qin, X.M. (1)H NMR-based metabolomics revealed the protective effects of Guilingji on the testicular dysfunction of aging rats. J. Ethnopharmacol. 2019, 238, 111839. [Google Scholar] [CrossRef]
- Gao, K.; Yang, R.; Zhang, J.; Wang, Z.; Jia, C.; Zhang, F.; Li, S.; Wang, J.; Murtaza, G.; Xie, H.; et al. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol. Res. 2018, 130, 93–109. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Zhan, R.; Zhang, L.; Wang, X. Integrated Systems Pharmacology, Urinary Metabonomics, and Quantitative Real-Time PCR Analysis to Uncover Targets and Metabolic Pathways of the You-Gui Pill in Treating Kidney-Yang Deficiency Syndrome. Int. J. Mol. Sci. 2019, 20, 3655. [Google Scholar] [CrossRef]
- Man, S.; Qiu, P.; Li, J.; Zhang, L.; Gao, W. Global metabolic profiling for the study of Rhizoma Paridis saponins-induced hepatotoxicity in rats. Environ. Toxicol. 2017, 32, 99–108. [Google Scholar] [CrossRef]
- Guo, P.; Wang, J.; Dong, G.; Wei, D.; Li, M.; Yang, M.; Kong, L. NMR-based metabolomics approach to study the chronic toxicity of crude ricin from castor bean kernels on rats. Mol. Biosyst. 2014, 10, 2426–2440. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Zhang, Q.; Ma, K.; Li, H.; Li, F.; Dong, F.; Yan, X. Metabonomics study of the effects of pretreatment with glycyrrhetinic acid on mesaconitine-induced toxicity in rats. J. Ethnopharmacol. 2014, 154, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Fang, Y.; Zhao, L.; Xiong, Z.; Zhang, Y.; Wang, Y.; Feng, C.; Yuan, M.; Wang, S.; Chen, M.; et al. (1)HNMR-based metabonomic study of sub-chronic hepatotoxicity induced by realgar. J. Ethnopharmacol. 2016, 192, 1–9. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Chen, G.; Li, W.; Xiang, R.; Zhang, X.; Pei, Y. A metabolic profiling analysis of the acute toxicological effects of the realgar (As2S2) combined with other herbs in Niuhuang Jiedu Tablet using 1H NMR spectroscopy. J. Ethnopharmacol. 2014, 153, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, X.; Wang, L.; Xu, L.; Zhang, Y.; Li, Z. Integrating candidate metabolites and biochemical factors to elucidate the action mechanism of Xue-sai-tong injection based on (1)H NMR metabolomics. J. Chromatogr. B 2016, 1026, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Zhang, C.; Liao, S.; Li, P.; Xu, D.; Lv, Y.; Yang, M.; Kong, L. The components of Huang-Lian-Jie-Du-Decoction act synergistically to exert protective effects in a rat ischemic stroke model. Oncotarget 2016, 7, 80872–80887. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, L.; Gao, J.; Ma, J.; Yu, H.; Zhang, Y.; Wang, T.; Han, L. Multiomics Integrative Analysis for Discovering the Potential Mechanism of Dioscin against Hyperuricemia Mice. J. Proteome Res. 2021, 20, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ning, K.; Shi, C.; Zhou, Y.F.; Meng, Y.; Pan, T.; Chen, Y.; Xie, Q.; Xiang, H. Xiaobugan decoction prevents CCl(4)-induced acute liver injury by modulating gut microbiota and hepatic metabolism. Phytomedicine 2024, 135, 156113. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yin, Q.; Tian, J.; Gao, X.; Qin, X.; Du, G.; Zhou, Y. Studies on the potential link between antidepressant effect of Xiaoyao San and its pharmacological activity of hepatoprotection based on multi-platform metabolomics. J. Ethnopharmacol. 2020, 249, 112432. [Google Scholar] [CrossRef]
- Jiang, N.; Liu, H.F.; Li, S.D.; Zhou, W.X.; Zhang, Y.X.; Zhang, Q.; Yan, X.Z. An integrated metabonomic and proteomic study on Kidney-Yin Deficiency Syndrome patients with diabetes mellitus in China. Acta Pharmacol. Sin. 2015, 36, 689–698. [Google Scholar] [CrossRef]
- Gong, M.J.; Han, B.; Wang, S.M.; Liang, S.W.; Zou, Z.J. Icariin reverses corticosterone-induced depression-like behavior, decrease in hippocampal brain-derived neurotrophic factor (BDNF) and metabolic network disturbances revealed by NMR-based metabonomics in rats. J. Pharm. Biomed. Anal. 2016, 123, 63–73. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, Q.; Lin, M.; Deng, X.; Zhang, P.; Yao, M.; Zhang, L.; Xie, Z. Combination of 1H NMR- and GC-MS-based metabonomics to study on the toxicity of Coptidis Rhizome in rats. PLoS ONE 2014, 9, e88281. [Google Scholar] [CrossRef]
- Dong, G.; Wang, J.; Guo, P.; Wei, D.; Yang, M.; Kong, L. Toxicity assessment of Arisaematis Rhizoma in rats by a (1)H NMR-based metabolomics approach. Mol. Biosyst. 2015, 11, 407–417. [Google Scholar] [CrossRef]
- Karjalainen, M.K.; Karthikeyan, S.; Oliver-Williams, C.; Sliz, E.; Allara, E.; Fung, W.T.; Surendran, P.; Zhang, W.; Jousilahti, P.; Kristiansson, K.; et al. Genome-wide characterization of circulating metabolic biomarkers. Nature 2024, 628, 130–138. [Google Scholar] [CrossRef]
- Masuda, R.; Lodge, S.; Nitschke, P.; Spraul, M.; Schaefer, H.; Bong, S.H.; Kimhofer, T.; Hall, D.; Loo, R.L.; Bizkarguenaga, M.; et al. Integrative Modeling of Plasma Metabolic and Lipoprotein Biomarkers of SARS-CoV-2 Infection in Spanish and Australian COVID-19 Patient Cohorts. J. Proteome Res. 2021, 20, 4139–4152. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H., Jr.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Punčochář, M.; et al. Author Correction: Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: A phase I trial. Nat. Med. 2024, 30, 604. [Google Scholar] [CrossRef]
- Xue, L.M.; Zhang, Q.Y.; Han, P.; Jiang, Y.P.; Yan, R.D.; Wang, Y.; Rahman, K.; Jia, M.; Han, T.; Qin, L.P. Hepatotoxic constituents and toxicological mechanism of Xanthium strumarium L. fruits. J. Ethnopharmacol. 2014, 152, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Lackner, S.; Mahnert, A.; Moissl-Eichinger, C.; Madl, T.; Habisch, H.; Meier-Allard, N.; Kumpitsch, C.; Lahousen, T.; Kohlhammer-Dohr, A.; Mörkl, S.; et al. Interindividual differences in aronia juice tolerability linked to gut microbiome and metabolome changes-secondary analysis of a randomized placebo-controlled parallel intervention trial. Microbiome 2024, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Fang, Y.; Zhang, Y.; Wang, Y.; Feng, C.; Yuan, M.; Wang, S.; Chen, M.; Jiang, H. Plasma metabolomics study of the hepatoprotective effect of glycyrrhetinic acid on realgar-induced sub-chronic hepatotoxicity in mice via (1)H NMR analysis. J. Ethnopharmacol. 2017, 208, 36–43. [Google Scholar] [CrossRef]
- Lv, M.; Wang, Y.; Qu, P.; Li, S.; Yu, Z.; Qin, X.; Liu, X. A combination of cecum microbiome and metabolome in CUMS depressed rats reveals the antidepressant mechanism of traditional Chinese medicines: A case study of Xiaoyaosan. J. Ethnopharmacol. 2021, 276, 114167. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Zhao, W.; Luo, B.X.; Zheng, Q.; Liu, Z.; Liu, W.; Ba, D.D.; Zhong, J.; Luo, H.; Wang, J.; et al. NMR-based metabolomics approach to evaluate the toxicological risks of Tibetan medicine ‘Ershiwuwei Shanhu’ pill in rats. J. Ethnopharmacol. 2022, 282, 114629. [Google Scholar] [CrossRef]
- Wu, D.L.; Wang, T.S.; Zhang, W.; Wang, J.S.; Peng, D.Y.; Kong, L.Y. NMR-based metabolomics approach to study the effects of Wu-Zi-Yan-Zong-Wan on triptolide-induced oligospermia in rats. J. Ethnopharmacol. 2021, 265, 113192. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, F.; Li, B.; Huang, C.; Xu, C.; Lin, K.; Lin, D. NMR-based metabolomic analysis for the effects of Huiyang Shengji extract on rat diabetic skin ulcers. J. Ethnopharmacol. 2020, 261, 112978. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Wang, G.; Qin, X. Material basis research for Huangqi Jianzhong Tang against chronic atrophic gastritis rats through integration of urinary metabonomics and SystemsDock. J. Ethnopharmacol. 2018, 223, 1–9. [Google Scholar] [CrossRef]
- Fan, G.; Li, Q.; Li, H.J.; Zhang, Y.S.; Xu, X.M.; Fang, G.; Ge, Y.M.; Du, L.L. Active Ingredients and Anti-Arthritic Mechanisms of Ba-Wei-Long-Zuan Granule Revealed by (1) H-NMR-Based Metabolomics Combined with Network Pharmacology Analysis. Chem. Biodivers. 2020, 17, e2000122. [Google Scholar] [CrossRef]
- Peng, W.; Du, H.; Liu, G.; Zhang, Q.; Kuang, T.; Wang, Z.; Fan, G. Antistress Effects of San-Huang-Xie-Xin Decoction on Restraint-Stressed Mice Revealed by (1)H NMR-Based Metabolomics and Biochemistry Analysis. Oxid. Med. Cell Longev. 2019, 2019, 5897675. [Google Scholar] [CrossRef]
- Guan, Z.; Wu, J.; Wang, C.; Zhang, F.; Wang, Y.; Wang, M.; Zhao, M.; Zhao, C. Investigation of the preventive effect of Sijunzi decoction on mitomycin C-induced immunotoxicity in rats by (1)H NMR and MS-based untargeted metabolomic analysis. J. Ethnopharmacol. 2018, 210, 179–191. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, J.; Peng, N.; Li, R.; Xie, D.J.; Zhang, R.X.; Sun, L.T.; Wang, J.L.; Ye, H. (1)H NMR-based metabolomic study of striatal injury in rats with copper-loaded Wilson’s disease by Chinese and Western medicine intervention. J. Pharm. Biomed. Anal. 2023, 229, 115355. [Google Scholar] [CrossRef]
- Li, A.; Li, B.; Cui, T.; Zhang, W.; Qin, X. Investigation of the Potential Material Basis and Mechanism of Astragali Radix Against Adriamycin-Induced Nephropathy Model Rat by (1)H NMR and MS-Based Untargeted Metabolomics Analysis. Biomed. Chromatogr. 2025, 39, e6054. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zheng, P.; Hu, J.; Liu, X.; Chen, K.; Cao, Y.; Liang, Y.; Lu, C.; Xie, J.; Ma, Y.; et al. Study on the Mechanism of the Leaves of Dimocarpus longan Lour. in the Management of Type 2 Diabetes based on Metabolomics. Comb. Chem. High. Throughput Screen. 2025, 28, 3105–3126. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Gu, X.; Wu, T.; Du, H.; Bai, C.; Yang, J.; Hu, K. Identifying Anti-NSCLC Bioactive Compounds in Scutellaria via 2D NMR-Based Metabolomic Analysis of Pharmacologically Classified Crude Extracts. Chem. Biodivers. 2024, 21, e202400258. [Google Scholar] [CrossRef]
- Zheng, W.; Shi, C.; Meng, Y.; Peng, J.; Zhou, Y.; Pan, T.; Ning, K.; Xie, Q.; Xiang, H. Integrated network analysis and metabolomics reveal the molecular mechanism of Yinchen Sini decoction in CCl(4)-induced acute liver injury. Front. Pharmacol. 2023, 14, 1221046. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cheng, B.; Guo, B.J.; Huang, Z.; Qin, J.H.; Wang, Q.Y.; Feng, L.L.; Nong, Y.Y.; Zhu, D.; Guo, H.W.; et al. Metabonomics and 16S rRNA gene sequencing to study the therapeutic mechanism of Danggui Sini decoction on collagen-induced rheumatoid arthritis rats with Cold Bi syndrome. J. Pharm. Biomed. Anal. 2023, 222, 115109. [Google Scholar] [CrossRef]
- Feng, Q.; Tong, L.; Lu, Q.; Liu, S.; Zhao, L.; Xiong, Z. (1)H NMR serum metabolomics and its endogenous network pharmacological analysis of Gushudan on kidney-yang-deficiency-syndrome rats. Anal. Biochem. 2022, 643, 114580. [Google Scholar] [CrossRef]
- Tong, L.; Feng, Q.; Lu, Q.; Zhang, J.; Xiong, Z. Combined (1)H NMR fecal metabolomics and 16S rRNA gene sequencing to reveal the protective effects of Gushudan on kidney-yang-deficiency-syndrome rats via gut-kidney axis. J. Pharm. Biomed. Anal. 2022, 217, 114843. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Q.; Liu, W.; Liu, Q.; Jia, W.; Chang, E.; Chen, F.; Liu, Z.; Guo, X.; Mo, H.; et al. Establishment of VCA and EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: A two-stage design with a preliminary performance study and a mass screening in southern China. Int. J. Cancer 2012, 131, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tang, T.; Li, N.; Zheng, Q.; Xiao, T.; Tian, Y.; Sun, J.; Zhang, L.; Wang, X.; Wang, Y.; et al. VLDL and LDL Subfractions Enhance the Risk Stratification of Individuals Who Underwent Epstein-Barr Virus-Based Screening for Nasopharyngeal Carcinoma: A Multicenter Cohort Study. Adv. Sci. 2024, 11, e2308765. [Google Scholar] [CrossRef]
- Tang, T.; Zhou, Z.; Chen, M.; Li, N.; Sun, J.; Chen, Z.; Xiao, T.; Wang, X.; Zhang, L.; Wang, Y.; et al. Plasma Metabolic Profiles-Based Prediction of Induction Chemotherapy Efficacy in Nasopharyngeal Carcinoma: Results of a Bidirectional Clinical Trial. Clin. Cancer Res. 2024, 30, 2925–2936. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, X.; Du, G.; Li, Z.; Qin, X. Brain metabonomics study of the antidepressant-like effect of Xiaoyaosan on the CUMS-depression rats by (1)H NMR analysis. J. Ethnopharmacol. 2019, 235, 141–154. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Wu, J.; Wang, B.; Gao, X.; Wang, L.; Ma, H. Cinnabar-induced subchronic renal injury is associated with increased apoptosis in rats. Biomed. Res. Int. 2015, 2015, 278931. [Google Scholar] [CrossRef]
- Wei, L.; Liao, P.; Wu, H.; Li, X.; Pei, F.; Li, W.; Wu, Y. Toxicological effects of cinnabar in rats by NMR-based metabolic profiling of urine and serum. Toxicol. Appl. Pharmacol. 2008, 227, 417–429. [Google Scholar] [CrossRef]
- Wang, D.; Yu, C.; Liu, B.; Wang, H. Traditional Chinese medicine Zhusha Anshen Wan: Protective effects on liver, kidney, and intestine of the individual drugs using (1)H NMR metabolomics. Front. Pharmacol. 2024, 15, 1353325. [Google Scholar] [CrossRef]
- Wei, L.; Liao, P.; Wu, H.; Li, X.; Pei, F.; Li, W.; Wu, Y. Metabolic profiling studies on the toxicological effects of realgar in rats by (1)H NMR spectroscopy. Toxicol. Appl. Pharmacol. 2009, 234, 314–325. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Jin, H.; Gu, D.; Wang, Q.; Liu, Y.; Zan, K.; Fan, J.; Wang, R.; Wei, F.; et al. Comparisons of physicochemical features and hepatoprotective potentials of unprocessed and processed polysaccharides from Polygonum multiflorum Thunb. Int. J. Biol. Macromol. 2023, 235, 123901. [Google Scholar] [CrossRef]
- Zeng, X.; Li, C.; Liu, Y.; Liu, W.; Hu, Y.; Chen, L.; Huang, X.; Li, Y.; Hu, K.; Ouyang, D.; et al. HLA-B*35:01-mediated activation of emodin-specific T cells contributes to Polygonum multiflorum thunb. -induced liver injury in mice. J. Ethnopharmacol. 2024, 334, 118523. [Google Scholar] [CrossRef]
- Luan, X.; Zhang, L.J.; Li, X.Q.; Rahman, K.; Zhang, H.; Chen, H.Z.; Zhang, W.D. Compound-based Chinese medicine formula: From discovery to compatibility mechanism. J. Ethnopharmacol. 2020, 254, 112687. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.G.; Li, Y.J.; Chen, Y.; Yang, Q.; Wang, Y.J.; Li, Q.; Cai, W.Y.; Zhu, X.X. Research initiative of new thought on “main effect” of TCM formulae--new thinking on mechanism of compound action and compatibility mechanism of Chinese herbal compound formulae. Zhongguo Zhong Yao Za Zhi 2018, 43, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, L.; Yang, J.; Yang, L.; Wang, Y.; Qu, J.; Zhang, C. Retrospective study of adverse reactions of Niuhuang Jiedu tablet (pill) and risk control based on literature analysis. Zhongguo Zhong Yao Za Zhi 2010, 35, 1342–1345. [Google Scholar] [CrossRef]
- Feng, Y.L.; Miao, J.W.; Li, J.; Sung, A.S.; Liu, J. Safety evaluation of niuhuang jiedu tablet. Zhongguo Zhong Yao Za Zhi 2014, 39, 3221–3225. [Google Scholar]
- Xu, W.; Wang, H.; Chen, G.; Li, W.; Xiang, R.; Pei, Y. (1)H NMR-based metabonomics study on the toxicity alleviation effect of other traditional Chinese medicines in Niuhuang Jiedu tablet to realgar (As2S2). J. Ethnopharmacol. 2013, 148, 88–98. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Zhang, T.; Liu, C. Research progress on quality markers of traditional Chinese medicine. J. Pharm. Biomed. Anal. 2022, 211, 114588. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ruan, L.Y.; Chen, C.; Fan, J.T.; Chen, J.F.; Zhao, W.L.; Meng, H.H.; Liu, W.Y.; Zhao, W.L.; Zheng, Q.; et al. Therapeutic assessment of fractions of Gastrodiae Rhizoma on chronic atrophic gastritis by (1)H NMR-based metabolomics. J. Ethnopharmacol. 2020, 254, 112403. [Google Scholar] [CrossRef]
- Blümich, B. Low-field and benchtop NMR. J. Magn. Reson. 2019, 306, 27–35. [Google Scholar] [CrossRef]
- Song, Z.; Ohnishi, Y.; Osada, S.; Gan, L.; Jiang, J.; Hu, Z.; Kumeta, H.; Kumaki, Y.; Yokoi, Y.; Nakamura, K.; et al. Application of Benchtop NMR for Metabolomics Study Using Feces of Mice with DSS-Induced Colitis. Metabolites 2023, 13, 611. [Google Scholar] [CrossRef]
- Silva Elipe, M.V.; Ndukwe, I.E.; Murray, J.I. Cryogen-free 400-MHz nuclear magnetic resonance spectrometer as a versatile tool for pharmaceutical process analytical technology. Magn. Reson. Chem. 2024, 62, 512–534. [Google Scholar] [CrossRef]
- Silva Elipe, M.V.; Ndukwe, I.E.; Navarro-Vázquez, A. Anisotropic NMR data acquisition with a prototype 400 MHz cryogen-free NMR spectrometer. Magn. Reson. Chem. 2023, 61, 530–543. [Google Scholar] [CrossRef]
- Joseph, D.; Griesinger, C. Optimal control pulses for the 1.2-GHz (28.2-T) NMR spectrometers. Sci. Adv. 2023, 9, eadj1133. [Google Scholar] [CrossRef] [PubMed]
- Wong, A. A roadmap to high-resolution standard microcoil MAS NMR spectroscopy for metabolomics. NMR Biomed. 2023, 36, e4683. [Google Scholar] [CrossRef]
- Thomas, J.N.; Ramaswamy, V.; Litvak, I.M.; Johnston, T.L.; Edison, A.S.; Brey, W.W. Progress Towards a Higher Sensitivity (13)C-Optimized 1.5 mm HTS NMR Probe. IEEE Trans. Appl. Supercond. 2021, 31, 1500504. [Google Scholar] [CrossRef]
- Thomas, J.N.; Johnston, T.L.; Litvak, I.M.; Ramaswamy, V.; Merritt, M.E.; Rocca, J.R.; Edison, A.S.; Brey, W.W. Implementing High Q-Factor HTS Resonators to Enhance Probe Sensitivity in (13)C NMR Spectroscopy. J. Phys. Conf. Ser. 2022, 2323, 012030. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Endo, Y.; Nemoto, T.; Bouzier-Sore, A.K.; Wong, A. High-resolution NMR-based metabolic detection of microgram biopsies using a 1 mm HRμMAS probe. Analyst 2015, 140, 8097–8100. [Google Scholar] [CrossRef]
- Lai, S.Y.; Sandulache, V.C.; Schellingerhout, D.; Fuller, C.D.; Chen, Y.; Walker, C.M.; Bankson, J.A. First-in-Human Hyperpolarized MRI for Tumor Metabolism in HNSCC. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, W.; Pi, X.; He, Z.; Liu, H. Advances in the Application of Traditional Chinese Medicine Using Artificial Intelligence: A Review. Am. J. Chin. Med. 2023, 51, 1067–1083. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, L.; Zhang, S.; Xu, Z.; Qin, J.; Shi, Y.; Yu, P.; Wang, Y.; Bao, Z.; Xia, Y.; et al. Development of a tongue image-based machine learning tool for the diagnosis of gastric cancer: A prospective multicentre clinical cohort study. EClinicalMedicine 2023, 57, 101834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Mao, R.; Chen, B.; Jiang, M. A novel data fusion strategy of LC-MS and NMR technologies using random forest model for emodin hepatotoxic metabolomics research. J. Pharm. Biomed. Anal. 2025, 264, 116990. [Google Scholar] [CrossRef] [PubMed]
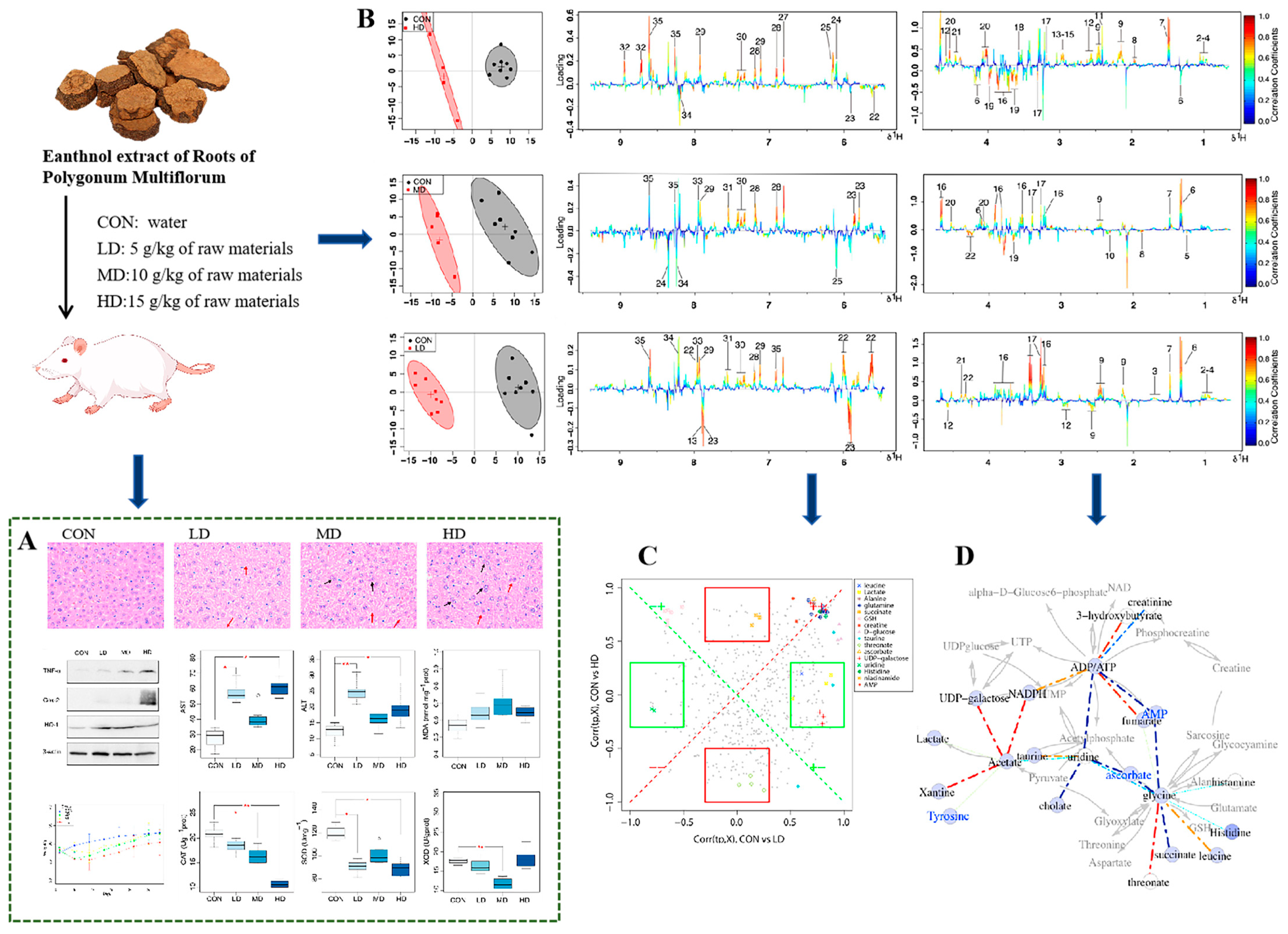
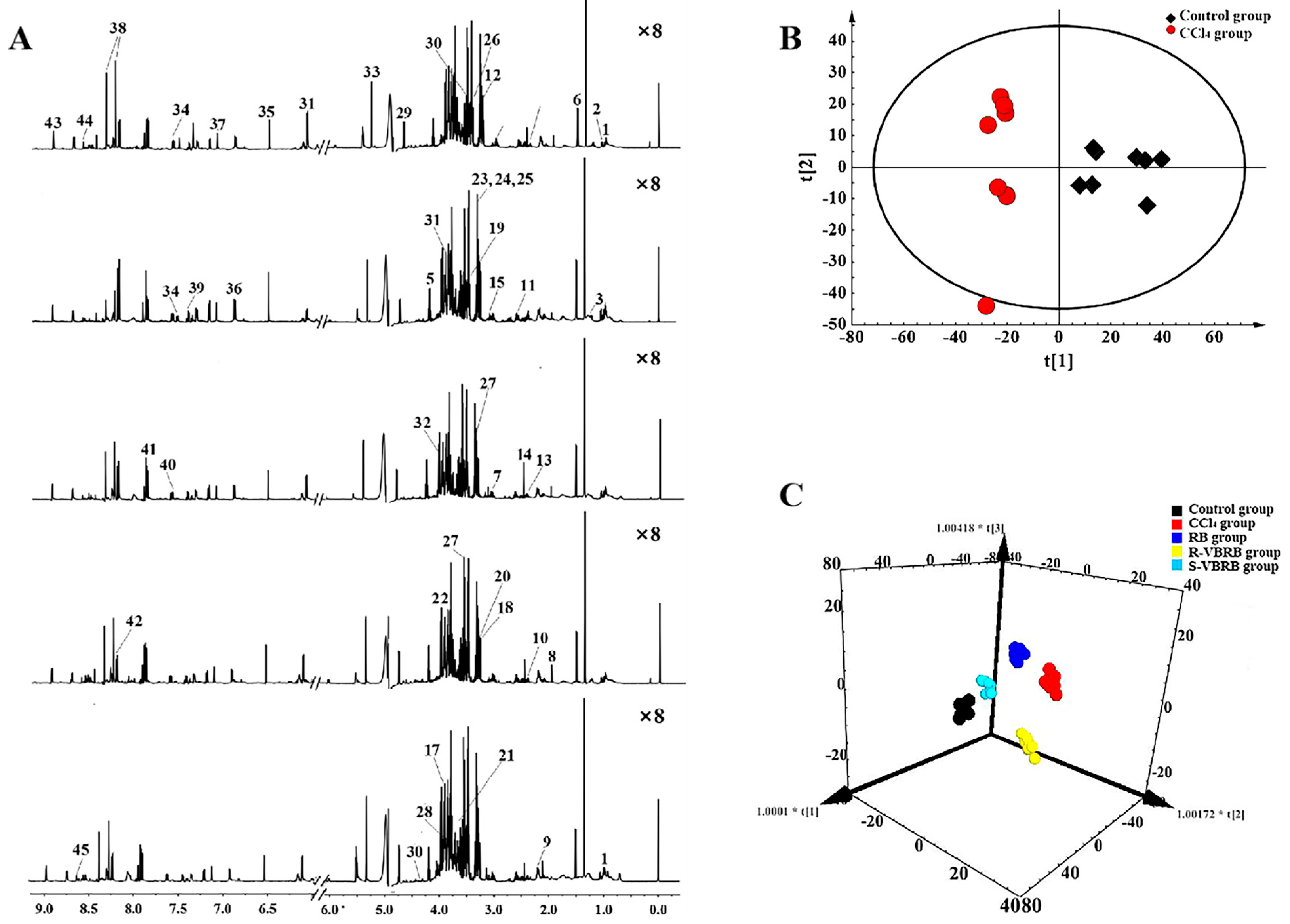
| Magnetic Field Strength (MHz) | Technique (s) | Sample | TCM(s) Involved | Research Objective | Reference |
|---|---|---|---|---|---|
| 600 | 1H, COSY, HSQC | Danshen injection | Danshen | Taking Danshen Injection as a case, establish a rapid and comprehensive method for evaluating the quality of Traditional Chinese Medicine injections. | [118] |
| 600 | 1H | fecal | Xiaoyao-San | Investigate the compatibility rule of the antidepressant effect of Xiao-Yao-San by using both the “Efficacy Compositions” research strategy and fecal metabolomics approach. | [119] |
| 400 | 1H | Extract of Ginseng | ginseng | Metabolomic quality control of commercial Asian ginseng and cultivated and wild American ginseng using 1H NMR and multi-step PCA. | [120] |
| 500 | 1H | serum | Polygonum multiflorum Thunb. | Study the mechanism of Polygonum multiflorum Thunb. induced hepatotoxicity and confirm the existence of a conversion between hepatotoxicity and hepatoprotection during the administration. | [121] |
| 600 | 1H | serum | Farfarae Flos | Comparison of the antitussive and expectorant effect of Farfarae Flos collected at different stages. | [122] |
| 600 | 1H | liver | Radix Bupleuri. | Compare the hepatoprotective efficacy of raw and vinegar-baked Radix Bupleuri. | [123] |
| 600 | 1H, HSQC, COSY | urine | HuangqiJianzhong-Tang | Research the mechanism of HuangqiJianzhong-Tang in combating chronic atrophic gastritis. | [124] |
| — | 1H | brain | Naozhenning granule | Reveal the protective effect and the potential active components of Naozhenning granule against traumatic brain injury. | [125] |
| 600 | 1H, COSY | urine | Rubus suavissimus S. Lee | Study the antidiabetic effects of Rubus suavissimus S. Lee in streptozotocin-induced type 1 diabetes mellitus rats. | [126] |
| 600 | 1H, HSQC, TOCSY | serum | HuangQi, DanShen | Explore the active mechanism of HuangQi-DanShen against cerebral ischemia. | [127] |
| 600 | 1H | serum, urine | Epimedii Folium | Investigate the efficacy and potential mechanism of icariin, the main prenylflavonoid of Epimedii Folium, in ameliorating chronic kidney disease. | [128] |
| 500 | 1H | plasma, urine | Moutan Cortex charcoal | Explore the protective effect of Moutan Cortex charcoal on blood-heat and hemorrhage in rats. | [129] |
| — | 1H | CSF | BuYangHuanWu Decoction | Multi-technology integration to reveal the effects of BuYangHuanWu Decoction on neurodegenerative diseases. | [130] |
| 500 | 1H, HSQC, COSY | plasma, urine, brain | Naodesheng | Determine the protective Effect of a combination of multiple components derived from Naodesheng on ischemic stroke in rats. | [95] |
| 600 | 1H | serum | — | Evaluate the metabolic profiles associated with different TCM syndromes of liver fibrosis related to Wilson’s disease and analyze the diagnostic and predictive capabilities of various metabolites. | [131] |
| 600 | 1H | liver, spleen, kidneys | Saffron essential oil | Study the effect and related molecular mechanisms of Saffron essential oil against depression. | [132] |
| 600 | 1H | serum, urine | Coptis chinensis Franch | Explore the protective effects of Coptis chinensis Franch and its main component, berberine, on cinnabar-induced hepatotoxicity and nephrotoxicity. | [133] |
| 500 | 1H, HSQC, TOCSY | plasma, urine | Baoyuan decoction | Delineates the therapeutic effects of Baoyuan decoction on isoproterenol-induced cardiac hypertrophy. | [96] |
| 600 | 1H | urine | Guilingji | Investigate the protective effects of Guilingji on the testicular dysfunction of aging rats, as well as its regulating effects on metabolic disorders in natural aging rats. | [134] |
| 600 | 1H, COSY, TOCSY, HMBC, JRES NMR | liver, kidney | Qijian mixture | Assess the safety and efficacy of Qijian mixture in the treatment of type 2 diabetes by metabonomics, gut microbiota, and system pharmacology. | [135] |
| 500 | 1H | urine | You-Gui Pill | Uncover the targets and metabolic pathways of the You-Gui Pill in Treating Kidney-Yang deficiency syndrome. | [136] |
| — | 1H | liver | Rhizoma Paridis saponins | Study the potential mechanism of Rhizoma Paridis saponins-induced hepatotoxicity in rats. | [137] |
| 500 | 1H | urine, serum | kernels of castor beans | Study the chronic toxicity of crude ricin from kernels of castor beans on rats. | [138] |
| 600 | 1H | urine | Rhizoma glycyrrhizae, Aconitum carmichaelii Debx. | Explain how Rhizoma glycyrrhizae alleviates the toxicity of Aconitum carmichaelii Debx. | [139] |
| 600 | 1H | plasma, urine | realgar | Elucidate the mechanism of sub-chronic hepatotoxicity induced by realgar | [140] |
| 600 | 1H | urine, serum | Niuhuang Jiedu Tablet | Analyze the metabolic profiling of the acute toxicological effects of the realgar (As2S2) combined with other herbs in Niuhuang Jiedu Tablet. | [141] |
| 600 | 1H, COSY, TOCSY, HSQC, HMBC, JRES NMR | serum | Xue-sai-tong injection | Integrate candidate metabolites and biochemical factors to elucidate the action mechanism of Xue-sai-tong injection. | [142] |
| 500 | 1H | brain | Huang-Lian-Jie-Du-Decoction | Elucidate the components of Huang-Lian-Jie-Du-Decoction that act synergistically to exert protective effects in a rat ischemic stroke model. | [143] |
| 600 | 1H, COSY, TOCSY, HSQC, HMBC, JRES NMR | plasma, urine | — | Discover the potential mechanism of dioscin against hyperuricemia in mice. | [144] |
| 600 | 1H | liver, small intestine | Xiaobugan decoction | Explore the prophylactic and hepatoprotective effects of Xiaobugan decoction and explore its related molecular mechanisms. | [145] |
| 600 | 1H | liver | Xiaoyao-San | Elucidate the potential link between the antidepressant and hepatoprotective effects of Xiaoyao-San. | [146] |
| 600 | 1H | urine | — | Investigate the specific changes in metabolites and proteins of Kidney-Yin Deficiency Syndrome patients with diabetes mellitus (DM) in China. | [147] |
| 500 | 1H | serum, urine, brain | Epimedium brevicornum Maxim. | Study the antidepressant-like effect and the possible mechanisms of icariin in a rat model of corticosterone-induced depression. | [148] |
| 500 | 1H | urine | Coptis chinensis Franch. | Combination of 1H NMR and GC-MS-based metabonomics to study the toxicity of Coptidis rhizome in rats. | [149] |
| 500 | 1H | urine, serum | Arisaematis rhizoma | Toxicity assessment of Arisaematis rhizoma in rats by an NMR-based metabolomics approach. | [150] |
| 500, 600 | 1H | serum, plasma | — | Brings new insights into human metabolic biology by using high-throughput metabolomics platforms for Genome-wide association analyses. | [151] |
| 600 | 1H | serum, plasma | — | Integrative Modeling of Plasma Metabolic and Lipoprotein Biomarkers of SARS-CoV-2 Infection in Spanish and Australian COVID-19 Patient Cohorts. | [152] |
| 600 | 1H | plasma | — | Confirm the feasibility of fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma. | [153] |
| 600 | 1H | urine, plasma | Xanthium strumarium L. | Revealing the hepatotoxic constituents and toxicological mechanism of Xanthium strumarium L. fruits. | [154] |
| 600 | 1H, JRES NMR | plasma | aronia | Investigated if aronia juice tolerability was associated with changes in intestinal microbiota and metabolites. | [155] |
| 600 | 1H | plasma | realgar | Explore the hepatoprotective effects of glycyrrhetinic acid on realgar-induced sub-chronic hepatotoxicity in mice. | [156] |
| 600 | 1H | cecal samples | Xiaoyao-san | Study the underlying mechanism of the anti-depressant effects of Xiaoyao san from the perspective of cecal microbiota and metabolites. | [157] |
| 500 | 1H | liver, kidney, serum | Ershiwuwei Shanhu Pill | Investigate the safety profile of Ershiwuwei Shanhu Pill, a classic Tibetan medicinal formulation. | [158] |
| 500 | 1H | kidney, serum, testicle | Wu-Zi-Yan-Zong-Wan | Elucidate the potential biomarkers and metabolic pathways involved in the treatment of oligozoospermia with Wu-Zi-Yan-Zong-Wan, providing a basis and guidance for its clinical application. | [159] |
| 600 | 1H, HSQC, TOCSY | skin tissues | Huiyang Shengji formula | Provide new insights into the mechanisms of the healing effects of the Huiyang Shengji formula in the treatment of diabetic skin ulcer. | [160] |
| 600 | 1H, HSQC, COSY | urine | Huangqi Jianzhong Tang | Integrate urine metabolomics and SystemsDock to screen out the material basis of Huangqi Jianzhong Tang against chronic atrophic gastritis. | [161] |
| 600 | 1H | serum | Ba-Wei-Long-Zuan Granule | Reveal the active ingredients and anti-arthritic Mechanisms of Ba-Wei-Long-Zuan Granule. | [162] |
| 600 | 1H | serum | San-Huang-Xie-Xin decoction | Combine 1H-NMR metabolomics and biochemical assays to investigate the anti-stress effects and underlying mechanisms of San-Huang-Xie-Xin decoction on restraint-stressed mice. | [163] |
| 600 | 1H | urine, plasma, spleen | Sijunzi decoction | Utilize NMR- and MS-based metabolomics technologies to investigate the preventive effect of Sijunzi decoction on mitomycin C-induced immunotoxicity. | [164] |
| 850 | 1H | striatum | Bushen Huoxue Huazhuo Recipe | Analyze the effects of two different interventions, penicillamine and Bushen Huoxue Huazhuo Recipe, on metabolites in striatal tissues of Wilson’s disease copper-loaded rats, providing valuable references for future integrated Western and Chinese medicine interventions targeting Wilson’s disease-related nerve injury. | [165] |
| 600 | 1H | serum | Astragali Radix | Elucidate the potential material basis and mechanism of Astragali Radix against nephrotic syndrome. | [166] |
| 600 | 1H | urine, serum | Dimocarpus longan Lour. | Reveal the therapeutic effect of the leaves of Dimocarpus longan Lour. on type 2 diabetes from a metabolomic perspective. | [167] |
| 700 | HSQC | Scutellaria crude extracts | Scutellaria | Identify anti-non-small cell lung cancer bioactive compounds in Scutellaria via NMR-Based Metabolomic analysis of pharmacologically classified crude extracts. | [168] |
| — | 1H | liver | Yinchen Sini decoction | Reveal the molecular mechanism of Yinchen Sini decoction in the treatment of acute liver injury using integrated network analysis and metabolomics. | [169] |
| 600 | 1H, COSY | feces | Danggui Sini decoction | Integrate NMR metabolomics and 16S rRNA gene sequencing techniques to clarify the intervention of Danggui Sini decoction on collagen-induced rheumatoid arthritis. | [170] |
| 600 | 1H | kidney | Xiaoyao-san | Reveal the compatibility of Xiaoyaosan from the perspective of the “gut-liver-kidney” axis based on the strategy of the “Efficacy Group”. | [15] |
| 600 | 1H | serum | Gushudan | Explore the preventive mechanism of Gushudan on kidney-yang-deficiency-syndrome through an integrated approach combining pharmacodynamics, 1H NMR serum metabolomics, and endogenous network pharmacology analysis. | [171] |
| 600 | 1H | feces | Gushudan | Combine 1H NMR fecal metabolomics and 16S rRNA gene sequencing to reveal the protective effects of Gushudan on kidney-yang-deficiency-syndrome rats via the gut–kidney axis. | [172] |
| No. | Metabolites | Chemical Shift (ppm) |
|---|---|---|
| 1 | isoleucine | 0.95 (t), 1.01 (d), 1.99 (m) |
| 2 | leucine | 0.96 (d), 0.97 (d) |
| 3 | valine | 0.99 (d), 1.04 (d) |
| 4 | β-hydroxybutyrate | 1.20 (d), 2.41 (d), 2.31 (d) |
| 5 | lactic acid | 1.33 (d), 4.12 (q) |
| 6 | alanine | 1.48 (d) |
| 7 | ornithine | 1.73 (m), 1.93 (m), 3.05 (t), 3.77 (t) |
| 8 | acetic acid | 1.92 (s) |
| 9 | glutamic acid | 2.05 (m), 2.34 (m), 3.75 (m) |
| 10 | glutamine | 2.14 (m), 2.44 (m), 3.77 (m) |
| 11 | methionine | 2.14 (s), 2.14 (m), 2.64 (t), 3.85 (m) |
| 12 | glutathione disulfide | 2.17 (m), 2.54 (m), 2.95 (m), 3.25 (m), 2.98 (dd), 3.32 (dd) |
| 13 | pyruvic acid | 2.37 (s) |
| 14 | succinic acid | 2.41 (s) |
| 15 | aspartic acid | 2.70 (dd), 2.82 (dd), 3.90 (dd) |
| 16 | trimethylamine | 2.88 (s) |
| 17 | creatine | 3.04 (s), 3.93 (s) |
| 18 | choline | 3.21 (s) |
| 19 | scyllo-inositol | 3.36 (s) |
| 20 | phosphatidylcholine | 3.22 (s) |
| 21 | glycerophosophocholine | 3.23 (s), 3.63 (m), 4.30 (m) |
| 22 | phosphoethanolamine | 3.23 (t), 3.99 (m) |
| 23 | trimethylamine N-oxide | 3.27 (s) |
| 24 | taurine | 3.27 (t), 3.43 (t) |
| 25 | betaine | 3.27 (s), 3.90 (s) |
| 26 | methanol | 3.36 (s) |
| 27 | glycine | 3.56 (s) |
| 28 | oxidized glutathione | 3.78 (t), 2.17 (m), 2.54 (m), 2.98 (dd) |
| 29 | β-glucose | 3.90 (dd), 4.65 (d) |
| 30 | adenosine | 4.28 (q),4.45 (t),6.10 (d),8.24 (s), 8.35 (s) |
| 31 | inosine | 4.28 (q), 4.45 (t), 6.10 (d) |
| 32 | mannose | 5.19 (d), 3.94 (m) |
| 33 | α-glucose | 5.23 (d) |
| 34 | uracil | 5.8 (d), 7.55 (d) |
| 35 | fumaric acid | 6.53 (s) |
| 36 | tyrosine | 6.91 (d), 7.20 (d) |
| 37 | histidine | 7.11 (s), 7.90 (s) |
| 38 | phenylalanine | 7.32 (m), 7.42 (m) |
| 39 | uracil | 7.55 (d) |
| 40 | nicotinurate | 7.60 (dd), 8.72 (d), 8.94 (s) |
| 41 | uridine | 7.88 (d), 5.92 (d), 4.36 (m), 4.24 (t) |
| 42 | hypoxanthine | 8.20 (s), 8.22 (s) |
| 43 | nicotinic acid | 8.24 (d), 8.72 (d), 8.94 (s) |
| 44 | inosine monophosphate | 8.58 (s) |
| 45 | adenosine monophosphate | 8.61 (s), 8.27 (s), 6.15 (d) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Liu, L.; Yan, G.; Bai, L.; Yang, L.; Kong, L.; Sun, H.; Liu, C.; Sun, Y.; Han, Y.; et al. Nuclear Magnetic Resonance Based Metabolomics—A Rising Star in Traditional Chinese Medicine Research. Pharmaceuticals 2025, 18, 1186. https://doi.org/10.3390/ph18081186
Hu Y, Liu L, Yan G, Bai L, Yang L, Kong L, Sun H, Liu C, Sun Y, Han Y, et al. Nuclear Magnetic Resonance Based Metabolomics—A Rising Star in Traditional Chinese Medicine Research. Pharmaceuticals. 2025; 18(8):1186. https://doi.org/10.3390/ph18081186
Chicago/Turabian StyleHu, Yu, Lei Liu, Guangli Yan, Luoning Bai, Le Yang, Ling Kong, Hui Sun, Chang Liu, Ye Sun, Ying Han, and et al. 2025. "Nuclear Magnetic Resonance Based Metabolomics—A Rising Star in Traditional Chinese Medicine Research" Pharmaceuticals 18, no. 8: 1186. https://doi.org/10.3390/ph18081186
APA StyleHu, Y., Liu, L., Yan, G., Bai, L., Yang, L., Kong, L., Sun, H., Liu, C., Sun, Y., Han, Y., & Wang, X. (2025). Nuclear Magnetic Resonance Based Metabolomics—A Rising Star in Traditional Chinese Medicine Research. Pharmaceuticals, 18(8), 1186. https://doi.org/10.3390/ph18081186








