Qifu Decoction Alleviates Lipopolysaccharide-Induced Myocardial Dysfunction by Inhibiting TLR4/NF-κB/NLRP3 Inflammatory Pathway and Activating PPARα/CPT Pathway
Abstract
1. Introduction
2. Results
2.1. Ingredient Identification of QFD by UPLC-QTOF-MS
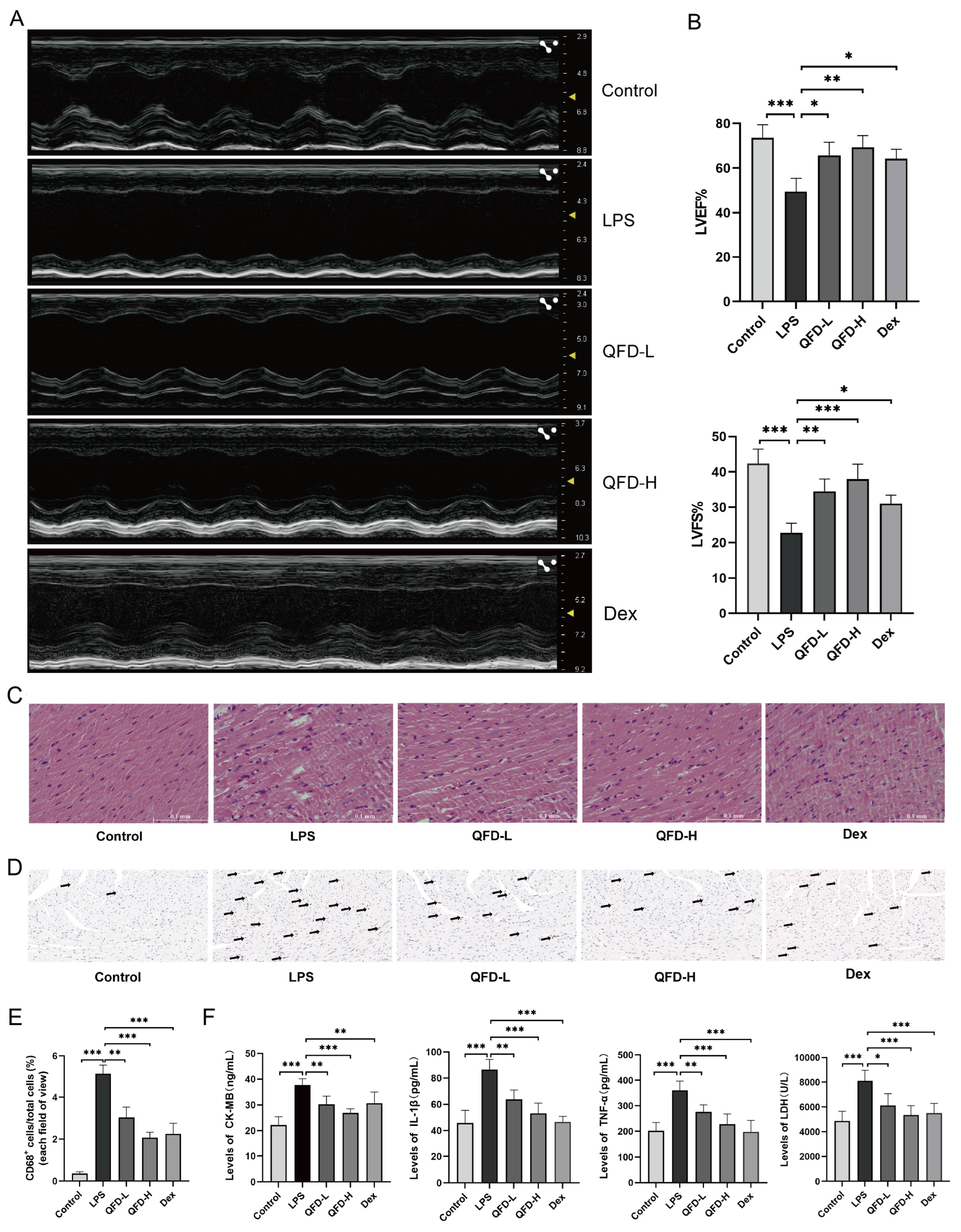
2.2. QFD Alleviated Symptoms of SIC in Mice
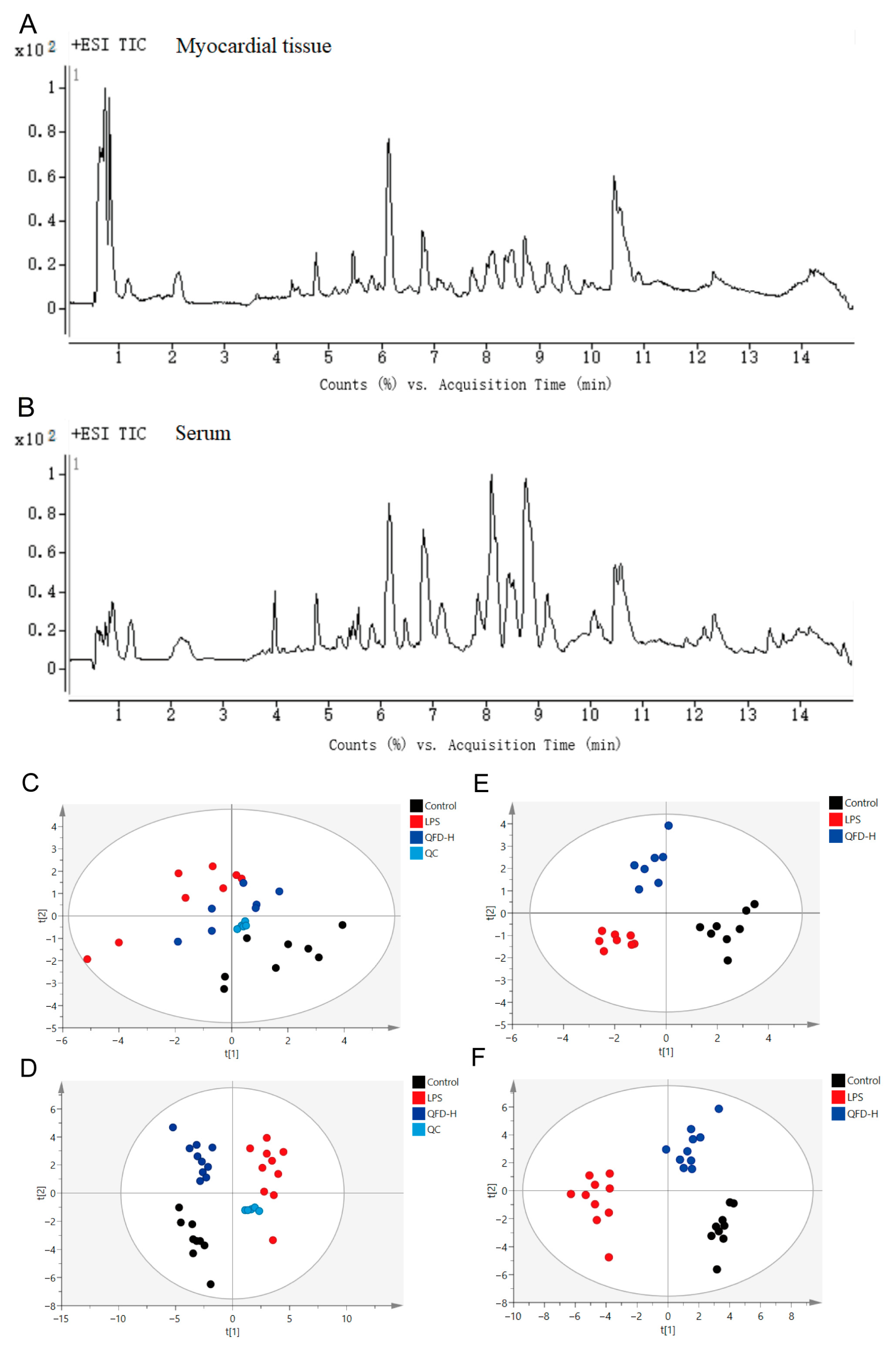
2.3. Metabolomic Analysis
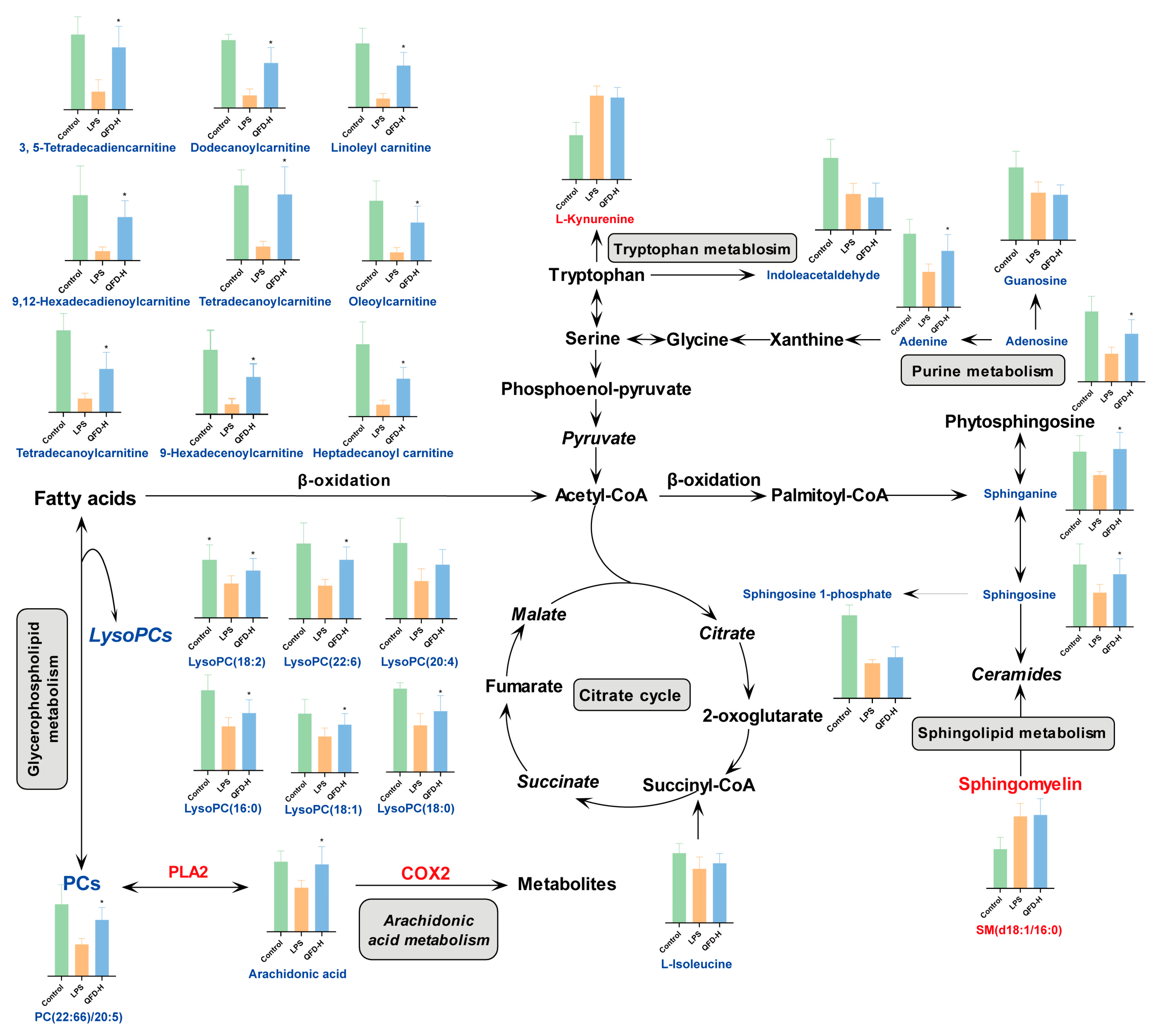
2.4. Identification of Differential Metabolites
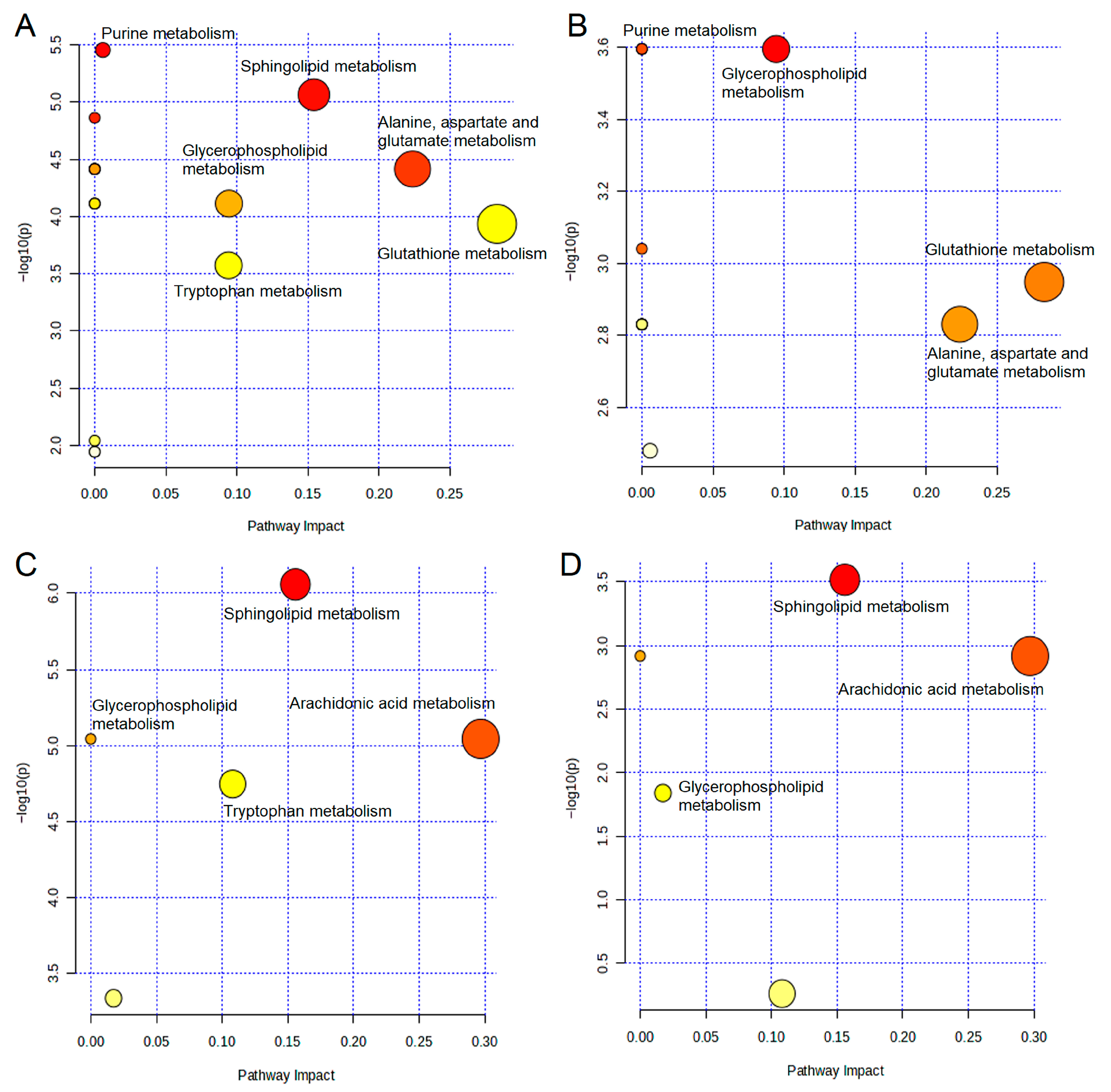
2.5. Metabolic Pathway Analysis
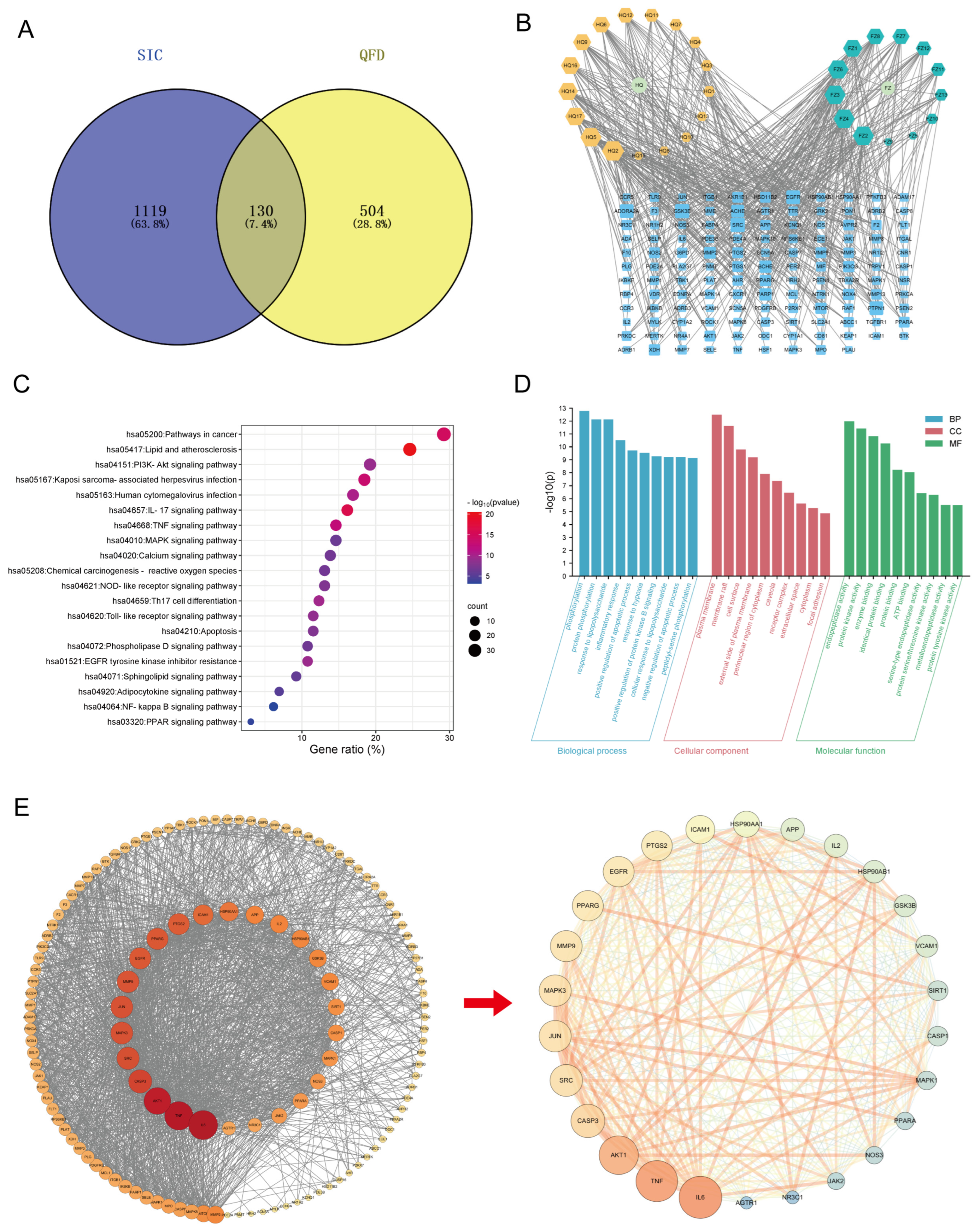
2.6. Network Pharmacology-Based Mechanism Analysis of QFD Against SIC
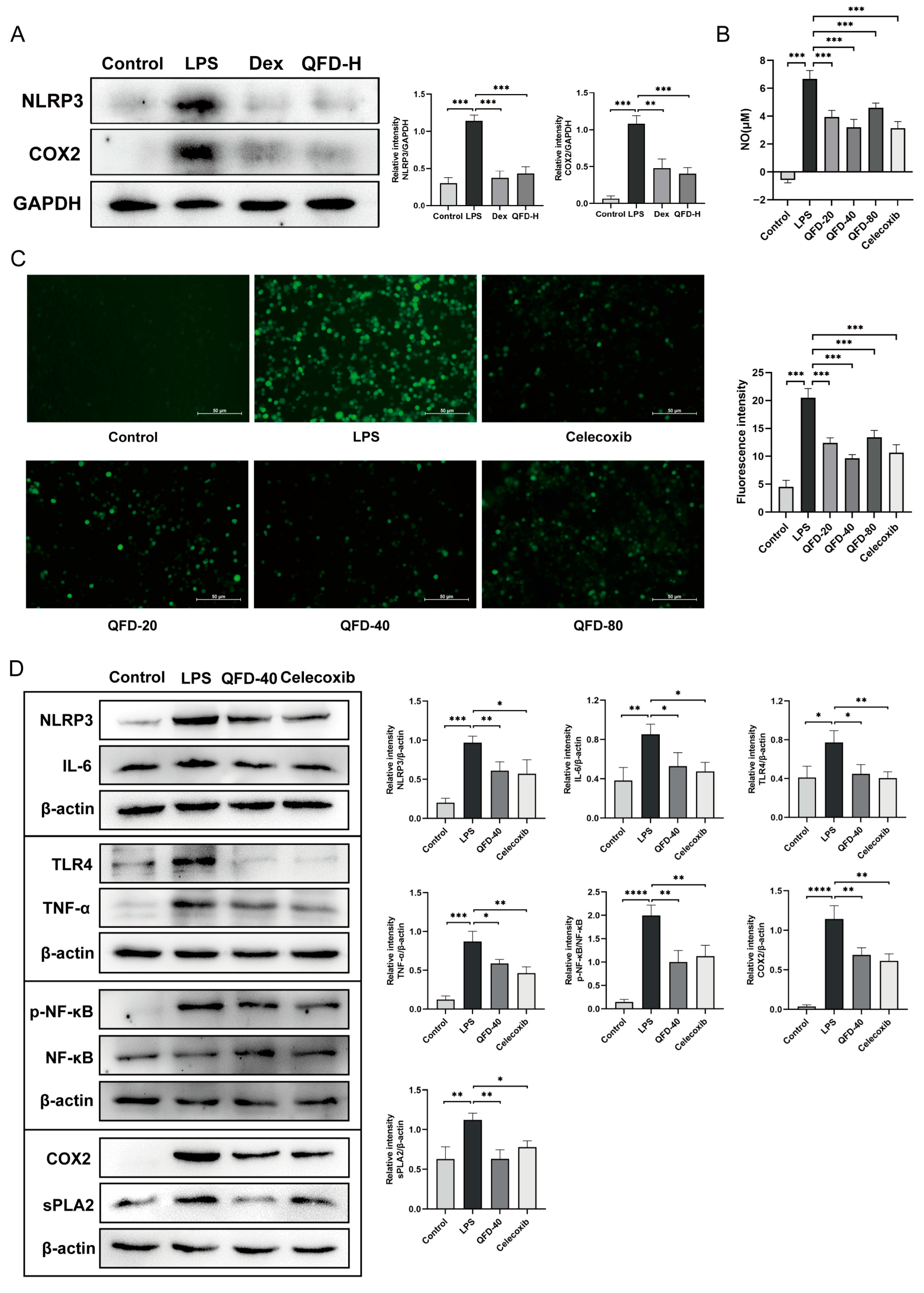
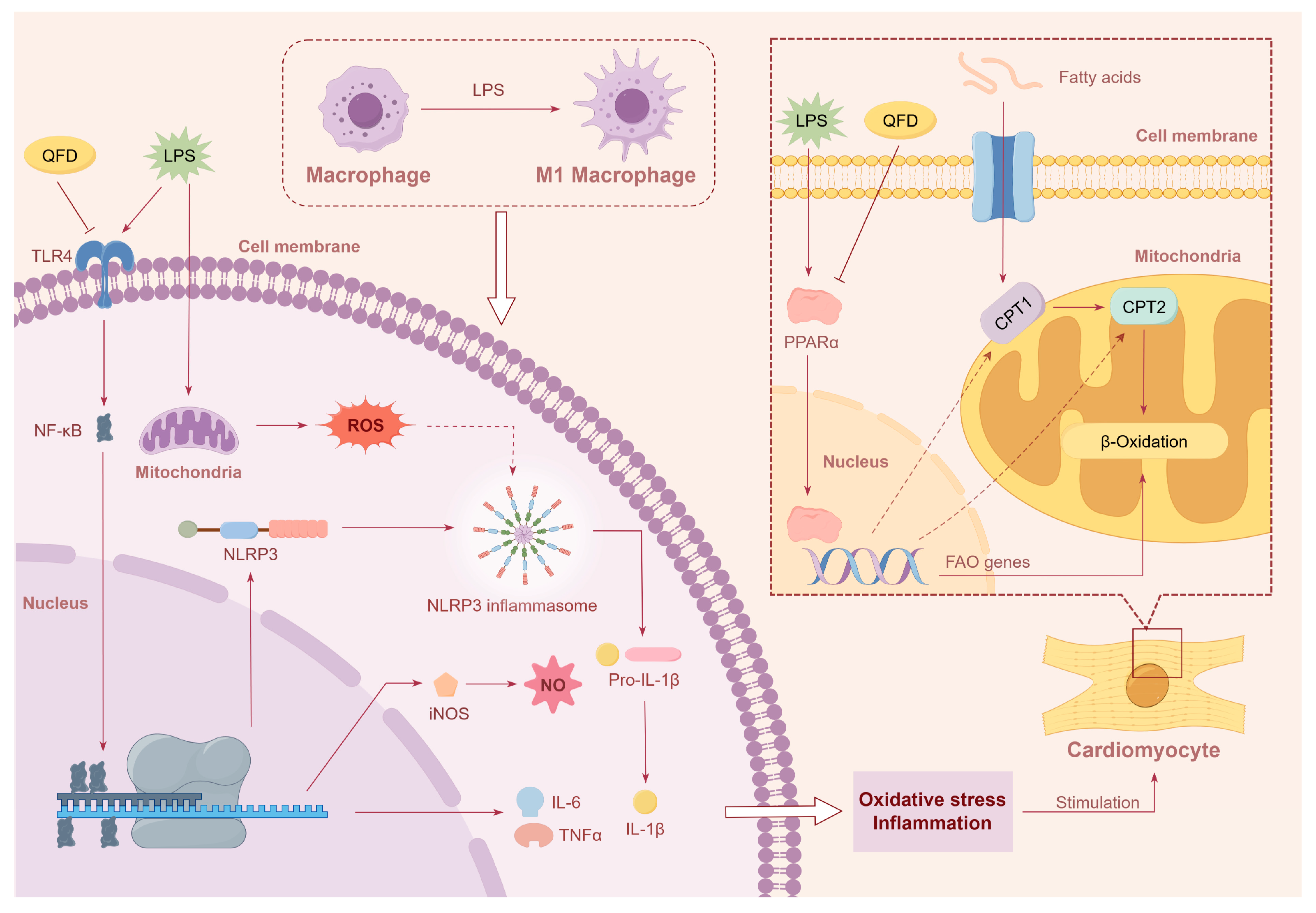
2.7. QFD Significantly Mitigated Inflammation in SIC Mice and RAW 264.7 Cells
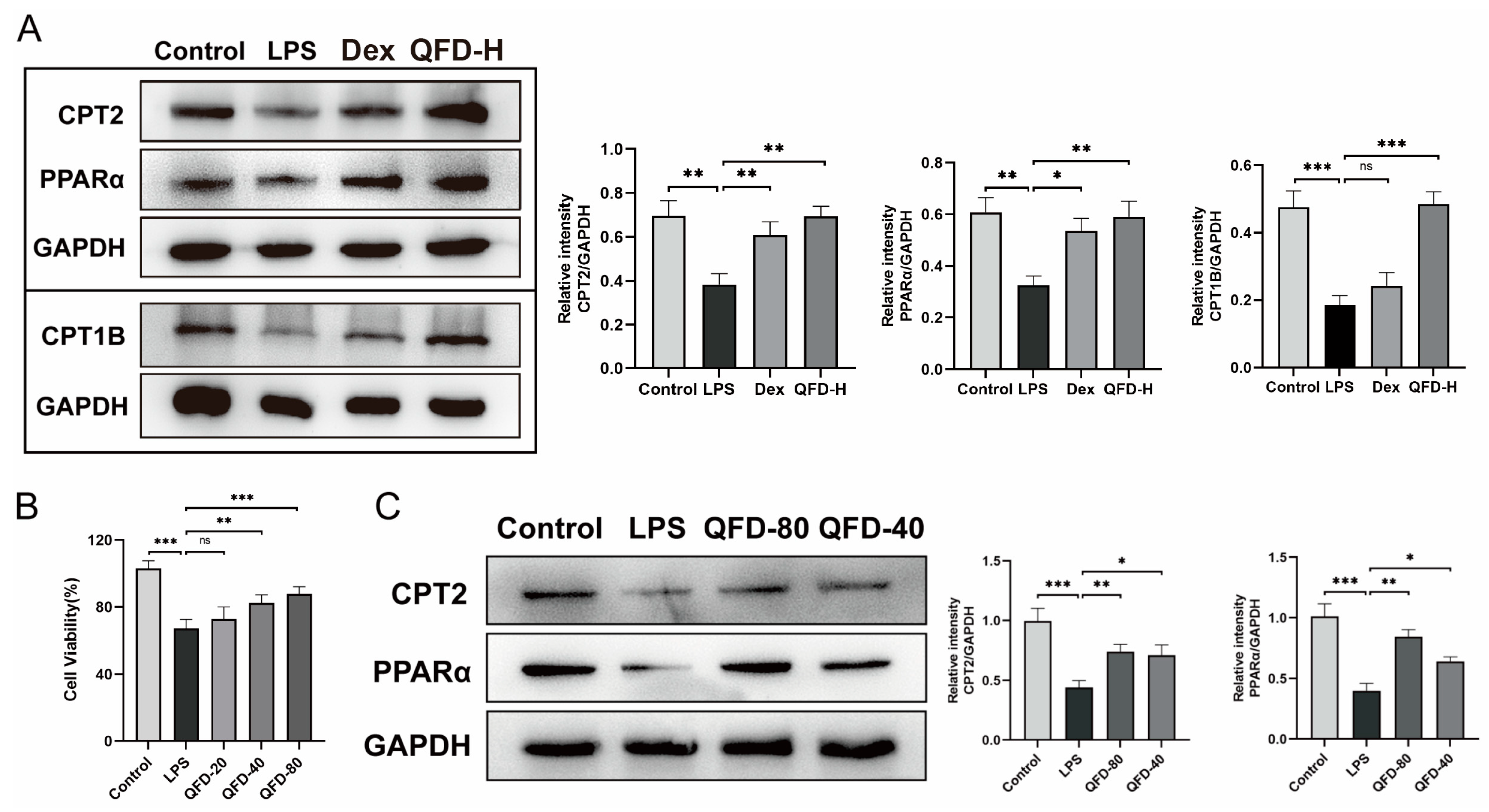
2.8. QFD Alleviated SIC by Modulating Impaired FAO Through Activation of PPARα/CPT Pathway
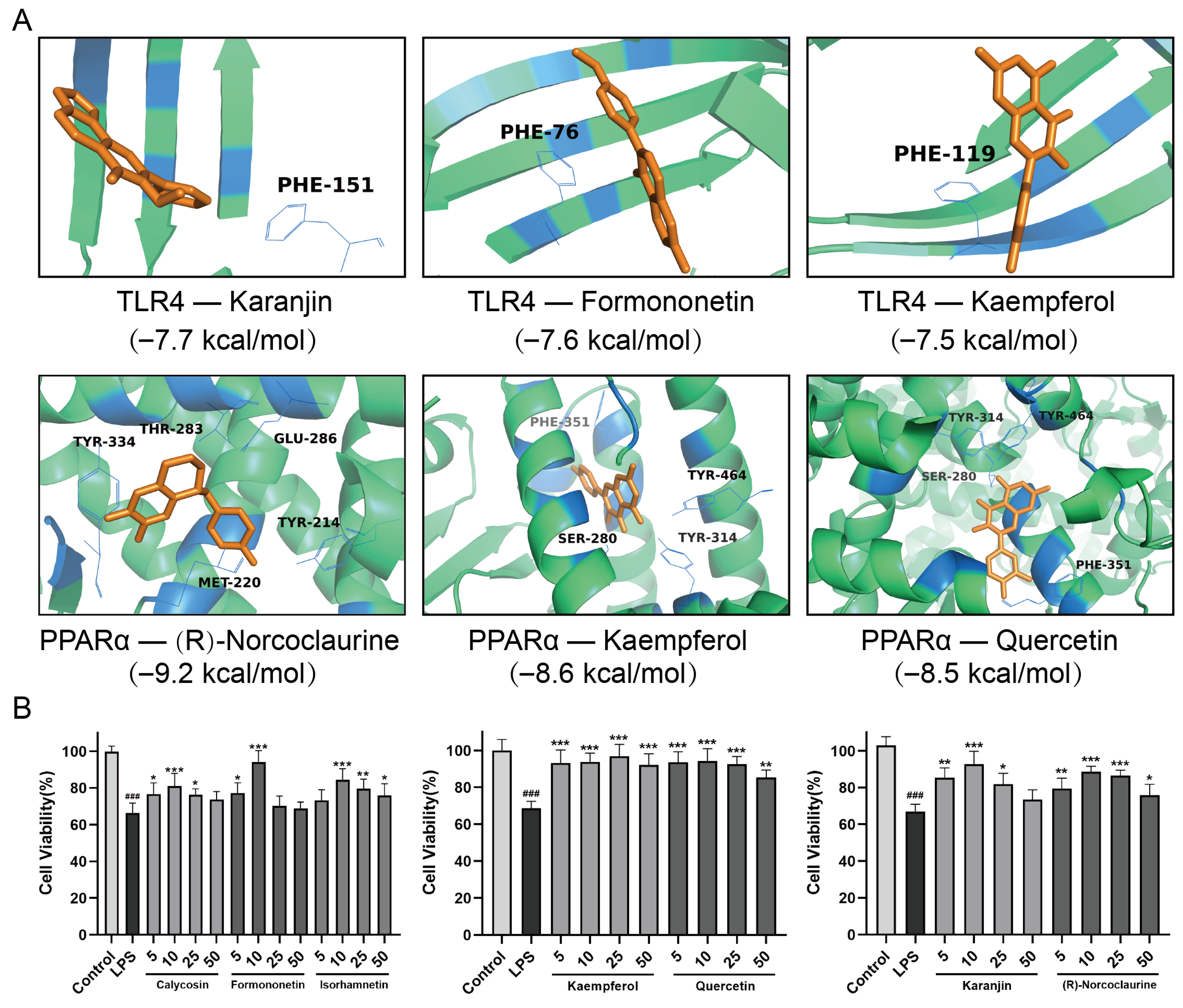
2.9. Molecular Docking Analysis and Efficacy Analysis of the Active Ingredients in QFD
3. Discussion
3.1. Inflammatory Pathway
3.2. Metabolic Homeostasis
4. Materials and Methods
4.1. Reagents
4.2. Preparation and Ingredient Identification of QFD
4.3. Animals
4.4. LPS-Induced Sepsis Model Construction and Treatment
4.5. Cardiac Function Measurements
4.6. Histopathological Analysis
4.7. Immunohistochemistry
4.8. Assessment of Biochemical Indicators and Inflammatory Factors
4.9. Sample Preparation for Metabolomic Analysis
4.10. Metabolomic Analysis
4.11. Network Pharmacology Analysis
4.12. Culture and Treatment of RAW 264.7 Cells and H9c2 Cells
4.13. Molecular Docking
4.14. Western Blot Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, N.; Sayed, M.A.; Poonsuph, C.J.; Sehgal, R.; Shirke, M.M.; Harky, A. Septic Cardiomyopathy: From Basics to Management Choices. Curr. Probl. Cardiol. 2021, 46, 100767. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 2021, 18, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Su, Z.; Zhao, K.; Li, P.; Xu, T. Ginkgolide A targets forkhead box O1 to protect against lipopolysaccharide-induced septic cardiomyopathy. Phytother. Res. 2023, 37, 3309–3322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, X.; Li, C.; Li, Q.; An, Y.A.; Luo, X.; Deng, Y.; Gillette, T.G.; Scherer, P.E.; Wang, Z.V. Integrated Stress Response Couples Mitochondrial Protein Translation with Oxidative Stress Control. Circulation 2021, 144, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.; Davidson, S.M.; Yellon, D.M. Does remote ischaemic conditioning reduce inflammation? A focus on innate immunity and cytokine response. Basic. Res. Cardiol. 2021, 116, 12. [Google Scholar] [CrossRef] [PubMed]
- Shvilkina, T.; Shapiro, N. Sepsis-Induced myocardial dysfunction: Heterogeneity of functional effects and clinical significance. Front. Cardiovasc. Med. 2023, 10, 1200441. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Q.; He, S.; Zhao, J.; Wang, N.; Han, X.; Guo, Y. Qiang-Xin 1 Formula Prevents Sepsis-Induced Apoptosis in Murine Cardiomyocytes by Suppressing Endoplasmic Reticulum- and Mitochondria-Associated Pathways. Front. Pharmacol. 2018, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, X.; Shang, Z.; Qiao, Y.; Liu, Y.; Zhou, L.; Liu, D. In vivo and in vitro study on the regulatory mechanism of XiaoChaiHu decoction on PANoptosis in sepsis-induced cardiomyopathy. J. Ethnopharmacol. 2024, 336, 118740. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.L.; Ji, M.M.; Chen, L.; Liao, Y.; Kong, X.Q.; Xu, X.Q.; Liao, Z.G.; Wilson, D.W. Traditional Chinese herbal medicine Astragalus Radix and its effects on intestinal absorption of aconite alkaloids in rats. Chin. Herb. Med. 2021, 13, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Tang, L.; Zhou, X.; Wang, T.; Kou, Z.; Wang, Z. A review on phytochemistry and pharmacological activities of the processed lateral root of Aconitum carmichaelii Debeaux. J. Ethnopharmacol. 2015, 160, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Jing, J.; Zhu, Z.; Lou, Z.; Li, W.; Zhao, L.; Zhang, G.; Chai, Y. Detection and identification of diterpenoid alkaloids, isoflavonoids and saponins in Qifu decoction and rat plasma by liquid chromatography-time-of-flight mass spectrometry. Biomed. Chromatogr. 2012, 26, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Guo, L.; Yu, X.; Guo, H.; Deng, X.; Yu, J.; Deng, X.; Xu, F.; Zhang, Z.; Huang, Y. Network-driven targeted analysis reveals that Astragali Radix alleviates doxorubicin-induced cardiotoxicity by maintaining fatty acid homeostasis. J. Ethnopharmacol. 2022, 287, 114967. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zou, X.; Zheng, Y.; Zhang, Y.; Cui, G.; Liu, S.; Sun, C.; Peng, C. Aconiti Lateralis Radix Praeparata ameliorates heart failure via PI3K/AKT/Bnip3 pathway. Front. Pharmacol. 2025, 16, 1526653. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, B.; Zhao, H.; Xu, C.; Xu, D.; Sieniawska, E.; Lin, X.; Kai, G. Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine 2022, 98, 153918. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Gao, M.; Weng, L.; Xu, T. Esculin targets TLR4 to protect against LPS-induced septic cardiomyopathy. Int. Immunopharmacol. 2024, 131, 111897. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.Z.; Hao, J.F.; Hou, M.X. Hmgcs2 regulates M2 polarization of macrophages to repair myocardial injury induced by sepsis. Aging 2023, 15, 7794–7810. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Shi, H.; Su, Y.; Han, B.; Shen, C.; Gao, S.; Chen, Z.; Xu, C. Photoactivated adenylyl cyclases attenuate sepsis-induced cardiomyopathy by suppressing macrophage-mediated inflammation. Front. Immunol. 2022, 13, 1008702. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, K.; Liao, X.; Hu, H.; Chen, L.; Meng, L.; Gao, W.; Li, Q. Carnitine Palmitoyltransferase System: A New Target for Anti-Inflammatory and Anticancer Therapy? Front. Pharmacol. 2021, 12, 760581. [Google Scholar] [CrossRef] [PubMed]
- Mhetre, N.M.; Bhatambrekar, A.L.; Priya, D.; Saravanan, V.; Kathiravan, M.; Shevate, K.S.; Rajagopal, K.; Asgaonkar, K.D.; Chitre, T.S. Rational design of some 1,3,4 trisubstituted pyrazole-thiazole derivatives to serve as MtInhA inhibitors using QSAR, ADMET, molecular docking, MM-GBSA, and molecular dynamics simulations approach. Chem. Phys. Impact 2024, 9, 100769. [Google Scholar] [CrossRef]
- Charpentier, J.; Luyt, C.E.; Fulla, Y.; Vinsonneau, C.; Cariou, A.; Grabar, S.; Dhainaut, J.F.; Mira, J.P.; Chiche, J.D. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit. Care Med. 2004, 32, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Hochstadt, A.; Meroz, Y.; Landesberg, G. Myocardial dysfunction in severe sepsis and septic shock: More questions than answers? J. Cardiothorac. Vasc. Anesth. 2011, 25, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Corredor, C.; Fletcher, N.; Landesberg, G.; Benedetto, U.; Foex, P.; Cecconi, M. Diastolic dysfunction and mortality in septic patients: A systematic review and meta-analysis. Intensive Care Med. 2015, 41, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.B.; Liu, B. Research Progress on the Mechanism and Management of Septic Cardiomyopathy: A Comprehensive Review. Emerg. Med. Int. 2023, 2023, 8107336. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Mihić, D.; Varžić, S.C.; Relatić, K.S.; Zibar, L.; Loinjak, D.; Ćurić, Ž.B.; Klobučar, L.; Maričić, L. Septic Cardiomyopathy. Rev. Cardiovasc. Med. 2024, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, H.; Qiu, L.Z.; Yue, L.X.; Zhang, G.J.; Deng, H.F.; Ni, Y.H.; Gao, Y. Cardiac efficacy and toxicity of aconitine: A new frontier for the ancient poison. Med. Res. Rev. 2021, 41, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Gao, Y.; Tan, G.; Wu, S.; Dong, X.; Lou, Z.; Zhu, Z.; Chai, Y. Myocardial lipidomics profiling delineate the toxicity of traditional Chinese medicine Aconiti Lateralis radix praeparata. J. Ethnopharmacol. 2013, 147, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhuo, L.; Zhang, B.; Zhu, L.; Xiang, X.; Zhang, C.; Liu, W.; Tan, G.; Liao, W. Untargeted metabolomics reveals the combination effects and mechanisms of Huangqi-fuzi herb-pair against doxorubicin-induced cardiotoxicity. J. Ethnopharmacol. 2023, 305, 116109. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Wang, S.H.; Liu, C.Y.; Gao, Y.L.; Meng, X.L.; Wei, W.; Shou, S.T.; Liu, Y.C.; Chai, Y.F. Losartan attenuates sepsis-induced cardiomyopathy by regulating macrophage polarization via TLR4-mediated NF-κB and MAPK signaling. Pharmacol. Res. 2022, 185, 106473. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Qiao, W.; Xiao, Y.J.; Cui, L.; Wang, X.; Ren, W.D. Naringin mitigates myocardial strain and the inflammatory response in sepsis-induced myocardial dysfunction through regulation of PI3K/AKT/NF-kappaB pathway. Int. Immunopharmacol. 2019, 75, 105782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-kappaB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef] [PubMed]
- Boaru, S.G.; Borkham-Kamphorst, E.; Van de Leur, E.; Lehnen, E.; Liedtke, C.; Weiskirchen, R. NLRP3 inflammasome expression is driven by NF-κB in cultured hepatocytes. Biochem. Biophys. Res. Commun. 2015, 458, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wang, G.; Huang, R.; Liu, C.; Yushanjiang, F.; Mao, T.; Li, J. Astilbin protects from sepsis-induced cardiac injury through the NRF2/HO-1 and TLR4/NF-κB pathway. Phytother. Res. 2024, 38, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yan, D.; Sun, Q.; Tao, J.; Xu, L.; Sun, H.; Zhao, H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J. Cell. Biochem. 2020, 121, 2994–3004. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, X.; Hu, J.; Tang, Z. Mild Hypothermia Alleviates CLP-induced Multiple Organ Dysfunction by Mitigating Pyroptosis Through the TLR4/NF-κB/NLRP3 Signaling Pathway. Arch. Med. Res. 2023, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Machado Dutra, J.; Espitia, P.J.P.; Andrade Batista, R. Formononetin: Biological effects and uses—A review. Food Chem. 2021, 359, 129975. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Zhang, Y.; Sun, Q.; Cao, J.; Tan, N.; Yang, S.; Lu, L.; Zhang, Q.; Wei, P.; et al. Calycosin as a Novel PI3K Activator Reduces Inflammation and Fibrosis in Heart Failure Through AKT-IKK/STAT3 Axis. Front. Pharmacol. 2022, 13, 828061. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, S.; Mutavdzin Krneta, S.; Djuric, D.; Milosevic, V.; Milenkovic, D. Plant Polyphenols as Heart’s Best Friends: From Health Properties, to Cellular Effects, to Molecular Mechanisms of Action. Int. J. Mol. Sci. 2025, 26, 915. [Google Scholar] [CrossRef] [PubMed]
- Mesaros, C.; Blair, I.A. Targeted chiral analysis of bioactive arachidonic Acid metabolites using liquid-chromatography-mass spectrometry. Metabolites 2012, 2, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Peretó, J.; López-García, P.; Moreira, D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem. Sci. 2004, 29, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Q.; Zhang, Y.; Zhang, N.; Lu, L.; Wu, Y.; Zhang, Q.; Wang, W.; Wang, Y.; et al. Qishen granules inhibit myocardial inflammation injury through regulating arachidonic acid metabolism. Sci. Rep. 2016, 6, 36949. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.Y.; Li, Z.Y.; Du, G.H.; Qin, X.M. (1)H NMR based metabolomic profiling revealed doxorubicin-induced systematic alterations in a rat model. J. Pharm. Biomed. Anal. 2016, 118, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Jian, C.; Peng, Q.; Hou, T.; Wu, K.; Shang, B.; Zhao, M.; Wang, Y.; Zheng, W.; Ma, Q.; et al. Prohibitin 2 deficiency impairs cardiac fatty acid oxidation and causes heart failure. Cell Death Dis. 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Standage, S.W.; Bennion, B.G.; Knowles, T.O.; Ledee, D.R.; Portman, M.A.; McGuire, J.K.; Liles, W.C.; Olson, A.K. PPARα augments heart function and cardiac fatty acid oxidation in early experimental polymicrobial sepsis. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H239–H249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.X.; Wang, X.; Jiao, S.Y.; Liu, Y.; Shi, L.; Xu, Q.; Wang, J.J.; Chen, Y.E.; Zhang, Q.; Song, Y.T.; et al. Cardiomyocyte peroxisome proliferator-activated receptor alpha prevents septic cardiomyopathy via improving mitochondrial function. Acta Pharmacol. Sin. 2023, 44, 2184–2200. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhou, Q.M.; Guo, L.; Dai, O.; Meng, C.W.; Miao, L.L.; Liu, J.; Lin, Q.; Peng, C.; Xiong, L. Cardioprotective effects and concentration-response relationship of aminoalcohol-diterpenoid alkaloids from Aconitum carmichaelii. Fitoterapia 2021, 149, 104822. [Google Scholar] [CrossRef] [PubMed]
- Barrero, M.J.; Camarero, N.; Marrero, P.F.; Haro, D. Control of human carnitine palmitoyltransferase II gene transcription by peroxisome proliferator-activated receptor through a partially conserved peroxisome proliferator-responsive element. Biochem. J. 2003, 369, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Fraser, F.; Corstorphine, C.G.; Zammit, V.A. Topology of carnitine palmitoyltransferase I in the mitochondrial outer membrane. Biochem. J. 1997, 323 Pt 3, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Li, Q.; Song, G.; Chen, B.; Luo, M.; Miao, J.; Liu, B. CPT2-mediated fatty acid oxidation inhibits tumorigenesis and enhances sorafenib sensitivity via the ROS/PPARγ/NF-κB pathway in clear cell renal cell carcinoma. Cell Signal. 2023, 110, 110838. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Esser, V.; Brown, N.F.; Cowan, A.T.; Foster, D.W.; McGarry, J.D. Expression of a cDNA isolated from rat brown adipose tissue and heart identifies the product as the muscle isoform of carnitine palmitoyltransferase I (M-CPT I). M-CPT I is the predominant CPT I isoform expressed in both white (epididymal) and brown adipocytes. J. Biol. Chem. 1996, 271, 6972–6977. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Pierro, A.; Spitz, L.; Eaton, S. Differential effects of neonatal endotoxemia on heart and kidney carnitine palmitoyl transferase I. J. Pediatr. Surg. 2002, 37, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.; Fukumoto, K.; Stefanutti, G.; Spitz, L.; Zammit, V.A.; Pierro, A. Myocardial carnitine palmitoyltransferase I as a target for oxidative modification in inflammation and sepsis. Biochem. Soc. Trans. 2003, 31, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Gao, Y.; Shou, S.; Chai, Y. The roles of macrophage polarization in the host immune response to sepsis. Int. Immunopharmacol. 2021, 96, 107791. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Yu, M.M.; Shou, S.T.; Chai, Y.F. Sepsis-Induced Cardiomyopathy: Mechanisms and Treatments. Front. Immunol. 2017, 8, 1021. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Ji, X.; Qin, Y.; Zhang, X.; Wan, X.; Zhu, C.; Lv, C.; Hu, C.; Zhou, J.; Lu, L.; et al. Harmine Alleviated Sepsis-Induced Cardiac Dysfunction by Modulating Macrophage Polarization via the STAT/MAPK/NF-κB Pathway. Front. Cell Dev. Biol. 2021, 9, 792257. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, Z.; Zhou, Y.; Su, M.; Zhang, H.; Yu, L.; Li, M. Atractylenolide I ameliorates sepsis-induced cardiomyocyte injury by inhibiting macrophage polarization through the modulation of the PARP1/NLRP3 signaling pathway. Tissue Cell 2024, 89, 102424. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, T.; Liu, Y.; Yin, L.; Xiao, L.; Fu, L.; Zhu, Y.; Chen, H.; Wang, K.; Xiao, X.; et al. HSF1 Protects Sepsis-Induced Acute Lung Injury by Inhibiting NLRP3 Inflammasome Activation. Front. Immunol. 2022, 13, 781003. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Zhou, Q.; Xu, M.; Ding, X.; Zhang, X.; Zhang, Y.; Tang, Y.; Tan, G. Sini decoction alleviates inflammation injury after myocardial infarction through regulating arachidonic acid metabolism. Chin. Herb. Med. 2025, 17, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tan, K.S.; Beng, H.; Liu, F.; Huang, J.; Kuai, Y.; Zhang, R.; Tan, W. Protective effect of isosteviol sodium against LPS-induced multiple organ injury by regulating of glycerophospholipid metabolism and reducing macrophage-driven inflammation. Pharmacol. Res. 2021, 172, 105781. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Jia, H.; Xu, L.; Cao, Y.; Zhai, M.; Li, K.; Xia, L.; Jiang, L.; Li, X.; et al. Tetrahydrocurcumin improves lipopolysaccharide-induced myocardial dysfunction by inhibiting oxidative stress and inflammation via JNK/ERK signaling pathway regulation. Phytomedicine 2022, 104, 154283. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yuan, L.; Luo, Z.; Tang, Y.; Lin, X.; Wang, S.; Liang, P.; Huang, L.; Jiang, B. COX-2 optimizes cardiac mitochondrial biogenesis and exerts a cardioprotective effect during sepsis. Cytokine 2024, 182, 156733. [Google Scholar] [CrossRef] [PubMed]
| No. | tR (Min) | Identification (In Vitro) | Formula | [M+H]+ m/z | [M+Na]+ m/z | MS/MS Fragments | Source | ||
|---|---|---|---|---|---|---|---|---|---|
| Detected | Expected | Error (ppm) | |||||||
| 1 | 2.691 | karakolidine | C22H35NO5 | 394.2594 | 394.2593 | 0.3 | 416.2415 | 376.2487, 378.2635, 360.2529 | FZ |
| 2 | 3.321 | chuanfumine | C22H35NO5 | 394.2597 | 394.2593 | 1.0 | 416.2391 | 376.2482, 358.2371 | FZ |
| 3 | 3.706 | senbusine A | C23H37NO6 | 424.2706 | 424.2699 | 1.6 | 446.2698 | 406.2588, 388.2485 | FZ |
| 4 | 4.019 | mesaconine | C24H39NO9 | 486.2704 | 486.2703 | 0.2 | 508.2523 | 436.2331, 404.2068, 454.2440 | FZ |
| 5 | 4.564 | kaempferol | C15H10O6 | 287.0551 | 287.0550 | 0.3 | 309.0370 | 287.0549, 289.0609, 286.0467 | HQ |
| 6 | 4.799 | 16-β-hydroxycardiopetaline | C21H33NO4 | 364.2489 | 364.2488 | 0.3 | 386.2276 | 346.2380, 358.2378 | FZ |
| 7 | 4.912 | (R)-norcoclaurine | C16H17NO3 | 272.1280 | 272.1281 | −0.4 | 294.1101 | 136.0637, 282.1080 | FZ |
| 8 | 5.160 | senbusine B | C23H37NO6 | 424.2700 | 424.2699 | 0.2 | 446.2505 | 406.2587, 388.2485 | FZ |
| 9 | 5.307 | karakoline | C22H35NO4 | 378.2643 | 378.2639 | 1.1 | 400.2458 | 360.2541, 356.2220 | FZ |
| 10 | 5.476 | isotalatizidine | C23H37NO5 | 408.2753 | 408.2750 | 0.7 | 430.2560 | 390.2640, 372.2533 | FZ |
| 11 | 5.634 | aconine | C25H41NO9 | 500.2858 | 500.2860 | −0.4 | 522.2730 | 450.2487, 468.2590 | FZ |
| 12 | 5.960 | songorine | C22H31NO3 | 358.2382 | 358.2382 | 0 | 380.2220 | 340.2317, 342.2411 | FZ |
| 13 | 6.164 | hetisine | C20H27NO3 | 330.2070 | 330.2069 | 0.3 | 352.1800 | 310.1799, 328.1909 | FZ |
| 14 | 6.638 | hypaconine | C24H39NO8 | 470.2754 | 470.2754 | 0 | 492.2528 | 438.2487, 439.2519 | FZ |
| 15 | 7.044 | fuziline | C24H39NO7 | 454.2808 | 454.2805 | 0.7 | 476.2640 | 404.2431, 436.2695 | FZ |
| 16 | 7.495 | neoline | C24H39NO6 | 438.2852 | 438.2856 | −0.9 | 460.2676 | 420.2749, 388.2482, 154.1226 | FZ |
| 17 | 7.540 | 14-acetylkarakoline | C24H37NO5 | 420.2754 | 420.2750 | 0.9 | 442.2834 | 388.2485, 102.0912, 402.2641 | FZ |
| 18 | 8.454 | guan fu base H | C22H33NO2 | 344.2597 | 344.2590 | 2.0 | 366.1071 | 390.2643, 372.2532 | FZ |
| 19 | 8.476 | talatisamine | C24H39NO5 | 422.2912 | 422.2906 | 1.4 | 444.2697 | 342.2427 | FZ |
| 20 | 9.435 | 14-acetyneoline | C26H41NO7 | 480.2964 | 480.2961 | 0.6 | 502.2722 | 462.2854, 331.0813 | FZ |
| 21 | 9.694 | isorhamnetin | C16H12O7 | 317.0661 | 317.0656 | 1.6 | 339.0475 | 153.0191, 217.0489, 203.034 | HQ |
| 22 | 9.920 | calycosin-7-O-β-D-glucoside | C22H22O10 | 447.1294 | 447.1291 | 0.7 | 469.1120 | 285.0758 | HQ |
| 23 | 10.551 | 14-acetyltalatizamine | C26H41NO6 | 464.3015 | 464.3012 | 0.6 | 486.2557 | 432.2746 | FZ |
| 24 | 11.323 | karanjin | C18H12O4 | 293.0813 | 293.0808 | 1.7 | 315.0628 | 293.0797, 278.0583, 277.0468 | FZ |
| 25 | 13.590 | benzoylmesaconitine | C31H43NO10 | 590.2967 | 590.2965 | 0.3 | 612.2778 | 540.2596, 558.2701 | FZ |
| 26 | 13.925 | formononetin-O-β-D-glucoside | C22H22O9 | 431.1343 | 431.1342 | 0.2 | 453.1165 | 269.0806 | HQ |
| 27 | 14.209 | calycosin-7-O-β-D-glc-6′′-O-acetate | C24H24O11 | 489.1403 | 489.1397 | 1.2 | 511.1214 | 371.2285 | HQ |
| 28 | 14.747 | benzoylaconine | C32H45NO10 | 604.3128 | 604.3122 | 1.0 | 626.2895 | 554.2752, 572.2857 | FZ |
| 29 | 15.134 | 9,10-dimethoxy-pterocarpan-3-O-β-D-glucoside | C23H26O10 | 463.1606 | 463.1604 | 0.4 | 485.1432 | 299.0911, 160.0713 | HQ |
| 30 | 15.330 | benzoylhypaconine | C31H43NO9 | 574.3022 | 574.3016 | 1.0 | 596.2847 | 542.2753, 570.3064 | FZ |
| 31 | 15.864 | 2′-hydroxy-3′,4′-dimethoxyisoflavan-7-O-β-D-glucoside | C23H28O10 | 465.1764 | 465.1761 | 0.6 | 487.1581 | 167.0700 | HQ |
| 32 | 16.090 | calycosin | C16H12O5 | 285.0766 | 285.0763 | 1.0 | 307.0588 | 225.0546, 253.0495, 137.0230 | HQ |
| 33 | 17.024 | benzoyldeoxyaconine | C32H45NO9 | 588.3177 | 588.3173 | 0.7 | 610.2787 | 556.2908 | FZ |
| 34 | 17.297 | quercetin | C15H10O7 | 303.0506 | 303.0499 | 2.3 | 325.0319 | 257.0440, 285.0390, 247.0596 | HQ |
| 35 | 17.567 | beiwudine | C31H41NO8 | 556.2909 | 556.2905 | 0.7 | 578.2724 | 524.2648 | FZ |
| 36 | 17.759 | formononetin-7-O-β-D-glc-6′-β-O-acetate | C24H24O10 | 473.1449 | 473.1448 | 0.2 | 495.1262 | 270.0842, 139.1112 | HQ |
| 37 | 18.289 | 9,10-dimethoxy-pterocHQpan-3-O-β-D-glc-6′-O-acetate | C25H28O11 | 505.1714 | 505.1710 | 0.8 | 527.1530 | 487.3125, 311.2218 | HQ |
| 38 | 18.616 | 2′-hydroxy-3′,4′-dimethoxyisoflavan-7-O-β-D-glc-6″-O-acetate | C25H30O11 | 507.1871 | 507.1866 | 0.9 | 529.1675 | 442.2586, 167.0711 | HQ |
| 39 | 18.650 | hypaconitine | C33H45NO10 | 616.3123 | 616.3122 | 0.2 | 638.7884 | 556.2898, 129.1019 | FZ |
| 40 | 19.406 | formononetin | C16H12O4 | 269.0815 | 269.0814 | 0.4 | 291.0631 | 213.0909, 237.0544, 118.0411 | HQ |
| 41 | 19.541 | isoastragalosideIV | C41H68O14 | - | 785.4687 | - | 807.4508 | 175.0597, 157.0491 | HQ |
| 42 | 19.620 | astragaloside IV | C41H68O14 | - | 785.4687 | - | 807.4516 | 437.3402, 455.3499, 419.3302 | HQ |
| 43 | 20.116 | 7,2′-Dihydroxy-3′,4′-dimethoxyisoflavan | C17H18O5 | 303.1233 | 303.1232 | 0.3 | 325.1065 | 133.0644, 161.0594 | HQ |
| 44 | 20.442 | soyasaponin I | C48H78O18 | 943.5264 | 943.5266 | −0.2 | 965.5077 | 441.3721, 599.3967, 797.4655 | HQ |
| 45 | 20.898 | astragaloside II | C43H70O15 | 827.4791 | 827.4793 | −0.2 | 849.4624 | 669.3980, 453.3356 | HQ |
| 46 | 21.820 | deoxyandrographolide | C20H30O4 | 335.2203 | 335.2217 | −4.1 | 357.2036 | 263.1314, 247.1543 | FZ |
| 47 | 21.978 | agroastragaloside III | C51H82O21 | 1031.5400 | 1031.5427 | −2.0 | 1053.5288 | 898.4067, 900.4128 | HQ |
| 48 | 22.451 | astragaloside I | C45H72O16 | 869.4886 | 869.4899 | −1.5 | 891.4728 | 217.0704, 143.1065, 139.0386 | HQ |
| 49 | 22.674 | isoastragaloside I | C45H72O16 | 869.4888 | 869.4899 | −1.3 | 891.4739 | 217.0704, 143.1065, 157.0491 | HQ |
| 50 | 24.267 | acetylastragaloside I | C47H74O17 | 911.5003 | 911.5004 | −0.1 | 933.4817 | 143.1064, 199.0597 | HQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, L.; Ma, M.; Zhang, J.; Zhou, J.; Zheng, Y.; Liang, A.; Sun, Q.; Liu, J.; Liao, W. Qifu Decoction Alleviates Lipopolysaccharide-Induced Myocardial Dysfunction by Inhibiting TLR4/NF-κB/NLRP3 Inflammatory Pathway and Activating PPARα/CPT Pathway. Pharmaceuticals 2025, 18, 1109. https://doi.org/10.3390/ph18081109
Zhuo L, Ma M, Zhang J, Zhou J, Zheng Y, Liang A, Sun Q, Liu J, Liao W. Qifu Decoction Alleviates Lipopolysaccharide-Induced Myocardial Dysfunction by Inhibiting TLR4/NF-κB/NLRP3 Inflammatory Pathway and Activating PPARα/CPT Pathway. Pharmaceuticals. 2025; 18(8):1109. https://doi.org/10.3390/ph18081109
Chicago/Turabian StyleZhuo, Lingxin, Mingxuan Ma, Jiayi Zhang, Jiayu Zhou, Yuqi Zheng, Aiyin Liang, Qingqing Sun, Jia Liu, and Wenting Liao. 2025. "Qifu Decoction Alleviates Lipopolysaccharide-Induced Myocardial Dysfunction by Inhibiting TLR4/NF-κB/NLRP3 Inflammatory Pathway and Activating PPARα/CPT Pathway" Pharmaceuticals 18, no. 8: 1109. https://doi.org/10.3390/ph18081109
APA StyleZhuo, L., Ma, M., Zhang, J., Zhou, J., Zheng, Y., Liang, A., Sun, Q., Liu, J., & Liao, W. (2025). Qifu Decoction Alleviates Lipopolysaccharide-Induced Myocardial Dysfunction by Inhibiting TLR4/NF-κB/NLRP3 Inflammatory Pathway and Activating PPARα/CPT Pathway. Pharmaceuticals, 18(8), 1109. https://doi.org/10.3390/ph18081109








