Abstract
Background/Objectives: Methadone maintenance treatment (MMT) is widely used in opioid use disorder (OUD). Its efficacy is influenced by its metabolism, primarily mediated by Cytochrome P450 (CYP450) enzymes in the liver. Genetic polymorphisms in CYP450 genes and other factors, such as age, sex, and concomitant treatments, contribute to interindividual variability in methadone response. This article addresses the relevance of pharmacokinetic biomarkers in methadone metabolism and its impact on treatment outcomes in European populations over the past 25 years. Methods: A systematic review was conducted using four databases (PsycINFO, PubMed, Scopus, and Web of Science) for studies published between 2000 and 2024 following the PRISMA 2020 guidelines (CRD42025641373 in PROSPERO). Two independent reviewers screened and assessed the study quality using NHLBI tools. Discrepancies were solved through consensus. Relevant data including sample size, genetic biomarkers, and key findings were extracted for each study. Data were synthesized and described in detail. Results: Fourteen studies on pharmacogenetic biomarkers influencing methadone metabolism in European populations were analyzed, encompassing a total of 3180 subjects. CYP2B6*6 was identified as a key variant associated with increased (S)-methadone plasma levels, potentially leading to cardiac complications, while the role of other pharmacokinetic genes, including ABCB1 and CYP2D6, was inconclusive. Conclusions: Genetic polymorphisms significantly influence methadone metabolism, with the CYP2B6*6 allele playing a key role in (S)-methadone metabolism and associated with cardiac risks. Pharmacogenetic studies integrating co-mediation—the principal cause of phenoconversion—as a potential variable alongside gender differences and encompassing adequate sample sizes could improve outcomes and establish the basis for personalized medicine of MMT.
Keywords:
metabolism; genes; methadone; biomarkers; opioid addiction; maintenance therapies; pharmacogenetics 1. Introduction
Addiction is a chronic disease with a neurological basis characterized by relapses [1,2]. In this line, the neurobiological foundations of craving, a key component of dependence syndromes and considered as a central element of the motivational drive in addiction, is considered an interesting research field [3]. Furthermore, craving is a common symptom across various substance use disorders, including those related to alcohol, nicotine, cannabis, cocaine, and other psychoactive drugs [4]. In this context, several theories have been proposed to explain different aspects of neuroadaptation in addiction, including the opponent process theory, inhibitory control theory, reward deficiency theory, incentive sensitization theory, aberrant learning theory, and anti-reward theory [5].
Opioid use disorder (OUD), also known as opioid addiction, is a pervasive disease characterized by the compulsive or uncontrollable seeking and consumption of opioids. OUD represents a health-related problem with social and economic consequences for the individual and the entire society, deteriorating convivence and quality of life [6]. Metabolic Syndrome (MetS) is a complex condition that can arise in patients with OUD as a result of increased calorie consumption, decreased physical activity, or a combination of both factors [7]. Over the past decade, the prevalence of this disorder has increased. It was reported in 2017 that 0.6% of the Spanish population between 15 and 64 years old had consumed heroin at some time, and the number of people using opioids worldwide reached 60 million in 2022 [8,9].
This continuous growth in the number of opioid consumers highlights the necessity of understanding the mechanisms underlying this disorder. Related to this, it is known that the transition from a sporadic user of opioids to an addict is surrounded by risk factors from different sources, which include social, developmental, behavioral, and genetic elements [6,10,11,12,13]. Moreover, people with OUD usually suffer from other psychiatric and medical comorbidities, the most common being anxiety and depression [8,14]. These comorbidities could complicate its diagnosis and exacerbate related symptoms, such as cravings and withdrawal symptoms, which may have an impact on its treatment and prevention [15].
Therapeutic approaches for OUD management encompass both behavioral and pharmacological, the most effective strategy being the combination of both. Whereas behavioral techniques, such as Cognitive Behavioral Therapy (CBT) and Contingency Management, are based on reinforcing patients’ motivation and improving attitudes to confront substance use, pharmacological treatments consist in the prescription of drugs (such as methadone, buprenorphine, and naltrexone) to overcome related symptoms and prolong detoxification once achieved [4,6,16]. The most studied drug for OUD treatment is methadone [17].
Methadone is a synthetic opioid that acts as a full agonist of the µ-opioid receptor (MOR) and as an agonist of the N-methyl-D-aspartate (NMDA) receptor [18,19]. Methadone has potential serotonergic effects with serotonin and noradrenaline reuptake inhibition and high affinity for serotonin receptors (5-HT2A and 5-HT2C) [20]. It was developed in Germany during World War II to be used as an analgesic drug when it was not possible to obtain morphine. Later, methadone was used as a maintenance treatment for heroin dependence in the mid-1960s and then repurposed to treat OUD with great success [21]. Methadone is commercially available in different formulations, from oral to intravenous, as a racemic mixture composed of two enantiomers: (R)-methadone and (S)-methadone. This chimerism results in different properties, with (R)-methadone presenting a 10-fold higher affinity to MOR in vitro and, as a consequence, higher therapeutic efficacy in vivo [22,23]. Methadone maintenance treatment (MMT) can be administered orally or intravenously, and doses can vary depending on the patient’s level of addiction.
Methadone is biotransformed primarily in the liver by the monooxygenases of the Cytochrome P450 (CYP450) family, which play a key role in the first-pass metabolism. Specifically, it is first metabolized into an unstable metabolite, after N-demethylation and subsequent cyclization, called 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), which is quickly transformed into 2-ethyl-5-methyl-3,3-diphenyl-1-pyrroline (EMDP) due to its high instability [24]. In this line, it is worth mentioning that some CYP450 enzymes present stereo-selectivity for a specific enantiomer. It has been reported that CYP2C19, CYP3A7, and CYP2C8 enzymes have a preference for (R)-methadone, whereas CYP2B6, CYP2D6, and CYP2C18 metabolize (S)-methadone in the first place. Other enzymes, such as CYP3A4, are not stereo-specific [23,25,26] (Figure 1).
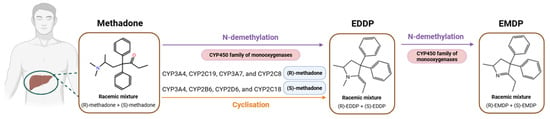
Figure 1.
Methadone metabolism in the liver. Created with BioRender (https://app.biorender.com/, accessed on 16 April 2025).
The relationship between the methadone dose, blood concentration, and clinical effect is complicated to assess due to pharmacological interactions and individual differences in terms of pharmacokinetics. For example, elevated plasma levels of (S)-methadone have been associated with cardiac complications, especially QT cardiac (QTc) interval prolongation, which may lead to arrhythmias in patients undergoing methadone maintenance treatment (MMT). In this line, genetic polymorphisms, such as single nucleotide polymorphisms (SNPs), present in genes involved in biotransformation and clearance of methadone, can cause different responses between patients [27,28,29,30]. It has been previously reported by Richards-Waugh et al. in 2014 that the following SNPs of the gene CYP3A4, rs2242480 and rs2740574, were overrepresented in patients suffering from methadone overdose fatalities compared with controls [31]; on the other hand, Yang et al. published a Genome-Wide Association Study (GWAS) in 2016, which highlights the association between CYP2B6, SPON1, and GSG1L haplotypes and (S)-methadone plasma concentration [32].
Considering the previous results, genetic polymorphisms bring complexity into the methadone prescription process, together with other factors such as age, body weight, liver and kidney function, as well as concomitant treatments. Methadone has been associated with serotonin toxicity when taken in concomitance with other serotonergic medicines; however, the risk appears low [33]. However, special attention should be given to serotonergic drugs, such as pethidine, monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs) administered concomitantly in order to avoid the risk of serotonin syndrome [34]. Recognizing these variants and elucidating their impact on methadone maintenance treatment (MMT) could help prevent a lack of effectiveness and the appearance of treatment-related toxicities, decreasing methadone dropout rates [35,36]. Methadone maintenance treatment (MMT) can be administered orally or intravenously, and doses can vary depending on the patient’s level of addiction. The Spanish Agency for Medicines and Health Products (AEMPS) recommends starting with a dose of 10 to 30 mg/day, and based on clinical progress, this dose may be increased to 40 to 60 mg/day over a period of one to two weeks. The final maintenance dose typically ranges from 60 to 100 mg/day, with increments of 10 mg/day each week [37].
To identify pharmacogenetic biomarkers involved in methadone pharmacokinetics and clarify their relationship with the outcome of the treatment in different patients, a systematic review was performed to compile the evidence published over the last twenty-five years (2000 to 2024, both included) on European population studies.
2. Materials and Methods
2.1. Desing and Registration
This systematic review has been designed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Guidelines [38]. A specific protocol has been developed for this purpose, which is available in the PROSPERO repository of systematic reviews, with the goal of ensuring transparency and methodological rigor. This systematic review has been registered under number CRD42025641373 since February 2025.
2.2. Search Strategy
A systematic literature search was performed across the scientific electronic databases PsycINFO, PubMed, Scopus, and Web of Science. The sequence of search included the following terms, separated using the Boolean operators “AND” and “OR”: (methadone) AND (metabolism) AND (gene* OR polymorphism* OR biomarker*). Syntex adjustments were included when necessary for each database (see Supplementary Table S1 for full search query syntax). As the automatic filters for the year and language of publication were available in all the beforementioned platforms, the search strategy involved English articles published during the last twenty-five years (2000 to 2024, both included).
2.3. Inclusion Criteria
This systematic review was restricted to research studies involving human subjects developed in European countries (Caucasians), which analyzed the impact of polymorphisms related to methadone’s metabolism (pharmacokinetics). Other types of articles, such as reviews, systematic reviews, letters, or similar, were excluded. Adult patients were selected regardless of sex, and the main pharmacological treatment to overcome opioid addiction in each patient should be methadone. Additionally, data from patients presenting special circumstances, such as pregnancy, were not extracted.
2.4. Screening Process and Study Selection
Two independent reviewers, S.R.-R. and J.G.-T., were responsible for compiling all the results from each database. J.G.-T. is a psychiatrist with expertise in addictions, while S.R.-R. is an expert in pharmacogenetic biomarkers in psychiatry, particularly in CYP450 genes. We considered the combination of their expertise to be a strong synergy for generating high-quality data for this systematic review. After duplicate elimination, titles and abstracts were revised in accordance with the defined eligibility criteria for first inclusion. Once this phase was completed, a full-text screening review was assessed in order to confirm the ethnicity of participants and the polymorphisms included in each article, as these aspects were not always specified in the title/abstract. When inconsistencies or disagreements regarding the articles included appeared, a meeting was held with the rest of the review team to reach a consensus.
2.5. Assessment of Study Quality
All studies meeting the inclusion criteria for full extraction were evaluated through a methodological quality assessment. The risk of bias was assessed independently by two reviewers (S.R.-R. and E.D.) using the Study Quality Assessment Tools designed by the National Heart, Lung, and Blood Institute (NHLBI) [39]. The questions assessed were different depending on the type of study: in the case of cross-sectional studies, the NHLBI Study Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used; in the case of case-control studies, the NHLBI Study Quality Assessment of Case-Control Studies was selected. Disagreements or inconsistencies regarding the rated quality were discussed with the rest of the review team.
2.6. Data Extraction
S.R.-R. and J.G.-T. performed independent data extraction and compiled this information into an Excel spreadsheet template, which was shared with the rest of the review team. The following information was extracted from each included article: (i) sample size, (ii) mean age, (iii) percentage of Caucasians or recruitment country, (iv) pharmacokinetic-related genes analyzed, (v) main findings, and (vi) association p-values. Supplementary Materials were revised to complete this information when necessary.
2.7. Data Analysis
The findings of this systematic review were synthesized in narrative (descriptive) form. Statistical association values were extracted from each included study, and the impact on methadone metabolism and clinical outcomes were descriptively assessed. Due to the presence of high heterogeneity of the articles finally included, it was not possible to perform a quantitative analysis. Additionally, no meta-analysis and no subgroup or subsect analyses were undertaken.
3. Results
Figure 2 summarizes the selection process of the articles included in this systematic review, according to the PRISMA statement. From the 1044 initial articles obtained from the four scientific databases (PsycINFO, PubMed, Scopus, and Web of Science), 206 records were removed due to duplication. During title and abstract screening, a total of 783 articles did not meet inclusion criteria for first-phase inclusion, leading to 55 articles for full-text assessment. Finally, 14 articles were included in this systematic review.
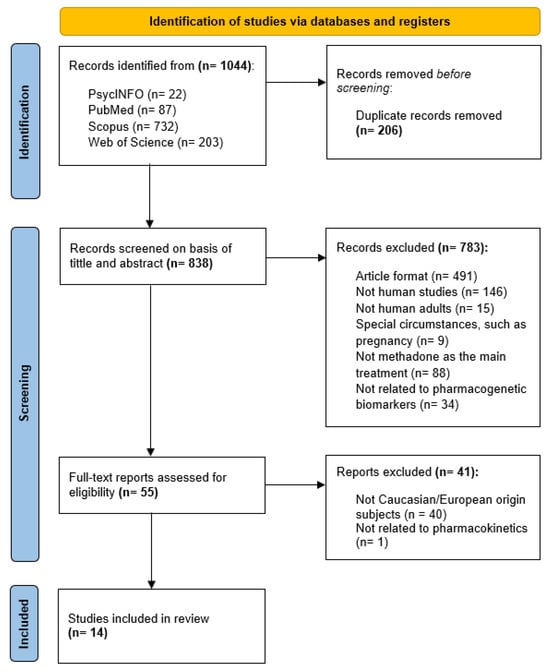
Figure 2.
PRISMA flow diagram of study selection.
The designed search strategy identified a total of 1044 articles from the four scientific databases chosen (PsycINFO, PubMed, Scopus, and Web of Science). After the removal of duplicates (206 records), title/abstract screening was performed, leading to the exclusion of a total of 783 articles for not meeting inclusion criteria. Full-text assessment ended up with 14 articles for final inclusion in this systematic review.
3.1. Characteristics of Included Articles
The screening process led to fourteen articles finally being included in this systematic review (Table 1). These articles, published between April 2001 and January 2021, could be categorized as cross-sectional studies (n = 10.71%) and case-control studies (n = 4.29%). All studies were carried out in European countries, except two that took place in the United States. Despite this difference, these articles were finally included, as most participants were Caucasians. Heterogeneity was present across the included studies as the evaluation of the impact of genetic polymorphisms was performed using different exposures: methadone plasma level concentrations, durability of QTc intervals, satisfaction with MMT, risk of death for opioid use, and treatment side effects.

Table 1.
Summary of findings from the final included studies in this systematic review.
3.2. Patients Included
The fourteen articles included in this systematic review analyzed a total of 3180 subjects, of which 709 were controls. The mean age was 3562 years, and the percentage of male subjects was higher than females in all articles except in Lötsch and collaborators (25 males and 26 females) [42]; in the case of Dobrinas et al., data segregated by sex were not available [48]. Additionally, Dobrinas et al.’s study used a different formulation for methadone: instead of administering the racemic mixture as in the rest of the studies, participants received levomethadone, which only contains the (R)-methadone enantiomer. Recruited participants from the 14 studies encompass a total of seven countries (Switzerland (n = 5), Germany (n = 2), Spain (n = 2), United Kingdom (n = 1), Denmark (n = 1), France (n = 1), and the United States of America, USA (n = 2)). Selection criteria include Caucasian/European patients, and in the case of the articles from the USA, the lowest percentage of Caucasians was detected in the study performed by Carlquist et al. (74%) [50].
3.3. Assessment of Risk of Bias
Specific quality assessment scales were used to evaluate the risk of bias in this systematic review according to the study type (see Supplementary Tables S2 and S3). In reference to cross-sectional studies (n = 10), a total of 14 questions were considered for assessing the risk of bias. Regarding sample size justification (question 5), most publications (9 out of 10) lacked justifications and equal inclusion criteria. It should also be noted that question 10, related to the number of times of exposure assessment, is marked negatively in this type of article. Concerning case-control studies (n = 4), sample size justification was also not included in most of the articles (3 out of 4), and an absence of similarity in some case-control populations was detected. Additionally, it was not always possible to find out whether the assessors were blinded to the exposure status of participants, with only one study reporting this aspect. Finally, unlike in cross-sectional studies, in which 9 out of 10 confounding variables were measured, most case-control studies (3 out of 4) did not specify if statistical corrections based on confounding variables (such as gender or concomitant medication) were performed.
3.4. Genetic Variants Analyzed
This systematic review obtained information for a total of ten relevant genes involved in methadone metabolism. Some of them showed interesting association results for genetic variants in relation to different exposures to methadone.
3.4.1. CYP2B6 Gene
Located on chromosome 19 (19q13.2), this gene encodes the CYP2B6 enzyme [54]. Regarding genetic variability, a total of forty-nine star alleles (*) have been identified for this gene [55], and six were analyzed in the articles reviewed. It is important to mention that CYP2B6*6/*6 diplotype was found to be associated with higher (R,S)-methadone plasma level concentrations (p = 0.006 [41], p = 0.013 [44]). When comparing both enantiomers separately, the association is presented in the case of (S)-methadone, reported by three articles (p = 0.0004 [41], p = 0.0001 [43], and p = 0.0004 [44]); however, (R)-methadone seems to not be affected equally (p = 0.14 [41], p = 0.18 [44], and p = 0.07 [43]), underlying possible stereo-selectivity for (S)-methadone. In line with this, the presence of the CYP2B6*6 allele has been reported to not have a significant association with a high methadone maintenance dose (p = 0.89 [49]), which could be explained by the main therapeutic activity relying on (R)-methadone. In terms of the risk of death, CYP2B6*6 presence was related to higher methadone concentration in post-mortem samples [46].
Other CYP2B6 alleles, such as *4, *5, *7, *9, *11, and *26, were also assessed and exhibited controversial results. The study of Dobrinas and coworkers highlighted that the CYP2B6*5 allele was overrepresented in the low (S)-methadone plasma level group (p = 0.005), whereas CYP2B6*9 was underrepresented (p < 0.05). Regarding CYP2B6*11, it was overrepresented in the high (S)-methadone plasma level group (p = 0.006). On the other hand, Ahmad et al. found that the CYP2B6*5/*5 genotype was associated with a significant increase in methadone blood concentration (p = 0.002), and CYP2B6*2 presence was related to a decreased metabolic ratio [52]. Other studies reported no association for these alleles [42,47,49,51].
3.4.2. ABCB1 Gene
This gene, located on chromosome 7, encodes the ABCB1 membrane-associated protein, also called P-glycoprotein, which belongs to the ATP-binding cassette (ABC) transporters [56]. Variants in this gene were widely studied in this systematic review, although with certain controversies. Crettol et al. reported lower levels of (R,S)-methadone, (R)-methadone, and (S)-methadone to be associated with rs1045642 (p = 0.01, p = 0.03, and p = 0.01, respectively) and rs9282564 (p = 0.01, p = 0.02, and p = 0.01, respectively), whereas rs2032582 was only associated with lower (R)-methadone levels (p = 0.04) [43]. According to Iwersen-Bergmann et al., rs1045642 homozygous patients presented higher medulla/blood methadone concentration ratios compared to heterozygous and non-carriers (p = 0.0019 and p = 0.0042, respectively), while rs1128503 and rs2032582 did not present any association (p = 0.5 and p = 0.12, respectively) [53]. On the other hand, SNP rs9282564 was underrepresented in a cohort of deceased patients due to opioid addiction in comparison with a cohort of living patients with opioid addiction, which has been reported to underly a possible protective role in the risk of death due to its consumption [51]. Other SNPs did not present any association with MMT [42,47,49,51].
3.4.3. CYP2D6 Gene
The CYP2D6 gene codes for the CYP2D6 enzyme, which is involved in the metabolism of many psychotropic drugs [57]. Located in position 22q13.2, CYP2D6 is a highly polymorphic gene, currently with over 170 star alleles (*) identified in PharmVar [55,58]. Eap and collaborators observed different (R)-methadone concentrations between ultrarapid metabolizers (UMs) and poor metabolizers (PMs) (p = 0.009); however, the association was not significant when compared with normal metabolizers (NMs) (p = 0.055 and p = 0.064, respectively) [40]. Crettol et al. described that UMs showed lower concentrations of (R,S)-methadone and (S)-methadone compared to NMs and intermediate metabolizers (IMs) (p = 0.03 and p = 0.04, respectively) but not with (R)-methadone (p = 0.08) [43], whereas the association for (R)-, (S)-, and (R,S)-methadone was reported by Fonseca et al. (p = 0.002 (R), p < 0.001, and p = 0.048) [47]. Additionally, Pérez de los Cobos et al. found that UM males reported lower satisfaction with MMT than females (p = 0.022 [45]). Other studies did not find statistically significant associations for other CYP2D6 variants, such as *2, *3, *4, *5, *10, and *11 alleles [42,51] or copy number variations (CNVs) [49]. The study by Lötsch et al., which investigated the influence of CYP2D6 polymorphisms alongside other genetic variants, did not find a significant association with levomethadone plasma concentrations [42]. Other studies, such as Eap et al. (2001) and Crettol et al. (2006), also examined the impact of different CYP2D6 metabolizer types on (R)-methadone, although statistical significance was not always achieved [40,43]. In this context, the authors suggested that it would be worthwhile to continue exploring these variants, including other genes such as ABCB1, even though the current results remain inconclusive.
3.4.4. Other Assessed Genes: CYP3A4, CYP3A5, CYP1A2, CYP2C8, CYP2C9, CYP2C19, and UGT2B7
Regarding the CYP3A gene family (CYP3A4 and CYP3A5), Crettol et al. reported that CYP3A4*1B’s presence was associated with higher (S)-methadone plasma levels but observed no significance with (R) enantiomer or the mixture of both [43]. Any association was reported for CYP3A5*3 [42,47,49,51].
Regarding the CYP2C family of genes (CYP2C8, CYP2C9, and CYP2C19), Carlquist and collaborators found that CYP2C19*2 carriers presented increased dose-corrected concentrations of EDDP, (S)-EDDP, and (R)-EDDP (p < 0.005, p < 0.004, and p < 0.003) [50]. However, CYP2C19*2 did not have an impact in other studies, in line with alleles *3, *4, and *17 [41,42,43,47,49,51]. Described alleles in the CYP2C9 gene (*2 and *3) and in CYP2C8 (*3 and *4) were not correlated with MMT outcomes either [41,42,43,47].
Finally, the impact of polymorphisms in CYP1A2 and UGT2B7 genes was also assessed by some studies in this systematic review, although potential associations were not found [42,43,51].
4. Discussion
Response to methadone treatment is characterized by a highly interindividual variability, in which several factors have been reported to play an important role. In order to elucidate the impact of pharmacogenetic polymorphisms on the outcomes of treatment, this systematic review, oriented to collect evidence of genetic biomarkers related to methadone metabolism in Europeans, was developed.
Specifically, ten different pharmacokinetic genes were analyzed across the fourteen articles included, the CYP2B6 gene being the most studied gene in relation to MMT. Within all CYP2B6 polymorphisms, the CYP2B6*6 allele seems to be crucially involved in methadone metabolism, especially in the case of the (S)-enantiomer, findings that are in line with other scientific publications [59,60]. This stereo-selectivity of methadone is important because high (S)-methadone plasma level concentrations were reported to be related to cardiac complications, with the possibility of prolonging the duration of the QTc intervals, leading to arrhythmia problems in patients under MMT [18,19].
The combination of CYP2B6 haplotypes can be translated into clinical phenotypes, which represent the metabolizer status of each individual [61]. Although forty-nine star alleles for the CYP2B6 gene have been identified, only six are included in the Clinical Pharmacogenetics Implementation Consortium (CPIC®) guideline for genotype-based recommendations for MMT. Concretely, two normal function alleles (*1 and *2), one increased function allele (*4), one decreased function allele (*6), and one no-function allele (*18). The *3 allele has been also mentioned; however, its functionality remains uncertain. Five categories of clinical phenotypes have been established by the Pharmacogenomics Knowledge Base (PharmGKB) and the CPIC®: normal metabolizers (NMs) for individuals carrying *1/*1, *1/*2, or *2/*2; rapid metabolizers (RMs) for *1/*4; ultrarapid metabolizers (UMs) for *4/*4; poor metabolizers (PMs) for *6/*6, *18/*18, or *6/*18; and intermediate metabolizers (IMs) for *1/*6 or *1/*18. The CPIC guideline for MMT establishes standard dosing, titration, and drug monitoring for RMs, NMs, Ims, and PMs, whereas genotype-based recommendations for UMs are currently not available [62].
It is also important to consider that, in addition to the individual genetic landscape, the presence of co-medications may contribute to phenoconversion. This phenomenon resulting from inhibition or induction with other drugs causes discrepancies between the patient’s genotype and the real patient’s phenotype, e.g., patients categorized as NMs converted into PMs phenotypically. The presence of potent inhibitors for the CYP2B6 enzyme, such as ticlopidine during MMT, may potentially reduce its metabolic activity and lead to interindividual variability in drug response. Conversely, the combination of MMT with CYP2B6 inducers or specific circumstances, like pregnancy, interfere by increasing its activity, leading to subtherapeutic methadone doses and nonadherence to treatment [63,64,65,66,67]. Therefore, although there are studies addressing the impact of co-medication during MMT [68,69,70], still further studies will need to include co-medication as a potential variable.
The role of the rest of the polymorphisms addressed remains unclear due to incongruencies between the studies included and other publications [29,71,72], thus underlining that the methadone metabolization process is still unknown and other non-metabolic related variants could have an impact on MMT outcomes. PharmGKB [73] compiles in vitro studies that indicate that approximately 63–74% of the drug is metabolized by the CYP3A4 gene [23]. However, there is still controversy, since many publications point out that the CYP2B6 gene is likely responsible for most of the metabolization of methadone [74,75].
The influence of other gene variants on methadone plasma levels has also been observed in other studies. The POR gene encodes the P450 oxidoreductase enzyme (POR), which plays a key role in electron transfer from the Nicotinamide Adenine Dinucleotide Phosphate (NADPH) to microsomal CYP450 enzymes, such as CYP3A4 or CYP3A5. Variants in this gene could potentially affect methadone metabolism [76,77,78]. It has been also described that polymorphisms in the Pregnane X receptor (PXR) could play a role in regulating CYP2B6 activity [29]. Other publications studied the role of pharmacodynamic variants, which mainly involve the opioid receptor gene (OPRM1) [42,46,79]. Therefore, further investigations taking into consideration these genes together might be of interest to create predictive models for methadone prescription.
Another aspect that should be taken into consideration for predictive models is gender differences in terms of methadone response. As reported by Pérez de los Cobos and collaborators, male UMs showed less satisfaction with MMT than female UMs [45]. In addition, other articles have reported that women required higher doses of methadone [80] and confirmed the existence of differences in relapse rates between males and females [81,82], underlining the possible role of sexual hormones in the outcome of MMT. Although the risk of bias assessment did not show the low methodology quality articles included in this review, the lack of balanced sex representation (only one of the articles presented similar sample sizes between males and females) may introduce the risk of bias. Therefore, findings might not accurately reflect the outcomes for both genders equally and should be taken into consideration.
In addition to the last point, it is important to highlight that this systematic review presents some limitations. On the one hand, some articles used reduced sample sizes, which may compromise the possibility of obtaining significant associations. Additionally, most articles did not include a control group (n = 10), which would be helpful to validate the results obtained in each case. Finally, although articles analyzing Caucasian and European patients were selected, the potential risk of bias cannot be excluded since no populational analysis has been performed on individuals; therefore, population stratification cannot be ruled out. Concerning the review process, the heterogeneity of study designs complicates the synthesis of findings. Furthermore, the authors are aware that the absence of subjectivity cannot be guaranteed.
5. Conclusions
MMT response varies significantly between individuals, and genetic polymorphisms can explain a part of these metabolic differences. This review highlights the importance of the CYP2B6*6 allele in (S)-methadone metabolism, encompassing clinical implications due to its association with the risk of cardiac complications. The CPIC® guideline for CYP2B6 genotype-based recommendations for MMT establishes standard dosing, titration, and drug monitoring for RMs, NMs, Ims, and PMs; however, it is important to take into consideration the co-medication of the patient when translating CYP2B6 genotypes into the expected phenotypes in order to avoid phenoconversion. Aligning future research with CPIC® nomenclature used in guidelines would not only facilitate consistent interpretation of genetic data across studies but also ensure an alignment with the established framework, facilitating the integration of pharmacogenetics into clinical practice. Concerning the rest of the genes included, inconsistencies have been detected in the role of other pharmacokinetic genes that are potentially relevant. The lack of consensus on the primary CYP450 enzyme responsible for methadone metabolism and the potential influence of pharmacodynamic variants, such as OPRM1, suggest that a more systematic approach is needed to optimize MMT. Additionally, gender differences in methadone response indicate that sex-based variations should be considered in future studies and treatment guidelines. Despite methodological limitations, such as small sample sizes and adequacy of control groups, pharmacogenetic research seems to promise an improvement in MMT outcomes. Currently, dosing continues to be primarily adjusted based on the severity of addiction; however, this approach might result in the underdosing or overdosing of the patient, leading to treatment interruptions and increasing both public healthcare system costs and health risks for patients, including relapse into substance use. In this context, this review highlights the potential of implementing genetic testing as a future tool, together with clinical outcomes to optimize dosing, in order to enable a more precise personalization of treatment from the outset.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph18050623/s1, Table S1: Search strategies for each database; Table S2: Risk of bias assessment of cross-sectional studies; Table S3: Risk of bias assessment of case-control studies. It is also possible to download the PRISMA 2020 Checklist for systematic reviews in Supplementary Materials.
Author Contributions
Conceptualization, M.A., A.C. and O.M.; methodology, S.R.-R., J.G.-T., A.G.-R., E.D., I.S.-M. and Á.R.-R.; analysis, S.R.-R., J.G.-T. and E.D.; writing—original draft preparation, S.R.-R., J.G.-T., A.G.-R., I.S.-M., Á.R.-R., A.B.-R. and A.R.-V.; writing—review and editing, E.D., J.d.L., M.A., A.C. and O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Instituto de Salud Carlos III (ISCIII) through the project PI22/01166 co-funded with European Union funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The full protocol of this systematic review is registered and available in PROSPERO under the registration code CRD42025641373. The raw data supporting the conclusions of this article will be made available by the authors on request. Requests to access these datasets should be directed to Olalla Maroñas, olalla.maronas@usc.es.
Acknowledgments
S.R.-R. acknowledges financial support from the Xunta de Galicia (Predoctoral Fellowship Program 2024), co-financed by European funds. A.G.-R. and A.B.-R. acknowledge the financial support of the “BioFRAM” project (PMP22/00056). Á.R.-R. and A.R.-V. acknowledge the financial support from IMPaCT-GENóMICA “IMP0009”, funded by Instituto de Salud Carlos III (ISCIII), with co-funding from European Union funds (ERDF).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OUD | Opioid use disorder |
| CBT | Cognitive Behavioral Therapy |
| CM | Contingency Management |
| MOR | µ-opioid receptor |
| NMDA | N-methyl-D-aspartate |
| CYP450 | Cytochrome P450 |
| EDDP | 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine |
| EMDP | 2-ethyl-5-methyl-3,3-diphenyl-1-pyrroline |
| SNP | Single Nucleotide Polymorphism |
| GWAS | Genome-Wide Association Study |
| MMT | Methadone Maintenance Treatment |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| NHLBI | National Heart, Lung, and Blood Institute |
| QTc | QT interval of the cardiac cycle |
| SD | Standard deviation |
| UMs | Ultrarapid metabolizers |
| RMs | Rapid metabolizers |
| PMs | Poor metabolizers |
| IMs | Intermediate metabolizers |
| NMs | Normal metabolizers |
| ABC | ATP-binding cassette |
| CPIC® | Clinical Pharmacogenetics Implementation Consortium |
| PharmGKB | Pharmacogenomics Knowledge Base |
| POR | Cytochrome P450 oxidoreductase |
| NADPH | Nicotinamide adenine dinucleotide phosphate oxidase |
| PXR | Pregnane X receptor |
| MetS | Metabolic syndrome |
| AEMPS | Spanish Agency of Drugs and Medical Products |
References
- Savage, S.R.; Joranson, D.E.; Covington, E.C.; Schnoll, S.H.; Heit, H.A.; Gilson, A.M. Definitions related to the medical use of opioids: Evolution towards universal agreement. J. Pain. Symptom Manag. 2003, 26, 655–667. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat. Neurosci. 2005, 8, 1442–1444. [Google Scholar] [CrossRef]
- Tiffany, S.T.; Wray, J.M. The clinical significance of drug craving. Ann. N. Y. Acad. Sci. 2012, 1248, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Bunachita, S.; Agarwal, A.A.; Lyon, A.; Patel, U.K. Opioid Use Disorder: Treatments and Barriers. Cureus 2021, 13, e13173. [Google Scholar] [CrossRef]
- Elman, I.; Borsook, D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron 2016, 89, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Strang, J.; Volkow, N.D.; Degenhardt, L.; Hickman, M.; Johnson, K.; Koob, G.F.; Marshall, B.D.L.; Tyndall, M.; Walsh, S.L. Opioid use disorder. Nat. Rev. Dis. Prim. 2020, 6, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Elman, I.; Howard, M.; Borodovsky, J.T.; Mysels, D.; Rott, D.; Borsook, D.; Albanese, M. Metabolic and Addiction Indices in Patients on Opioid Agonist Medication-Assisted Treatment: A Comparison of Buprenorphine and Methadone. Sci. Rep. 2020, 10, 5617. [Google Scholar] [CrossRef]
- United Nations: Office on Drugs and Crime. World Drug Report 2024—Special Points of Interest. Available online: www.unodc.org/unodc/en/data-and-analysis/wdr2024-special-points-of-interest.html (accessed on 26 January 2025).
- Ministerio de Sanidad, Consumo y Bienestar Social. Memoria Plan Nacional sobre Drogas 2017. Disponible en. Available online: https://pnsd.sanidad.gob.es/pnsd/memorias/docs/2019_MEMORIA_2017.pdf (accessed on 26 January 2025).
- Sharma, B.; Bruner, A.; Barnett, G.; Fishman, M. Opioid Use Disorders. Child Adolesc. Psychiatr. Clin. 2016, 25, 473–487. [Google Scholar] [CrossRef]
- Panday, S.K.; Shankar, V.; Lyman, R.A.; Alexov, E. Genetic Variants Linked to Opioid Addiction: A Genome-Wide Association Study. Int. J. Mol. Sci. 2024, 25, 12516. [Google Scholar] [CrossRef]
- Gelernter, J.; Kranzler, H.R.; Sherva, R.; Koesterer, R.; Almasy, L.; Zhao, H.; Farrer, L.A. Genome-wide association study of opioid dependence: Multiple associations mapped to calcium and potassium pathways. Biol. Psychiatry 2014, 76, 66–74. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008, 59, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Santo, T.; Campbell, G.; Gisev, N.; Martino-Burke, D.; Wilson, J.; Colledge-Frisby, S.; Clark, B.; Tran, L.T.; Degenhardt, L. Prevalence of mental disorders among people with opioid use disorder: A systematic review and meta-analysis. Drug Alcohol Depend. 2022, 238, 109551. [Google Scholar] [CrossRef] [PubMed]
- Altable, M.; De la Serna, J.M.; Akram, M. Comorbidity in Opioid Addiction: A Brief Review. J. Drug Alcohol. Res. 2023, 12, 3–5. [Google Scholar] [CrossRef]
- Sofuoglu, M.; DeVito, E.E.; Carroll, K.M. Pharmacological and Behavioral Treatment of Opioid Use Disorder. Psychiatr. Res. Clin. Pr. 2018, 1, 4–15. [Google Scholar] [CrossRef]
- Kreek, M.J.; Borg, L.; Ducat, E.; Ray, B. Pharmacotherapy in the Treatment of Addiction: Methadone. J. Addict. Dis. 2010, 29, 200–216. [Google Scholar] [CrossRef]
- Sheen, S.; Choo, J.; Huh, B.; Chung, M. The effect of oral methadone on the QTc interval in cancer pain patients. Pain Manag. 2025, 15, 21–25. [Google Scholar] [CrossRef]
- McClain, M.R.; Subramaniam, K.; Cheema, R.; Lavage, D.R.; Lin, H.H.S.; Sultan, I.; Sadhasivam, S.; Howard-Quijano, K. Intraoperative Methadone in Adult Cardiac Surgical Patients and Risks for Postoperative QTc Prolongation. J. Cardiothorac. Vasc. Anesth. 2024, 39, 406–413. [Google Scholar] [CrossRef]
- Rickli, A.; Liakoni, E.; Hoener, M.C.; Liechti, M.E. Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: Link to clinical reports of serotonin syndrome. Br. J. Pharmacol. 2018, 175, 532–543. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Action Collaborative on Countering the U.S. Opioid Epidemic; Health and Medicine Division; Board on Health Care Services; Board on Health Sciences Policy; Stroud, C.; Posey Norris, S.M.; Bain, L. (Eds.) The History of Methadone and Barriers to Access for Different Populations. In Methadone Treatment for Opioid Use Disorder: Improving Access Through Regulatory and Legal Change: Proceedings of a Workshop; National Academies Press (US): Washington, DC, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK585210/ (accessed on 26 January 2025).
- Hanna, V.; Senderovich, H. Methadone in Pain Management: A Systematic Review. J. Pain 2021, 22, 233–245. [Google Scholar] [CrossRef]
- Chang, Y.; Fang, W.B.; Lin, S.N.; Moody, D.E. Stereo-Selective Metabolism of Methadone by Human Liver Microsomes and cDNA-Expressed Cytochrome P450s: A Reconciliation. Basic Clin. Pharmacol. Toxicol. 2011, 108, 55–62. [Google Scholar] [CrossRef]
- Fredheim, O.M.S.; Moksnes, K.; Borchgrevink, P.C.; Kaasa, S.; Dale, O. Clinical pharmacology of methadone for pain. Acta Anaesthesiol. Scand. 2008, 52, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.G.; Rhodes, R.J.; Gal, J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality 2004, 16, 36–44. [Google Scholar] [CrossRef]
- Totah, R.A.; Sheffels, P.; Roberts, T.; Whittington, D.; Thummel, K.; Kharasch, E.D. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology 2008, 108, 363–374. [Google Scholar] [CrossRef]
- Eap, C.B.; Buclin, T.; Baumann, P. Interindividual Variability of the Clinical Pharmacokinetics of Methadone. Clin. Pharmacokinet. 2002, 41, 1153–1193. [Google Scholar] [CrossRef]
- Li, Y.; Kantelip, J.P.; Schieveen, P.G.; van Davani, S. Interindividual Variability of Methadone Response. Mol. Diagn. Ther. 2008, 12, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Wang, S.C.; Liu, S.W.; Ho, I.K.; Chang, Y.S.; Tsai, Y.T.; Lin, K.-M.; Liu, Y.-L. Assessment of CYP450 Genetic Variability Effect on Methadone Dose and Tolerance. Pharmacogenomics 2014, 15, 977–986. [Google Scholar] [CrossRef]
- Bart, G.; Lenz, S.; Straka, R.J.; Brundage, R.C. Ethnic and genetic factors in methadone pharmacokinetics: A population pharmacokinetic study. Drug Alcohol. Depend. 2014, 145, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Richards-Waugh, L.L.; Primerano, D.A.; Dementieva, Y.; Kraner, J.C.; Rankin, G.O. Fatal methadone toxicity: Potential role of CYP3A4 genetic polymorphism. J. Anal. Toxicol. 2014, 38, 541–547. [Google Scholar] [CrossRef]
- Yang, H.C.; Chu, S.K.; Huang, C.L.; Kuo, H.W.; Wang, S.C.; Liu, S.W.; Ho, I.-K.; Liu, Y.-L. Genome-Wide Pharmacogenomic Study on Methadone Maintenance Treatment Identifies SNP rs17180299 and Multiple Haplotypes on CYP2B6, SPON1, and GSG1L Associated with Plasma Concentrations of Methadone R- and S-enantiomers in Heroin-Dependent Patients. PLoS Genet. 2016, 12, e1005910. [Google Scholar] [CrossRef]
- Baldo, B.A.; Rose, M.A. The anaesthetist, opioid analgesic drugs, and serotonin toxicity: A mechanistic and clinical review. Br. J. Anaesth. 2020, 124, 44–62. [Google Scholar] [CrossRef]
- Perananthan, V.; Buckley, N.A. Opioids and antidepressants: Which combinations to avoid. Aust. Prescr. 2021, 44, 41–44. [Google Scholar] [CrossRef]
- Crist, R.C.; Li, J.; Doyle, G.A.; Gilbert, A.; Dechairo, B.M.; Berrettini, W.H. Pharmacogenetic analysis of opioid dependence treatment dose and dropout rate. Am. J. Drug Alcohol Abus. 2018, 44, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, J.Y.; Ferguson, C.S.; Zhu, A.Z.X.; Tyndale, R.F. Pharmacogenetics of Drug Dependence: Role of Gene Variations in Susceptibility and Treatment. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Ficha Técnica Eptadone 100 mg Solución Oral. Agencia Española de Medicamentos y Productos Sanitarios. Available online: https://cima.aemps.es/cima/pdfs/es/ft/69909/fichatecnica_69909.html.pdf (accessed on 7 February 2025).
- PRISMA Statement. PRISMA Statement. Available online: https://www.prisma-statement.org (accessed on 7 February 2025).
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 7 February 2025).
- Eap, C.B.; Broly, F.; Mino, A.; Hämmig, R.; Déglon, J.J.; Uehlinger, C.; Meili, D.; Chevalley, A.F.; Bertschy, G.; Zullino, D.; et al. Cytochrome P450 2D6 genotype and methadone steady-state concentrations. J. Clin. Psychopharmacol. 2001, 21, 229–234. [Google Scholar] [CrossRef]
- Crettol, S.; Déglon, J.J.; Besson, J.; Croquette-Krokkar, M.; Gothuey, I.; Hämmig, R.; Monnat, M.; Hüttemann, H.; Baumann, P.; Eap, C.B. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin. Pharmacol. Ther. 2005, 78, 593–604. [Google Scholar] [CrossRef]
- Lötsch, J.; Skarke, C.; Wieting, J.; Oertel, B.G.; Schmidt, H.; Brockmöller, J.; Geisslinger, G. Modulation of the central nervous effects of levomethadone by genetic polymorphisms potentially affecting its metabolism, distribution, and drug action. Clin. Pharmacol. Ther. 2006, 79, 72–89. [Google Scholar] [CrossRef]
- Crettol, S.; Déglon, J.J.; Besson, J.; Croquette-Krokar, M.; Hämmig, R.; Gothuey, I.; Monnat, M.; Eap, C. ABCB1 and cytochrome P450 genotypes and phenotypes: Influence on methadone plasma levels and response to treatment. Clin. Pharmacol. Ther. 2006, 80, 668–681. [Google Scholar] [CrossRef]
- Eap, C.B.; Crettol, S.; Rougier, J.S.; Schläpfer, J.; Sintra, G.L.; Déglon, J.J.; Besson, J.; Croquette-Krokar, M.; Carrupt, P.-A.; Abriel, H. Stereoselective block of hERG channel by (S)-methadone and QT interval prolongation in CYP2B6 slow metabolizers. Clin. Pharmacol. Ther. 2007, 81, 719–728. [Google Scholar] [CrossRef]
- Pérez de los Cobos, J.; Siñol, N.; Trujols, J.; del Río, E.; Bañuls, E.; Luquero, E.; Menoyo, A.; Queraltó, J.M.; Baiget, M.; Álvarez, E. Association of CYP2D6 ultrarapid metabolizer genotype with deficient patient satisfaction regarding methadone maintenance treatment. Drug Alcohol Depend. 2007, 89, 190–194. [Google Scholar] [CrossRef]
- Bunten, H.; Liang, W.J.; Pounder, D.; Seneviratne, C.; Osselton, M.D. CYP2B6 and OPRM1 gene variations predict methadone-related deaths. Addict. Biol. 2011, 16, 142–144. [Google Scholar] [CrossRef]
- Fonseca, F.; de la Torre, R.L.; Pastor, A.; Cuyàs, E.; Pizarro, N.; Khymenets, O.; Farré, M.; Torrens, M. Contribution of cytochrome P450 and ABCB1 genetic variability on methadone pharmacokinetics, dose requirements, and response. PLoS ONE 2011, 6, e19527. [Google Scholar] [CrossRef] [PubMed]
- Dobrinas, M.; Crettol, S.; Oneda, B.; Lahyani, R.; Rotger, M.; Choong, E.; Lubomirov, R.; Csajka, C.; Eap, C.B. Contribution of CYP2B6 alleles in explaining extreme (S)-methadone plasma levels: A CYP2B6 gene resequencing study. Pharmacogenetics Genom. 2013, 23, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Mouly, S.; Bloch, V.; Peoc’h, K.; Houze, P.; Labat, L.; Ksouda, K.; Simoneau, G.; Declèves, X.; Bergmann, J.F.; Scherrmann, J.; et al. Methadone dose in heroin-dependent patients: Role of clinical factors, comedications, genetic polymorphisms and enzyme activity. Br. J. Clin. Pharmacol. 2015, 79, 967–977. [Google Scholar] [CrossRef]
- Carlquist, J.F.; Moody, D.E.; Knight, S.; Johnson, E.G.; Fang, W.B.; Huntinghouse, J.A.; Rollo, J.S.; Webster, L.R.; Anderson, J.L. A Possible Mechanistic Link Between the CYP2C19 Genotype, the Methadone Metabolite Ethylidene-1,5-Dimethyl-3,3-Diphenylpyrrolidene (EDDP), and Methadone-Induced Corrected QT Interval Prolongation in a Pilot Study. Mol. Diagn. Ther. 2015, 19, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, D.J.; Damkier, P.; Feddersen, S.; Möller, S.; Thomsen, J.L.; Brasch-Andersen, C.; Brøsen, K. The ABCB1, rs9282564, AG and TT Genotypes and the COMT, rs4680, AA Genotype are Less Frequent in Deceased Patients with Opioid Addiction than in Living Patients with Opioid Addiction. Basic Clin. Pharmacol. Toxicol. 2016, 119, 381–388. [Google Scholar] [CrossRef]
- Ahmad, T.; Sabet, S.; Primerano, D.A.; Richards-Waugh, L.L.; Rankin, G.O. Tell-Tale SNPs: The Role of CYP2B6 in Methadone Fatalities. J. Anal. Toxicol. 2017, 41, 325–333. [Google Scholar] [CrossRef]
- Iwersen-Bergmann, S.; Plattner, S.; Hischke, S.; Müller, A.; Andresen-Streichert, H.; Jungen, H.; Erb, R.; Beer-Sandner, B. Brain/blood ratios of methadone and ABCB1 polymorphisms in methadone-related deaths. Int. J. Leg. Med. 2021, 135, 473–482. [Google Scholar] [CrossRef]
- PubChem. CYP2B6—Cytochrome P450 Family 2 Subfamily B Member 6 (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/CYP2B6/human (accessed on 7 February 2025).
- PharmVar. Available online: https://www.pharmvar.org/gene/CYP2B6 (accessed on 7 February 2025).
- ABCB1 ATP Binding Cassette Subfamily B Member 1 [Homo Sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/5243 (accessed on 7 February 2025).
- Nofziger, C.; Turner, A.J.; Sangkuhl, K.; Whirl-Carrillo, M.; Agúndez, J.A.G.; Black, J.L.; Dunnenberger, H.M.; Ruano, G.; Kennedy, M.A.; Phillips, M.S.; et al. PharmVar GeneReview: CYP2D6. Clin. Pharmacol. Ther. 2020, 107, 154–170. [Google Scholar] [CrossRef]
- CYP2D6 Cytochrome P450 Family 2 Subfamily D Member 6 (Gene/Pseudogene) [Homo Sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/1565 (accessed on 7 February 2025).
- Kharasch, E.D.; Regina, K.J.; Blood, J.; Friedel, C. Methadone pharmacogenetics: CYP2B6 polymorphisms determine plasma concentrations, clearance and metabolism. Anesthesiology 2015, 123, 1142–1153. [Google Scholar] [CrossRef]
- Kringen, M.K.; Chalabianloo, F.; Bernard, J.P.; Bramness, J.G.; Molden, E.; Høiseth, G. Combined Effect of CYP2B6 Genotype and Other Candidate Genes on a Steady-State Serum Concentration of Methadone in Opioid Maintenance Treatment. Ther. Drug Monit. 2017, 39, 550–555. [Google Scholar] [CrossRef]
- Desta, Z.; El-Boraie, A.; Gong, L.; Somogyi, A.A.; Lauschke, V.M.; Dandara, C.; Klein, K.; Miller, N.A.; Klein, T.E.; Tyndale, R.F.; et al. PharmVar GeneFocus: CYP2B6. Clin. Pharmacol. Ther. 2021, 110, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Eum, S.; Desta, Z.; Tyndale, R.F.; Gaedigk, A.; Crist, R.C.; Haidar, C.E.; Myers, A.L.; Samer, C.F.; Somogyi, A.A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2B6 Genotype and Methadone Therapy. Clin. Pharmacol. Ther. 2024, 116, 932–938. [Google Scholar] [CrossRef]
- PharmGKB. Gene-Specific Information Tables for CYP2B6. Available online: https://www.pharmgkb.org/page/cyp2b6RefMaterials (accessed on 7 February 2025).
- Shah, R.R.; Smith, R.L. Addressing phenoconversion: The Achilles’ heel of personalized medicine. Br. J. Clin. Pharmacol. 2015, 79, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Mangó, K.; Kiss, Á.F.; Fekete, F.; Erdős, R.; Monostory, K. CYP2B6 allelic variants and non-genetic factors influence CYP2B6 enzyme function. Sci. Rep. 2022, 12, 2984. [Google Scholar] [CrossRef]
- De Leon, J. The effects of antiepileptic inducers in neuropsychopharmacology, a neglected issue. Part II: Pharmacological issues and further understanding. Rev. Psiquiatr. Salud Ment. 2015, 8, 167–188. [Google Scholar] [CrossRef] [PubMed]
- McCance-Katz, E.F. Drug interactions associated with methadone, buprenorphine, cocaine, and HIV medications: Implications for pregnant women. Life Sci. 2011, 88, 953–958. [Google Scholar] [CrossRef]
- McCance-Katz, E.F.; Sullivan, L.E.; Nallani, S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: A review. Am. J. Addict. 2010, 19, 4–16. [Google Scholar] [CrossRef]
- Begré, S.; von Bardeleben, U.; Ladewig, D.; Jaquet-Rochat, S.; Cosendai-Savary, L.; Golay, K.P.; Kosel, M.; Baumann, P.; Eap, C.B. Paroxetine increases steady-state concentrations of (R)-methadone in CYP2D6 extensive but not poor metabolizers. J. Clin. Psychopharmacol. 2002, 22, 211–215. [Google Scholar] [CrossRef]
- Uehlinger, C.; Crettol, S.; Chassot, P.; Brocard, M.; Koeb, L.; Brawand-Amey, M.; Eap, C.B. Increased (R)-methadone plasma concentrations by quetiapine in cytochrome P450s and ABCB1 genotyped patients. J. Clin. Psychopharmacol. 2007, 27, 273–278. [Google Scholar] [CrossRef]
- Hamilton, S.P.; Nunes, E.V.; Janal, M.; Weber, L. The effect of sertraline on methadone plasma levels in methadone-maintenance patients. Am. J. Addict. 2000, 9, 63–69. [Google Scholar] [CrossRef]
- Irwin, M.N.; Ellingrod, V.L.; Smith, M.A. Pharmacogenetics of Methadone for Pain Management in Palliative Care. J. Pain Symptom Manag. 2022, 63, e142–e145. [Google Scholar] [CrossRef]
- Grimsrud, K.N.; Davis, R.R.; Tepper, C.G.; Palmieri, T.L. Pharmacogenetic Gene-Drug Associations in Pediatric Burn and Surgery Patients. J. Burn. Care Res. 2022, 43, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, E.D.; Stubbert, K. Role of cytochrome P4502B6 in methadone metabolism and clearance. J. Clin. Pharmacol. 2013, 53, 305–313. [Google Scholar] [CrossRef]
- Oneda, B.; Crettol, S.; Jaquenoud Sirot, E.; Bochud, M.; Ansermot, N.; Eap, C.B. The P450 oxidoreductase genotype is associated with CYP3A activity in vivo as measured by the midazolam phenotyping test. Pharmacogenetics Genom. 2009, 19, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Rojas Velazquez, M.N.; Therkelsen, S.; Pandey, A.V. Exploring Novel Variants of the Cytochrome P450 Reductase Gene (POR) from the Genome Aggregation Database by Integrating Bioinformatic Tools and Functional Assays. Biomolecules 2023, 13, 1728. [Google Scholar] [CrossRef]
- Wang, P.F.; Sharma, A.; Montana, M.; Neiner, A.; Juriga, L.; Reddy, K.N.; Tallchief, D.; Blood, J.; Kharasch, E.D. Methadone pharmacogenetics in vitro and in vivo: Metabolism by CYP2B6 polymorphic variants and genetic variability in paediatric disposition. Br. J. Clin. Pharmacol. 2022, 88, 4881–4893. [Google Scholar] [CrossRef]
- Levran, O.; Peles, E.; Hamon, S.; Randesi, M.; Adelson, M.; Kreek, M.J. CYP2B6 SNPs are associated with methadone dose required for effective treatment of opioid addiction. Addict. Biology 2013, 18, 709–716. [Google Scholar] [CrossRef]
- Takemura, M.; Niki, K.; Okamoto, Y.; Kawamura, T.; Kohno, M.; Matsuda, Y.; Ikeda, K. Comparison of the Effects of OPRM1 A118G Polymorphism Using Different Opioids: A Prospective Study. J. Pain Symptom Manag. 2024, 67, 39–49.e5. [Google Scholar] [CrossRef]
- Chiang, Y.; Wang, R.; Huang, C.; Chen, S.; Ho, W.; Lane, H.; Ho, I.; Yang, H.; Ma, W. Reduced dosing and liability in methadone maintenance treatment by targeting oestrogen signal for morphine addiction. J. Cell. Mol. Med. 2017, 21, 3552–3564. [Google Scholar] [CrossRef]
- Huhn, A.S.; Berry, M.S.; Dunn, K.E. Review: Sex-based Differences in Treatment Outcomes for Persons with Opioid Use Disorder. Am. J. Addict. 2019, 28, 246–261. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).