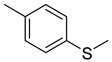
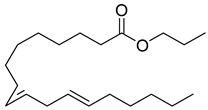
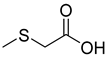
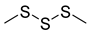Abstract
Background/Objectives: Medicinal plants are used around the globe to treat and/or manage various medical conditions, including respiratory diseases such as tuberculosis, which affect the lower respiratory tract, with its related symptoms being treated and/or managed using medicinal plants. This review collates the available literature pertaining to the medicinal uses and phytochemistry of Carpobrotus edulis, Drosera capensis, Pelargonium reniforme, and Tulbaghia violacea used for the treatment and management of tuberculosis in South Africa. The abovementioned plants were selected based on their long history of use, anecdotal evidence, and the scientific data available. Methods: Data to compile this review article were sourced and analyzed from Google Scholar, Pubmed, ScienceDirect, and textbooks published from 2000 to 2022. The search terms included the plant and genus names of each species, tuberculosis, and Mycobacterium tuberculosis. Results: The data obtained indicate that the plants do not only have an effect on Mycobacterium tuberculosis, but also on other conditions, including cough, colds, eczema, infections, and asthma, which are differential diagnoses in suspected tuberculosis cases. The literature indicates that extracts from the four plants under review have antimicrobial activity, with MICs ranging between 0.20 and 50.00 mg/mL. The major classes of phytochemicals identified from the four medicinal plants included flavonoids, naphthoquinone, terpenoids, and sulfur-containing compounds. Conclusions: The literature review on the plants reveals that they are also used to treat other lower-respiratory ailments, including cough and fever, which may be signs and symptoms of TB. The literature review reveals that medicinal plants contain valuable phytochemicals which may be strong drug leads to combat the tuberculosis epidemic.
1. Introduction
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis; it can cause a silent, latent, or progressive active infection [1]. In a 2022 report, the World Health Organization estimated that a quarter of the world’s population (approximately 2 billion people) are infected with tuberculosis, with about 2–3 million people dying from TB each year [1,2]. However, some people will not develop the TB disease, and thus will not transmit it, and some will clear the infection [3]. In 2020 alone, it was reported that a total of 1.5 million people died from TB [3]. In 2021, 10.6 million people fell ill with TB and approximately 1.6 million people died [3].
The United States Food and Drug Administration (US-FDA) has ten approved drugs for TB, and, of those, four drugs form a part of the first-line treatment of TB, namely isoniazid, rifampicin, ethambutol, and pyrazinamide [4,5]. The latest drug approved for use by the FDA was approved in 2019, a drug called pretomanid, which is also the third new drug approved by the administration in over 40 years [6]. The WHO recommends that new TB patients presumed to have drug-susceptible TB should receive six months of drug treatment with the first two months, being intensive treatment followed by a four-month continuation treatment, as summarized in Table 1 [7]. The initial phase is the bactericidal phase, which ensures that the Mycobacterium with a high rate of replication are eradicated, thus reducing symptoms and resolving clinical signs [8]. The continuation phase is the sterilizing phase when semi-dormant bacteria are eliminated, thus lowering the probability of the emergence of drug-resistant Mycobacterium [8]. Multi-drug-resistant tuberculosis (MDR-TB), however, can be treated up to a period of two years [9]. The intensive treatment involves taking isoniazid, rifampicin, pyrazinamide, and ethambutol. The continuation treatment involves taking isoniazid and rifampicin. It is vital that the TB drugs are taken together at a given time due to the possibility of quickly developing drug resistance [7]. The treatment should be taken every day for six months, and the course should be completed. The treatment is rendered ineffective if it is interrupted or stopped early due to the ability of M. tuberculosis to develop resistance rapidly against TB drugs [7]. The drug regimen, as well as its frequency, is dependent on whether the patient is an adult or child, whether the patient is infected with Human Immunodeficiency Virus (HIV) or not, and whether the patient has cavitation on an initial chest x-ray and positive cultures at completion of two months of therapy [7]; these factors will determine which patient receives which regimen. Table 1 and Table 2 adopted from the Center for Disease Control and Prevention (CDC) indicate the recommended initial and continuation phase drug regimen for drug susceptible pulmonary TB.

Table 1.
Different drug regimens for drug-susceptible pulmonary TB for adults [7].

Table 2.
Recommended dose in adults and children [7].
An illustration of the mechanism of action for current TB drugs is depicted in Figure 1.
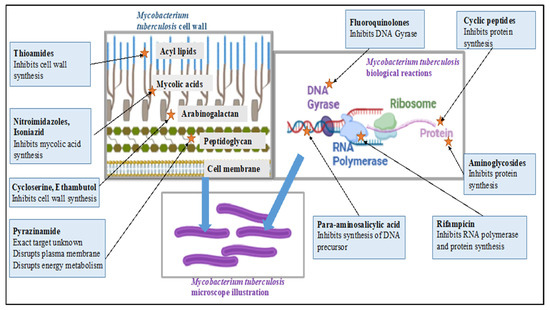
Figure 1.
Summary of the mechanisms of action for current TB drugs. Figure was illustrated using BioRender® and inspired by [10].
Ethnobotanically, traditional practitioners ‘diagnose’ tuberculosis by visual examination of the signs they observe visible of the patients and symptoms reported by patients such as cough, weight loss, and night sweats [11]. The aim of the review is to highlight the traditional uses of the selected four plants, C. edulis, D. capensis, P. reniforme, and T. violacea, based on the reported studies conducted on the plant extracts in the literature, specifically antimycobacterium studies including isolated or present phytochemicals.
2. Literature Search Strategy
The applications of four medicinal plants were sourced from different sources, including Google Scholar, Pubmed, Pubchem, and Sciencedirect, as well as textbooks dated between 2000 and 2022, with one report from 1992 that specified isolated compounds. This period was selected in order to obtain sufficient and relevant data, as well as to highlight the improvements in research over the years. A desktop review was conducted using the following approach: Firstly, scientific publications were screened. In appraising the available body of knowledge, the keywords tuberculosis, phytochemistry, Carpobrotus edulis (L.) Bolus., Drosera capensis, Pelargonium reniforme, Tulbaghia violacea, and toxicology had to be contained within the publications reviewed. The software ChemDraw Professional® v15.0 was used to illustrate the chemical structures of the phytochemicals present in the different plant extracts. The Mendeley referencing tool was used to cite the in-text references, as well as to compile the bibliography. Section 2.1, Section 2.2, Section 2.3 and Section 2.4 provide the botanical descriptions, medicinal uses, pharmacological effects, phytochemistry (including isolated compounds), and studies performed on the plant extracts (including in vivo, in vitro, and clinical data) where available for the four selected plants.
2.1. Botanical Description of Carpobrotus edulis (L.) Bolus
Carpobrotus edulis (L.) Bolus is an edible succulent that belongs to the family Aizoaceae or Masembryantgemaceae naturally distributed in the Western, Eastern, and Northern Cape [12,13] The family comprises 143 genera and about 2300 species across the tropical and sub-tropical regions [14]. The plant C. edubilis is commonly known as sour fig, Cape fig, and Hottentots fig in English, ikhambi-lamabulawo and umgongozi in IsiZulu, igcukuma in Xhosa, ghaukum in Khoi, and ghoenavy, hottentotsvy, kaapsevy, perdevy, rankvy, suurvy, and vyerank in Afrikaans [12,13,15]. It is a perennial mat-like creeper succulent with smoothly upright and triangular-shaped fleshy leaves, as visualized in Figure 2. It has large and fleshy yellow flowers that develop into aromatic fleshy fruits with a jelly like sour-sweet fruit pulp and a multitude of small brown seeds. The ripe fruits are famously used for jams and curry dishes and are sold in the Cape’s street markets [13].

Figure 2.
Carpobrotus edulis (L.) Bolus [16].
2.1.1. Ethnobotanical Uses of Carpobrotus edulis
The leaf juice is traditionally used to treat tuberculosis and is gargled to treat mouth and throat infections [13,17]. It is taken orally to treat digestive ailments and dysentery. The plant juice is used as a diuretic and a styptic, applied on the source to treat eczema, wounds, and burns. The leaf pulp is reported to treat toothache, earache, and oral and vaginal thrush [13]. The plant is also used to treat tuberculosis, diabetes, high blood pressure, toothache, headaches, oral and vaginal thrush, intestinal worms, constipation, sores, and infections of HIV/AIDS [15]. Other reported uses of the plant include sinusitis, diarrhea, spider and tick bites, infantile eczema, and fungal and bacterial infections [18]. The traditional uses are summarized in Table 3.
2.1.2. Biological Effects of Carpobrotus edulis
Antimicrobial, antioxidant, and antifungal effects are reported for C. edulis. Aqueous leaf extracts reportedly show antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa, with minimum inhibitory concentration (MIC) values between 4.00 and 6.50 mg/mL, as well as antioxidant activity when using the DPPH method [13,17]. It is reported that antibacterial MIC values for extracts are considered significant when equal or less than 0.1 mg/mL, moderate at greater than 0.1 mg/mml, but less than 0.625 mg/mL, and weak if greater than 0.625 mg/mL [19], and, thus, the reported MICs indicate weak efficacy or lack of efficacy thereof. Methanolic extracts below a toxic level have shown activity against multidrug-resistant Mycobacterium tuberculosis, as well as methicillin-resistant S. aureus [20]. The extracts inhibit the growth of multidrug-resistant M. tuberculosis within three days of culture and methicillin-resistant S. aureus within six hours of culture [20]. In another study, the antimicrobial effect of methanolic leaf extract against Moraxella catarrhalis was reported to have a concentration of 50.00 mg/mL using an agar plate diffusion assay [21]. Cytotoxicity studies were performed on Raw 264.7, Vero Kidney, and HepG2 cell lines, with the results showing LC50 ranging between 89.98 ± 10.29 to 849.86 ± 7.13 µg/mL, thus indicating a safe profile for use in humans [22].
2.1.3. Phytochemistry of Carpobrotus edulis
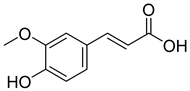
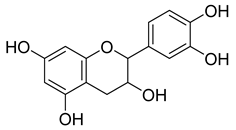
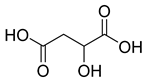
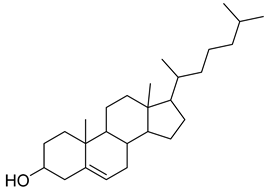
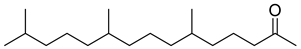
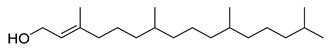
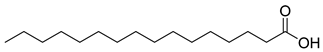
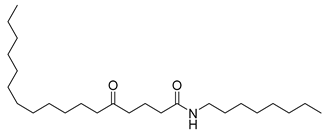
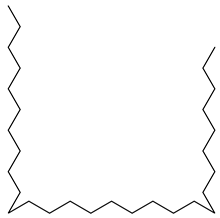
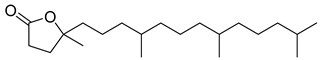
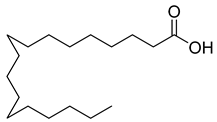
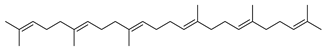
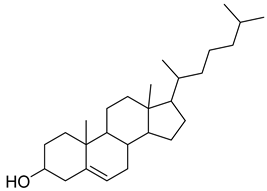
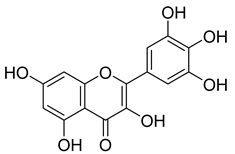
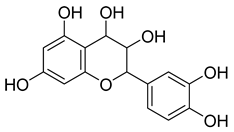
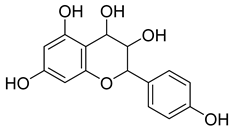
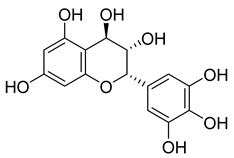
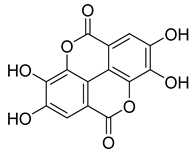
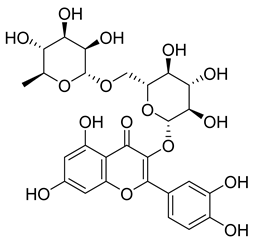
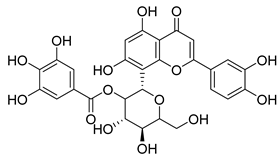
Carpobrotus edulis is reported to contain alkaloids, flavonoids, flavonols, phenolics, proanthocyanidins, saponins, and tannins [13,17]. The presence of tannins supports the use of the plant as an antiseptic and strong astringent. Other active components that are reportedly present in the plant are catechin, malic acid, citric acid, ferulic acid, hyperoside, rutin, and neohesperidin [13]. A report by Omoruyi, Bradley, and Afolayan, 2012, indicates the strong presence of phenolics, tannins, and proanthocyanidins, as well as a moderate presence of alkaloids and saponins [15]. The essential oils analyzed using gas chromatography–mass spectrometry (GC-MS) revealed a composition of monoterpenes, sesquiterpenes, diterpenes, and fatty acids [23]. Another report revealed isolated compounds from the methanolic extract using column chromatography and further purified using reverse-phase high-performance liquid chromatography, including β-amyrin (1) oleanolic acid (2), uvaol (3), monogalactosyldiacylglycerol (MGDG) (7), catechin (6), epicatechin (4), and procyanidin B5 (13), which were identified using NMR and a comparison of the spectral data with those published in the literature [24]. Table 4 summarizes the isolated and identified compounds; however, there are no reports for mechanism of action of compounds on the M. tuberculosis pathogen.

Table 4.
Phytoconstituents identified in C. edulis.

Table 3.
The uses, biological effects, and phytochemistry of Carpobrotus edulis.
Table 3.
The uses, biological effects, and phytochemistry of Carpobrotus edulis.
| Plant Part | Uses | Extraction | Biological Effect | Phytochemistry | References | ||
|---|---|---|---|---|---|---|---|
| Traditional | Method | Type of Extract | Analysis/Profile | Bioactive Components | |||
| Leaf juice Leaf pulp | Mouth and throat infections, dysentery, digestive troubles, TB, diuretic and styptic, eczema, wounds and burns, toothache, earache, oral and vaginal thrush Wounds and infections | NR | NR | Antimicrobial activity | NR | Catechin (6), malic acid (9), citric acid (8), ferulic acid (5) | [13] |
| Leaves | TB, sore throat, lung infections | NR | NR | Antimicrobial activity | TLC | Tannins and flavonoids, Hyperoside, rutin, neohesperidin | [13,17] |
| Centrifugation | Methanolic extract | Inhibits the growth of multidrug-resistant M. tuberculosis within three days of culture and methicillin-resistant S. aureus within six hours of culture below toxic levels | NR | NR | [20] | ||
| Maceration with stirring followed by centrifuging | Methanolic extract | Antimicrobial activity against M. catarrhalis with concentration of 50 mg/mL | NR | NR | [21] | ||
| Methanolic extract | Antiproliferative activity | Column chromatography | β-amyrin (1), oleanolic acid (2), uvaol (3), monogalactosyldiacylglycerol (7) (MGDG), catechin (6), epicatechin (4), and procyanidin B5 (13) | [25] | |||
NR—Not reported at this time.
2.2. Botanical Description of Drosera capensis L.
Drosera capensis belongs to the family Droseraceae and is distributed in the Eastern and Western Cape, with 160 species within the Drosera genus [17,61]. D. capensis is a small upright perennial plant (Figure 3), commonly known as Cape sundew in English, and is native to the Cape of South Africa [62,63]. The cape sundew is a carnivorous plant that uses sticky tentacles to capture their prey [62].

Figure 3.
Drosera capensis [64].
2.2.1. Ethnobotanical Uses of Drosera capensis
Drosera capensis is used as a traditional remedy for fever and tuberculosis [17]. It has also been used to treat warts, corns, sunburn, asthma, coughs, eye and ear infections, liver pain, morning sickness, stomach conditions, syphilis, toothache, and intestinal problems [64].
2.2.2. Biological Effects of Drosera capensis
The antimicrobial activity of the leaf ethanol extract was investigated against Mycobacterium smegmatis as well as Mycobacterium tuberculosis, with inhibition indicated in M. smegmatis with MIC 3.12 mg/mL and no activity in M. tuberculosis [65]. Drosera capensis is reportedly not toxic; however, when taken in large quantities, it can result in the irritation of the digestive tract lining, thus causing stomach pains or gastritis [66]. However, there is no reports that states recommended dose. The cytotoxicity of D. capensis was investigated on Vero cells with IC50 of 141.40 µg/mL [65]. The traditional uses of the plants and their biological effects are summarized in Table 5.

Table 5.
Drosera capensis uses, biological effects, and phytochemistry.
2.2.3. Phytochemistry of Drosera capensis
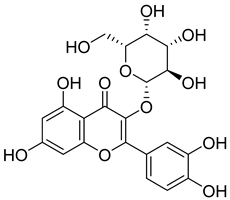
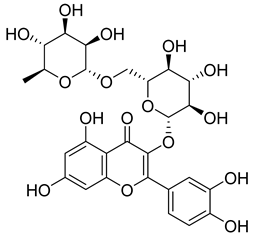
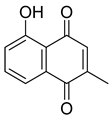
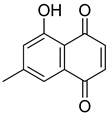
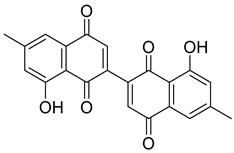
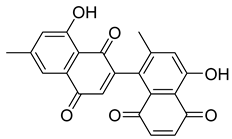
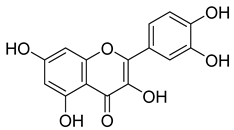
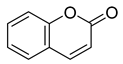
Flavonoids, including Quercetin, Myricetin, and Leucocyanidin, are reported as the bioactive components in the plant [17]. Compounds identified in D. capensis and the activity against M. tuberculosis are summarized in Table 6, including Plumbagin, which was identified in the methanolic extracts [67]. Other phytochemicals identified in D. capensis included 7-Methyljuglone (45), Mamegakinone (46), Neodiospyrin (47), Quercetin (48), Myricetin (49), Leucocyanidin (50), Leucopelargonidin (51), Leucodelphinidin (52), and Ellagic acid (53) [68]. The antimycobacterium mechanism of action was reported in two compounds—Plumbagin, a Naphthoquinone, and Quercetin, a flavonoid—both of which have anti-inflammatory properties [69,70]. TB infection in the lungs cause mild inflammation [71], thus confirming that the plant can help in the management of TB.

Table 6.
Phytoconstituents identified in Drosera capensis.
2.3. Botanical Decription of Pelargonium reniforme Curtis
Pelargonium reniforme Curtis belongs to the Geraniaceae family, which consists of 5 genera and 830 species [81,82]. The plant is commonly found in the Eastern Cape, ranging from Knysna to Umtata. It is commonly known as kidney-leaved pelargonium in English, rooirabas in Afrikaans, and iyeza lesikhali and umsongelo in IsiXhosa [81]. It is a small upright perennial shrublet with tuberous roots that grow about 300–400 mm in height, but which have been known to reach 1 m. They have kidney- or heart-shaped leaves, with the mat-like hairs on the leaves being responsible for the plant’s velvety texture and gray-green color, as observed in Figure 4 [81].

Figure 4.
Pelargonium reniforme Curtis [81].
2.3.1. Ethnobotanical Uses of Pelargonium reniforme Curtis
The plant is used traditionally as a remedy for stomach ailments, bronchitis, and bloody stools [81,83]. The Xhosa and Zulu tribes in South Africa use the plant to treat cough, tuberculosis, dysentery, and diarrhea [17,84,85]. The plant is also used to manage menstrual complaints [86].
2.3.2. Biological Effects of Pelargonium reniforme
The antimicrobial effects of the tuber against M. tuberculosis were investigated for the acetone, chloroform, and ethanol extracts by evaluating the minimum inhibitory concentration (MIC) of the plant extracts with an MIC value of 10.30 mg/mL [17], as summarized in Table 7. The antioxidant activity of the isolated bioactive compounds was investigated using the DPPH method, with IC50 ranging from 2.60 to 32.90 µM, with ascorbic acid standard IC50 of 40.0 µM [87]. The toxicology of the plant was investigated on the aqueous extract, which indicated no possibility of the toxicity of the hematological parameters in rats, and thus could be safe for use as a traditional medicine [86].

Table 7.
Pelargonium reniforme uses, pharmacological effects, and phytochemistry.
2.3.3. Phytochemistry of Pelargonium reniforme
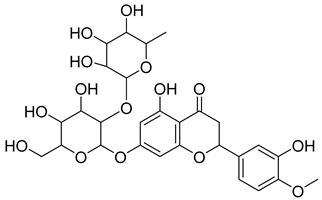
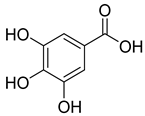
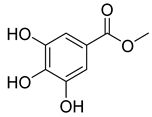
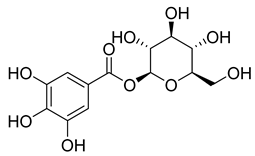
Phenolic compounds, including coumarins and scopoletin, are reported as bioactive compounds in P. reniforme [17]. Bioactive components isolated (Table 8) from P. reniforme included gallic acid (54), methyl gallate (55), glucogallin (56), corilagin (57), vitexin (58), isovitexin (59), orientin (60), isoorientin (61), vitexin 2″-gallate (62), quercetin (63), isoquercitrin (64), and rutin (65) [87]. Table 8 also summarizes the biological activity of the various compounds.

Table 8.
Phytoconstituents identified in Pelargonium reniforme.
2.4. Botanical Description of Tulbaghia violacea Harv.
Tulbaghia violacea belongs to the Alliaceae family and is distributed in the Eastern Cape as well as southern Kwa-Zulu Natal [13]. Tulbaghia genus has 63 species, with 21–30 species mostly found in Southern Africa. The species in this genus are characterized by an onion or garlic odor that comes from the leaves when they are cut [100,101]. The plant T. violacea is commonly known as wild garlic in English, isihaqa in Zulu, and wilde knoffel in Afrikaans. It is a bulbous plant that has hairless narrow leaves growing from white fleshy bases. The plant has a strong garlic smell when bruised. It has purple flowers, as depicted in Figure 5, which occur as a group at the top of the plant’s stalk [13].

Figure 5.
Tulbaghia violacea (picture captured with cellphone camera).
2.4.1. Ethnobotanical Uses of Tulbaghua violacea
The bulbs and leaves of the plant are used as a traditional remedy for fever and colds, as well as asthma, lung ulcerations, sinusitis, and tuberculosis [13,102]. A decoction of the plant, prepared by boiling the bulbs in water, is used as an enema for stomach problems, and the leaves, which can be eaten as a vegetable, are used to treat esophagus cancer [13,17]. The leaves can also be used as a tick, flea, and mosquito repellent [103]. Furthermore, the plant is used by Zulu traditional healers to treat bronchitis and asthma [104].
2.4.2. Biological Effects of Tulbaghia violacea
Antibacterial, antifungal, and antihypertensive effects were reported for T. violaceae. The dichloromethane bulb extract indicated antibacterial effects against Klebsiella pneumonia and S. aureus, with a minimum inhibitory concentration value of 0.195 mg/mL [13,17]. The bulb of the plant indicated an antifungal effect on Candida albicans [105]. The antibacterial activity of essential oils isolated from T. violacea exhibited activity against Pseudomonas aeruginosa, Streptococcus faecalis, Acinetobacter calcoaceticus anitratus, Bacillus subtilis, Enterococcus faecalis, Staphylococcus aureus, and Streptococcus viridans [104]. Cytotoxicity studies have been conducted on the plant, including its effect against Vero cells, which yielded results of 0.4909 ± 0.034 mg/mL, indicating a non-toxic profile against normal cells [106].
2.4.3. Phytochemistry of Tulbaghia violacea
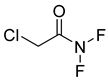
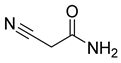
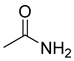
The plant reportedly contains sulfur compounds, allin, and S-(methylthiomethyl)-cysteine-4-oxide, which is the main compound in the intact plant and is broken down to marasmicin [13]. Sulfur-containing compounds were isolated and characterized from the plant [107]. Essentials oils extracted from T. violacea were subjected to GC-MS, revealing a number of volatile constituents including acetamide, 2-cyano (69), chlorodifluoro acetamide (68), σ-xylene, (E)-2-heptenoic acid (70), ρ-xylol, ρ-xylene thiodiglycol (72), 2,4-dithiapentane (73), chloromethylmethyl sulfide (74), acetamide (71), phthalic acid 2-ethylhexyl isobutyl ester (77), phthalic acid (75), phthalic acid heptyl2-methylallyl ester (76), nonadecane (78), heptacosane (79), and tetracosane (80) [104]. The biological activities of the plant are provided in Table 9. However, these are mainly on crude extracts. Table 10 indicates the compound names of the compounds identified in the plant; however, the phytochemical profile, biological activity, specific anti-TB assay data, and the proposed mechanism of action are unavailable, or are otherwise scarse, for this plant. The activity of specific compounds, especially against M. tuberculosis, requires further investigation.

Table 9.
Tulbaghia violacea uses, pharmacological effects, and phytochemistry.

Table 10.
Phytochemical constituents identified in T. violacea.
3. Conclusions
The long-term use of medicinal plants for generations clearly indicates the importance of these naturally occurring agents in our society.
- From this review, it can be concluded that the four plants are not only used to treat or manage tuberculosis. They are also used to treat other lower-respiratory ailments, including cough and fever, which may be signs and symptoms of TB.
- From the four commonly used plants, the important phytochemicals were identified from different plants, including flavonoids, phenols, terpenes, naphthoquinones, and phenolics. The mentioned phytochemicals are generally abundant in nature and are also found in other plants.
- Carpobrotus edulis and Tulbaghi violacea are edible plants, and, because of this fact, it may be safe to conclude that the plant can be taken or formulated as supplements or nutraceuticals. Many more plants are taken as teas, such as Senna, Green tea, and Rooibos. The teas contain phytochemicals such as flavonoids. Therefore, the plants may most probably be taken as tea, if edible, to manage illnesses.
- The South African Health Products Regulatory Authority (SAHPRA) has a committee dedicated to evaluate complementary medicine. It will then be recommended to submit products for evaluation with the safety and efficacy profiles.
- There are reports that provide evidence that phytochemicals, including alkaloids, flavones, phenols, terpenoids, and some fatty acids, are effective against Mycobacterium strains [111]. Most of these phytochemicals were identified in the four plants, and thus there is scientific evidence that these plants and the isolated compounds from them could serve as potential drug candidates for new anti-TB drugs. However, there are no reports beyond the potential drug candidates. It is important to note that basic research has a great impact in assembling knowledge and there is, therefore, a need to report data in a systemic manner.
- Plumbagin (44) is one of the most effective isolated compounds and was identified in D. capensis as per the review. Plumbagin (44) is effective against MDR and XDR tuberculosis [112].
- From Table 10, it is clear that further research can be conducted on compounds identified in T. violacea to investigate their efficacy against TB, as well as their mode of action. In fact, Table 4, Table 6, Table 8 and Table 10 indicate that there is an opportunity to further investigate which specific compounds are responsible for the effects against M. tuberculosis. Further research into these plants may provide treatments for TB, as well as the management or treatment of the signs and symptoms of TB, including the clinical safety and efficacy aspects.
- Many plants are commercialized without any scientific evidence, which poses a danger to society. It is therefore important for SAHPRA, as well as other Medicine Regulatory Authorities (MRAs), around the globe to develop frameworks that guide the assessment of the safety, efficacy, and quality of traditional medicines, as well as to have a harmonized regulatory standard amongst the various MRAs.
- Generally, there is an assumption that the use of traditional medicine is safer than modern medicine. Therefore, there is a need to educate the public regarding the safe use of medicinal plants. Some plants are toxic and can be fatal when taken in large quantities.
- Moreover, there is a need to inform and educate healthcare professionals regarding the use of traditional medicine. Some patients take traditional medicine and do not inform their healthcare provider. This is largely due to the stigma around the use of traditional medicine, thus resulting in drug–herb interactions.
- TB is an opportunistic infection, the risk of infection increasing in HIV-positive patients. There is, therefore, a need to conduct drug interactions, especially to ensure the safe use of traditional medicine in HIV-positive patients. This thus creates a gap in pharmacovigilance studies to develop criteria for each countries’ MRA.
- For most isolated compounds, there was no progress made from the study of extracts, phytochemical profiling to isolation, and in vitro studies, as well as little progress made in few in vivo studies, to identify compounds. Most studies end there; however, there is a need for basic research that will enable further higher-level studies to be performed, such as clinical studies using animal models, pharmacodynamic and pharmacokinetic studies, and quality assurance of traditional medicines in general.
- The number of deaths from TB remains high, despite all of the interventions such as the direct observed therapy (DOT) program, which involves healthcare workers, or other designated people, making sure that patients take their medicine correctly, thereby ensuring adherence and tolerability. According to the World Health Organization, the identification of TB cases increased after the Coronavirus Disease 2019 (COVID-19) pandemic due to the renewed attention toward infectious diseases other than COVID-19.
- TB deaths, however, remain high, especially in economically burdened countries. Governments may provide treatment; however, there are challenges around food insecurity and access. Without effective nutrition, the immune system is weakened and this increases the risk of active TB. Adherence to TB treatment thus proves to be difficult due to lack of food and the multiple drugs they have to take, resulting in resistance and, consequently, death.
Author Contributions
Conceptualization: M.P.M. and N.P.M.; methodology, M.P.M.; investigation, M.P.M.; resources, M.P.M.; data curation, M.P.M.; writing—original draft preparation, M.P.M.; writing—review and editing, M.P.M., N.P.M., B.T. and X.S.N.; supervision, N.P.M. and X.S.N.; project administration, N.P.M.; funding acquisition, M.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation (NRF) under grant number MND210415594929, the Department of Higher Education and Training (DHET) and Health and Welfare Sector Education and Training Authority (HWSETA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the manuscript.
Acknowledgments
The author expresses gratitude to the National Research Foundation (NRF), the Department of Higher Education and Training (DHET), Health and Welfare Sector Education and Training Authority (HWSETA) and the Department of Pharmaceutical Sciences at Sefako Makgatho Health Sciences University for support to conduct the study.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| COVID-19 | Coronavirus Disease 2019 |
| DOT | direct observed therapy |
| TB | Tuberculosis |
| HIV | Human Immunodeficiency Virus |
| MRAs | Medicine Regulatory Authorities |
| SAHPRA | South African Health Products Authority |
| MDR | multi-drug resistant |
| XRD | extensive-drug resistant |
References
- Wells, B.G.; Dipiro, J.T.; Dipiro, C.V.; Schwinghammer, T.L. Pharmacotherapy Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 2009; ISBN 9780071643269. [Google Scholar]
- World Health Organization. Use of Alternative Interferon-Gamma Release Assays for the Diagnosis of TB Infection: WHO Policy Statement; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 25 November 2022).
- Centers for Disease Control and Prevention. Treatment for TB Disease. Available online: https://www.cdc.gov/tb/topic/treatment/tbdisease.htm (accessed on 28 June 2021).
- Seung, K.J.; Keshavjee, S.; Rich, M.L. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a017863. [Google Scholar] [CrossRef] [PubMed]
- TB Alliance. FDA Approves New Treatment for Highly Drug-Resistant Forms of Tuberculosis. Available online: https://www.tballiance.org/news-fda-approves-new-treatment-highly-drug-resistant-forms-tuberculosis/ (accessed on 12 March 2023).
- Centers for Disease Control and Prevention. Treatment of Tuberculosis Disease; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013; pp. 139–187. [Google Scholar]
- Sotgiu, G.; Centis, R.; D’Ambrosio, L.; Battista Migliori, G. Tuberculosis Treatment and Drug Regimens. Cold Spring Harb. Perspect. Med. 2015, 5, a017822. [Google Scholar] [CrossRef] [PubMed]
- Leavens, J. TB Online—How TB Is Treated. Available online: https://www.tbonline.info/posts/2011/9/2/how-tb-treated/ (accessed on 29 June 2022).
- National Institute of Allergy and Infectious Diseases. Tuberculosis Drugs and Mechanisms of Action. Available online: https://www.niaid.nih.gov/diseases-conditions/tbdrugs (accessed on 15 April 2024).
- Thinwa, J. Indigenous Healing Practices and Their Effect on TB and HIV/TB Patients’ Utilization and Compliance with Anti-TB Medication. Indep. Study Proj. Collect. 2004, 498, 4–48. [Google Scholar]
- van Wyk, B.-E.; van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa, 2nd ed.; Briza Publications: Pretoria, South Africa, 2017. [Google Scholar]
- Akinyede, K.A.; Ekpo, O.E.; Oguntibeju, O.O. Ethnopharmacology, Therapeutic Properties and Nutritional Potentials of Carpobrotus edulis: A Comprehensive Review. Sci. Pharm. 2020, 88, 39. [Google Scholar] [CrossRef]
- Rocha, M.I.; Rodrigues, M.J.; Pereira, C.; Pereira, H.; da Silva, M.M.; da Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Biochemical Profile and in Vitro Neuroprotective Properties of Carpobrotus edulis L., a Medicinal and Edible Halophyte Native to the Coast of South Africa. S. Afr. J. Bot. 2017, 111, 222–231. [Google Scholar] [CrossRef]
- El-Raouf, H.S.A. Taxonomic Significance of Leaves in Family Aizoaceae. Saudi J. Biol. Sci. 2021, 28, 512–522. [Google Scholar] [CrossRef]
- Omoruyi, B.E.; Bradley, G.; Afolayan, A.J. Antioxidant and Phytochemical Properties of Carpobrotus edulis (L.) Bolus Leaf Used for the Management of Common Infections in HIV/AIDS Patients in Eastern Cape Province. BMC Complement. Altern. Med. 2012, 12, 215. [Google Scholar] [CrossRef]
- Malan, C.; Notten, A. Carpobrotus Edulis. PlantZAfrica. Available online: http://pza.sanbi.org/carpobrotus-edulis (accessed on 26 July 2021).
- Semenya, S.S.; Maroyi, A. Data on Medicinal Plants Used to Treat Respiratory Infections and Related Symptoms in South Africa. Data Br. 2018, 21, 419–423. [Google Scholar] [CrossRef]
- Awouafack, M.D.; McGaw, L.J.; Gottfried, S.; Mbouangouere, R.; Tane, P.; Spiteller, M.; Eloff, J.N. Antimicrobial Activity and Cytotoxicity of the Ethanol Extract, Fractions and Eight Compounds Isolated from Eriosema robustum (Fabaceae). BMC Complement. Altern. Med. 2013, 13, 289. [Google Scholar] [CrossRef]
- Martins, M.; Ordway, D.; Kristiansen, M.; Viveiros, M.; Leandro, C.; Molnar, J.; Amaral, L. Inhibition of the Carpobrotus edulis Methanol Extract on the Growth of Phagocytosed Multidrug-Resistant Mycobacterium tuberculosis and Methicillin-Resistant Staphylococcus aureus. Fitoterapia 2005, 76, 96–99. [Google Scholar] [CrossRef]
- Van Der Watt, E.; Pretorius, J.C. Purification and Identification of Active Antibacterial Components in Carpobrotus edulis L. J. Ethnopharmacol. 2001, 76, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Mulaudzi, R.B.; Aremu, A.O.; Rengasamy, K.R.R.; Adebayo, S.A.; McGaw, L.J.; Amoo, S.O.; Van Staden, J.; Du Plooy, C.P. Antidiabetic, Anti-Inflammatory, Anticholinesterase and Cytotoxicity Determination of Two Carpobrotus Species. S. Afr. J. Bot. 2019, 125, 142–148. [Google Scholar] [CrossRef]
- Omoruyi, B.E.; Afolayan, A.J.; Bradley, G. Chemical Composition Profiling and Antifungal Activity of the Essential Oil and Plant Extracts of Mesembryanthemum edule (L.) Bolus Leaves. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 19–30. [Google Scholar] [CrossRef]
- Martins, A.; Vasas, A.; Schelz, Z.; Viveiros, M.; Molnár, J.; Hohmann, J.; Amaral, L. Constituents of Carpobrotus edulis Inhibit P-Glycoprotein of MDR1-Transfected Mouse Lymphoma Cells. Anticancer Res. 2010, 30, 829–836. [Google Scholar] [PubMed]
- Martins, A.; Vasas, A.; Viveiros, M.; Molnár, J.; Hohmann, J.; Amaral, L. Antibacterial Properties of Compounds Isolated from Carpobrotus edulis. Int. J. Antimicrob. Agents 2011, 37, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Viet, T.D.; Xuan, T.D.; Anh, L.H. α-Amyrin and β-Amyrin Isolated from Celastrus hindsii Leaves and Their Antioxidant, Anti-Xanthine Oxidase, and Anti-Tyrosinase Potentials. Molecules 2021, 26, 7248. [Google Scholar] [CrossRef]
- Santos, F.A.; Frota, J.T.; Arruda, B.R.; De Melo, T.S.; André, A.; Almeida, D.C.; Anne, G.; Brito, D.C.; Chaves, M.H.; Rao, V.S. Antihyperglycemic and Hypolipidemic Effects of α, β-Amyrin, a Triterpenoid Mixture from Protium heptaphyllum in Mice. Lipids Health Dis. 2012, 11, 98. [Google Scholar]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Carmo, J.; Cavalcante-Araújo, P.; Silva, J.; Ferro, J.; Correia, A.C.; Lagente, V.; Barreto, E. Uvaol Improves the Functioning of Fibroblasts and Endothelial Cells and Accelerates the Healing of Cutaneous Wounds in Mice. Molecules 2020, 25, 4982. [Google Scholar] [CrossRef]
- Du, S.Y.; Huang, H.F.; Li, X.Q.; Zhai, L.X.; Zhu, Q.C.; Zheng, K.; Song, X.; Xu, C.S.; Li, C.Y.; Li, Y.; et al. Anti-Inflammatory Properties of Uvaol on DSS-Induced Colitis and LPS-Stimulated Macrophages. Chinese Med. 2020, 15, 43. [Google Scholar] [CrossRef]
- Bernatova, I. Biological Activities of (−)-Epicatechin and (−)-Epicatechin-Containing Foods: Focus on Cardiovascular and Neuropsychological Health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential Applications of Ferulic Acid from Natural Sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Raj, N.D.; Singh, D. A Critical Appraisal on Ferulic Acid: Biological Profile, Biopharmaceutical Challenges and Nano Formulations. Health Sci. Rev. 2022, 5, 100063. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Fan, F.Y.; Sang, L.X.; Jiang, M.; McPhee, D.J. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Foseid, L.; Devle, H.; Naess-Andresen, C.F.; Ekeberg, D. Laminaria hyperborea as a Source of Valuable Glyceroglycolipids—A Characterization of Galactosyldiacilglycerols in Stipe and Blade by HPLC-MS/MS. AppliedChem 2022, 2, 185–198. [Google Scholar] [CrossRef]
- Basnet, R.; Zhang, J.; Hussain, N.; Shu, Q. Characterization and Mutational Analysis of a Monogalactosyldiacylglycerol Synthase Gene OsMGD2 in Rice. Front. Plant Sci. 2019, 10, 992. [Google Scholar] [CrossRef]
- ELİUZ, E. Antimicrobial Activity of Citric Acid against Escherichia Coli, Staphylococcus Aureus and Candida Albicans as a Sanitizer Agent. Eurasian J. For. Sci. 2020, 8, 295–301. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H. Bin Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef]
- Park, J.Y.; Han, X.; Piao, M.J.; Oh, M.C.; Fernando, P.M.D.J.; Kang, K.A.; Ryu, Y.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Hyperoside Induces Endogenous Antioxidant System to Alleviate Oxidative Stress. J. Cancer Prev. 2016, 21, 41–47. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A Flavonoid as an Effective Sensitizer for Anticancer Therapy; Insights into Multifaceted Mechanisms and Applicability for Combination Therapy. Evid.-Based Complement. Altern. Med. 2021, 2021, 9913179. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance Its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Sasikumar, K.; Ghosh, A.R.; Dusthackeer, A. Antimycobacterial Potentials of Quercetin and Rutin against Mycobacterium Tuberculosis H37Rv. 3 Biotech 2018, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.d.C.; Fideles, S.O.M.; Reis, C.H.B.; Bellini, M.Z.; Pereira, E.d.S.B.M.; Pilon, J.P.G.; de Marchi, M.Â.; Detregiachi, C.R.P.; Flato, U.A.P.; Trazzi, B.F.d.M.; et al. Therapeutic Effects of Citrus Flavonoids Neohesperidin, Hesperidin and Its Aglycone, Hesperetin on Bone Health. Biomolecules 2022, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Chen, Y.; Agarwal, R. Anti-Tumor-Promoting Activity of a Polyphenolic Fraction Isolated from Grape Seeds in the Mouse Skin Two-Stage Initiation-Promotion Protocol and Identification of Procyanidin B5-3’-Gallate as the Most Effective Antioxidant Constituent. Carcinogenesis 1999, 20, 1737–1745. [Google Scholar] [CrossRef]
- MedChemExpress. Hexahydrofarnesyl Acetone. Available online: https://www.medchemexpress.com/hexahydrofarnesyl-acetone.html#:~:text=COA HandlingInstructions-,Hexahydrofarnesylacetone (6%2C10%2C14-Trimethyl-2,nociceptiveandanti-inflammationactivities (accessed on 9 April 2023).
- Nakatsuji, T.; Kao, M.C.; Fang, J.Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.M. Antimicrobial Property of Lauric Acid against Propionibacterium Acnes: Its Therapeutic Potential for Inflammatory Acne Vulgaris. J. Investig. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A Review of Biomedical Activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Siswadi, S.; Saragih, G.S. Phytochemical Analysis of Bioactive Compounds in Ethanolic Extract of Sterculia quadrifida R.Br. AIP Conf. Proc. 2021, 2353, 030098. [Google Scholar]
- Choudhary, D.; Shekhawat, J.K.; Kataria, V. GC-MS Analysis of Bioactive Phytochemicals in Methanol Extract of Aerial Part and Callus of Dipterygium Glaucum Decne. Pharmacogn. J. 2019, 11, 1055–1063. [Google Scholar] [CrossRef]
- ChEBI Octadecanoic Acid. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:28842#:~:text=A%20C18%20straight%2Dchain%20saturated,making%20cosmetics%2C%20candles%20and%20plastics (accessed on 12 April 2023).
- Tsai, F.S.; Lin, L.W.; Wu, C.R. Lupeol and Its Role in Chronic Diseases. In Drug Discovery from Mother Nature; Spring: Berlin/Heidelberg, Germany, 2016; ISBN 9783319413426. [Google Scholar]
- Gallo, M.B.C.; Sarachine, M.J. Biological Activities of Lupeol. Int. J. Biomed. Pharm. Sci. 2009, 3, 46–66. [Google Scholar] [CrossRef]
- MacAbeo, A.P.G.; Vidar, W.S.; Chen, X.; Decker, M.; Heilmann, J.; Wan, B.; Franzblau, S.G.; Galvez, E.V.; Aguinaldo, M.A.M.; Cordell, G.A. Mycobacterium Tuberculosis and Cholinesterase Inhibitors from Voacanga Globosa. Eur. J. Med. Chem. 2011, 46, 3118–3123. [Google Scholar] [CrossRef]
- El-shahaby, O.A.; El-zayat, M.M.; El-fattah, G.A.; El-hefny, M.M. Evaluation of the Biological Activity of Capparis Spinosa Var. Aegyptiaca Essential Oils and Fatty Constituents as Anticipated Antioxidant and Antimicrobial Agents. Prog. Chem. Biochem. Res. 2019, 2, 211–221. [Google Scholar] [CrossRef]
- Kalaimagal, C. Identification of Bioactive Compounds in Flower of Tabernaemontana divaricata (L.) Using Gas Chromatography–Mass Spectrometry Analysis. Asian J. Pharm. Clin. Res. 2019, 12, 129–132. [Google Scholar] [CrossRef]
- Zahumenicka, P.; Sysova, B.; Holik, A.; Fernandez, E.C. In Vitro Induced Mitotic Polyploidy in Drosera capensis L. Agric. Trop. Subtrop. 2013, 46, 107–110. [Google Scholar] [CrossRef]
- Krausko, M.; Perutka, Z.; Šebela, M.; Šamajová, O.; Šamaj, J.; Novák, O.; Pavlovič, A. The Role of Electrical and Jasmonate Signalling in the Recognition of Captured Prey in the Carnivorous Sundew Plant Drosera Capensis. New Phytol. 2017, 213, 1818–1835. [Google Scholar] [CrossRef]
- Pavlovič, A.; Krausko, M.; Libiaková, M.; Adamec, L. Feeding on Prey Increases Photosynthetic Efficiency in the Carnivorous Sundew Drosera Capensis. Ann. Bot. 2014, 113, 69–78. [Google Scholar] [CrossRef]
- McQuillan, M. Drosera Capensis. PlantZAfrica. Available online: http://pza.sanbi.org/drosera-capensis (accessed on 3 August 2021).
- Mativandlela, S.P.N.; Meyer, J.J.M.; Hussein, A.A.; Houghton, P.J.; Hamilton, C.J.; Lall, N. Activity against Mycobacterium Smegmatis and M. Tuberculosis by Extract of South African Medicinal Plants. Phyther. Res. 2008, 22, 841–845. [Google Scholar] [CrossRef]
- Botanical Online. Sundew Toxicity. Available online: https://www.botanical-online.com/en/medicinal-plants/sundew-toxicity (accessed on 10 November 2021).
- Marczak, Ł.; Kawiak, A.; Łojkowska, E.; Stobiecki, M. Secondary Metabolites in in Vitro Cultured Plants of the Genus Drosera. Phytochem. Anal. 2005, 16, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.A.; Van Der Kooy, F. Phytochemistry of the Carnivorous Sundew Genus Drosera (Droseraceae)—Future Perspectives and Ethnopharmacological Relevance. Chem. Biodivers. 2013, 10, 1774–1790. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, J.; Chen, L.; Guo, Q.; Yang, B.; Zhang, W.; Kang, W. Anticancer Effects and Mechanisms of Action of Plumbagin: Review of Research Advances. BioMed Res. Int. 2020, 2020, 6940953. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Sasindran, S.J.; Torrelles, J.B. Mycobacterium Tuberculosis Infection and Inflammation: What Is Beneficial for the Host and for the Bacterium? Front. Microbiol. 2011, 2, 2. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, S.; Shaw, R.; Patra, M.M.; Calcuttawala, F.; Mukherjee, N.; Gupta, S.K.D. Mycobacterium tuberculosis Thymidylate Synthase (ThyX) Is a Target for Plumbagin, a Natural Product with Antimycobacterial Activity. PLoS ONE 2020, 15, e0228657. [Google Scholar] [CrossRef]
- Mathew, R.; Kruthiventi, A.K.; Prasad, J.V.; Kumar, S.P.; Srinu, G.; Chatterji, D. Inhibition of Mycobacterial Growth by Plumbagin Derivatives. Chem. Biol. Drug Des. 2010, 76, 34–42. [Google Scholar] [CrossRef]
- Yadav, A.M.; Bagade, M.M.; Ghumnani, S.; Raman, S.; Saha, B.; Kubatzky, K.F.; Ashma, R. The Phytochemical Plumbagin Reciprocally Modulates Osteoblasts and Osteoclasts. Biol. Chem. 2022, 403, 211–229. [Google Scholar] [CrossRef]
- Mahapatra, A.; Mativandlela, S.P.N.; Binneman, B.; Fourie, P.B.; Hamilton, C.J.; Meyer, J.J.M.; van der Kooy, F.; Houghton, P.; Lall, N. Activity of 7-Methyljuglone Derivatives against Mycobacterium tuberculosis and as Subversive Substrates for Mycothiol Disulfide Reductase. Bioorganic Med. Chem. 2007, 15, 7638–7646. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Kuete, V. Review of the Chemistry and Pharmacology of 7-Methyljugulone. Afr. Health Sci. 2014, 14, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Review of Ethnomedicinal Uses, Phytochemistry and Pharmacological Properties of Euclea natalensis A.DC. Molecules 2017, 22, 2128. [Google Scholar] [CrossRef]
- van der Kooy, F.; Meyer, J.J.M.; Lall, N. Antimycobacterial Activity and Possible Mode of Action of Newly Isolated Neodiospyrin and Other Naphthoquinones from Euclea Natalensis. S. Afr. J. Bot. 2006, 72, 349–352. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin Bioactive Effects: Moving from Preclinical Evidence to Potential Clinical Applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Ascacio, A.; Rodríguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. Ellagic Acid: Biological Properties and Biotechnological Development for Production Processes. Afr. J. Biotechnol. 2011, 10, 4518–4523. [Google Scholar] [CrossRef]
- Jones, G.; Adams, T. Pelargonium Reniforme. PlantZAfrica. Available online: http://pza.sanbi.org/pelargonium-reniforme (accessed on 4 August 2021).
- Jeiter, J.; Hilger, H.H.; Smets, E.F.; Weigend, M. The Relationship between Nectaries and Floral Architecture: A Case Study in Geraniaceae and Hypseocharitaceae. Ann. Bot. 2017, 120, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Latté, K.P.; Kayser, O.; Tan, N.; Kaloga, M.; Kolodziej, H. Unusual Coumarin Patterns of Pelargonium Species Forming the Origin of the Traditional Herbal Medicine Umckaloabo. Z. Für Naturforsch. C 2000, 55, 528–533. [Google Scholar]
- Mativandlela, S.P.N.; Lall, N.; Meyer, J.J.M. Antibacterial, Antifungal and Antitubercular Activity of (the Roots of) Pelargonium Reniforme (CURT) and Pelargonium Sidoides (DC) (Geraniaceae) Root Extracts. S. Afr. J. Bot. 2006, 72, 232–237. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kiderlen, A.F. In Vitro Evaluation of Antibacterial and Immunomodulatory Activities of Pelargonium Reniforme, Pelargonium Sidoides and the Related Herbal Drug Preparation EPs® 7630. Phytomedicine 2007, 14, 18–26. [Google Scholar] [CrossRef]
- Adewusi, E.A.; Afolayan, A.J. Safety Evaluation of the Extract from the Roots of Pelargonium Reniforme Curtis in Male Wistar Rats. Afr. J. Pharm. Pharmacol. 2009, 3, 368–373. [Google Scholar]
- Latté, K.P.; Kolodziej, H. Antioxidant Properties of Phenolic Compounds from Pelargonium Reniforme. J. Agric. Food Chem. 2004, 52, 4899–4902. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Rehberg, N.; Omeje, E.; Ebada, S.S.; van Geelen, L.; Sureechatchayan, P.; Kassack, M.U.; Loerger, T.R.; Proksch, P.; Kalscheuer, R. 3-O-Methyl-Alkylgallates Inhibit Fatty Acid Desaturation in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2019, 63, 1–13. [Google Scholar]
- Cechinel-Zanchett, C.C.; Bolda Mariano, L.N.; Schlickmann, F.; Cechinel-Filho, V.; de Souza, P. In Vitro Effects of Two Bioactive Compounds, Gallic Acid and Methyl Gallate, on Urolithiasis. Actas Urológicas Españolas 2021, 45, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, A.K.; Kumar, R.; Ganguly, R.; Rana, H.K.; Pandey, P.K.; Sethi, G.; Bishayee, A.; Pandey, A.K. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules 2019, 24, 3399. [Google Scholar] [CrossRef]
- Abdulai, I.L.; Kwofie, S.K.; Gbewonyo, W.S.; Boison, D.; Puplampu, J.B.; Adinortey, M.B. Multitargeted Effects of Vitexin and Isovitexin on Diabetes Mellitus and Its Complications. Sci. World J. 2021, 2021, 6641128. [Google Scholar] [CrossRef]
- Peng, Y.; Gan, R.; Li, H.; Yang, M.; McClements, D.J.; Gao, R.; Sun, Q. Absorption, Metabolism, and Bioactivity of Vitexin: Recent Advances in Understanding the Efficacy of an Important Nutraceutical. Crit. Rev. Food Sci. Nutr. 2021, 61, 1049–1064. [Google Scholar] [CrossRef]
- Liu, S.; Lyu, Y.; Yu, S.; Cheng, J.; Zhou, J. Efficient Production of Orientin and Vitexin from Luteolin and Apigenin Using Coupled Catalysis of Glycosyltransferase and Sucrose Synthase. J. Agric. Food Chem. 2021, 69, 6578–6587. [Google Scholar] [CrossRef]
- de Jesus, C.C.M.; de Araújo, M.H.; Simão, T.L.B.V.; Lasunskaia, E.B.; Barth, T.; Muzitano, M.F.; Pinto, S.C. Natural Products from Vitex Polygama and Their Antimycobacterial and Anti-Inflammatory Activity. Nat. Prod. Res. 2022, 36, 1337–1341. [Google Scholar] [CrossRef]
- Mohammed, R.S.; Abou Zeid, A.H.; El Hawary, S.S.; Sleem, A.A.; Ashour, W.E. Flavonoid Constituents, Cytotoxic and Antioxidant Activities of Gleditsia triacanthos L. Leaves. Saudi J. Biol. Sci. 2014, 21, 547–553. [Google Scholar] [CrossRef]
- Kim, S.; Woo, E.R.; Lee, D.G. Synergistic Antifungal Activity of Isoquercitrin: Apoptosis and Membrane Permeabilization Related to Reactive Oxygen Species in Candida Albicans. IUBMB Life 2019, 71, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Mazlun, M.H.; Sabran, S.F.; Mohamed, M.; Bakar, M.F.A.; Abdullah, Z. Phenolic Compounds as Promising Drug Candidates in Tuburculosis Theraphy. Molecules 2019, 24, 2449. [Google Scholar] [PubMed]
- Çakır, D.K.; Zannou, O.; Koca, I. Scopoletin Contents and Antioxidant Properties of Some Edible Plants of Black Sea Regions. Discov. Food 2022, 2, 7. [Google Scholar] [CrossRef]
- Notten, A. Tulbaghia Simmleri. Available online: http://pza.sanbi.org/tulbaghia-simmleri (accessed on 21 April 2021).
- Takaidza, S. Phytochemical Analysis and Biological Activities of Crude Extracts from Selected Tulbhaghia Species. Ph.D. Thesis, Vaal University of Technology, Vanderbijlpark, South Africa, 2018. [Google Scholar]
- Bungu, L.; Van De Venter, M.; Frost, C. Evidence for an in Vitro Anticoagulant and Antithrombotic Activity in Tulbaghia Violacea. Afr. J. Biotechnol. 2008, 7, 681–688. [Google Scholar]
- Madike, L.N.; Takaidza, S.; Pillay, M. Preliminary Phytochemical Screening of Crude Extracts from the Leaves, Stems, and Roots of Tulbaghia Violacea. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 1300–1308. [Google Scholar] [CrossRef]
- Soyingbe, O.S.; Oyedeji, A.O.; Basson, A.K.; Singh, M.; Opoku, A.R. Chemical Composition, Antimicrobial and Antioxidant Properties of the Essential Oils of Tulbaghia Violacea Harv L.F. Afr. J. Microbiol. Res. 2013, 7, 1787–1793. [Google Scholar] [CrossRef]
- Motsei, M.L. Screening of Traditionally Used South African Plants against Candida albicans. Master’s Thesis, University of Natal, Pietermaritzburg, South Africa, 2003. [Google Scholar]
- Abd’quadri-Abojukoro, A.N.; Nkadimeng, S.M.; Mcgaw, L.J.; Nsahlai, I. V Phytochemical Composition and Cytotoxicity of Ethanolic Extracts of Some Selected Plants. J. Appl. Anim. Res. 2022, 50, 656–665. [Google Scholar] [CrossRef]
- Burton, S.G.; Kaye, P.T. Isolation and Characterisation of Sulphur Compounds from Tulbaghia Violacea. Planta Med. 1992, 58, 295–296. [Google Scholar] [CrossRef]
- Moodley, K.; Joseph, K.; Naidoo, Y.; Islam, S.; Mackraj, I. Antioxidant, Antidiabetic and Hypolipidemic Effects of Tulbaghia violacea Harv. (Wild Garlic) Rhizome Methanolic Extract in a Diabetic Rat Model. BMC Complement. Altern. Med. 2015, 15, 408. [Google Scholar] [CrossRef]
- Olorunnisola, O.S.; Bradley, G.; Afolayan, A.J. Chemical Composition, Antioxidant Activity and Toxicity Evaluation of Essential Oil of Tulbaghia Violacea Harv. J. Med. Plants Res. 2012, 6, 2340–2347. [Google Scholar] [CrossRef]
- Takaidza, S.; Kumar, A.M.; Ssemakalu, C.C.; Natesh, N.S.; Karanam, G.; Pillay, M. Anticancer Activity of Crude Acetone and Water Extracts of Tulbaghia Violacea on Human Oral Cancer Cells. Asian Pac. J. Trop. Biomed. 2018, 8, 456–462. [Google Scholar] [CrossRef]
- Veluthoor, S.; Badi, P.; Mukharjee, K.; Mandal, V. Phytochemicals: In Pursuit of Antitubercular Drugs. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 38, pp. 417–463. ISBN 9780444595300. [Google Scholar]
- Nayak, N.; Bajpai, M.; Razdan, B. Plumbagin Analogs-Synthesis, Characterization, and Antitubercular Activity. J. Adv. Pharm. Technol. Res. 2014, 5, 28–32. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).