Abstract
The rising prevalence of fungal infections, especially those caused by Candida species, presents a major risk to global health. With approximately 1.5 million deaths annually, the urgency for effective treatment options has never been greater. Candida spp. are the leading cause of invasive infections, significantly impacting immunocompromised patients and those in healthcare settings. C. albicans, C. parapsilosis and the emerging species C. auris are categorized as highly dangerous species because of their pathogenic potential and increasing drug resistance. This review comparatively describes the formation of microbial biofilms of both bacterial and fungal origin, including major pathogens, thereby creating a novel focus. Biofilms can further complicate treatment, as these structures provide enhanced resistance to antifungal therapies. Traditional antifungal agents, including polyenes, azoles and echinocandins, have shown effectiveness, yet resistance development continues to rise, necessitating the exploration of novel therapeutic approaches. Antimicrobial peptides (AMPs) such as the anti-biofilm peptides Pom-1 and Cm-p5 originally isolated from snails represent promising candidates due to their unique mechanisms of action and neglectable cytotoxicity. This review article discusses the challenges posed by Candida infections, the characteristics of important species, the role of biofilms in virulence and the potential of new therapeutic options like AMPs.
1. Candida and Candidiasis
The incidence of invasive fungal infections in humans has reached alarming levels. Worldwide, billions of people are infected with these eukaryotic pathogens and about 1.5 million patients die every year. Different species of the genera Candida, Aspergillus and Cryptococcus are responsible for 90% of cases of fungal infections with high mortality rates [1]. In fact, four species of these pathogens are classified by the World Health Organization (WHO) in the critical priority group [2]. Among these three highly dangerous microorganisms, Candida spp. is characterized by its high pathogenicity, which has been identified as the most common cause of invasive infections. As a result of this and the dramatic increase in infections associated with drug-resistant Candida spp., the US Centers for Disease Control and Prevention have identified this pathogen as a major risk to public health [1].
Candida spp. occurs naturally on the skin, in the gastrointestinal (GI) tract and on mucosal surfaces, including the oral and vaginal cavities of up to 70% of healthy individuals [3,4]. Under certain conditions, however, this fungus can overgrow and change its lifestyle into a pathogenic type, leading to primary infections in those body areas in which it naturally inhabits [5]. Risk factors for the development of a so-called “candidiasis” are prolonged stay in the intensive care unit (ICU), use of prosthetic material (e.g., central venous catheters), surgery on the GI tract (increased permeability of the intestinal epithelium can facilitate the translocation of the organism from the intestine to the bloodstream), polytrauma, advanced age, immunosuppression, neutropenia, solid tumors and hematological malignancies, as well as various drug treatments with agents such as antibiotics or corticosteroids [1,6,7,8,9,10,11,12,13,14,15]. The spectrum of resulting diseases ranges from superficial infections of the mucous membranes, such as oropharyngeal or vulvovaginal candidiasis, to deep-seated, life-threatening diseases caused by dissemination, such as invasive candidiasis (secondary infections affecting organs like the heart, lungs, bones and brain) [16,17].
As one of the most widespread fungal infections, candidiasis affects approximately 250,000 to 700,000 individuals worldwide each year, with an incidence rate of 2 to 14 cases per 100,000 people. The mortality rate is between 40% and 55%, and about 79 cases are diagnosed daily [17,18,19]. New risk groups emerged during the COVID-19 pandemic, which potentially increased the incidence rate mentioned above [20]. In the United States, Candida infections are the fourth most common hospital-related bloodstream infection [21], with numerous cases in certain clinical areas such as the ICU (60%) as well as in cancer and transplant facilities (13%) [22,23]. The number of Candida spp. infections outside of hospital settings is even higher. For example, between 50 and 70% of women in their childbearing years will experience vulvovaginal candidiasis at least once, and 5 to 8% will suffer from recurrent infections [24].
These serious diseases are triggered by around 15 species of Candida, which are part of the roughly 200 species that have been documented so far [8,24]. Among these, the six organisms Candida albicans, Candida parapsilosis, Nakaseomyces glabratus (formerly Candida glabrata), Candida tropicalis, Pichia kudriavzevii (formerly Candida krusei) and, in some regions of the world already, Candida auris are the most common pathogens causing about 95% of invasive disease [25,26,27,28,29,30]. C. albicans and C. parapsilosis, particularly, are well adapted to various host environments due to their frequent coexistence with different microbiota members and their genetic, morphological and biochemical flexibility, significantly influencing the course and outcome of disease. Certain Candida species are linked to specific risk groups, suggesting that differences in their colonization and survival strategies only result in infections under particular conditions. For example, C. parapsilosis is more often linked to infections in neonates than in adults and is a common pathogen in catheter-related infections [31].
1.1. C. albicans
The species most frequently isolated and known for its virulence is C. albicans [21]. With its remarkable ability to grow both as round budding yeast cells and as pseudo or true hyphae [32,33,34], this polymorphic yeast is a natural member of the human microbiome, colonizing areas such as the oropharynx, genitals, and gastrointestinal mucosa in healthy individuals [35,36,37]. The genetic instability of the naturally diploid genome of C. albicans leads to this phenotypic diversity, making it one of the key factors contributing to its virulence [38]. This pathogen’s unique features allow it to flourish in host niches with changing environmental conditions, including nutrient availability, pH, O2 and CO2 levels, and immune cell presence, due to its flexibility and capacity to rapidly adjust to environmental changes [39]. Additionally, it can specialize in certain micro-niches to optimize the use of available resources [40]. C. albicans also enhances its virulence through adherence to biological and inert surfaces [5,40,41,42,43] and the secretion of hydrolases that allow it to exploit host components for growth and nutrition [44,45,46]. Even though C. albicans is the most common species in most areas of the world, the frequency of non-albicans diagnoses has been on the rise in recent decades [19,20,47,48], being responsible for more than 50% of cases [49].
1.2. C. parapsilosis
C. parapsilosis, first isolated in 1928 from the stool of a patient with diarrhea in Puerto Rico [50], is also present in non-human environments such as domestic animals, insects, soil, and marine ecosystems [51]. This diploid yeast has eight chromosome pairs and a genome size of 13.1 Mb, with only 1.83% of its genome characterized so far [52], as its biology has not been as extensively explored as that of the closely related species C. albicans. Unlike C. albicans, C. parapsilosis does not form true hyphae and exists only as yeast or in pseudo-hyphal forms [53] and colonizes the human skin and mucosal membranes as a commensal microorganism [51,54]. The virulence of C. parapsilosis is primarily attributed to its ability to adhere to both biotic and abiotic surfaces, a crucial feature for biofilm formation [55,56]. Like other Candida species, this yeast produces and secretes several hydrolytic enzymes (lipases (LIPs), secreted aspartyl proteases and phospholipases), which are closely linked to its pathogenic features such as adhesion, cell damage, and tissue invasion [55]. Additionally, its ability to grow in hyperalimentation solutions increases the infection risk posed by this pathogen [57]. In southern Europe, southern America, India and Pakistan, C. parapsilois is more common as a cause of candidiasis [58,59] and it has mainly been responsible for the increasing incidences of non-albicans Candida infections in the past few years [16]. Infections related to this pathogen are common among neonates with low birth weights, immunocompromised individuals such as HIV and surgical patients (especially those with GI tract surgery), and patients with central venous catheters or other indwelling devices, where C. parapsilosis can adhere to [56,60,61]. It accounts for one-third of neonatal Candida infections, with a mortality rate of around 10%, and poses a particularly high risk for low-birth-weight neonates [62].
1.3. C. auris
Another Candida species of increasing interest is C. auris. Although this novel and emerging pathogen was first identified in Japan in 2009, according to the European Center for Disease Prevention and Control (ECDC), C. auris poses an emerging threat to public health (systems) [63,64]. In 2014, five years after the discovery of the pathogen, C. auris bloodstream infections were already reported in South Korea, India and South Africa [65,66,67]. Further instances of rapidly spreading, high-mortality infections have been documented in regions including Europe (the United Kingdom, Spain, Italy), Asia (India, Pakistan), Latin America (Colombia, Venezuela, Panama), and the USA. In 2016, the ECDC requested all local, state and national health departments to report all emerging cases of C. auris infections to highlight the emerging threat posed by this pathogen. In September 2017, 127 confirmed and 27 potential cases were reported across 10 states [68,69,70]. In the meantime, the pathogen was isolated in numerous countries, including South Africa, Kuwait, Malaysia, Kenya, Norway, Germany, Oman, Spain, Israel, Venezuela, Brazil, the United States and Canada [65,67,68,71,72,73,74,75,76,77,78,79]. This rapid spread of the fungus is mainly enabled by its ability to persist on human skin and environmental surfaces for weeks, thus causing large outbreaks especially in healthcare facilities through easy skin-to-skin transmission and facilitating inter- and intra-hospital clonal transmission [64,80,81,82,83]. Another alarming finding in the context of C. auris infections is the rapid development of (multi-) drug-resistant species, including isolates that show lower sensitivities to all three classes of antifungal drugs [84]. This represents an unprecedented challenge in the treatment of fungal infections [84]. As this pathogen has spread impressively fast since its first discovery to such a considerable extent, the underlying development of mechanisms of resistance is still poorly understood [85]. However, it has recently been shown in studies that azole resistance is linked to clade-specific mutations [86]. Furthermore, the presence of resistance genes on different alleles suggests that the development of C. auris resistance is more likely to develop through acquisition than being innate [87]. Morphologically, C. auris shows a high similarity to C. parapsilosis and other closely related species, which has already led to misidentification of the pathogen, using commercial biochemical diagnostic methods and ultimately to a high rate of failed treatment. In comparison to other Candida spp. infections, this resulted in longer ICU stays, underlying respiratory conditions, vascular surgery, prior exposure to antifungal drugs, and lower APACHE II scores in the case of C. auris bloodstream infections [88]. Also, C. auris shows, in comparison to other Candida species, a typical ovoid, ellipsoidal or elongated form and no hyphae [63,65,66,89,90,91,92,93,94]. This fungus, however, is capable of undergoing a tristable phenotypic switch between the regular yeast form, filamentation-competent yeast, and filamentous cells, which is induced by passage through the murine model of systemic candidiasis [92]. Filamentous cells of C. auris appear similar to the true hyphae produced by C. albicans, but they have distinct biological properties. In terms of metabolism, these cells are more active than yeast cells [92]. Furthermore, this fungus can occur individually or as aggregates. Aggregates are generally more tolerant to antifungal agents, whereas single cells show a higher virulence [95]. Ploidy switching has also been observed under certain conditions. The switch from a haploid to a diploid yeast allows a high degree of adaptation to different environments, as it leads to alterations in various biological characteristics, such as colony size, cellular appearance, color and global gene expression profile. In addition, diploid cells show a higher virulence in mouse models than haploid ones [96]. In contrast to the typical virulence factors of other Candida spp., such as phospholipase and proteinase production, germination, adherence, and biofilm formation [97], this pathogen is able to produce phospholipase and proteinase [98,99], but the fungus does not form germ tubes, pseudohyphae or chlamydospores [66,73,95] and has a significantly reduced adherence and biofilm formation [99,100], as this pathogen prefers to grow in so-called “clumps” (i.e., aggregates) [95].
2. Biofilm
Biofilms represent one of the most ancient life forms on Earth. The discovery of this microbial lifestyle dates to the late 19th century, when scientists like Antonie van Leeuwenhoek first observed microorganisms in dental plaque. However, the significance of biofilms was not fully appreciated until the 1970s when research started to reveal their prevalence and importance in various environments, including natural aquatic systems, industrial processes, and medical settings [101]. The understanding of biofilms evolved through advances in microscopy and molecular techniques. Early studies focused on the physical and chemical properties of biofilms [102], while later research highlighted their ecological roles and implications for human health [103,104,105,106]. Around 80% of all microorganisms are known to attach to biotic or abiotic surfaces and form a sessile community. This term describes an organized structure of microbial cells enclosed in an extracellular matrix (ECM) [107]. Living in a community like this can protect the pathogenic cells from the host’s immune defense and antibacterial and antifungal drugs and provides a certain degree of stability in a self-controlled microenvironment [108]. These biofilms can consist of either a single species or can host a mixed culture of bacteria and yeasts [109] with poorly understood microbial interactions between the different biofilm members. The coexistence of different organisms within a biofilm complicates the treatment of such infections, as antimicrobials are often specifically directed against one species, non-target organisms still thrive, and infection continues during treatment [110]. Biofilms composed of mixed cultures, including Candida spp. and pathogenic bacteria such as Pseudomonas aeruginosa and Staphylococcus aureus, have been found on implanted medical devices like urinary bladder catheters and central venous catheters [111].
Methods to analyze microbial biofilms at different complexity levels (see Table 1) have advanced significantly over the years and are used for both bacterial and yeast biofilms. Traditional approaches include culture-based techniques, which often underestimate biofilm presence due to the difficulty of culturing microorganisms in a biofilm state [112]. More recent methods utilize molecular techniques, such as polymerase chain reaction (PCR) and next-generation sequencing, to quantitatively analyze biofilm communities [113,114]. These methods enable researchers to identify the diversity and composition of microbial populations within biofilms, providing insights into their ecological dynamics. Additionally, imaging techniques like confocal laser scanning microscopy (CLSM) allow for the visualization of biofilm architecture and composition in situ, providing insights into their complex structures [115,116,117]. Other advanced imaging techniques, such as scanning electron microscopy (SEM) and atomic force microscopy (AFM), further elucidate the physical characteristics and interactions within biofilms [118,119]. These tools have been crucial for understanding biofilm morphology, thickness, and the spatial organization of different microbial species [120].
An important question is the feasibility of analysis methods in clinical settings, i.e., which degree of efficiency a technique can reach with respect to required personnel and equipment for the diagnosis and analysis of biofilms ideally at the bedside of patients. Taking this into account, two groups of methods originating from different eras of diagnostic research appear to be mentioned. Traditional plate count methods (on selective media) offer an undoubted high level of applicability in every microbiological laboratory in clinics but may underrepresent biofilm load in certain clinical samples with low to moderate expected sensitivities and specificities [121]. In contrast, qPCR requires specific (and economically expensive) equipment and personnel with more specific training, but this quantification method can be regarded as highly sensitive for detecting Candida biofilms and monitoring treatment effects combined with a high specificity, especially when biofilm-relevant genes are measured, qualifying it as the method of choice for quantification and monitoring in both research and clinical use [122,123,124]. In light of this, it appears clear that there is an urgent need to implement or develop novel reliable and precise diagnostic tools capable of detecting biofilms at the bedside; unfortunately, until now, this has been challenging as most methods are demanding, highly specialized and technically complex.

Table 1.
Detection methods for microbial biofilms (nicely reviewed in [125]).
Table 1.
Detection methods for microbial biofilms (nicely reviewed in [125]).
| Type | Method | Description | Reference |
|---|---|---|---|
| Cell staining assays | Crystal violet (CV) assay | CV binds to negatively charged molecules. After staining, the adsorbed CV is eluted using a solvent. The amount of dye solubilized by the solvent (measured by optical absorbance at 590 nm) is directly proportional to biofilm size. | [126,127] |
| 1,9-dimethyl methylene blue (DMMB) assay | DMMB binds to the biofilm EPS, which is the intercellular polysaccharide adhesin (PIA), composed of poly-b-1,6-linked-N-acetylglucosamine. After complexation of DMMB with polysaccharides of biofilm, the addition of a decomplexation solution enables the quantification of the released amount of DMMB dye spectrophotometrically. | [128] | |
| Fluorescein-di-acetate (FDA) assay | After uptake into the cell, FDA is hydrolyzed by cellular esterizes to fluorescein, which can be measured spectrophotometrically. | [129,130] | |
| LIVE/DEAD BacLight assay | This assay to discriminate live from dead cells is based on the use of two different nucleic acid binding stains. The first dye is the green-fluorescent Syto9, able to cross membranes and bind to DNA. Propidium iodide, the second dye, is red-fluorescent and penetrates only damaged bacterial membranes. The stained samples are analyzed by fluorescent optical microscopy to distinguish between live and dead bacterial populations. | [131,132] | |
| Resazurin assay | Resazurin (7-hydroxy-3H-phenoxazin-3-one-10-oxide) is a blue, non-fluorescent dye that is reduced by cellular metabolic processes into pink-fluorescent resorufin. The fluorescence of resorufin can be measured spectrophotometrically. These characteristics make resazurin a valuable tool for detecting viable microorganisms and determining the number of viable cells in biofilms. | [133,134,135] | |
| XTT assay | Using a redox indicator, the XTT method allows for spectrophotometric enumeration of viable cells in biofilms. | [129] | |
| BioTimer assay (BTA) | Colorimetric assay allowing counting of viable bacteria or yeasts in biofilms. The BTA contains phenol red. Microbial products of primary fermentative metabolism cause a color change from red to yellow. The time required for color switch correlates to initial bacterial or yeast concentration. | [136] | |
| Genetic assays | PCR; qRT-PCR | This PCR-based method allows identification of specific genetic sequences related to individual species. One of the most sensitive and powerful gene analysis methods today is “Real-Time Quantitative Reverse-Transcription PCR” (qRT-PCR). In this method, the fluorescent signal is measured in real time at each amplification cycle and is directly proportional to the amount of amplicons generated. | [137] |
| FISH | Fluorescence in situ Hybridization (FISH) is a genetic technique that utilizes oligonucleotide probes tagged with fluorescent dyes. These probes can be specifically designed to bind rRNA, a specific molecule that indicates a target of interest. | [138] | |
| Physical assays | MS; DESI | Mass spectrometry (MS) is a technique used to quantify known substances and to determine the chemical properties of various molecules. In this process, the substance is exposed to an electron beam, ionizing the molecules and producing gaseous ions. These ions are then separated in the mass spectrometer and identified based on their mass-to-charge ratios and relative abundances. The resulting data provide a mass spectrum that is characteristic of each compound and directly reflects its chemical structure. MS offers both qualitative and quantitative capabilities, making it useful for identifying and quantifying unknown compounds. The Desorption Electro-Spray Ionization (DESI) assay enables direct, non-destructive analysis of complex samples, facilitating the chemical characterization of microbial biofilms in various growth states and conditions. | [139] |
| CLSM | Using confocal laser scanning microscopy (CLSM) technology, thick biological samples, such as microbial biofilms, can be scanned by capturing images in a line-by-line fashion along the X, Y, and Z axes. | [140,141,142] | |
| CRM (Confocal RAMAN Microscopy) | The sample is exposed to an electromagnetic laser beam with a known wavelength. By measuring the scattered radiation and energy shifts, the chemical characteristics of the sample can be identified. This method facilitates the capture of the chemical fingerprints of different biofilms. | [143,144,145] | |
| EM | Electron microscopy (EM) methods exploit the high resolution provided by electron beams, which utilize short-wavelength, high-energy radiation. Transmission electron microscopy (TEM) is particularly effective for imaging the interior of biofilms and their intracellular components. Scanning electron microscopy (SEM) is widely used to visualize the surfaces of microcolonies and mature biofilms. Coupling SEM with focused ion beam (FIB) technology allows for the examination of biofilm interiors by removing surface layers or cutting cross-sections. Both SEM-FIB and TEM can be complemented with energy-dispersive X-ray spectroscopy (EDX), which enables the acquisition of local compositional spectra and maps of bacterial cells and biofilms. | [146,147] | |
| XM | In X-ray microscopy (XM), the sample is exposed to soft X-ray radiation, either mono- or poly-chromatic, which is focused for high-resolution imaging and compositional mapping. This technique enables detailed analysis of biological samples with minimal preparation and less radiation damage. | [148] | |
| SPM | Scanning probe microscopy (SPM) reconstructs topographical details of the sample by analyzing the signal from a sharp, nanometer-scale probe that scans the sample near its surface. | [149,150,151,152] |
In the context of Candida spp. infections, biofilms are one of the main virulence-associated physiological traits and a serious threat to the infected individual [47,153,154,155,156,157]. Compared to infections caused by Candida strains preferring the planktonic lifestyle, those pathogens living in these sessile communities show at least twice-as-high mortality rates [158,159] and pose a serious threat as a permanent source of infection [160], causing spread of infection into the bloodstream [161] and increased resistance to antifungal therapies [162].
The potential of biofilm formation varies widely between different Candida strains. Various studies have been performed to investigate the formation of biofilm among different Candida isolates, showing significantly less biofilm formation among less pathogenic C. parapsilosis isolates than among isolates belonging to the highly pathogenic species C. albicans [163]. However, differences in biofilm formation were also observed between the isolates within the same species, which directly correlated with the pathogenicity of the respective isolate [164]. Furthermore, it has been observed that an increased pathogenicity of C. albicans isolates resulted not only from the increased amount of biofilm formed, but also from the heterogenic composition of this complex formed by conventional yeast cells, hyphae and pseudohyphae [165,166].
2.1. Biofilm Formation
The formation of a biofilm is a highly regulated process and usually occurs over a period of 38 to 72 h. This process is significantly dependent on the species undergoing its development. In general, however, biofilm formation can be divided into three main phases: First, a planktonic cell adheres to a biotic or abiotic surface, followed by proliferation of the cells and formation of a mature biofilm (Figure 1). The dispersion of cells from the biofilm into the surrounding tissue to colonize new host niches also plays an important role during infection [55].
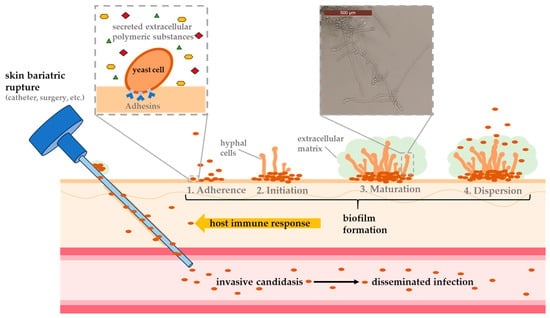
Figure 1.
Schematic representation of biofilm development based on the four main steps (adhesion, initiation, maturation and dispersion) and an exemplary illustration showing typical invasive candidiasis infection caused by skin lesions upon the use of, e.g., medical devices.
During the first stage of biofilm formation, lasting 10 to 12 h, planktonic cells adhere to a surface, aggregate into microcolonies, and form a basal monolayer [167,168]. It depends on the species to which surface the cells can attach to the best. C. albicans, for example, can adhere much more efficiently to epithelial cells of the GI, urinary tract or blood vessels [169], whereas C. parapsilosis tends to form biofilms on abiotic materials like central venous catheters [170,171]. This process is enabled by adhesins (glycoproteins located on the cell wall), which mediate interactions between cells and surfaces [169,172,173]. After the formation of the first layer, present cells undergo species-dependent morphological changes (forming yeast cells, filamentous cells and young hyphae), which can last up to 19 h. During this period, the number of cells increases and macrocolonies are formed. This process is strongly dependent by the species’ capacity to produce extracellular polymeric substances (EPSs), like polysaccharides, lipids and proteins [167]. Approximately 72 h after biofilm formation begins, this process is complete. The result is a complex 3D structure of several layers of polymorphic cells embedded in an ECM formed by exopolimeric material. This network includes numerous pores and water channels, which ensure the smooth circulation of molecules supplying the biofilm components. EPS also plays an important role at this stage. Depending on the carbon source available, these molecules ensure the integrity of the ECM (40% polysaccharide content), which protects the cells from phagocytosis and drug diffusion [174]. During biofilm formation, the detachment of round daughter cells occurs mainly during the maturation phase, resulting in the release of these cells into the surrounding tissue, ready to establish themselves in new host niches [55]. However, such detachment can also occur during the entire formation of a biofilm, leading to the formation of even more robust biofilms than those formed by initial planktonic mother cells [161]. As biofilms mature through successive generations, their virulence potential can grow, presenting a serious threat to both patient treatment and public health [175].
Biofilm development is shaped by mechanisms and factors from both the pathogen and the host, such as the host’s immune status and the yeast’s capacity to interact with the host’s homeostasis [167,176]. In addition, various factors are crucial for the adhesion of the pathogen to a surface of biotic or abiotic nature. These include, for example, the species-specific cell surface hydrophobicity [177], which depend on the glycoproteins on the fibrillar layer (the site of the first contact between the pathogen and the surface) of the cell wall [178,179]. Moreover, the substrate on which the pathogen adheres is another important factor affecting the architecture, morphology and thickness of the biofilm [163]. The interaction of the pathogenic yeast cells with each other, as well as with the substrate, is influenced by physiological conditions such as fluid flow, pH, oxygen concentration and available nutrients [167,180,181]. The fluid flow at the infected site in particular plays a crucial role in nutrient exchange and the integrity of the biofilm by influencing ECM formation [182,183,184,185,186]. Furthermore, the Candida species that forms the biofilm and the presence of other microorganisms such as other yeasts or bacteria within the biofilm is important [187]. Several studies using gene disruption, microarray-based transcriptomics, proteomics and genomics have also demonstrated roles of various genes, proteins, DNA and metabolites in the maturation of Candida biofilms [188]. These include alcohol dehydrogenase, which controls ethanol acetaldehyde conversion and can thus modulate the biofilm [185].
2.2. Biofilm-Specific Resistance Mechanisms
Candida spp. infections have been categorized as a high risk to public health due to the high number of resistant planktonic and biofilm-forming isolates against all classes of antifungal therapy causing them [1,189,190,191,192,193,194]. The emergence of such (multi-) resistant pathogens is closely linked to biofilm formation [195,196]. While planktonic cells acquire their resistance by increasing the activity of efflux pumps and mutations in genes encoding the drug target [197,198], biofilm-forming pathogens exhibit additional mechanisms for resistance development which are influenced by the phase of the biofilm within an organism (see Figure 2). For example, planktonic C. albicans cells do not show increased sensitivity to antifungal agents, whereas cells of the same isolate forming biofilms show increased tolerance to amphotericin B, nystatin, chlorhexidine and fluconazole [199]. Like planktonic cells, those that form biofilms can overexpress the genes for ATP binding cassette transporters (CDR1 and CDR2) and major facilitator transporter (MDR1) to increase the activity of these efflux pumps and thus prevent the aggregation of azoles (especially fluconazole) in the cells [198,200,201,202,203,204]. Furthermore, biofilm-forming cells have a significantly lower concentration of ergosterol in their cell membrane, especially in the later stages of biofilm formation (cells in mature biofilms contain about half as much ergosterol as planktonic cells) due to an altered transcriptional profile of the sterol pathway to maintain better membrane fluidity [204,205]. However, ergosterol is the target of many azoles and amphotericin B; as such, these cells show a lower sensitivity to those drugs [206]. Moreover, the cell density in the biofilm, but also of cells in general, is supposed to influence antifungal resistance [207,208]. Another key factor contributing to the increased tolerance to antifungal agents is the ECM of the biofilm, shielding the cells from the environment while allowing diffusion of required nutrients, enzymes and water [209,210,211]. Other experiments have shown that biofilms formed under continuous flow have a higher level of ECM than those grown under stagnant or shaking conditions, which is directly associated with increased resistance to amphotericin B, fluconazole and flucytosine [186]. In addition, the ECM has been shown to have the ability to bind or sequester various drugs and thus develop resistance to these [162,212,213,214,215]. Transition to persistent cells is species- and strain-specific, resulting in a change in the cell wall and cell membrane. These cells embedded deep in the biofilm show the highest tolerance to antifungal agents [216,217].
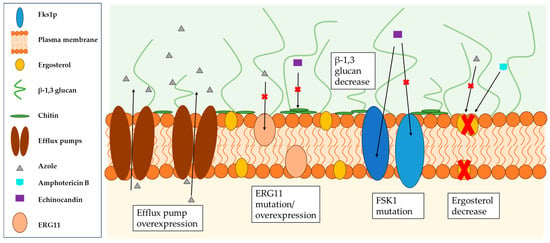
Figure 2.
Schematic representation of the biofilm-specific reduced sensitivity of Candida species to azoles, amphotericin B and echinocandins due to efflux pump overexpression, ERG11 mutation/overexpression (gene encodes lanosterol 14α-demethylase), the mutation of Fsk1 (gene encodes (1,3)-β-D-glucan synthase) and decreased levels of ergosterol and β-1,3 glycans.
3. Detection and Treatment
To initiate a Candida infection, these pathogens will face mechanical barriers (e.g., the epithelium), chemical, physical and biochemical antagonists (e.g., pH and antimicrobial peptides), competition among microbes, and the host’s initial and adaptive immune response [218]. As a consequence of their specific cell wall composition, pathogenic Candida cells are recognized by the host [219], leading to various immune responses such as the secretion of proinflammatory mediators recruiting specific phagocytes such as polymorphonuclear neutrophils (PMNs), monocytes/macrophages and dendritic cells [220,221,222,223]. However, pathogens of the Candida species have developed many preventive mechanisms to avoid recognition by the host, and various adaptations of the pathogen are known to evade clearance by the host immune system [224]. Mononuclear cells in the peripheral blood like macrophages and monocytes have the ability to phagocytize and degrade C. albicans cells upon their recognition during infection [225]. However, Candida biofilms are resistant to this immune response. In fact, monocytes can become embedded within biofilms, inadvertently strengthening the biofilm structure [226,227]. Additionally, biofilm cells hinder macrophage migration and induce a cytokine response in macrophages that differs from the response triggered by planktonic cells [30]. Interestingly, unlike the neutrophil response, the disruption of Candida albicans glycosylation (and consequently ECM disruption) does not affect macrophage migration, suggesting that biofilm cells, rather than the extracellular matrix, are key in influencing macrophage activity [226,227,228]. Moreover, although biofilms trigger elevated levels of proinflammatory cytokines like IL-1β and MCP-1, these do not enhance anti-biofilm activity [229]. Similarly to neutrophils, biofilms also provoke an IL-10 response in macrophages, which exacerbates the issue by promoting biofilm persistence [230]. C. auris, which is an example of a non-naturally filamentous yeast, can still form strong multicellular aggregates during infection. These biofilm-like structures show increased resistance to macrophages and host-derived antimicrobial peptides compared to the sensitive planktonic yeast form [231]. Disrupting C. auris clumps into single cells reduces this resistance, allowing more efficient macrophage phagocytosis. This emphasizes the fact that large biofilm-associated cell aggregates—regardless of whether they are filamentous—render immune cells less effective at combating biofilm formation [226]. The fact that the organism is already present in small numbers in the host’s healthy gut is an advantage, as various Candida spp. are individually adapted to different niches [232,233]. Furthermore, C. albicans can reduce phagocytosis by the host’s immune cells through their morphogenesis alone [234]. If a Candida cell is recognized and phagocytosed despite these mentioned mechanisms, the environment within the phagosome triggers a stress response [233]. Using their metabolic flexibility, Candida spp. can quickly adapt to different available nutrients and switch their metabolism accordingly [233,235,236]. In addition, they have developed effective and extended redundant mechanisms to deal with oxidative, nitrosative and osmotic stress, tightly regulated to respond immediately and strongly to avoid clearance [237]. Taking this into account, it is clear why the US Centers for Disease Control and Prevention classify Candida spp. as a serious threat to human health [1].
3.1. Detection
A timely diagnosis of Candida infections and rapid treatment can be life-critical [238,239]. This includes identifying not only the Candida species causing the infection but also the type of infection to determine the mode and length of treatment [240,241,242]. Currently, the gold standard for diagnosing candidiasis is the culture of blood and sterile sites [243], with a sensitivity of approximately 5% depending on the Candida species, and pre-treatment of patients with antifungal agents [244]. The major disadvantage of these methods is the time they take to report a positive result, which is 2 to 3 days [238,239,244]. This causes a delay in treatment, as Candida has a growth time of 24 h [27]. The varying degree of sensitivity also shows the urgency of other diagnostic methods [242]. Faster, non-culture methods for identifying Candida infections include mannan and anti-mannan antibody detection [30,242,245], β-D-glucan (BDG) detection [230], C. albicans germ tube antibody (CAGTA) detection [246], PCR detection of Candida DNA [247] and the T2 magnetic resonance (T2MR) Candida test [239,240]. These methods also have their limitations; therefore, a combined diagnosis is recommendable [243,248,249,250].
3.2. Classic Therapeutic Options
The classic fungicides used for anti-Candida treatment include polyenes, azoles, echinocandins and (rarely used) flucytosine [251].
3.2.1. Polyenes
Amphotericin B (AmB) has been the most commonly used polyene for over 55 years [252]. The function of AmB consists in interacting with the ergosterol on the lipid layer of the fungal cell. Binding to ergosterol activates a cascade of events, including the formation of pores in the cell membrane. The resulting increase in membrane permeability causes potassium ions and other intracellular components to escape, ultimately causing cell lysis [253]. The primary limitation of this agent is its low solubility and the high toxicity it presents to host cells, as ergosterol’s structural similarity to cholesterol in mammalian membranes drastically restricts its long-term use [254]. However, promising studies have shown that lipid-based polyenes, such as liposomal amphotericin B (LAmB), exhibit less toxicity towards host cells and are therefore becoming first-line treatment for several types of invasive fungal infections [255]. Furthermore, lower sensitivities to this drug have already been demonstrated in several Candida isolates, especially isolates of the species C. parapsilosis and C. auris [256,257]. The underlying resistance mechanisms are still poorly understood and therefore not as clear as those of echinocandins and azoles.
3.2.2. Echinocandins
Echinocandins represent a newer class of antifungal agents used for treating invasive fungal infections. Compared to polyenes and azoles, echinocandins offer the best clinical outcomes for Candida infections, with an efficacy rate of 70 to 75% [258,259,260,261]. The significantly higher survival probability after echinocandin treatment is associated with a high fungicidal activity against most commonly Candida species, a low drug–drug interaction, a high safety profile and a lower incidence of acquired resistance compared to the other drug groups [27,262]. This class of drugs, which includes caspofungin, micafungin, and anidulafungin [30,258,263], is known for its high safety profile and potent antifungal activity. This is attributed to their non-competitive inhibition of (1,3)-β-D-glucan synthase, an enzyme crucial for the synthesis of (1,3)-β-D-glucan, an essential fungal cell wall component [264,265]. Despite their narrower antifungal spectrum, echinocandins are highly effective against the most common Candida species, including those that have developed resistance to azole medications [201,266]. However, reports of resistant isolates are also increasing against this class of antifungal agents, including C. parapsilosis and C. albicans isolates, with raised minimum inhibitory concentrations (MICs) against echinocandins in vitro [266,267,268,269,270,271,272]. The reasons for high MICs include an increase in the chitin content of the cell wall [273,274] or mutations in the genes coding for Fks1p, the target of echinocandin treatment [256,272,275].
3.2.3. Azoles
Azoles, the largest class of antifungal drugs in clinical use, are popular for their broad-spectrum efficacy against Candida species, good safety profile, and high bioavailability [275]. As a result, azoles like fluconazole (FLC), voriconazole (VRC), posaconazole (PSC), itraconazole, and isavuconazole are commonly used to treat invasive candidiasis [276]. One of the reasons for the low toxicity of azoles to human somatic cells is their specific mechanism of action, which involves the inhibition of lanosterol 14α-demethylase (encoded by the ERG11 gene), a key enzyme in ergosterol synthesis [277]. As an essential part of the fungal cell membrane, ergosterol’s synthesis inhibition results in the buildup of the toxic 14α-methyl sterol, compromising membrane integrity and the function of membrane-bound proteins. Although the drug class of azoles shows about 15% lower efficacy than echinocandins, as a first-line therapeutic agent, azoles exhibit higher penetration than echinocandins in some forms of deep-seated candidiasis [278]. Furthermore, azoles are inexpensive and can be administered both orally and intravenously, whereas echinocandins are scarce in resource-limited settings and require once-daily intravenous administration. Moreover, azoles are generally better tolerated than echinocandins, which often show strong side effects such as nephrotoxicity, which can be reduced but not completely avoided by lipid-based forms [279,280]. The main challenge in dealing with this antifungal agent is the high incidence of resistant Candida species caused by the widespread use of this class of drugs [281]. Azole resistance develops due to a combination of factors, such as mutations in the ERG11 gene (which encodes the target enzyme), point mutations in the ERG3 gene that modify the ergosterol biosynthesis pathway to create less toxic 14α-methyl fecosterol, and the increased expression of multidrug efflux pumps like CDR1, CDR2, and MDR1, which export azoles from the fungal cell [282,283,284].
3.2.4. 5-Flucytosine
5-Flucytosine is carried into the cell by a cytosine permease and metabolized into a toxic version of uridine triphosphate by a cytosine deaminase or converted into an inhibitor of thymidylate synthase, which reduces the availability of nucleotides for DNA synthesis [285,286]. The greatest threat to this antifungal agent is also the increasing number of Candida isolates with secondary acquired resistance of up to 8% after monotherapy [287]. The root causes are mutations in the FCY2 gene, which encodes cysteine permease, or in the FCY1 gene, which codes for cysteine deaminase [287]. Currently, flucytosine is only given in combination with AmB to prevent the further development of resistance [30].
3.3. Treatment Options and Promising Approaches
Although the healthcare system and ICU care have generally improved in recent decades, as well as new developments in various fungicides and microbial techniques having taken place, the mortality rates associated with invasive candidiasis have not decreased significantly [262]. This is primarily driven by the rising global incidence of multidrug-resistant isolates linked to invasive candidiasis, which results in reduced efficacy of established treatment options [288]. Because of this, the constant development of new therapeutic options, especially against biofilm-forming Candida spp., is essential. Due to the mechanical protection offered by the ECM and other factors, Candida spp. generally have lower therapeutic sensitivity within biofilms, highlighting the importance of preventing biofilm formation and targeting biofilm cells for the development of novel treatments [289]. Conventional fungicides such as lipid-based formulations of AmB and echinocandins, including caspofungin, also show anti-biofilm effects, but new therapeutic options should be established due to several factors, such as resistance development and increased toxicities, to enable a therapy that is individually customized according to the type of infection, the pathogen and the patient [290]. Several experimental agents are already under investigation as potential new agents against Candida spp., but compared to anti-biofilm research against bacteria, that against yeasts is lagging behind (see Table 2). These include substances like chlorhexidine, filastatin, sodium hypochlorite, zosteric acid, gentian violet, EDTA/ethanol catheter lock solutions, and essential oils [291,292,293,294,295,296,297,298,299,300,301,302,303], but also physical methods such as low-level laser [304], photodynamic therapy [305,306,307,308] and antimicrobial coating of catheters [309,310,311,312]. Probiotics are also currently being investigated as a preventive therapy to boost the patient’s immune system, but also as a supportive treatment during a Candida infection, as different types of lactobacilli, for example, exert a strong inhibitory effect on C. albicans pathogens [313,314]. However, in most cases, an effect against Candida biofilms has not been investigated so far.

Table 2.
Number of publications on the treatment of biofilms of major bacterial and yeast pathogens.
Antimicrobial Peptides
Antimicrobial peptides (AMPs) represent a promising approach for new therapeutic options, not only for biofilm-forming resistant Candida strains, but also for pathogens in general (including bacteria, fungi and viruses) [314,315,316,317]. This class of molecules is characterized by its broad antimicrobial spectrum of activity [318,319]. Due to its occurrence in almost all living organisms as a preserved defense mechanism, such as plants, mammals, arthropods and many others, it is an almost unlimited source of potential new therapeutics against various highly dangerous infectious diseases [320]. Furthermore, these molecules are less susceptible to resistance development than conventional therapeutics due to their special mode of action, distributed growth and modulation of the host immune system [321]. Currently, around 50 AMPs are in clinical trials (see Table 3), including up to 20 against Gram-positive bacteria, about 15 against Gram-negative bacteria and only a few against yeasts such as Candida spp. These numbers highlighted the urgent need for investigation into antimicrobial peptides against fungi such as Candida spp.

Table 3.
Overview of AMPs in clinical trials (nicely reviewed in [322,323]).
Defensins, found in plants, are critical in defending against microbial infections. These small peptides, which are rich in cysteine, are capable of combating many pathogens, including fungi and bacteria [366,367]. Their primary function is to prevent microbial invasion of plant tissues, making them a vital defense mechanism. Research has shown that plant defensins are generally non-toxic to human cells, which makes them attractive candidates for developing new antimicrobial agents [368,369,370,371,372].
In humans, AMPs such as human β-defensins are mainly expressed in epithelial tissues and play a role in diminishing pathogen virulence by either inhibiting growth or modulating the immune response [373,374,375]. Among these, HBD-3 is recognized for its potent antifungal activity against C. albicans, while HBD-1 and HBD-2 also display antimicrobial properties, but to a lesser extent. Interestingly, studies have demonstrated that reducing the disulfide bridges in HBD-1 can enhance its effectiveness against Candida species [376,377,378,379,380,381].
Insects and arachnids also contribute to the pool of AMPs, with arthropods being one of the largest sources of these peptides. For instance, the 18-amino-acid peptide gomesin, extracted from the tarantula Acanthoscurria gomesiana, exhibits a broad spectrum of activity against various pathogens, including fungi and bacteria. Its low toxicity and effectiveness make it a promising candidate for treating infections such as vulvovaginal candidiasis [382,383,384,385,386,387,388,389].
Moreover, AMPs can be isolated from other organisms, such as amphibians and mollusks. The exploration of these peptides is essential, particularly in light of the drawbacks associated with conventional antifungal drugs, which often carry significant side effects and require long-term therapy [390,391,392]. The discovery of new AMPs is imperative, as they may offer effective alternatives with reduced cytotoxicity. Pom-1 and Cm-p5 are two noteworthy AMPs that have garnered attention for their antifungal properties, particularly against Candida species [393,394,395]. The peptide Pom-1, isolated from the freshwater snail Pomacea poeyana, exhibits a unique α-helical structure when in a membrane-like environment. This peptide demonstrates significant activity against various pathogens, including Pseudomonas aeruginosa, Klebsiella pneumoniae, and Listeria monocytogenes, while also showcasing antifungal activity against Candida species [395,396]. Research indicates that while Pom-1 has low activity against planktonic cells of Candida, its derivatives, Pom-1A to Pom-1F, display enhanced antifungal effects, particularly against biofilm formation of C. albicans [397]. The ability of these derivatives to inhibit biofilm development is particularly promising, as biofilms contribute to the persistence and resistance of fungal infections. The low cytotoxicity of Pom-1 and its derivatives indicates that their mode of action is not based on traditional pore formation (Figure 3). It is assumed that these novel AMPs do not interact with membran lipids, but possibly bind to other membrane epitopes such as membrane proteins, which are essential for biofilm formation. Hence, it has been suggested that, similar to neutralizing antibodies, these peptides simply act by concealing those epitopes from productive interactions of cell–cell or cell–substratum contacts [398]. This model proposes that the peptides bind to specific targets on the pathogenic membrane, disrupting cell–cell interactions and inhibiting biofilm formation, which opens avenues for further research into their precise mechanisms of action [398].
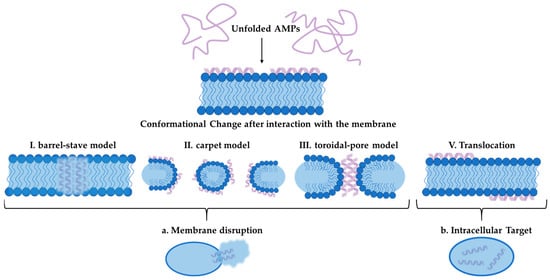
Figure 3.
Illustration of AMP mechanism of action. Unfolded AMP interacts with cell membrane and undergoes a conformational change. Afterwards, the peptide can be translocated into the cell to act intracellularly or distribute the membrane following the barrel-stave model, the carped model or the toroidal pore model.
Cm-p5, another compelling AMP, is derived from the coastal tropical mollusk Cenchritis muricatus [399]. This peptide has demonstrated specific antifungal activity against C. auris and C. albicans, including strains resistant to conventional treatments [400,401,402]. The ability of Cm-p5 derivatives to inhibit biofilm formation and affect various Candida isolates, including FLC-resistant mutants, makes it a significant candidate in the fight against opportunistic fungal infections. Studies show that Cm-p5 acts by targeting the fungal membranes, leading to alterations that prevent the establishment and growth of biofilms. It has been described that Cm-p5 interacts with C. albicans lipid bilayers in a fungistatic mode of action without causing significant perturbation or pore formation, probably fitting the carpet model of action better [400]. This may suggest, although not yet clear in detail, that surface structures required for productive cell–cell or cell–substratum interactions may be sequestered or concealed by the peptide, causing modification of the membrane. This peptide’s effectiveness, combined with its low toxicity, positions it as a promising alternative to existing antifungal therapies [394]. Both Pom-1 and Cm-p5 exemplify the potential of AMPs in combating fungal infections, particularly in an era where antibiotic resistance is a growing concern. Their unique structures and mechanisms of action highlight the importance of exploring natural sources for the development of new, effective antimicrobial agents. As research continues to unveil the intricacies of these peptides, their role in therapeutic applications may significantly impact the management of fungal diseases.
Synergistic effects of AMPs with traditional antibiotics against, e.g., P. aeruginosa and other bacterial pathogens, leading to improved antibiotic activity or overcoming bacterial resistance, were described decades ago and have been recognized as a valuable additional option in the treatment of infections, as is nicely reviewed in the paper of Taheri-Araghi [403]. A similar situation has also been described for fungal pathogens like Candida species, for which synergistic effects have been observed for AMPs in combination with traditional antifungals like AmB and FLC (nicely reviewed by Mhlongo et al. [404]). Additional recent evidence comes from Kissmann et al. [398], who found that neutralizing peptides lead to reduced biofilm formation and hence enhanced growth in the planktonic phase, rendering the planktonic cells more accessible for traditional AmB and FLC, resulting, in turn, in a “rescuing” of the antifungal agents, and overcoming resistance by even multi-resistant clinical isolates [398].
Overall, the study of AMPs across different species provides valuable insights into their mechanisms of action, therapeutic potential, and the possibility of overcoming antibiotic resistance, making them a focal point for future research in the development of novel antimicrobial therapies. Fascinating new opportunities appear to be opened at the moment by the introduction of artificial intelligence and machine learning approaches for the design of novel (optimized) sequences, likely representing the next generation of highly active molecules [405,406,407].
4. Conclusions
In conclusion, the rise in Candida infections and their associated morbidity and mortality rates highlight the urgent need for improved diagnostic and therapeutic strategies. The pathogenicity of species like C. albicans, C. parapsilosis, and C. auris, combined with their ability to form resilient biofilms, complicates treatment efforts and underscores the significance of antibiotic resistance. While traditional antifungal therapies have been effective, their limitations and the emergence of resistant strains necessitate the exploration of alternative approaches. AMPs, exemplified by Pom-1 and Cm-p5, offer a promising avenue for combating these infections due to their broad-spectrum activity and unique mechanisms that circumvent traditional resistance pathways. Continued research into the mechanisms of action of these peptides and their potential integration into clinical practice could significantly enhance our ability to manage and treat fungal infections effectively. As the landscape of infectious diseases evolves, the development of innovative therapeutic options will be crucial in safeguarding public health against the growing threat of drug-resistant pathogens.
Author Contributions
Conceptualization, F.R.; writing—original draft preparation, V.A. and A.-K.K.; writing—review and editing, V.A., A.-K.K., C.F. and F.R.; funding acquisition, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and the German Research Society (DFG) project 465229237 and the by the Austrian Research Promotion Agency (FFG) within the COMET Project “PI-SENS” (Project No 915477) as well as by the Federal Provinces of Lower Austria and Tirol.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.I.; Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Hube, B. Fungal adaptation to the host environment. Curr. Opin. Microbiol. 2009, 12, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Izumikawa, K.; Kohno, S. Candidiasis. Nihon Rinsho. 2008, 66, 2341–2344. (In Japanese) [Google Scholar] [PubMed]
- Spellberg, B.; Lipsky, B.A. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Bueler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Gamaletsou, M.N.; Walsh, T.J.; Zaoutis, T.; Pagoni, M.; Kotsopoulou, M.; Voulgarelis, M.; Panayiotidis, P.; Vassilakopoulos, T.; Angelopoulou, M.K.; Marangos, M.; et al. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin. Microbiol. Infect. 2014, 20, O50–O57. [Google Scholar] [CrossRef]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierichini, A.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar]
- Mochon, A.B.; Ye, J.; Kayala, M.A.; Wingard, J.R.; Clancy, C.J.; Nguyen, M.H.; Felgner, P.; Baldi, P.; Liu, H. Serological profiling of a Candida albicans protein microarray reveals permanent host-pathogen interplay and stage-specific responses during candidemia. PLoS Pathog. 2010, 6, e1000827. [Google Scholar] [CrossRef]
- Sipsas, N.V.; Lewis, R.E.; Tarrand, J.; Hachem, R.; Rolston, K.V.; Raad, I.I.; Kontoyiannis, D.P. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): Stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 2009, 115, 4745–4752. [Google Scholar] [CrossRef]
- Manolakaki, D.; Velmahos, G.; Kourkoumpetis, T.; Chang, Y.; Alam, H.B.; De Moya, M.M.; Mylonakis, E. Candida infection and colonization among trauma patients. Virulence 2010, 1, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Perlroth, J.; Choi, B.; Spellberg, B. Nosocomial fungal infections: Epidemiology, diagnosis, and treatment. Med. Mycol. 2007, 45, 321–346. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Logan, C.; Martin-Loeches, I.; Bicanic, T. Invasive candidiasis in critical care: Challenges and future directions. Intensive Care Med. 2020, 46, 2001–2014. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Samantaray, S.; Karan, P.; Sharma, A.; Nag, V.; Dutt, N.; Garg, M.K.; Bhatia, P.K.; Misra, S. Prevalence, Presentation and Outcome of Secondary Bloodstream Infections among COVID-19 Patients. Infect. Disord. Drug Targets 2022, 22, e180422203723. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, G.; Alangaden, G.J. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect. Dis. Clin. N. Am. 2021, 35, 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Ebbers, J.; Geurtz, L.; Stefanik, D.; Major, Y.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Candida spp. in the USA: Species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents 2014, 43, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, O.; Bille, J.; Fluckiger, U.; Eggimann, P.; Ruef, C.; Garbino, J.; Calandra, T.; Glauser, M.P.; Täuber, M.G.; Pittet, D.; et al. Epidemiology of candidemia in Swiss tertiary care hospitals: Secular trends, 1991–2000. Clin. Infect. Dis. 2004, 38, 311–320. [Google Scholar] [CrossRef]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.; Benjamin, D. Treatment of neonatal fungal infections. Adv. Exp. Med. Biol. 2010, 659, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Lanternier, F.; Dellière, S.; Groll, A.H.; Mueller, N.J.; Alastruey-Izquierdo, A.; Slavin, M.A.; ECCMID study groups on Invasive Fungal Infection and Infection in Immunocompromised Hosts. Invasive fungal disease in the immunocompromised host: Changing epidemiology, new antifungal therapies, and management challenges. Clin. Microbiol. Infect. 2025, 31, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 19–37. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Hsueh, P.R.; Mendes, R.E.; Pfaller, M.A.; Rolston, K.V.; Sader, H.S.; Jones, R.N. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2019, 63, e00355-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewis, R.E. Overview of the changing epidemiology of candidemia. Curr. Med. Res. Opin. 2009, 25, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar]
- Diekema, D.J. Healthcare-associated fungal infections: Beyond Candida and Aspergillus. South Med. J. 2007, 100, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Sudbery, P. Candida albicans, a major human fungal pathogen. J. Microbiol. 2011, 49, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C.; Webster, C.E.; Mayuranathan, P.; Simmons, P.D. Candida concentrations in the vagina and their association with signs and symptoms of vaginal candidosis. J. Med. Vet. Mycol. 1988, 26, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [Google Scholar] [CrossRef]
- Barton, R.C.; Scherer, S. Induced chromosome rearrangements and morphologic variation in Candida albicans. J. Bacteriol. 1994, 176, 756–763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Odds, F.C. Candida infections: An overview. Crit. Rev. Microbiol. 1987, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Ren, B.; Cheng, L. The regulation of hyphae growth in Candida albicans. Virulence 2020, 11, 337–348. [Google Scholar] [CrossRef]
- Latgé, J.P.; Calderone, R. Host-microbe interactions: Fungi invasive human fungal opportunistic infections. Curr. Opin. Microbiol. 2002, 5, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Yoshijima, Y.; Murakami, K.; Kayama, S.; Liu, D.; Hirota, K.; Ichikawa, T.; Miyake, Y. Effect of substrate surface hydrophobicity on the adherence of yeast and hyphal Candida. Mycoses 2010, 53, 221–226. [Google Scholar] [CrossRef]
- Sundstrom, P. Adhesion in Candida spp. Cell. Microbiol. 2002, 4, 461–469. [Google Scholar] [CrossRef]
- Wächtler, B.; Wilson, D.; Haedicke, K.; Dalle, F.; Hube, B. From attachment to damage: Defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS ONE 2011, 6, e17046. [Google Scholar] [CrossRef]
- Naglik, J.; Albrecht, A.; Bader, O.; Hube, B. Candida albicans proteinases and host/pathogen interactions. Cell. Microbiol. 2004, 6, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Stehr, F.; Felk, A.; Gacser, A.; Kretschmar, M.; Mahnss, B.; Neuber, K.; Hube, B.; Schäfer, W. Expression analysis of the Candida albicans lipase gene family during experimental infections and in patient samples. FEMS Yeast Res. 2004, 4, 401–408. [Google Scholar] [CrossRef]
- Klingspor, L.; Tortorano, A.M.; Peman, J.; Willinger, B.; Hamal, P.; Sendid, B.; Velegraki, A.; Kibbler, C.; Meis, J.F.; Sabino, R.; et al. Invasive Candida infections in surgical patients in intensive care units: A prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008). Clin. Microbiol. Infect. 2015, 21, 87.e1–87.e10. [Google Scholar] [CrossRef]
- Maubon, D.; Garnaud, C.; Calandra, T.; Sanglard, D.; Cornet, M. Resistance of Candida spp. to antifungal drugs in the ICU: Where are we now? Intensive Care Med. 2014, 40, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and invasive candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef]
- Ashford, B.K. Certain conditions of the gastrointestinal tract in Puerto Rico and their relation to tropical sprue. Am. J. Trop. Med. Hyg. 1928, 8, 507–538. [Google Scholar] [CrossRef]
- van Asbeck, E.C.; Clemons, K.V.; Stevens, D.A. Candida parapsilosis: A review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit. Rev. Microbiol. 2009, 35, 283–309. [Google Scholar] [CrossRef]
- Skrzypek, M.S.; Binkley, J.; Binkley, G.; Miyasato, S.R.; Simison, M.; Sherlock, G. The Candida Genome Database (CGD): Incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res. 2017, 45, D592–D596. [Google Scholar] [CrossRef] [PubMed]
- Laffey, S.F.; Butler, G. Phenotype switching affects biofilm formation by Candida parapsilosis. Microbiology 2005, 151, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Bonassoli, L.A.; Bertoli, M.; Svidzinski, T.I. High frequency of Candida parapsilosis on the hands of healthy hosts. J. Hosp. Infect. 2005, 59, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- Nemeth, T.; Toth, A.; Szenzenstein, J.; Horvath, P.; Nosanchuk, J.D.; Grozer, Z.; Toth, R.; Papp, C.; Hamari, Z.; Vagvolgyi, C.; et al. Characterization of virulence properties in the C. parapsilosis sensu lato species. PLoS ONE 2013, 8, e68704. [Google Scholar] [CrossRef]
- Trofa, D.; Gacser, A.; Nosanchuk, J.D. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 2008, 21, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Messer, S.A.; Rhomberg, P.R.; Pfaller, M.A. Antifungal susceptibility patterns of a global collection of fungal isolates: Results of the SENTRY antifungal surveillance program (2013). Diagn. Microbiol. Infect. Dis. 2016, 85, 200–204. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Moet, G.J.; Messer, S.A.; Jones, R.N.; Castanheira, M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: Report from the SENTRY antimicrobial surveillance program (2008 to 2009). J. Clin. Microbiol. 2011, 49, 396–399. [Google Scholar] [CrossRef]
- Ramage, G.; Martinez, J.P.; Lopez-Ribot, J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006, 6, 979–986. [Google Scholar] [CrossRef]
- Cuellar-Cruz, M.; Lopez-Romero, E.; Villagomez-Castro, J.C.; Ruiz-Baca, E. Candida species: New insights into biofilm formation. Future Microbiol. 2012, 7, 755–771. [Google Scholar] [CrossRef]
- Pammi, M.; Holland, L.; Butler, G.; Gacser, A.; Bliss, J.M. Candida parapsilosis is a Significant Neonatal Pathogen: A Systematic Review and Meta-Analysis. Pediatr. Infect. Dis. J. 2013, 32, e206–e216. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New clonal strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Magobo, R.E.; Corcoran, C.; Seetharam, S.; Govender, N.P. Candida auris–associated candidemia, South Africa. Emerg. Infect. Dis. 2014, 20, 1250–1251. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjadins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Emergence of Candida auris: An international call to arms. Clin. Infect. Dis. 2017, 64, 141–143. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Isolates of the emerging pathogen Candida auris present in the UK have several geographic origins. Med. Mycol. 2017, 55, 563–567. [Google Scholar] [CrossRef]
- Calvo, B.; Melo, A.S.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Anil Kumar, V.; Sharma, C.; Prakash, A.; Agarwal, K.; Babu, R.; Dinesh, K.R.; Karim, S.; Singh, S.K.; Hagen, F.; et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Emara, M.; Ahmad, S.; Khan, Z.; Joseph, L.; Al-Obaid, I.; Purohit, P.; Bafna, R. Candida auris candidemia in Kuwait, 2014. Emerg. Infect. Dis. 2015, 21, 1091–1092. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, J.; Hagen, F.; Al-Balushi, Z.A.M.; de Hoog, G.S.; Chowdhary, A.; Meis, J.F.; Al-Hatmi, A.M.S. The first cases of Candida auris candidaemia in Oman. Mycoses 2017, 60, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Morales-López, S.E.; Parra-Giraldo, C.M.; Ceballos-Garzon, A.; Martinez, H.P.; Rodriguez, G.J.; Alvarez-Moreno, C.A.; Rodriguez, J.Y. Invasive infections with multidrug-resistant yeast Candida auris, Colombia. Emerg. Infect. Dis. 2017, 23, 162–164. [Google Scholar] [CrossRef]
- Ruiz Gaitán, A.C.; Moret, A.; Lopez Hontangas, J.L.; Molina, J.M.; Aleixandre Lopez, A.I.; Cabezas, A.H.; Mollar Maseres, J.; Arcas, R.C.; Gomez Ruiz, M.D.; Chiveli, M.A.; et al. Nosocomial fungemia by Candida auris: First four reported cases in continental Europe. Rev. Iberoam Micol. 2017, 34, 23–27. [Google Scholar] [CrossRef]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Kallen, A.; Tsay, S.; Chow, N.; Welsh, R.; Kerins, J.; Kemble, S.K.; Pacilli, M.; Black, S.R.; Landon, E.; et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. Am. J. Transplant. 2017, 17, 296–299. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Candida auris Incident Management Team; Manuel, R.; Brown, C.S. Candida auris: A review of the literature. Clin. Microbiol. Rev. 2018, 31, e00029-17. [Google Scholar] [CrossRef]
- Spivak, E.S.; Hanson, K.E. Candida auris: An emerging fungal pathogen. J. Clin. Microbiol. 2018, 56, e01588-17. [Google Scholar] [CrossRef]
- Alanio, A.; Snell, H.M.; Cordier, C.; Desnos-Olivier, M.; Dellière, S.; Aissaoui, N.; Sturny-Leclère, A.; Da Silva, E.; Eblé, C.; Rouveau, M.; et al. First patient-to-patient intrahospital transmission of clade I Candida auris in France revealed after a two-month incubation period. Microbiol. Spectr. 2022, 10, e0183322. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hurabielle, C.; Drummond, R.A.; Bouladoux, N.; Desai, J.V.; Sim, C.K.; Belkaid, Y.; Lionakis, M.S.; Segre, J.A. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe 2020, 29, 210–221.e6. [Google Scholar] [CrossRef] [PubMed]
- Ostrowsky, B.; Greenko, J.; Adams, E.; Quinn, M.; O’Brien, B.; Chaturvedi, V.; Berkow, E.; Vallabhaneni, S.; Forsberg, K.; Chaturvedi, S.; et al. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. Morb. Mortal. Wkly. Rep. 2020, 69, 6–9. [Google Scholar] [CrossRef]
- Kordalewska, M.; Perlin, D.S. Identification of drug resistant Candida auris. Front. Microbiol. 2019, 10, 1918. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.A.; Munoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 2020, 11, e03364. [Google Scholar] [CrossRef]
- Escandon, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin. Infect. Dis. 2019, 68, 15–21. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-resistant Candida auris misidentified as Candida haemulonii: Characterization by matrix-assisted laser desorption ionization-time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by VITEK 2, CLSI broth microdilution, and Etest method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [PubMed]
- Bravo Ruiz, G.; Ross, Z.K.; Gow, N.A.R.; Lorenz, A. Pseudohyphal growth of the emerging pathogen Candida auris is triggered by genotoxic stress through the S phase checkpoint. mSphere 2020, 5, e00151. [Google Scholar] [CrossRef]
- Santana, D.J.; O’Meara, T.R. Forward and reverse genetic dissection of morphogenesis identifies filament-competent Candida auris strains. Nat. Commun. 2021, 12, 7197. [Google Scholar] [CrossRef]
- Yue, H.; Bing, J.; Zheng, Q.; Zhang, Y.; Hu, T.; Du, H.; Wang, H.; Huang, G. Filamentation in Candida auris, an emerging fungal pathogen of humans: Passage through the mammalian body induces a heritable phenotypic switch. Emerg. Microbes Infect. 2018, 7, 188. [Google Scholar]
- Borman, A.M.; Fraser, M.; Johnson, E.M. CHROMagarTM Candida Plus: A novel chromogenic agar that permits the rapid identification of Candida auris. Med. Mycol. 2021, 59, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Sasoni, N.; Maidana, M.; Latorre-Rapela, M.G.; Morales-Lopez, S.; Berrio, I.; Gamarra, S.; Garcia-Effron, G. Candida auris and some Candida parapsilosis strains exhibit similar characteristics on CHROMagarTM Candida Plus. Med. Mycol. 2022, 60, myac062. [Google Scholar]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. mSphere 2016, 1, e00189. [Google Scholar] [PubMed]
- Fan, S.; Li, C.; Bing, J.; Huang, G.; Du, H. Discovery of the diploid form of the emerging fungal pathogen Candida auris. ACS Infect. Dis. 2020, 6, 2641–2646. [Google Scholar] [CrossRef]
- Chatterjee, S.; Alampalli, S.V.; Nageshan, R.K.; Chettiar, S.T.; Joshi, S.; Tatu, U.S. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genomics 2015, 16, 686. [Google Scholar] [CrossRef]
- Kumar, D.; Banerjee, T.; Pratap, C.B.; Tilak, R. Itraconazole-resistant Candida auris with phospholipase, proteinase and hemolysin activity from a case of vulvovaginitis. J. Infect. Dev. Ctries 2015, 9, 435–437. [Google Scholar] [CrossRef]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The emerging pathogen Candida auris: Growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Costerton, J.W.; Geesey, G.G.; Cheng, G.K. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef]
- Characklis, W.G.; Marshall, K.C. Biofilms: A basis for an interdisciplinary approach. In Biofilms; Characklis, W.G., Marshall, K.C., Eds.; John Wiley & Sons: New York, NY, USA, 1990; pp. 3–15. [Google Scholar]
- Ferguson, D.J.P.; McColm, A.A.; Ryan, D.M.; Acred, P. A morphological study of experimental staphylococcal endocarditis and aortitis. II. Interrelationship of bacteria, vegetation and cardiovasculature in established infections. Br. J. Exp. Pathol. 1986, 67, 679–686. [Google Scholar] [PubMed]
- Nickel, J.C.; Costerton, J.W. Coagulase-negative staphylococcus in chronic prostatitis. J. Urol. 1992, 147, 398–401. [Google Scholar] [CrossRef]
- Raad, I.; Costerton, W.; Sabharwal, U.; Sacilowski, M.; Anaissie, W.; Bodey, G.P. Ultrastructural analysis of indwelling vascular catheters: A quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 1993, 168, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Stickler, D.; Morris, N.; Moreno, M.-C.; Sabbuba, N. Studies on the formation of crystalline bacterial biofilms on urethral catheters. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 649–652. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- El-Azizi, M.A.; Starks, S.E.; Khardori, N. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J. Appl. Microbiol. 2004, 96, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Mitchell, A.P. Fungal biofilms. PLoS Pathog. 2012, 8, e1002585. [Google Scholar] [CrossRef]
- Potera, C. Forging a link between biofilms and disease. Science 1999, 283, 1837–1839. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.G.; Ramage, G.; Lopez_Ribot, J.L. Biofilms and implant infections. In Microbial Biofilms; Ghannoum, M., O’Toole, G., Eds.; ASM Press: Washington, DC, USA, 2004; pp. 269–293. [Google Scholar]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Chung, P.H.; Leong, J.Y.; Wallen, J.J.; Stanton, W.; Diaz, N.; Phillips, C.D.; Henry, G.D. Molecular testing with next-generation sequencing appears to identify biofilm on penile prostheses better than traditional cultures: The new gold standard? Can J. Urol. 2022, 29, 11348–11354. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, W.; You, C.; Liu, Z. Development of a Multiplex PCR Assay for Detection of Pseudomonas fluorescens with Biofilm Formation Ability. J. Food Sci. 2017, 82, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Condinho, M.; Carvalho, B.; Arraiano, C.M.; Pobre, V.; Pinto, S.N. The two weapons against bacterial biofilms: Detection and treatment. Antibiotics 2021, 10, 1482. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dhaouadi, Y.; Stoodley, P.; Ren, D. Sensing the unreachable: Challenges and opportunities in biofilm detection. Curr. Opin. Biotechnol. 2020, 64, 79–84. [Google Scholar] [CrossRef]
- Leoney, A.; Karthigeyan, S.; Asharaf, A.S.; Felix, A.J.W. Detection and Categorization of Biofilm-forming Staphylococcus aureus, Viridans streptococcus, Klebsiella pneumoniae, and Escherichia coli Isolated from Complete Denture Patients and Visualization Using Scanning Electron Microscopy. J. Int. Soc. Prev. Community Dent. 2020, 10, 627–633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papi, M.; Maiorana, A.; Bugli, F.; Torelli, R.; Posteraro, B.; Maulucci, G.; De Spirito, M.; Sanguinetti, M. Detection of biofilm-grown Aspergillus fumigatus by means of atomic force spectroscopy: Ultrastructural effects of alginate lyase. Microsc. Microanal. 2012, 18, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, A.; Gupta, P. Real-time biofilm detection techniques: Advances and applications. Future Microbiol. 2024, 19, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm Dispersal: Mechanisms, Clinical Implications, and Potential Therapeutic Uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Short, B.; Delaney, C.; McKloud, E.; Brown, J.L.; Kean, R.; Litherland, G.J.; Williams, C.; Martin, S.L.; MacKay, W.G.; Ramage, G. Investigating the Transcriptome of Candida albicans in a Dual-Species Staphylococcus aureus Biofilm Model. Front. Cell. Infect. Microbiol. 2021, 11, 791523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alonso, G.C.; Pavarina, A.C.; Sousa, T.V.; Klein, M.I. A quest to find good primers for gene expression analysis of Candida albicans from clinical samples. J. Microbiol. Methods 2018, 147, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fragkioudakis, I.; Konstantopoulos, G.; Kottaridi, C.; Doufexi, A.E.; Sakellari, D. Quantitative assessment of Candida albicans, Staphylococcus aureus and Staphylococcus epidermidis in peri-implant health and disease: Correlation with clinical parameters. J. Med. Microbiol. 2024, 73, 001933. [Google Scholar] [CrossRef] [PubMed]
- Pantanella, F.; Valenti, P.; Natalizi, T.; Passeri, D.; Berlutti, F. Analytical techniques to study microbial biofilm on abiotic surfaces: Pros and cons of the main techniques currently in use. Ann. Ig. 2013, 25, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Toté, K.; Vanden Berghe, D.; Maes, L.; Cos, P. A new colorimetric microtitre model for the detection of Staphylococcus aureus biofilms. Lett. Appl. Microbiol. 2008, 46, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Honraet, K.; Goetghebeur, E.; Nelis, H.J. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J. Microbiol. Methods 2005, 63, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Tawakoli, P.N.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin. Oral Investig. 2013, 17, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L.; Prévost, M.; Barbeau, B.; Coallier, J.; Desjardins, R. LIVE/DEAD BacLight: Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 1999, 37, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, T.; Samaranayake, Y.H.; Fang, H.H.; Yip, H.K.; Samaranayake, L.P. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia 2005, 159, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Punithavathy, P.M.; Nalina, K.; Menon, T. Antifungal susceptibility testing of Candida tropicalis biofilms against fluconazole using calorimetric indicator resazurin. Indian J. Pathol. Microbiol. 2012, 55, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, A.; Lopez-Gigosos, R.M.; Carnero-Varo, M.; Fernandez-Crehuet, J. Fluorescent assay based on resazurin for detection of activity of disinfectants against bacterial biofilm. Appl. Microbiol. Biotechnol. 2009, 82, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Skogman, M.E.; Vuorela, P.M.; Fallarero, A. Combining biofilm matrix measurements with biomass and viability assays in susceptibility assessments of antimicrobials against Staphylococcus aureus biofilms. J. Antibiot. 2012, 65, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Pantanella, F.; Valenti, P.; Frioni, A.; Natalizi, T.; Coltella, L.; Berlutti, F. BioTimer Assay, a new method for counting Staphylococcus spp. in biofilm without sample manipulation applied to evaluate antibiotic susceptibility of biofilm. J. Microbiol. Methods 2008, 75, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Thompson, A.; Kashleva, H.; Dongari-Bagtzoglou, A. A quantitative real-time RT-PCR assay for mature C. albicans biofilms. BMC Microbiol. 2011, 11, 93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thurnheer, T.; Gmür, R.; Guggenheim, B. Multiplex FISH analysis of a six-species bacterial biofilm. J. Microbiol. Methods 2004, 56, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lawrence, J.R.; Korber, D.R.; Hoyle, B.D.; Costerton, J.W.; Caldwell, D.E. Optical sectioning of microbial biofilms. J. Bacteriol. 1991, 173, 6558–6567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorman, S.P.; Adair, C.G.; Mawhinney, W.M. Incidence and nature of peritoneal catheter biofilm determined by electron and confocal laser scanning microscopy. Epidemiol. Infect. 1994, 112, 551–559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karygianni, L.; Follo, M.; Hellwig, E.; Burghardt, D.; Wolkewitz, M.; Anderson, A.; Al-Ahmad, A. Microscope-based imaging platform for large-scale analysis of oral biofilms. Appl. Environ. Microbiol. 2012, 78, 8703–8711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chao, Y.; Zhang, T. Surface-enhanced Raman scattering (SERS) revealing chemical variation during biofilm formation: From initial attachment to mature biofilm. Anal. Bioanal. Chem. 2012, 404, 1465–1475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandt, C.; Smith-Palmer, T.; Pink, J.; Brennan, L.; Pink, D. Confocal Raman microspectroscopy as a tool for studying the chemical heterogeneities of biofilms in situ. J. Appl. Microbiol. 2007, 103, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, R.; Keuntje, M.; Anders-von Ahlften, A. A new approach to non-destructive analysis of biofilms by confocal Raman microscopy. Anal. Bioanal. Chem. 2006, 386, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Alhede, M.; Qvortrup, K.; Liebrechts, R.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Combination of microscopic techniques reveals a comprehensive visual impression of biofilm structure and composition. FEMS Immunol. Med. Microbiol. 2012, 65, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wallace, P.K.; Arey, B.; Mahaffee, W.F. Subsurface examination of a foliar biofilm using scanning electron- and focused-ion-beam microscopy. Micron 2011, 42, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Dynes, J.J.; Tyliszczak, T.; Araki, T.; Lawrence, J.R.; Swerhone, G.D.; Leppard, G.G.; Hitchcock, A.P. Speciation and quantitative mapping of metal species in microbial biofilms using scanning transmission X-ray microscopy. Environ. Sci. Technol. 2006, 40, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, Y.F. Application of atomic force microscopy to microbial surfaces: From reconstituted cell surface layers to living cells. Micron 2001, 32, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Huang, Q.; Cai, P. Application of atomic force microscopy (AFM) to study bacterial biofilms. Sheng Wu Gong Cheng Xue Bao 2017, 33, 1399–1410. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Núñez, M.E.; Martin, M.O.; Chan, P.H.; Spain, E.M. Predation, death, and survival in a biofilm: Bdellovibrio investigated by atomic force microscopy. Colloids Surf. B Biointerfaces 2005, 42, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Razatos, A.; Ong, Y.L.; Sharma, M.M.; Georgiou, G. Molecular determinants of bacterial adhesion monitored by atomic force microscopy. Proc. Natl. Acad. Sci. USA 1998, 95, 11059–11064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pohl, C.H. Recent advances and opportunities in the study of Candida albicans polymicrobial biofilms. Front. Cell. Infect. Microbiol. 2022, 12, 836379. [Google Scholar] [CrossRef]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef]
- Vitális, E.; Nagy, F.; Tóth, Z.; Forgács, L.; Bozó, A.; Kardos, G.; Majoros, L.; Kovács, R. Candida biofilm production is associated with higher mortality in patients with candidaemia. Mycoses 2020, 63, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Gayosso, P.; Hernandez-Hernandez, F.; Zavala-Velasquez, N.; Mendez-Tovar, L.J.; Naquid-Narvaez, J.M.; Torres-Rodriguez, J.M.; Lopez-Martinez, R. Candiduria in type 2 diabetes mellitus patients and its clinical significance. Candida spp. antifungal susceptibility. Rev. Med. Inst. Mex. Seguro Soc. 2008, 46, 603–610. [Google Scholar]
- Cleveland, A.A.; Harrison, L.H.; Farley, M.M.; Hollick, R.; Stein, B.; Chiller, T.M.; Lockhart, S.R.; Park, B.J. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: Results from population-based surveillance. PLoS ONE 2015, 10, e0120452. [Google Scholar] [CrossRef]
- Tsay, S.V.; Mu, Y.; Williams, S.; Epson, E.; Nadle, J.; Bamberg, W.M.; Barter, D.M.; Johnston, H.L.; Farley, M.M.; Harb, S.; et al. Burden of candidemia in the United States. 2017. Clin. Infect. Dis. 2020, 71, e449–e453. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J. Candida biofilm resistance. Drug Resist. Updat. 2004, 7, 301–309. [Google Scholar]
- Blankenship, J.R.; Mitchell, A.P. How to build a biofilm: A fungal perspective. Curr. Opin. Microbiol. 2006, 9, 588–594. [Google Scholar] [CrossRef]
- Uppuluri, P.; Chaturvedi, A.K.; Srinivasan, A.; Banerjee, M.; Ramasubramaniam, A.K.; Köhler, J.R.; Kadosh, D.; Lopez-Ribot, J.L. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010, 6, e1000828. [Google Scholar]
- Taff, H.T.; Nett, J.E.; Zarnowski, R.; Ross, K.M.; Sanchez, H.; Cain, M.T.; Hamaker, J.; Mitchell, A.P.; Andres, D.R. A Candida biofilm-induced pathway for matrix glucan delivery: Implications for drug resistance. PLoS Pathog. 2012, 8, e1002848. [Google Scholar] [CrossRef]
- Hawser, S.P.; Douglas, L.J. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 1994, 62, 915–921. [Google Scholar]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Sharma, S.; Shrivastva, P. Multi-species biofilm of Candida albicans and non-Candida albicans Candida species on acrylic substrate. J. Appl. Oral Sci. 2012, 20, 70–75. [Google Scholar] [CrossRef]
- d’Enfert, C. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr. Drug Targets 2006, 7, 465–470. [Google Scholar] [CrossRef]
- Chandra, J.; Mukherjee, P. Candida biofilms: Development, architecture, and resistance. Microbiol. Spectr. 2015, 3, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Jin, L.; Samaranayake, L.P. Biofilm lifestyle of Candida: A mini review. Oral Dis. 2008, 14, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Las Penčz de, A.; Pan, S.J.; CastaĖo, I.; Alder, J.; Cregg, R.; Cormack, B.P. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP-1 and SIRdependent transcriptional silencing. Genes Dev. 2003, 17, 2245–2258. [Google Scholar] [CrossRef]
- Yamin, D.H.; Husin, A.; Harun, A. Risk factors of Candida parapsilosis catheter-related bloodstream infection. Front. Public Health 2021, 9, 631865. [Google Scholar] [CrossRef]
- Harrington, R.; Kindermann, S.L.; Hou, Q.; Taylor, R.J.; Azie, N.; Horn, D.L. Candidemia and invasive candidiasis among hospitalized neonates and pediatric patients. Curr. Med. Res. Opin. 2017, 33, 1803–1812. [Google Scholar] [CrossRef]
- Dranginis, A.M.; Rauceo, J.M.; Coronado, J.E.; Lipke, P.N. A biochemical guide to yeast adhesins: Glycoproteins for social and antisocial occasions. Microb. Mol. Biol. Rev. 2007, 71, 282–294. [Google Scholar] [CrossRef]
- Weig, M.; Jänsch, L.; Gross, U.; De Koster, C.G.; Klis, F.M.; De Groot, P.W. Systematic identification in silico of covalently bound cell wall proteins and analysis of proteinpolysaccharide linkages of the human pathoge Candida glabrata. Microbiology 2004, 150, 3129–3144. [Google Scholar] [CrossRef]
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Tejera, E.; Machado, A. Prevalence of biofilms in Candida spp. bloodstream infections: A meta-analysis. PLoS ONE 2022, 17, e0263522. [Google Scholar] [CrossRef]
- Silva, S.; Rodrigues, C.F.; Araujo, D.; Rodrigues, M.E.; Henriques, M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi 2017, 3, 8. [Google Scholar] [CrossRef]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single-and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Panagoda, G.J.; Ellepola, A.N.; Samaranayake, L.P. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses 2001, 44, 29–35. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Mora-Montes, H.M. Current aspects in the biology, pathogeny, and treatment of Candida krusei, a neglected fungal pathogen. Infect. Drug Resist. 2020, 13, 1673–1689. [Google Scholar] [CrossRef] [PubMed]
- Berila, N.; Subík, J. Opportunistic pathogen Candida glabrata and the mechanisms of its resistance to antifungal drugs. Epidemiol. Mikrobiol. Imunol. 2010, 59, 67–79. [Google Scholar]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Estivill, D.; Arias, A.; Torres-Lana, A.; Carrillo-Munoz, A.J.; Arevalo, M.P. Biofilm formation by five species of Candida on three clinical materials. J. Microbiol. Methods 2011, 86, 238–242. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar]
- Hall-Stoodley, L.; Stoodley, P. Developmental regulation of microbial biofilms. Curr. Opin. Biotechnol. 2002, 13, 228–233. [Google Scholar]
- Kolenbrander, P.E. Oral microbial communities: Biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 2000, 54, 413–437. [Google Scholar] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar]
- Al-Fattani, M.A.; Douglas, L.J. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J. Med. Microbiol. 2006, 55, 999–1008. [Google Scholar] [CrossRef]
- Holmes, A.R.; Cannon, R.D.; Jenkinson, H.F. Interactions of Candida albicans with bacteria and salivary molecules in oral biofilms. J. Ind. Microbiol. 1995, 15, 208–213. [Google Scholar]
- Desai, J.V.; Mitchell, A.P. Candida albicans biofilm development and its genetic control. In Microbial Biofilms, 2nd ed.; Ghannoum, M., Parsek, M., Whiteley, M., Mukherjee, P., Eds.; ASM Press: Washington, DC, USA, 2015. [Google Scholar]
- Mukherjee, P.K.; Mohamed, S.; Chandra, J.; Kuhn, D.; Liu, S.; Antar, O.S.; Munyon, R.; Mitchell, A.P.; Andes, D.; Chance, M.R.; et al. Alcohol dehydrogenase restricts the ability of the pathogen Candida albicans to form a biofilm on catheter surfaces through an ethanol-based mechanism. Infect. Immun. 2006, 74, 3804–3816. [Google Scholar] [PubMed]
- Baillie, G.S.; Douglas, L.J. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 1999, 310, 644–656. [Google Scholar]
- Baillie, G.S.; Douglas, L.J. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 1998, 42, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Douglas, L.J. Iron-limited biofilms of Candida albicans and their susceptibility to amphotericin B. Antimicrob. Agents Chemother. 1998, 42, 2146–2149. [Google Scholar]
- Hawser, S.P.; Douglas, L.J. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 1995, 39, 2128–2133. [Google Scholar] [CrossRef]
- Kalya, A.V.; Ahearn, D.G. Increased resistance to antifungal antibiotics of Candida spp. adhered to silicone. J. Ind. Microbiol. 1995, 14, 451–455. [Google Scholar] [CrossRef]
- Guinea, J.; Arendrup, M.C.; Canton, R.; de la Pedrosa, E.G.G.; Hare, R.K.; Orden, B.; Sanguinetti, M.; Pemán, J.; Posteraro, B.; Ruiz-Gaitán, A.; et al. Genotyping reveals high clonal diversity and widespread genotypes of Candida causing candidemia at distant geographical areas. Front. Cell. Infect. Microbiol. 2020, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Balashov, S.V.; Park, S.; Perlin, D.S. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob. Agents Chemother. 2006, 50, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim. Biophys. Acta 2002, 1587, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.K.; Leidich, S.D.; Faddoul, F.F.; Hoyer, L.L.; Douglas, L.J.; Ghannoum, M.A. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 2001, 80, 903–908. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef]
- Bachmann, S.P.; Patterson, T.F.; Lopez-Ribot, J.L. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J. Clin. Microbiol. 2002, 40, 2228–2230. [Google Scholar] [CrossRef]
- Niimi, K.; Maki, K.; Ikeda, F.; Holmes, A.R.; Lamping, E.; Niimi, M.; Monk, B.C.; Cannon, R.D. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1148–1155. [Google Scholar] [CrossRef]
- Silver, P.M.; Oliver, B.G.; White, T.C. Characterization of caspofungin susceptibilities by broth and agar in Candida albicans clinical isolates with characterized mechanisms of azole resistance. Med. Mycol. 2008, 46, 231–239. [Google Scholar] [CrossRef]
- Nett, J.E.; Lepak, A.J.; Marchillo, K.; Andes, D.R. Time course global gene expression analysis of an in vivo Candida biofilm. J. Infect. Dis. 2009, 200, 307–313. [Google Scholar] [CrossRef]
- Finkel, J.S.; Mitchell, A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011, 9, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef]
- Perumal, P.M.S.; Chaffin, W.L. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob. Agents Chemother. 2007, 7, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Jin, L.J.; Samaranayake, Y.H.; Samaranayake, L.P. Cell density and cell aging as factors modulating antifungal resistance of Candida albicans biofilms. Antimicrob. Agents Chemother. 2008, 8, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; Lopez-Ribot, J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Baillie, G.S.; Douglas, L.J. Matrix polymers of Candida biofilms and their possible resistance to antifungal agents. J. Antimicrob. Chemother. 2000, 46, 397–403. [Google Scholar] [CrossRef]
- Nett, J.; Lincoln, L.; Marchillo, K.; Massey, R.; Holoyda, K.; Hoff, B.; VanHandel, M.; Andres, D. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 2007, 51, 510–520. [Google Scholar] [CrossRef]
- Nett, J.E.; Sanchez, H.; Cain, M.T.; Andes, D.R. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J. Infect. Dis. 2010, 202, 171–175. [Google Scholar] [CrossRef]
- Vediyappan, G.; Rossignol, T.; d’Enfert, C. Interaction of Candida albicans biofilms with antifungals: Transcriptional response and binding of antifungals to β-glucans. Antimicrob. Agents Chemother. 2010, 54, 2096–2111. [Google Scholar] [CrossRef]
- Nett, J.E.; Crawford, K.; Marchillo, K.; Andes, D.R. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob. Agents Chemother. 2010, 54, 3505–3508. [Google Scholar] [PubMed]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [PubMed]
- Lewis, K. Multidrug tolerance of biofilms and persister cells. Curr. Top. Microbiol. Immunol. 2008, 332, 107–131. [Google Scholar]
- Klis, F.M.; de Groot, P.; Hellingwerf, K. Molecular organization of the cell wall of Candida albicans. Med. Mycol. 2001, 39 (Suppl. S1), 1–8. [Google Scholar] [CrossRef]
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67–78. [Google Scholar] [CrossRef]
- Linden, J.R.; Maccani, M.A.; Laforce-Nesbitt, S.S.; Bliss, J.M. High efficiency opsonin-independent phagocytosis of Candida parapsilosis by human neutrophils. Med. Mycol. 2010, 48, 355–364. [Google Scholar] [PubMed]
- Lindemann, R.A.; Franker, C.K. Phagocyte-mediated killing of Candida tropicalis. Mycopathologia 1991, 113, 81–87. [Google Scholar] [CrossRef]
- Kowanko, I.C.; Ferrante, A.; Harvey, D.P.; Carman, K.L. Granulocyte-macrophage colony-stimulating factor augments neutrophil killing of Torulopsis glabrata and stimulates neutrophil respiratory burst and degranulation. Clin. Exp. Immunol. 1991, 83, 225–230. [Google Scholar]
- Van‘t Wout, J.W.; Linde, I.; Leijh, P.C.; van Furth, R. Contribution of granulocytes and monocytes to resistance against experimental disseminated Candida albicans infection. Eur. J. Clin. Microbiol. Infect. Dis. 1988, 7, 736–741. [Google Scholar]
- Fradin, C.; De Groot, P.; MacCallum, D.; Schaller, M.; Klis, F.; Odds, F.C.; Hube, B. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 2005, 56, 397–415. [Google Scholar]
- Kernien, J.F.; Snarr, B.D.; Sheppard, D.C.; Nett, J.E. The Interface between Fungal Biofilms and Innate Immunity. Front. Immunol. 2018, 8, 1968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mannan, M.; Nabeela, S.; Mishra, R.; Uppuluri, P. Host immune response against fungal biofilms. Curr. Opin. Microbiol. 2024, 81, 102520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alonso, M.F.; Gow, N.A.R.; Erwig, L.P.; Bain, J.M. Macrophage Migration Is Impaired within Candida albicans Biofilms. J. Fungi 2017, 3, 31. [Google Scholar] [CrossRef]
- Johnson, C.J.; Cabezas-Olcoz, J.; Kernien, J.F.; Wang, S.X.; Beebe, D.J.; Huttenlocher, A.; Ansari, H.; Nett, J.E. The Extracellular Matrix of Candida albicans Biofilms Impairs Formation of Neutrophil Extracellular Traps. PLoS Pathog. 2016, 12, e1005884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandra, J.; McCormick, T.S.; Imamura, Y.; Mukherjee, P.K.; Ghannoum, M.A. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect. Immun. 2007, 75, 2612–2620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelletier, C.; Shaw, S.; Alsayegh, S.; Brown, A.J.P.; Lorenz, A. Candida auris undergoes adhesin-dependent and -independent cellular aggregation. PLoS Pathog. 2024, 20, e1012076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Svobodova, E.; Staib, P.; Losse, J.; Hennicke, F.; Barz, D.; Józsi, M. Differential interaction of the two related fungal species Candida albicans and Candida dubliniensis with human neutrophils. J. Immunol. 2012, 189, 2502–2511. [Google Scholar]
- Lorenz, M.C.; Bender, J.A.; Fink, G.R. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 2004, 3, 1076–1087. [Google Scholar]
- Lewis, L.E.; Bain, J.M.; Lowes, C.; Gillespie, C.; Rudkin, F.M.; Gow, N.A.R.; Erwig, L.P. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012, 8, e1002578. [Google Scholar]
- Vylkova, S.; Carman, A.J.; Danhof, H.A.; Collette, J.R.; Zhou, H.; Lorenz, M.C. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio 2011, 2, e00055-11. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Navarathna, D.H.; Roberts, D.D.; Cooper, J.T.; Atkin, A.L.; Petro, T.M.; Nickerson, K.W. Arginine-induced germ tube formation in Candida albicans is essential for escape from murine macrophage line RAW 264.7. Infect. Immun. 2009, 77, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Ma, B.; Cormack, B.P. A family of glycosylphosphatidylinositol-linked aspartyl proteases is required for virulence of Candida glabrata. Proc. Natl. Acad. Sci. USA 2007, 104, 7628–7633. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Roberts, J.A.; Antonelli, M.; Bassetti, M.; Vincent, J.-L. Diagnosis and management of invasive candidiasis in the ICU: An updated approach to an old enemy. Crit Care 2016, 20, 125. [Google Scholar] [CrossRef]
- Tang, D.L.; Chen, X.; Zhu, C.-G.; Li, Z.W.; Guo, X.-G. Pooled analysis of T2 Candida for rapid diagnosis of candidiasis. BMC Infect. Dis. 2019, 19, 798. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Pappas, P.G.; Vazquez, J.; Judson, M.A.; Kontoyiannis, D.P.; Thompson, G.R., III; Garey, K.W.; Reboli, A.; Greenberg, R.N.; Apewokin, S.; et al. Detecting infections rapidly and easily for candidemia trial, part 2 (DIRECT2): A prospective, multicenter study of the T2 Candida panel. Clin. Infect. Dis. 2018, 66, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Jimenez, M.C.; Munoz, P.; Guinea, J.; Valerio, M.; Alonso, R.; Escribano, E.; Bouza, E. Potential role of Candida albicans germ tube antibody in the diagnosis of deep-seated candidemia. Med. Mycol. 2014, 52, 270–275. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Diagnosing invasive candidiasis. J. Clin. Microbiol. 2018, 56, e01909-17. [Google Scholar] [CrossRef]
- Lagunes, L.; Rello, J. Invasive candidiasis: From mycobiome to infection, therapy, and prevention. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1221–1226. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Verweij, P.E.; Arendrup, M.C.; Arikan-Akdagli, S.; Bille, J.; Donnelly, J.P.; Jensen, H.E.; Lass-Flörl, C.; Richardson, M.D.; Akova, M.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Diagnostic procedures. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 9–18. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Finding the ‘missing 50%’ of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wu, T.; Wu, Y.; Ming, D.; Zhu, X. Diagnostic accuracy of Candida albicans germ tube antibody for invasive candidiasis: Systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2019, 93, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Camp, I.; Spettel, K.; Willinger, B. Molecular methods for the diagnosis of invasive candidiasis. J. Fungi 2020, 6, 101. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Mikulska, M.; Calandra, T.; Sanguinetti, M.; Poulain, D.; Viscoli, C.; the Third European Conference on Infections in Leukemia Group. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: Recommendations from the Third European Conference on Infections in Leukemia. Crit. Care 2010, 14, R222. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, C.; Le Bihan, C.; Maubon, D.; Calvet, L.; Ruckly, S.; Schwebel, C.; Bouadma, L.; Azoulay, E.; Cornet, M.; Timsit, J.-F.; et al. Performance of repeated measures of (1-3)-beta-D-glucan, mannan antigen, and antimannan antibodies for the diagnosis of invasive candidiasis in ICU patients: A preplanned ancillary analysis of the EMPIRICUS randomized clinical trial. Open Forum. Infect. Dis. 2021, 8, ofab080. [Google Scholar] [CrossRef]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug. Discov. 2017, 16, 603–616. [Google Scholar]
- Ben-Ami, R.; Kontoyiannis, D.P. Resistance to Antifungal Drugs. Infect. Dis. Clin. N. Am. 2021, 35, 279–311. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes-Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Fanos, V.; Cataldi, L. Amphotericin B-induced nephrotoxicity: A review. J. Chemother. 2000, 12, 463–470. [Google Scholar] [CrossRef]
- Groll, A.H.; Rijnders, B.J.A.; Walsh, T.J.; Adler-Moore, J.; Lewis, R.E.; Bruggemann, R.J.M. Clinical Pharmacokinetics, Pharmacodynamics, Safety and Efficacy of Liposomal Amphotericin B. Clin. Infect. Dis. 2019, 68, S260–S274. [Google Scholar] [PubMed]
- Yamin, D.; Akanmu, M.H.; Al Mutair, A.; Alhumaid, S.; Rabaan, A.A.; Hajissa, K. Global Prevalence of Antifungal-Resistant Candida parapsilosis: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 188. [Google Scholar] [CrossRef]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [PubMed]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Reboli, A.C.; Shorr, A.F.; Rotstein, C.; Pappas, P.G.; Kett, D.H.; Schlamm, H.T.; Reisman, A.L.; Biswas, P.; Walsh, T.J. Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: A multivariate analysis of factors associated with improved outcome. BMC Infect. Dis. 2011, 11, 261. [Google Scholar] [CrossRef]
- Pappas, P.G.; Rotstein, C.M.; Betts, R.F.; Nucci, M.; Talwar, D.; De Waele, J.J.; Vazquez, J.A.; Dupont, B.F.; Horn, D.L.; Ostrosky-Zeichner, L.; et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 2007, 45, 883–893. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Viscoli, C.; Pappas, P.G.; Vazquez, J.A.; Ostrosky-Zeichner, L.; Rotstein, C.; Sobel, J.D.; Herbrecht, R.; Rahav, G.; Jaruratanasirikul, S.; et al. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive Candida infections: The ACTIVE trial. Clin. Infect. Dis. 2019, 68, 1981–1989. [Google Scholar] [CrossRef]
- Demir, K.K.; Butler-Laporte, G.; Del Corpo, O.; Ekmejian, T.; Sheppard, D.C.; Lee, T.C.; Cheng, M.P. Comparative effectiveness of amphotericin B, azoles and echinocandins in the treatment of candidemia and invasive candidiasis: A systematic review and network meta-analysis. Mycoses 2021, 64, 1098–1110. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar]
- Chen, S.A.; Slavin, M.; Sorrell, T. Echinocandin Antifungal Drugs in Fungal Infections. Drugs 2011, 71, 11–41. [Google Scholar]
- Beyda, N.D.; Lewis, R.E.; Garey, K.W. Echinocandin Resistance in Candida Species: Mechanisms of Reduced Susceptibility and Therapeutic Approaches. Ann. Pharmacother. 2012, 46, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Current perspectives on echinocandin class drugs. Future Microbiol. 2011, 6, 441–457. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Rex, J.H.; Pappas, P.G.; Hamill, R.J.; Larsen, R.A.; Horowitz, H.W.; Powderly, W.G.; Hyslop, N.; Kauffman, C.A.; Cleary, J.; et al. Antifungal Susceptibility Survey of 2,000 Bloodstream Candida Isolates in the United States. Antimicrob. Agents Chemother. 2003, 47, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 2007, 10, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Effron, G.; Canton, E.; Pemán, J.; Dilger, A.; Romá, E.; Perlin, D.S. Epidemiology and echinocandin susceptibility of Candida parapsilosis sensu lato species isolated from bloodstream infections at a Spanish university hospital. J. Antimicrob. Chemother. 2012, 67, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Siopi, M.; Papadopoulos, A.; Spiliopoulou, A.; Paliogianni, F.; Abou-Chakra, N.; Arendrup, M.C.; Damoulari, C.; Tsioulos, G.; Giannitsioti, E.; Frantzeskaki, F.; et al. Pan-Echinocandin Resistant C. parapsilosis Harboring an F652S Fks1 Alteration in a Patient with Prolonged Echinocandin Therapy. J. Fungi 2022, 8, 931. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Xiao, M.; Perlin, D.S.; Zhao, Y.; Lu, M.; Li, Y.; Luo, Z.; Dai, R.; Li, S.; Xu, J.; et al. Decreased echinocandin susceptibility in Candida parapsilosis causing candidemia and emergence of a pan-echinocandin resistant case in China. Emerg. Microbes Infect. 2023, 12, 2153086. [Google Scholar] [CrossRef]
- Katiyar, S.; Pfaller, M.; Edlind, T. Candida albicans and Candida glabrata Clinical Isolates Exhibiting Reduced Echinocandin Susceptibility. Antimicrob. Agents Chemother. 2006, 50, 2892–2894. [Google Scholar] [CrossRef]
- Singh, S.D.; Robbins, N.; Zaas, A.K.; Schell, W.A.; Perfect, J.R.; Cowen, L.E. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009, 5, e1000532. [Google Scholar] [CrossRef]
- Munro, C.A.; Selvaggini, S.; De Bruijn, I.; Walker, L.; Lenardon, M.D.; Gerssen, B.; Milne, S.; Brown, A.J.P.; Gow, N.A.R. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 2007, 63, 1399–1413. [Google Scholar] [CrossRef]
- Lee, K.K.; Maccallum, D.M.; Jacobsen, M.D.; Walker, L.A.; Odds, F.C.; Gow, N.A.; Munro, C.A. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob. Agents Chemother. 2012, 56, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular Evolution of Antifungal Drug Resistance. Annu. Rev. Microbiol. 2017, 71, 753–775. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Andes, D.; Arendrup, M.C.; Diekema, D.J.; Espinel-Ingroff, A.; Alexander, B.; Brown, S.D.; Chaturvedi, V.; Fowler, C.L.; Ghannoum, M.A.; et al. Clinical breakpoints for voriconazole and Candida spp. revisited: Review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn. Microbiol. Infect. Dis. 2011, 70, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Kuse, E.R.; Chetchotisakd, P.; da Cunha, C.A.; Ruhnke, M.; Barrios, C.; Raghunadharao, D.; Sekhon, J.S.; Freire, A.; Ramasubramanian, V.; Demeyer, G.; et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: A phase III randomised double-blind trial. Lancet 2007, 369, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Ma, J.; Saliba, F.; Dupont, B.; Wingard, J.R.; Hachem, R.Y.; Mattiuzzi, G.N.; Chandrasekar, P.H.; Kontoyiannis, D.P.; Rolston, K.V.; et al. Drug-induced nephrotoxicity caused by amphotericin B lipid complex and liposomal amphotericin B: A review and meta-analysis. Medicine 2010, 89, 236–244. [Google Scholar] [CrossRef]
- Xiao, L.; Madison, V.; Chau, A.S.; Loebenberg, D.; Palermo, R.E.; McNicholas, P.M. Three-Dimensional Models of Wild-Type and Mutated Forms of Cytochrome P450 14α-Sterol Demethylases from Aspergillus fumigatus and Candida albicans Provide Insights into Posaconazole Binding. Antimicrob. Agents Chemother. 2004, 48, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: Traditional and alternative antifungal agents. BioMed. Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 2, 2–13. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Perfect, J.R. Resistance to antifungal agents: Mechanisms and clinical impacts. Antimicrobiol. Resist. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- White, T.C.; Marr, K.A.; Bowden, R.A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.; Tabernero, L.; Denning, D.W.; Anderson, M.J. Molecular mechanisms of primary resistance to flucytosice in Candida albicans. Antimicrob. Agents Chemother. 2004, 48, 4377–4386. [Google Scholar] [CrossRef]
- Sanglard, D.; Odds, F.C. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2002, 2, 73–85. [Google Scholar]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [PubMed]
- Cateau, E.; Levasseur, P.; Borgonovi, M.; Imbert, C. The effect of aminocandin (HMR 3270) on the in-vitro adherence of Candida albicans to polystyrene surfaces coated with extracellular matrix proteins or fibronectin. Clin. Microbiol. Infect. 2007, 13, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Suci, P.A.; Tyler, B.J. Action of chlorhexidine digluconate against yeast and filamentous forms in an early-stage Candida albicans biofilm. Antimicrob. Agents Chemother. 2002, 46, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.C.; Willcox, M.D.; Thomas, C.J.; Harty, D.W.; Knox, K.W. The effect of sodium hypochlorite on potential pathogenic traits of Candida albicans and other Candida species. Oral Microbiol. Immunol. 1995, 10, 334–341. [Google Scholar] [CrossRef]
- Bersan, S.M.; Galvao, L.C.; Goes, V.F.; Sartoratto, A.; Figueira, G.M.; Rehder, V.L.; Alencar, S.M.; Duarte, R.M.; Rosalen, P.L.; Duarte, M.C. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. BMC Complement. Altern. Med. 2014, 14, 451. [Google Scholar] [CrossRef]
- da Silva, P.M.; Acosta, E.J.; Pinto Lde, R.; Graeff, M.; Spolidorio, D.M.; Almeida, R.S.; Porto, V.C. Microscopical analysis of Candida albicans biofilms on heat-polymerised acrylic resin after chlorhexidine gluconate and sodium hypochlorite treatments. Mycoses 2011, 54, e712–e717. [Google Scholar]
- Fazly, A.; Jain, C.; Dehner, A.C.; Issi, L.; Lilly, E.A.; Ali, A.; Cao, H.; Fidel, P.L., Jr.; Rao, R.P.; Kaufman, P.D. Chemical screening identifies filastatin, a small molecule inhibitor of Candida albicans adhesion, morphogenesis, and pathogenesis. Proc. Natl. Acad. Sci. USA. 2013, 110, 13594–13599. [Google Scholar] [CrossRef] [PubMed]
- Furletti, V.F.; Teixeira, I.P.; Obando-Pereda, G.; Mardegan, R.C.; Sartoratto, A.; Figueira, G.M.; Duarte, R.M.; Rehder, V.L.; Duarte, M.C.; Hofling, J.F. Action of Coriandrum sativum L. essential oil upon oral Candida albicans biofilm formation. Evid. -Based Complement. Altern. Med. 2011, 2011, 985832. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, H.S.; Kim, K.H.; Kwon, T.Y.; Hong, S.H. Effect of garlic on bacterial biofilm formation on orthodontic wire. Angle Orthod. 2011, 81, 895–900. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Lima, R.; Alves, E.P.; Rosalen, P.L.; Ruiz, A.L.; Teixeira Duarte, M.C.; Goes, V.F.; de Medeiros, A.C.; Pereira, J.V.; Godoy, G.P.; Melo de Brito Costa, E.M. Antimicrobial and antiproliferative potential of Anadenanthera colubrina (Vell.) Brenan. Evid. -Based Complement. Altern. Med. 2014, 2014, 802696. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, A.; Gaspar, C.; Palmeira-de-Oliveira, R.; Silva-Dias, A.; Salgueiro, L.; Cavaleiro, C.; Pina-Vaz, C.; Martinez-de-Oliveira, J.; Queiroz, J.A.; Rodrigues, A.G. The anti-Candida activity of Thymbra capitata essential oil: Effect upon pre-formed biofilm. J. Ethnopharmacol. 2012, 140, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Shinde, R.B.; Chauhan, N.M.; Karuppayil, S.M. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling 2013, 29, 87–96. [Google Scholar] [CrossRef]
- Sudjana, A.N.; Carson, C.F.; Carson, K.C.; Riley, T.V.; Hammer, K.A. Candida albicans adhesion to human epithelial cells and polystyrene and formation of biofilm is reduced by sub-inhibitory Melaleuca alternifolia (tea tree) essential oil. Med. Mycol. 2012, 50, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Traboulsi, R.S.; Mukherjee, P.K.; Chandra, J.; Salata, R.A.; Jurevic, R.; Ghannoum, M.A. Gentian violet exhibits activity against biofilms formed by oral Candida isolates obtained from HIV-infected patients. Antimicrob. Agents Chemother. 2011, 55, 3043–3045. [Google Scholar] [CrossRef]
- Villa, F.; Pitts, B.; Stewart, P.S.; Giussani, B.; Roncoroni, S.; Albanese, D.; Giordano, C.; Tunesi, M.; Cappitelli, F. Efficacy of zosteric acid sodium salt on the yeast biofilm model Candida albicans. Microb Ecol. 2011, 62, 584–598. [Google Scholar] [CrossRef]
- Basso, F.G.; Oliveira, C.F.; Fontana, A.; Kurachi, C.; Bagnato, V.S.; Spolidorio, D.M.; Hebling, J.; de Souza Costa, C.A. In vitro effect of low-level laser therapy on typical oral microbial biofilms. Braz. Dent J. 2011, 22, 502–510. [Google Scholar] [CrossRef]
- Chabrier-Rosello, Y.; Foster, T.H.; Perez-Nazario, N.; Mitra, S.; Haidaris, C.G. Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob. Agents Chemother. 2005, 49, 4288–4295. [Google Scholar] [PubMed]
- Lopes, M.; Alves, C.T.; Rama Raju, B.; Goncalves, M.S.; Coutinho, P.J.; Henriques, M.; Belo, I. Application of benzo[a]phenoxazinium chlorides in antimicrobial photodynamic therapy of Candida albicans biofilms. J. Photochem. Photobiol. B 2014, 141, 93–99. [Google Scholar]
- Machado-de-Sena, R.M.; Correa, L.; Kato, I.T.; Prates, R.A.; Senna, A.M.; Santos, C.C.; Picanco, D.A.; Ribeiro, M.S. Photodynamic therapy has antifungal effect and reduces inflammatory signals in Candida albicans-induced murine vaginitis. Photodiagnosis Photodyn. Ther. 2014, 11, 275–282. [Google Scholar]
- Rossoni, R.D.; Barbosa, J.O.; de Oliveira, F.E.; de Oliveira, L.D.; Jorge, A.O.; Junqueira, J.C. Biofilms of Candida albicans serotypes A and B differ in their sensitivity to photodynamic therapy. Lasers Med. Sci. 2014, 29, 1679–1684. [Google Scholar]
- Farber, B.F.; Wolff, A.G. Salicylic acid prevents the adherence of bacteria and yeast to silastic catheters. J. Biomed. Mater. Res. 1993, 27, 599–602. [Google Scholar] [CrossRef]
- Maki, D.G.; Stolz, S.M.; Wheeler, S.; Mermel, L.A. Prevention of central venous catheter-related bloodstream infection by use of an anti-septic-impregnated catheter. A randomized, controlled trial. Ann. Intern. Med. 1997, 127, 257–266. [Google Scholar] [PubMed]
- Raad, I.; Darouiche, R.; Hachem, R.; Sacilowski, M.; Bodey, G.P. Antibiotics and prevention of microbial colonization of catheters. Antimicrob. Agents Chemother. 1995, 39, 2397–2400. [Google Scholar] [CrossRef]
- Zhou, L.; Tong, Z.; Wu, G.; Feng, Z.; Bai, S.; Dong, Y.; Ni, L.; Zhao, Y. Parylene coating hinders Candida albicans adhesion to silicone elastomers and denture bases resin. Arch Oral Biol. 2010, 55, 401–409. [Google Scholar] [PubMed]
- Elmer, G.W.; Surawicz, C.M.; McFarland, L.V. Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA 1996, 275, 870–876. [Google Scholar]
- Hatakka, K.; Ahola, A.J.; Yli-Knuuttila, H.; Richardson, M.; Poussa, T.; Meurman, J.H.; Korpela, R. Probiotics reduce the prevalence of oral candida in the elderly--a randomized controlled trial. J. Dent. Res. 2007, 86, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal Peptides: To Be or Not to Be Membrane Active. Biochimie 2016, 130, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Reassessing the host defense peptide landscape. Front. Chem. 2019, 7, 43. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide Antimicrobial Agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Aoki, W.; Ueda, M. Characterization of Antimicrobial Peptides toward the Development of Novel Antibiotics. Pharmaceuticals 2013, 6, 1055–1081. [Google Scholar] [CrossRef]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E.W. Multifunctional Cationic Host Defence Peptides and Their Clinical Applications. Cell. Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lesiuk, M.; Paduszyńska, M.; Greber, K.E. Synthetic Antimicrobial Immunomodulatory Peptides: Ongoing Studies and Clinical Trials. Antibiotics 2022, 11, 1062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anumudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent Advances in the Application of the Antimicrobial Peptide Nisin in the Inactivation of Spore-Forming Bacteria in Foods. Molecules 2021, 26, 5552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zerfas, B.L.; Joo, Y.; Gao, J. Gramicidin A Mutants with Antibiotic Activity against Both Gram-Positive and Gram-Negative Bacteria. ChemMedChem 2016, 11, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Li, J.; Cars, O.; Couet, W.; Dudley, M.N.; Kaye, K.S.; Mouton, J.W.; Paterson, D.L.; Tam, V.H.; Theuretzbacher, U.; et al. Framework for optimisation of the clinical use of colistin and polymyxin B: The Prato polymyxin consensus. Lancet Infect. Dis. 2015, 15, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjya, S.; Zhang, Z.; Ramamoorthy, A. LL-37: Structures, Antimicrobial Activity, and Influence on Amyloid-Related Diseases. Biomolecules 2024, 14, 320. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Sun, C.; Xu, N.; Liu, W. The current landscape of the antimicrobial peptide melittin and its therapeutic potential. Front. Immunol. 2024, 15, 1326033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vértesy, L.; Ehlers, E.; Kogler, H.; Kurz, M.; Meiwes, J.; Seibert, G.; Vogel, M.; Hammann, P. Friulimicins: Novel lipopeptide antibiotics with peptidoglycan synthesis inhibiting activity from Actinoplanes friuliensis sp. nov. II. Isolation and structural characterization. J. Antibiot. 2000, 53, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Armaganidis, A.; Frantzeskaki, F.; Diakaki, C.; Apostolopoulou, O.; Zakynthinos, S.; Ischaki, E.; Mandragos, C.; Katsenos, C.; Paraschos, M.; Patrani, M.; et al. Pharmacokinetic and efficacy analysis of murepavadin (POL7080) coadministered with standard-of-care (SOC) in a phase II study in patients with ventilator-associated pneumonia (VAP) due to suspected or documented Pseudomonas aeruginosa infection, abstr 2720. In Proceedings of the 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017. [Google Scholar]
- Domingues, M.M.; Santos, N.C.; Castanho, M.A. Antimicrobial peptide rBPI21: A translational overview from bench to clinical studies. Curr. Protein. Pept. Sci. 2012, 13, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Giles, F.J.; Redman, R.; Yazji, S.; Bellm, L. Iseganan HCl: A novel antimicrobial agent. Expert Opin. Investig. Drugs 2002, 11, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Knight-Connoni, V.; Mascio, C.; Chesnel, L.; Silverman, J. Discovery and development of surotomycin for the treatment of Clostridium difficile. J. Ind. Microbiol. Biotechnol. 2016, 43, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; MacDonald, D.L.; Holroyd, K.J.; Thornsberry, C.; Wexler, H.; Zasloff, M. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob Agents Chemother. 1999, 43, 782–788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Fedler, K.A.; Rennie, R.P.; Stevens, S.; Jones, R.N. Omiganan pentahydrochloride (MBI 226), a topical 12-amino-acid cationic peptide: Spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrob Agents Chemother. 2004, 48, 3112–3118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crowther, G.S.; Baines, S.D.; Todhunter, S.L.; Freeman, J.; Chilton, C.H.; Wilcox, M.H. Evaluation of NVB302 versus vancomycin activity in an in vitro human gut model of Clostridium difficile infection. J. Antimicrob. Chemother. 2013, 68, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Huang, J.A. The Antibacterial Effects of Antimicrobial Peptides OP-145 against Clinically Isolated Multi-Resistant Strains. Jpn. J. Infect. Dis. 2017, 70, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Woong, S.J.; Xuewei, S.L.; Sun, J.N.; Edgerton, M. The P-113 fragment of histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob. Agents Chemother. 2008, 52, 497–504. [Google Scholar] [CrossRef]
- Isaksson, J.; Brandsdal, B.O.; Engqvist, M.; Flaten, G.E.; Svendsen, J.S.M.; Stensen, W. A synthetic antimicrobial peptidomimetic (LTX 109): Stereochemical impact on membrane disruption. J. Med. Chem. 2011, 54, 5786–5795. [Google Scholar] [CrossRef] [PubMed]
- Van Groenendael, R.; Kox, M.; Van Eijk, L.T.; Pickkers, P. Immunomodulatory and kidney-protective effects of the human chorionic gonadotropin derivate EA-230. Nephron 2018, 140, 148–151. [Google Scholar] [CrossRef]
- Kudrimoti, M.; Curtis, A.; Azawi, S.; Worden, F.; Katz, S.; Adkins, D.; Bonomi, M.; Elder, J.; Sonis, S.T.; Straube, R.; et al. Dusquetide: A novel innate defense regulator demonstrating a significant consistent reduction in the duration of oral mucositis in preclinical data a randomized placebo-controlled Phase 2a clinical study. J. Biotechnol. 2016, 239, 115–125. [Google Scholar] [CrossRef]
- Nibbering, P.H.; Ravensbergen, E.; Welling, M.M.; van Berkel, L.A.; van Berkel, P.H.C.; Pauwels, E.K.J.; Nuijens, J.H. Human lactoferrin and peptides derived from Its N terminus are highly effective against infections with antibioticresistant bacteria. Infect. Immun. 2001, 69, 1469–1476. [Google Scholar] [CrossRef]
- Guo, L.; Mclean, J.S.; Yang, Y.; Eckert, R.; Kaplan, C.W.; Kyme, P.; Sheikh, O.; Varnum, B.; Lux, R.; Shi, W.; et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. USA 2015, 112, 7569–7574. [Google Scholar] [CrossRef]
- Mercer, D.K.; Robertson, J.C.; Miller, L.; Stewart, C.S.; O’Neil, D.A. NP213 (Novexatin R©): A unique therapy candidate for onychomycosis with a differentiated safety and efficacy profile. Med. Mycol. 2020, 58, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Fulco, P.; Wenzel, R.P. Ramoplanin: A topical lipoglycodepsipeptide antibacterial agent. Expert Rev. Anti. Infect. Ther. 2006, 4, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Bulger, E.M.; Maier, R.V.; Sperry, J.; Joshi, M.; Henry, S.; Moore, F.A.; Moldawer, L.L.; Demetriades, D.; Talving, P.; Schreiber, M.; et al. A novel drug for treatment of necrotizing soft-tissue infections: A randomized clinical trial. JAMA Surg. 2014, 149, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Muchintala, D.; Suresh, V.; Raju, D.; Sashidhar, R.B. Synthesis and characterization of cecropin peptide-based silver nanocomposites: Its antibacterial activity and mode of action. Mater. Sci. Eng. C 2020, 110, 110712. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS ONE 2019, 14, e0215703. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Hossain, K.R.; Chen, R.; Ho, K.K.K.; Kuppusamy, R.; Clarke, R.J.; Kumar, N.; Willcox, M.D.P. Mechanism of action of surface immobilized antimicrobial peptides against Pseudomonas aeruginosa. Front. Microbiol. 2020, 10, 3053. [Google Scholar] [CrossRef]
- Leeds, J.A.; Sachdeva, M.; Mullin, S.; Dzink-Fox, J.; LaMarche, M.J. Mechanism of action of and mechanism of reduced susceptibility to the novel anti-Clostridium difficile compound LFF571. Antimicrob. Agents Chemother. 2012, 56, 4463–4465. [Google Scholar] [CrossRef]
- Travis, S.; Yap, L.M.; Hawkey, C.; Warren, B.; Lazarov, M.; Fong, T.; Tesi, R.J. RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm. Bowel Dis. 2005, 11, 713–719. [Google Scholar] [CrossRef]
- Håkansson, J.; Ringstad, L.; Umerska, A.; Johansson, J.; Andersson, T.; Boge, L.; Rozenbaum, R.T.; Sharma, P.K.; Tollbäck, P.; Björn, C.; et al. Characterization of the in vitro, ex vivo, and in vivo efficacy of the antimicrobial peptide DPK-060 used for topical treatment. Front. Cell. Infect. Microbiol. 2019, 9, 174. [Google Scholar] [CrossRef]
- Peyrusson, F.; Butler, D.; Tulkens, P.M.; Van Bambeke, F. Cellular pharmacokinetics and intracellular activity of the novel peptide deformylase inhibitor GSK1322322 against Staphylococcus aureus laboratory and clinical strains with various resistance phenotypes: Studies with human THP-1 monocytes and J774 murine macrophages. Antimicrob. Agents Chemother. 2015, 59, 5747–5760. [Google Scholar] [CrossRef]
- Edsfeldt, S.; Holm, B.; Mahlapuu, M.; Reno, C.; Hart, D.A.; Wiig, M. PXL01 in sodium hyaluronate results in increased PRG4 expression: A potential mechanism for anti-adhesion Ups. J. Med. Sci. 2017, 122, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Hu, X.; Yuen, P.S.; Leelahavanichkul, A.; Yasuda, H.; Kim, S.M.; Schnermann, J.; Jonassen, T.E.; Frøkiaer, J.; Nielsen, S.; et al. AP214, an analogue of α-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008, 73, 1266–1274. [Google Scholar] [CrossRef]
- Mensa, B.; Howell, G.; Scott, R.; DeGrado, W. Comparative mechanistic studies of brilacidin, daptomycin, and the antimicrobial peptide LL16. Antimicrob. Agents Chemother. 2014, 58, 5136–5145. [Google Scholar] [CrossRef] [PubMed]
- Ooi, N.; Miller, K.; Hobbs, J.; Rhys-Williams, W.; Love, W.; Chopra, I. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J. Antimicrob. Chemother. 2009, 64, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Csato, M.; Kenderessy, A.S.; Dobozy, A. Enhancement of Candida albicans killing activity of separated human epidermal cells by α-melanocyte stimulating hormone. Br. J. Dermatol. 1989, 121, 145–147. [Google Scholar] [CrossRef]
- Gualillo, O.; Lago, F.; Gómez-Reino, J.; Casanueva, F.F.; Dieguez, C. Ghrelin, a widespread hormone: Insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 2003, 552, 105–109. [Google Scholar] [CrossRef]
- Itoh, H.; Tokumoto, K.; Kaji, T.; Paudel, A.; Panthee, S.; Hamamoto, H.; Sekimiza, K.; Inoue, M. Total synthesis biological mode of action of WAP-8294A2: Amenaquinone-targeting antibiotic. J. Org. Chem. 2017, 83, 6924–6935. [Google Scholar] [CrossRef]
- Miyake, O.; Ochiai, A.; Hashimoto, W.; Murata, K. Origin diversity of alginate lyases of families PL-5 and -7 in Sphingomonas sp strain A1. J. Bacteriol. 2004, 186, 2891–2896. [Google Scholar] [CrossRef]
- Yu, H.B.; Kielczewska, A.; Rozek, A.; Takenaka, S.; Li, Y.; Thorson, L.; Hancock, R.E.W.; Guarna, M.M.; North, J.R.; Foster, L.J.; et al. Sequestosome-1/p62 is the key intracellular target of innate defense regulator peptide. J. Biol. Chem. 2009, 284, 36007–36011. [Google Scholar] [CrossRef]
- Nilsson, E.; Björn, C.; Sjöstrand, V.; Lindgren, K.; Münnich, M.; Mattsby-Baltzer, I.; Ivarsson, M.L.; Olmarker, K.; Mahlapuu, M. A novel polypeptide derived from human lactoferrin in sodium hyaluronate prevents postsurgical adhesion formation in the rat. Ann. Surg. 2009, 250, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.T.; Anderson, M.A. Defensins–components of the innate immune system in plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.M.; François, I.E.; Cammue, B.P.; Thevissen, K. The mode of antifungal action of plant, insect and human defensins. Cell. Mol. Life Sci. 2008, 65, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Osborn, R.W.; De Samblanx, G.W.; Thevissen, K.; Goderis, I.; Torrekens, S.; Van Leuven, F.; Attenborough, S.; Rees, S.B.; Broekaert, W.F. Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett. 1995, 368, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.S.; Cabral, K.M.; Zingali, R.B.; Kurtenbach, E. Characterization of two novel defense peptides from pea (Pisum sativum) seeds. Arch. Biochem. Biophys. 2000, 378, 278–286. [Google Scholar] [CrossRef]
- Thevissen, K.; Kristensen, H.H.; Thomma, B.P.; Cammue, B.P.; François, I.E. Therapeutic potential of antifungal plant and insect defensins. Drug Discov. Today. 2007, 12, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Mello, E.O.; Ribeiro, S.F.; Carvalho, A.O.; Santos, I.S.; Da Cunha, M.; Santa-Catarina, C.; Gomes, V.M. Antifungal activity of PvD1 defensin involves plasma membrane permeabilization, inhibition of medium acidification, and induction of ROS in fungi cells. Curr. Microbiol. 2011, 62, 1209–1217. [Google Scholar] [CrossRef]
- Tavares, P.M.; Thevissen, K.; Cammue, B.P.; François, I.E.; Barreto-Bergter, E.; Taborda, C.P.; Marques, A.F.; Rodrigues, M.L.; Nimrichter, L. In vitro activity of the antifungal plant defensin RsAFP2 against Candida isolates and its in vivo efficacy in prophylactic murine models of candidiasis. Antimicrob. Agents Chemother. 2008, 52, 4522–4525. [Google Scholar] [CrossRef]
- Schneider, J.J.; Unholzer, A.; Schaller, M.; Schäfer-Korting, M.; Korting, H.C. Human defensins. J. Mol. Med. 2005, 83, 587–595. [Google Scholar] [CrossRef]
- Weinberg, A.; Krisanaprakornkit, S.; Dale, B.A. Epithelial antimicrobial peptides: Review and significance for oral applications. Crit. Rev. Oral Biol. Med. 1998, 9, 399–414. [Google Scholar] [CrossRef]
- Mathews, M.; Jia, H.P.; Guthmiller, J.M.; Losh, G.; Graham, S.; Johnson, G.K.; Tack, B.F.; McCray, P.B., Jr. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect. Immun. 1999, 67, 2740–2745. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schröder, J.M. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- Joly, S.; Maze, C.; McCray, P.B.; Guthmiller, J.M., Jr. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J. Clin. Microbiol. 2004, 42, 1024–1029. [Google Scholar] [CrossRef]
- Feng, Z.; Jiang, B.; Chandra, J.; Ghannoum, M.; Nelson, S.; Weinberg, A. Human beta-defensins: Differential activity against candidal species and regulation by Candida albicans. J. Dent. Res. 2005, 84, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Li, X.S.; Berner, J.C.; Edgerton, M. Distinct antifungal mechanisms: Beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob. Agents Chemother. 2006, 50, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Nayyar, N.; Li, W.; Edgerton, M. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob. Agents Chemother. 2007, 51, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Wu, Z.; Nuding, S.; Groscurth, S.; Marcinowski, M.; Beisner, J.; Buchner, J.; Schaller, M.; Stange, E.F.; Wehkamp, J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 2011, 469, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.I.; Daffre, S., Jr.; Bulet, P. Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the tachyplesin family. J. Biol. Chem. 2000, 275, 33464–33470. [Google Scholar] [CrossRef]
- Fukuzawa, A.H.; Vellutini, B.C.; Lorenzini, D.M.; Silva, P.I.; Mortara, R.A., Jr.; da Silva, J.M.; Daffre, S. The role of hemocytes in the immunity of the spider Acanthoscurria gomesiana. Dev. Comp. Immunol. 2008, 32, 716–725. [Google Scholar] [CrossRef]
- Barbosa, F.M.; Daffre, S.; Maldonado, R.A.; Miranda, A.; Nimrichter, L.; Rodrigues, M.L. Gomesin, a peptide produced by the spider Acanthoscurria gomesiana, is a potent anticryptococcal agent that acts in synergism with fluconazole. FEMS Microbiol. Lett. 2007, 274, 279–286. [Google Scholar] [CrossRef]
- Troeira Henriques, S.; Lawrence, N.; Chaousis, S.; Ravipati, A.S.; Cheneval, O.; Benfield, A.H.; Elliott, A.G.; Kavanagh, A.M.; Cooper, M.A.; Chan, L.Y.; et al. Redesigned spider peptide with improved antimicrobial and anticancer properties. ACS Chem. Biol. 2017, 12, 2324–2334. [Google Scholar] [CrossRef]
- Moreira, C.K.; Rodrigues, F.G.; Ghosh, A.; Varotti Fde, P.; Miranda, A.; Daffre, S.; Jacobs-Lorena, M.; Moreira, L.A. Effect of the antimicrobial peptide gomesin against different life stages of Plasmodium spp. Exp. Parasitol. 2007, 116, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.G.; Dobroff, A.S.; Cavarsan, C.F.; Paschoalin, T.; Nimrichter, L.; Mortara, R.A.; Santos, E.L.; Fázio, M.A.; Miranda, A.; Daffre, S.; et al. Effective topical treatment of subcutaneous murine B16F10-Nex2 melanoma by the antimicrobial peptide gomesin. Neoplasia 2008, 10, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Soletti, R.C.; del Barrio, L.; Daffre, S.; Miranda, A.; Borges, H.L.; Moura-Neto, V.; Lopez, M.G.; Gabilan, N.H. Peptide gomesin triggers cell death through L-type channel calcium influx, MAPK/ERK, PKC and PI3K signaling and generation of reactive oxygen species. Chem. Biol. Interact. 2010, 186, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.C.; Muñoz, J.E.; Carvalho, D.D.; Belmonte, R.; Faintuch, B.; Borelli, P.; Miranda, A.; Taborda, C.P.; Daffre, S. Therapeutic use of a cationic antimicrobial peptide from the spider Acanthoscurria gomesiana in the control of experimental candidiasis. BMC Microbiol. 2012, 12, 28. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef]
- Tóth, L.; Kele, Z.; Borics, A.; Nagy, L.G.; Váradi, G.; Virágh, M.; Takó, M.; Vágvölgyi, C.; Galgóczy, L. NFAP2, a novel cysteine-rich anti-yeast protein from Neosartorya fischeri NRRL 181: Isolation and characterization. AMB Express. 2016, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Kovács, L.; Virágh, M.; Takó, M.; Papp, T.; Vágvölgyi, C.; Galgóczy, L. Isolation and characterization of Neosartorya fischeri antifungal protein (NFAP). Peptides 2011, 32, 1724–1731. [Google Scholar] [CrossRef]
- Amann, V.; Kissmann, A.K.; Krämer, M.; Krebs, I.; Perez-Erviti, J.A.; Otero-Gonzalez, A.J.; Morales-Vicente, F.; Rodríguez, A.; Ständker, L.; Weil, T.; et al. Increased Activities against Biofilms of the Pathogenic Yeast Candida albicans of Optimized Pom-1 Derivatives. Pharmaceutics 2022, 14, 318. [Google Scholar] [CrossRef]
- Amann, V.; Kissmann, A.K.; Mildenberger, V.; Krebs, I.; Perez-Erviti, J.A.; Martell-Huguet, E.M.; Otero-Gonzalez, A.J.; Morales-Vicente, F.; Rodríguez-Castaño, G.P.; Firacative, C.; et al. Cm-p5 Peptide Dimers Inhibit Biofilms of Candida albicans Clinical Isolates, C. parapsilosis and Fluconazole-Resistant Mutants of C. auris. Int. J. Mol. Sci. 2023, 24, 9788. [Google Scholar] [CrossRef]
- Bhosale, V.B.; Koparde, A.A.; Thorat, V.M. Vulvovaginal candidiasis-an overview of current trends and the latest treatment strategies. Microb. Pathog. 2025, 200, 107359. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Garcia, M.; Rodriguez, A.; Alba, A.; Vazquez, A.A.; Morales Vicente, F.E.; Perez-Erviti, J.; Spellerberg, B.; Stenger, S.; Grieshober, M.; Conzelmann, C.; et al. New Antibacterial Peptides from the Freshwater Mollusk Pomacea poeyana (Pilsbry, 1927). Biomolecules 2020, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Häring, M.; Amann, V.; Kissmann, A.K.; Herberger, T.; Synatschke, C.; Kirsch-Pietz, N.; Perez-Erviti, J.A.; Otero-Gonzalez, A.J.; Morales-Vicente, F.; Andersson, J.; et al. Combination of Six Individual Derivatives of the Pom-1 Antibiofilm Peptide Doubles Their Efficacy against Invasive and Multi-Resistant Clinical Isolates of the Pathogenic Yeast Candida albicans. Pharmaceutics 2022, 14, 1332. [Google Scholar] [CrossRef]
- Kissmann, A.-K.; Mildenberger, V.; Krämer, M.; Alpízar-Pedraza, D.; Martell-Huguet, E.M.; Perez-Erviti, J.A.; Cetinkaya, A.; Pietrasik, J.; Otero-Gonzalez, A.J.; Firacative, C.; et al. Anti-biofilm peptides can rescue fluconazole and amphotericin B efficacies against Candida albicans. Res. Sq. 2024. [Google Scholar] [CrossRef]
- López-Abarrategui, C.; McBeth, C.; Mandal, S.M.; Sun, Z.J.; Heffron, G.; Alba-Menéndez, A.; Migliolo, L.; Reyes-Acosta, O.; García-Villarino, M.; Nolasco, D.O.; et al. Cm-P5: An Antifungal Hydrophilic Peptide Derived from the Coastal Mollusk Cenchritis muricatus (Gastropoda: Littorinidae). FASEB J. 2015, 29, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, M.; Bertrand, B.; Martell-Huguet, E.M.; Espinosa-Romero, J.F.; Vázquez, R.F.; Morales-Vicente, F.; Rosenau, F.; Standker, L.H.; Franco, O.L.; Otero-Gonzalez, A.J.; et al. Cm-p5, a molluscan-derived antifungal peptide exerts its activity by a membrane surface covering in a non-penetrating mode. Peptides 2024, 182, 171313. [Google Scholar] [CrossRef] [PubMed]
- Kubiczek, D.; Flaig, C.; Raber, H.; Dietz, S.; Kissmann, A.K.; Heerde, T.; Bodenberger, N.; Wittgens, A.; González-Garcia, M.; Kang, F.; et al. A Cerberus-Inspired Anti-Infective Multicomponent Gatekeeper Hydrogel against Infections with the Emerging “Superbug” Yeast Candida auris. Macromol. Biosci. 2020, 20, 2000005. [Google Scholar] [CrossRef]
- González-García, M.; Morales-Vicente, F.; Pico, E.D.; Garay, H.; Rivera, D.G.; Grieshober, M.; Olari, L.R.; Groß, R.; Conzelmann, C.; Krüger, F.; et al. Antimicrobial Activity of Cyclic-Monomeric and Dimeric Derivatives of the Snail-Derived Peptide Cm-P5 against Viral and Multidrug-Resistant Bacterial Strains. Biomolecules 2021, 11, 745. [Google Scholar] [CrossRef]
- Taheri-Araghi, S. Synergistic action of antimicrobial peptides and antibiotics: Current understanding and future directions. Front. Microbiol. 2024, 15, 1390765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mhlongo, J.T.; Waddad, A.Y.; Albericio, F.; de la Torre, B.G. Antimicrobial Peptide Synergies for Fighting Infectious Diseases. Adv. Sci. 2023, 10, e2300472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.; Kim, J.S.; Bai, X.; Zhang, J.; Park, M.; Lee, U.; Lee, J.; Bahn, Y.S.; Xu, Y.; Ha, N.C. Development of Miniprotein-Type Inhibitors of Biofilm Formation in Candida albicans and Candida auris. J. Microbiol. Biotechnol. 2025, 35, e2411076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, Y.; Yin, K.; Zhang, J.; Du, L.; Wang, S.; Zheng, D.; Li, R. ML-AMPs designed through machine learning show antifungal activity against C. albicans and therapeutic potential on mice model with candidiasis. Life Sci. 2025, 366, 123485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, M.; Liu, F.; Liang, Z.; Hong, R.; Dong, Y.; Luan, H.; Fu, X.; Yuan, W.; Fang, W.; et al. Artificial intelligence using a latent diffusion model enables the generation of diverse and potent antimicrobial peptides. Sci. Adv. 2025, 11, eadp7171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).