Type I Arabinogalactan and Methyl-Esterified Homogalacturonan Polysaccharides from Tamarillo (Solanum betaceum cav.) Fruit Pulp Ameliorate DSS-Induced Ulcerative Colitis
Abstract
1. Introduction
2. Results
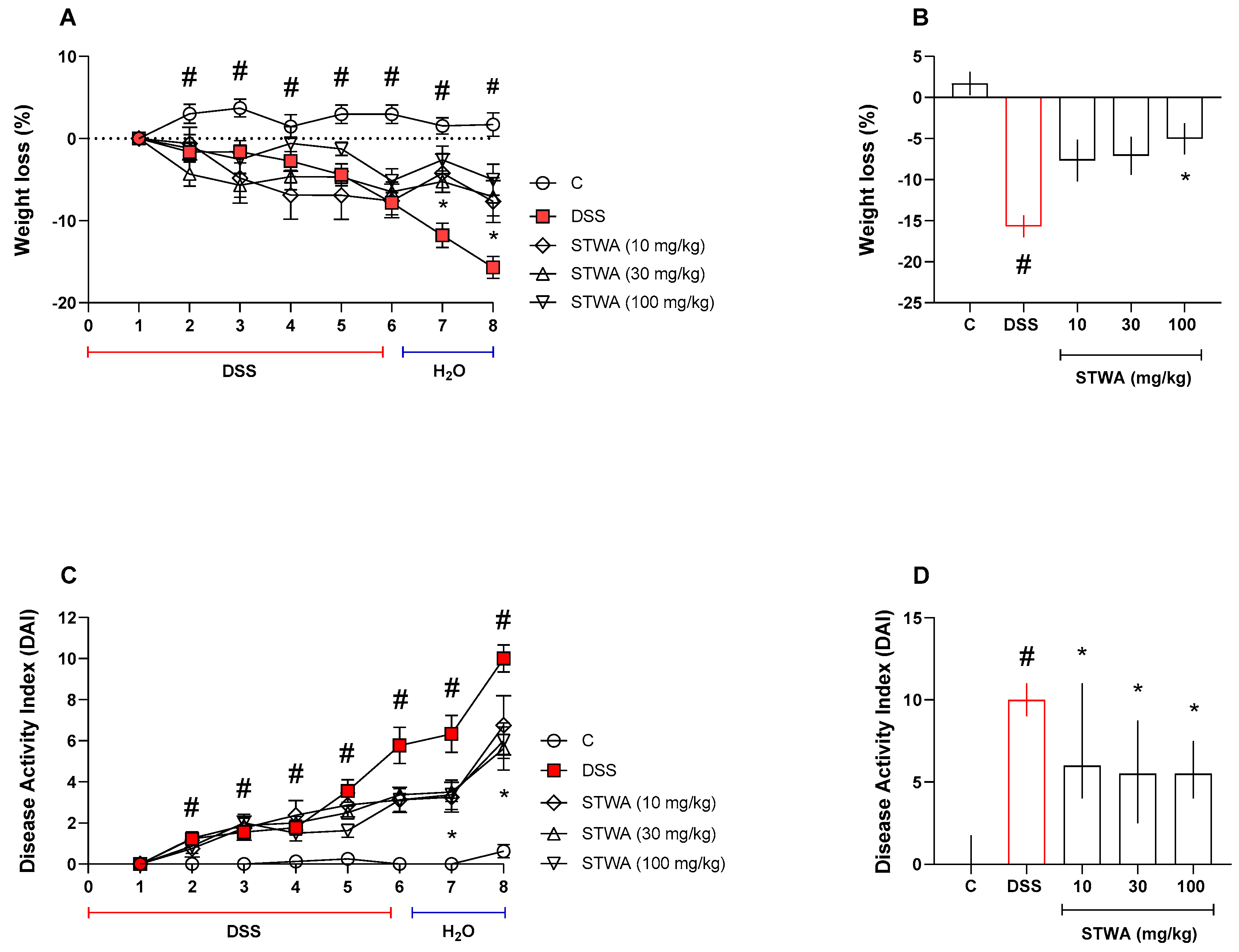
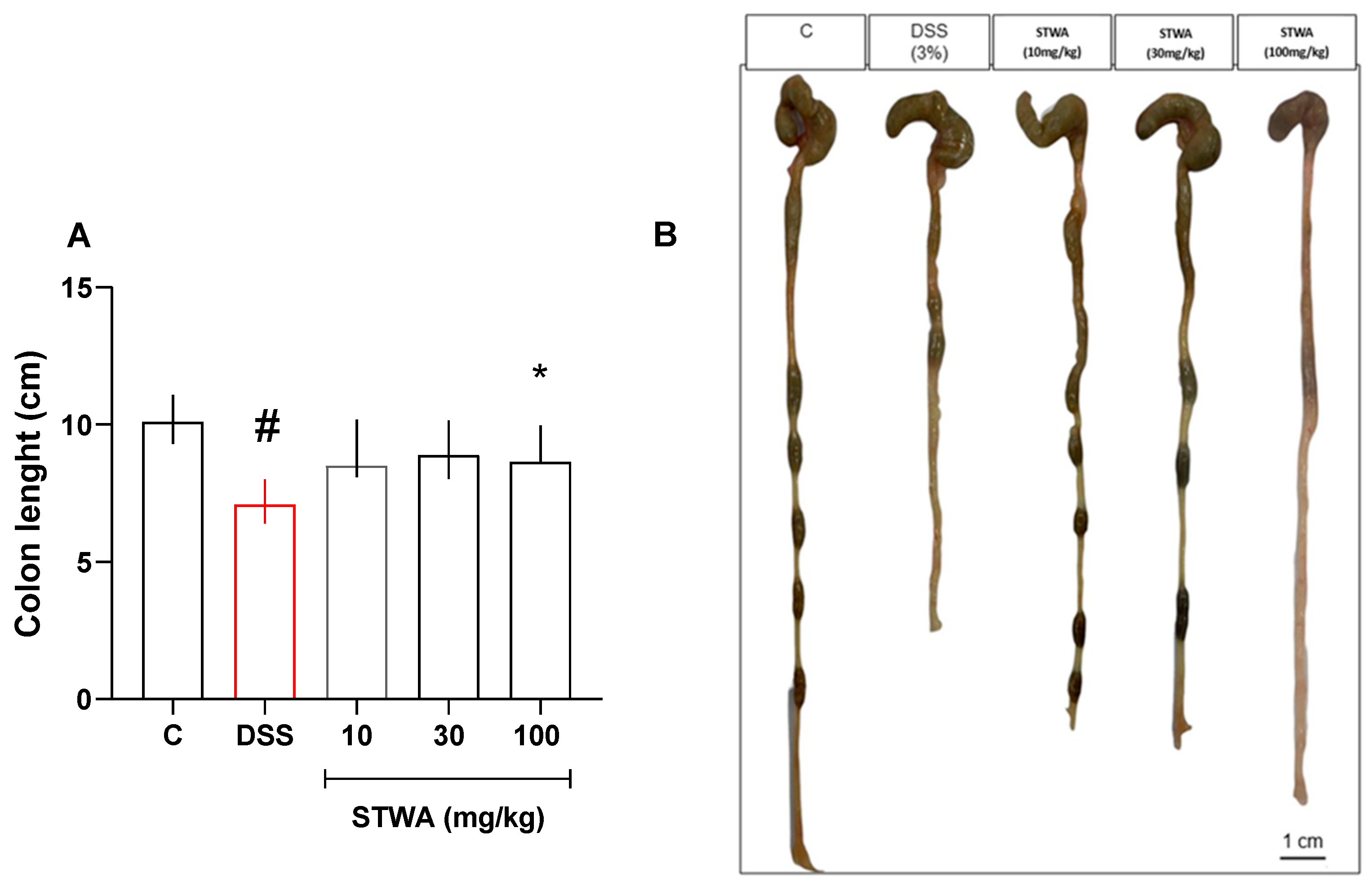
2.1. Treatment with STWA Attenuates DSS-Induced Colitis
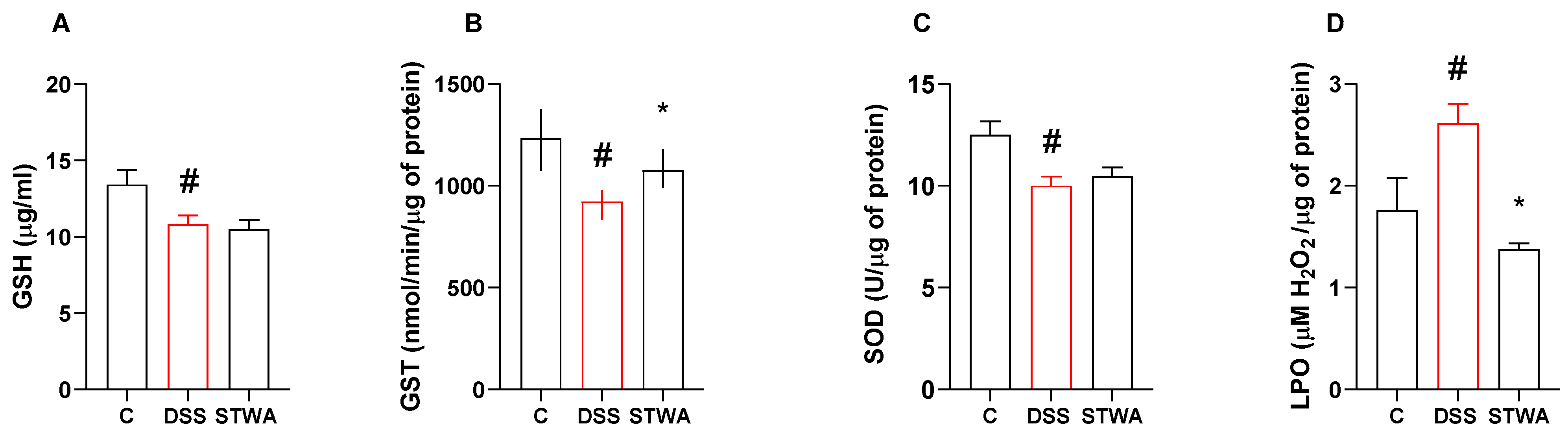
2.2. STWA Reduces Oxidative Stress
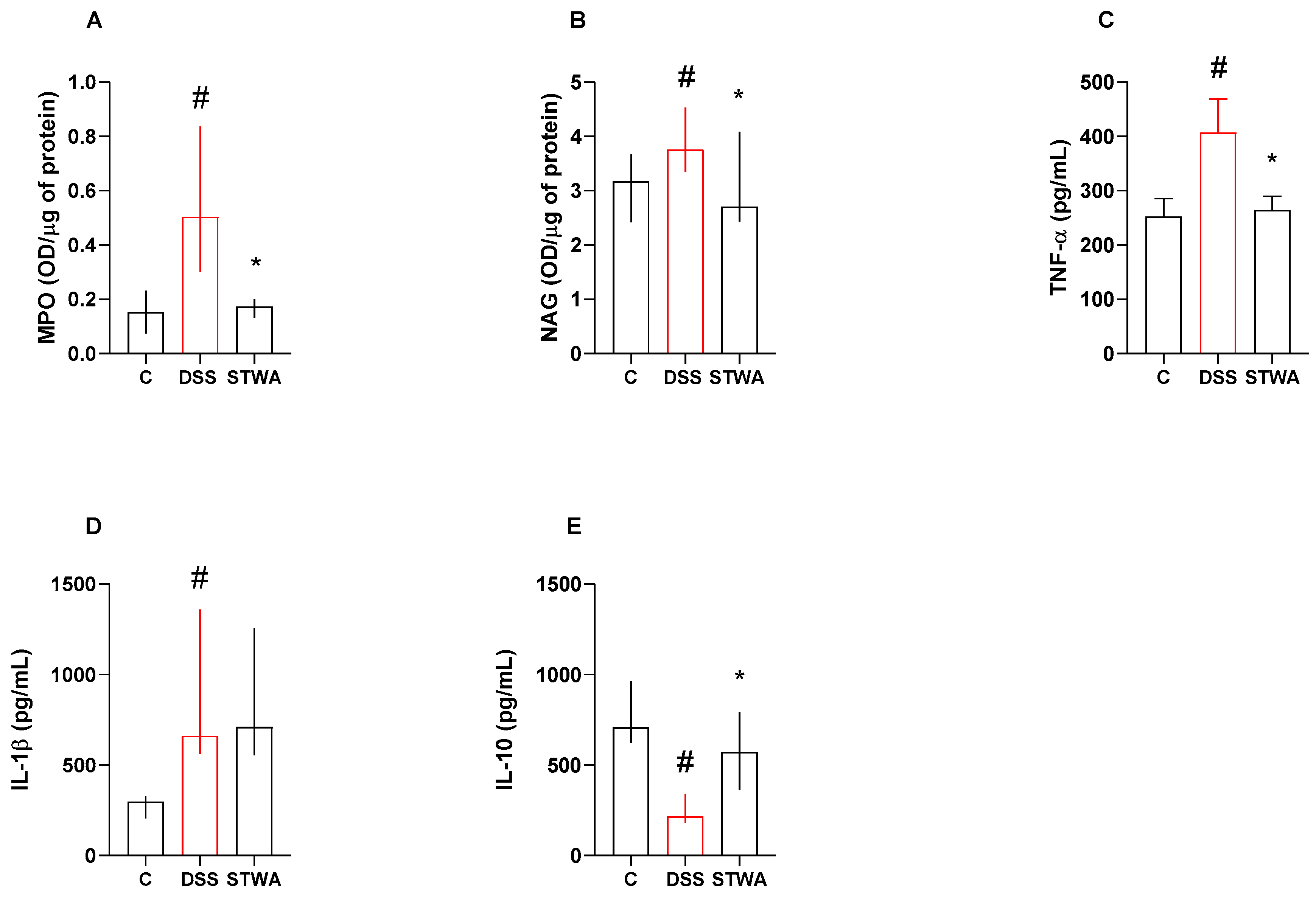
2.3. Effect of STWA on Inflammatory Parameters
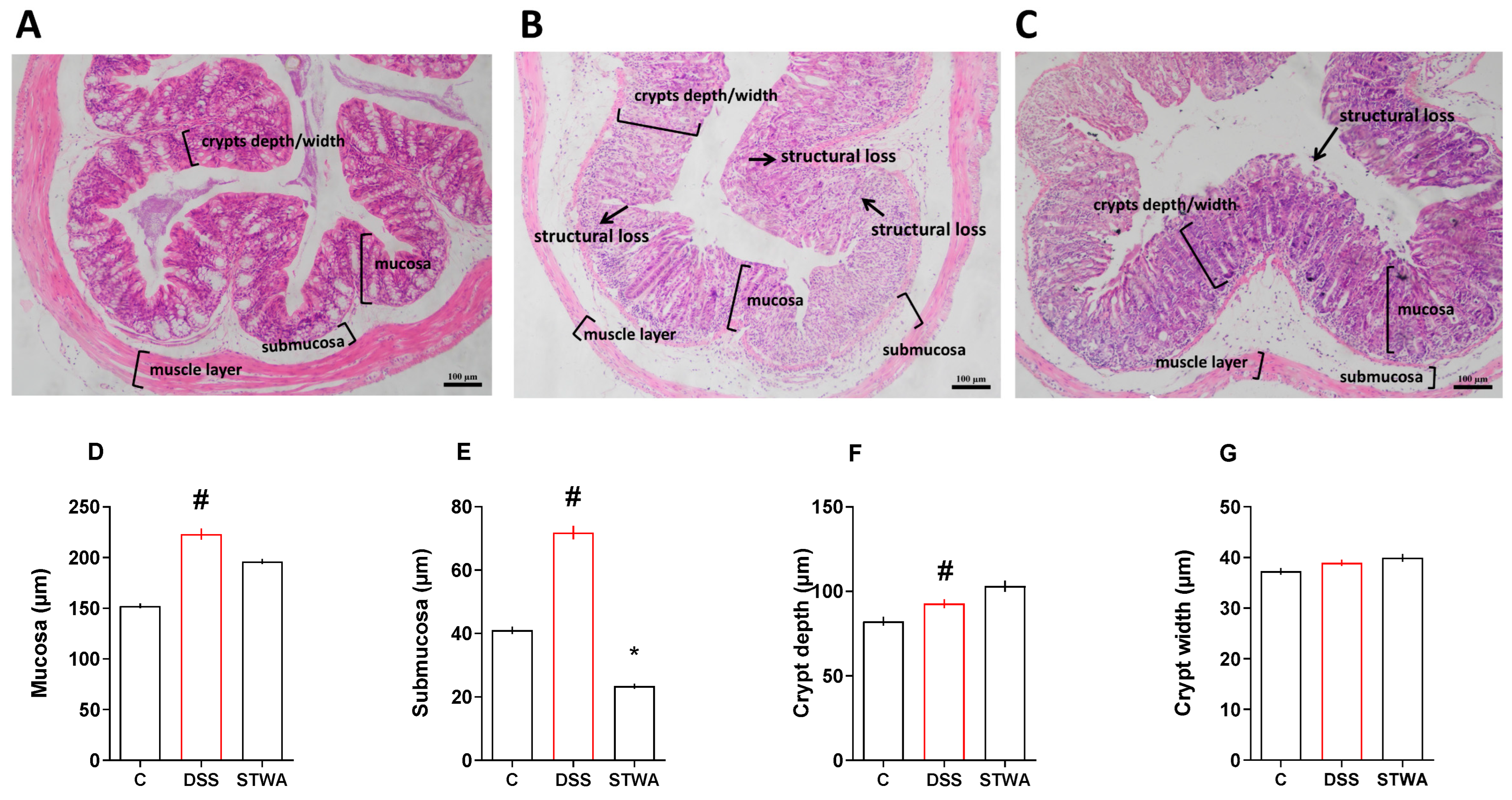
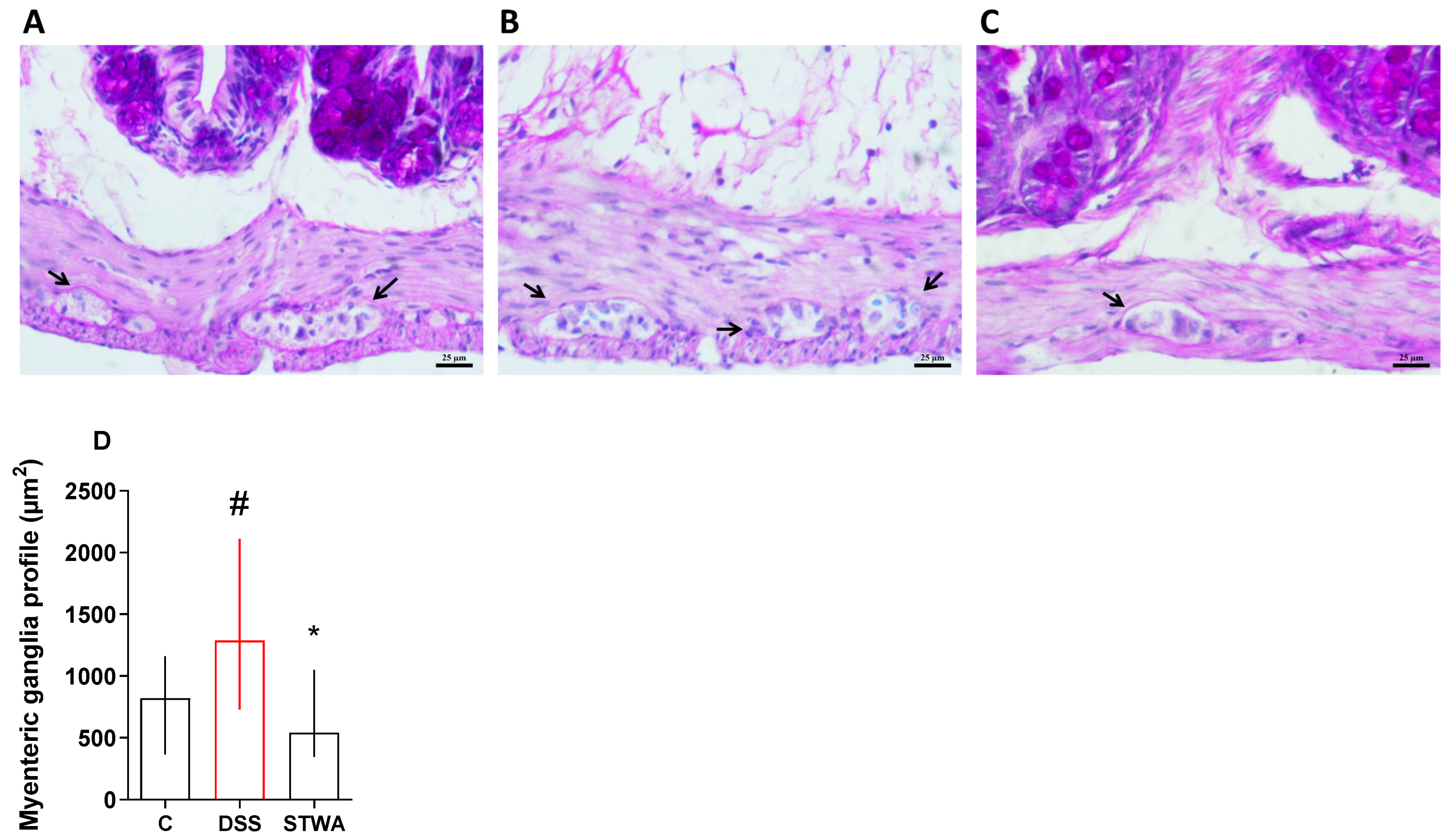
2.4. Effect of STWA on Histological Parameters
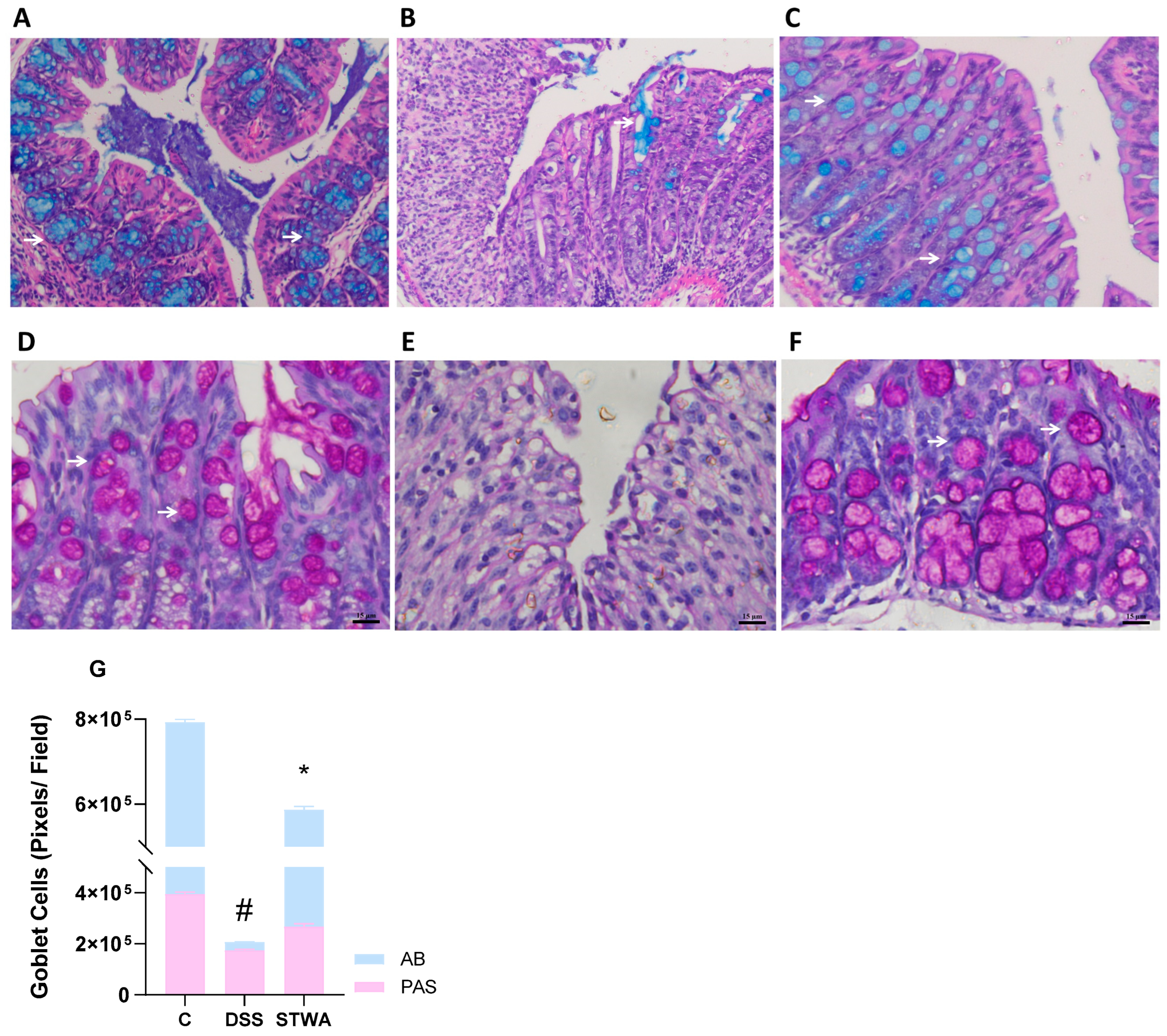
2.5. PAS and Alcian Blue Staining Show Mucosal Recovery with STWA
3. Discussion
4. Materials and Methods
4.1. Pectic Polysaccharides from Tamarillo Fruit Pulp
4.2. Animals
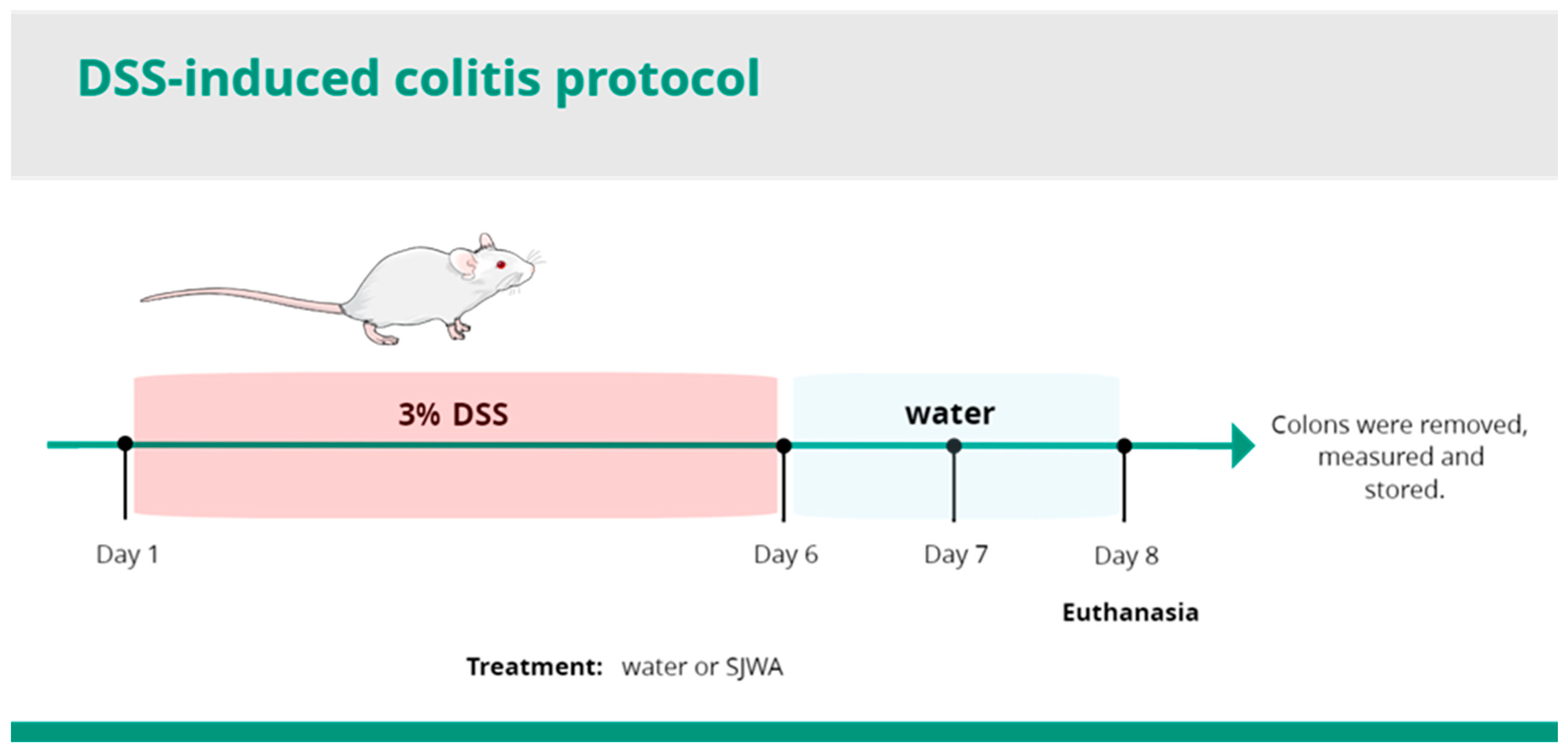
4.3. Induction and Evaluation of DSS Ulcerative Colitis
4.4. Pharmacological Treatment and DAI Measurement
4.5. Measurement of Occult Blood
4.6. Preparation of the Tissue
4.7. Quantification of the Proteins
4.8. Determination of the GSH Content
4.9. Determination of GST Activity
4.10. Determination of SOD Activity
4.11. Determination of LPO Levels
4.12. Quantification of MPO and NAG
4.13. Evaluation of the Cytokine Levels
4.14. Histological Examination
4.15. Histopathological Score
4.16. Quantification of Goblet Cells
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muzammil, M.A.; Fariha, F.N.U.; Patel, T.; Sohail, R.; Kumar, M.; Khan, E.; Khanam, B.; Kumar, S.; Khatri, M.; Varrassi, G.; et al. Advancements in Inflammatory Bowel Disease: A Narrative Eview of Diagnostics, Management, Epidemiology, Prevalence, Patient Outcomes, Quality of Life, and Clinical Presentation. Cureus 2023, 15, E41120. [Google Scholar] [PubMed]
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD—Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar]
- do Carmo Carvalho, L.; da Silva, E.S.; Roma, A.L.M.; Lauriano, J.E.G.; dos Reis, S.C.; Costa, F.V.S.; de Carvalho Bezerra, R.O.; Rocha, M.F.Q.; Mariano Filho, V.Q.; de Sousa Machado, L.C. Doenças Inflamatórias Intestinais: Uma Abordagem Geral. Rev. Eletrônica Acervo Médico 2022, 2, E9650. [Google Scholar]
- Liu, D.; Saikam, V.; Skrada, K.A.; Merlin, D.; Iyer, S.S. Inflammatory Bowel Disease Biomarkers. Med. Res. Rev. 2022, 42, 1856–1887. [Google Scholar]
- Higashiyama, M.; Hokari, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2023, 104, 74–81. [Google Scholar]
- Jeruc, J. Histomorphological Diagnosis of Ulcerative Colitis and Associated Conditions. In Ulcerative Colitis—Etiology, Diagnosis, Diet, Special Populations, and The Role of Interventional Endoscopy; IntechOpen: London, UK, 2022. [Google Scholar]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative Colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Hagel, S.; Bruns, T. Adverse Effects of Biologics Used for Treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 167–182. [Google Scholar] [PubMed]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Sharara, A.I.; Su, C.; Modesto, I.; Mundayat, R.; Gunay, L.M.; Salese, L.; Sands, B.E. Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 591–601.E8. [Google Scholar]
- Parigi, T.L.; Solitano, V.; Peyrin-Biroulet, L.; Danese, S. Do Jak Inhibitors Have a Realistic Future in Treating Crohn’s Disease? Expert Rev. Clin. Immunol. 2022, 18, 181–183. [Google Scholar]
- Herrador-López, M.; Martín-Masot, R.; Navas-López, V.M. Dietary Interventions in Ulcerative Colitis: A Systematic Review of the Evidence with Meta-Analysis. Nutrients 2023, 15, 4194. [Google Scholar] [CrossRef]
- Popov, S.V.; Markov, P.A.; Nikitina, I.R.; Petrishev, S.; Smirnov, V.; Ovodov, Y.S. Preventive effect of a pectic polysaccharide of the common cranberry Vaccinium oxycoccos L. on acetic acid-induced colitis in mice. World J. Gastroenterol. 2006, 12, 6646–6651. [Google Scholar]
- Cao, C.; Zhu, B.; Liu, Z.; Wang, X.; Ai, C.; Gong, G.; Hu, M.; Huang, L.; Song, S. An arabinogalactan from Lycium barbarum attenuates DSS-induced chronic colitis in C57BL/6J mice associated with the modulation of intestinal barrier function and gut microbiota. Food Funct. 2021, 12, 9829–9843. [Google Scholar]
- Zhao, N.; Liu, C.; Li, N.; Zhou, S.; Guo, Y.; Yang, S.; Liu, H. Role of Interleukin-22 in ulcerative colitis. Biomed. Pharmacother. 2023, 159, 114273. [Google Scholar]
- Zhang, S.; Ding, C.; Liu, X.; Zhao, Y.; Li, S.; Ding, Q.; Zhao, T.; Ma, S.; Li, W.; Liu, W. New resource food-arabinogalactan improves DSS-induced acute colitis through intestinal flora and NLRP3 signaling pathway. Int. J. Biol. Macromol. 2024, 258, 129118. [Google Scholar]
- Zhang, Y.; Ji, W.; Qin, H.; Chen, Z.; Zhou, Y.; Zhou, Z.; Wang, J.; Wang, K. Astragalus polysaccharides alleviate DSS-induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner. Carbohydr. Polym. 2025, 349, 122829. [Google Scholar] [PubMed]
- Osorio, C.; Hurtado, N.; Dawid, C.; Hofmann, T.; Heredia-Mira, F.J.; Morales, A.L. Chemical Characterisation of Anthocyanins in Tamarillo (Solanum betaceum Cav.) and Andes Berry (Rubus glaucus Benth.) Fruits. Food Chem. 2012, 132, 1915–1921. [Google Scholar]
- Do Nascimento, G.E.; Corso, C.R.; De Paula Werner, M.F.; Baggio, C.H.; Iacomini, M.; Cordeiro, L.M. Structure of an Arabinogalactan from the Edible Tropical Fruit Tamarillo (Solanum betaceum) and Its Antinociceptive Activity. Carbohydr. Polym. 2015, 116, 300–306. [Google Scholar]
- Cao, C.; Wang, L.; Ai, C.; Gong, G.; Wang, Z.; Huang, L.; Song, S.; Zhu, B. Impact of Lycium barbarum arabinogalactan on the fecal metabolome in a DSS-induced chronic colitis mouse model. Food Funct. 2022, 13, 8703–8716. [Google Scholar] [PubMed]
- Wang, Q.; Wang, Z.; Song, J.; Xu, K.; Tian, W.; Cai, X.; Mo, J.; Cao, Y.; Xiao, J. Homogalacturonan enriched pectin based hydrogel enhances 6-gingerol’s colitis alleviation effect via NF-κB/NLRP3 axis. Int. J. Biol. Macromol. 2023, 245, 125282. [Google Scholar]
- Gong, X.; Cai, W.; Yang, D.; Wang, W.; Che, H.; Li, H. Effect of the arabinogalactan from Ixeris chinensis (Thunb.) Nakai. Attenuates DSS-induced colitis and accompanying depression-like behavior. Int. J. Biol. Macromol. 2025, 286, 138525. [Google Scholar]
- Chen, J.; Mei, M.-S.; Yu, Y.; Zhao, Y.; Gong, H.; Chen, W.; Qiu, B.; Shi, S.; Dilixiati, M.; Wang, S.; et al. Elegant approach to intervention of homogalacturonan from the fruits of Ficus pumila L. in colitis: Unraveling the role of methyl esters and acetyl groups. Int. J. Biol. Macromol. 2024, 283 Pt 2, 137793. [Google Scholar]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.-F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 2018, 154, 1037–1046.e2. [Google Scholar]
- Batista, J.A.; Magalhães, D.d.A.; Sousa, S.G.; Ferreira, J.d.S.; Pereira, C.M.C.; Lima, J.V.D.N.; de Albuquerque, I.F.; Bezerra, N.L.S.D.; de Brito, T.V.; Monteiro, C.E.d.S.; et al. Polysaccharides Derived from Morinda citrifolia Linn Reduce Inflammatory Markers During Experimental Colitis. J. Ethnopharmacol. 2020, 248, 112303. [Google Scholar]
- Guo, X.; Liu, L.; Zhao, W.; Li, X.; Wang, X.; Ning, A.; Cao, J.; Zhang, W.; Cao, L.; Zhong, M. The protective effect of Schisandra chinensis (Turcz.) Baill. polysaccharide on DSS-induced ulcerative colitis in mice via the modulation of gut microbiota and inhibition of NF-κB activation. J. Sci. Food Agric. 2024, 104, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Maria-Ferreira, D.; Nascimento, A.M.; Cipriani, T.R.; Santana-Filho, A.P.; Watanabe, P.d.S.; Sant’ana, D.d.M.G.; Luciano, F.B.; Bocate, K.C.P.; Wijngaard, R.M.v.D.; Werner, M.F.d.P.; et al. Rhamnogalacturonan, a chemically-defined polysaccharide, improves intestinal barrier function in DSS-induced colitis in mice and human Caco-2 cells. Sci. Rep. 2018, 8, 12261. [Google Scholar] [CrossRef] [PubMed]
- Do, H.J.; Kim, Y.-S.; Oh, T.W. Effect of Polycan, a β-Glucan from Aureobasidium pullulans SM-2001, on Inflammatory Response and Intestinal Barrier Function in DSS-Induced Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 14773. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Chasaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Poritz, L.S.; Garver, K.I.; Green, C.; Fitzpatrick, L.; Ruggiero, F.; Koltun, W.A. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 2007, 140, 12–19. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Unveiling Colitis: A Journey through the Dextran Sodium Sulfate-induced Model. Inflamm. Bowel Dis. 2024, 30, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Egger, B.; Bajaj-Elliott, M.; Macdonald, T.T.; Inglin, R.; Eysselein, V.E.; Büchler, M.W. Characterisation of acute murine dextran sodium sulphate colitis: Cytokine profile and dose dependency. Digestion 2000, 62, 240–248. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef]
- Bamias, G.; Nyce, M.R.; De La Rue, S.A.; Cominelli, F. New concepts in the pathophysiology of inflammatory bowel disease. Ann. Intern. Med. 2005, 143, 895–904. [Google Scholar] [PubMed]
- Li, B.; Alli, R.; Vogel, P.; Geiger, T. IL-10 Modulates DSS-Induced Colitis Through a Macrophage-Ros-No Axis. Mucosal Immunol. 2014, 7, 869–878. [Google Scholar]
- Neurath, M.F. Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar]
- Liu, J.; Gao, Y.; Zhou, J.; Tang, X.; Wang, P.; Shen, L.; Chen, S. Changes in Serum Inflammatory Cytokine Levels and Intestinal Flora in A Self-Healing Dextran Sodium Sul-fate-Induced Ulcerative Colitis Murine Model. Life Sci. 2020, 263, 118587. [Google Scholar]
- Tavakoli, P.; Vollmer-Conna, U.; Hadzi-Pavlovic, D.; Grimm, M.C. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021, 42, 1603990. [Google Scholar]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory Feedback Control of NF-κB Signalling in Health and Disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [PubMed]
- Ashique, S.; Mishra, N.; Garg, A.; Sibuh, B.Z.; Taneja, P.; Rai, G.; Djearamane, S.; Wong, L.S.; Al-Dayan, N.; Roychoudhury, S.; et al. Recent Updates on Correlation Between Reactive Oxygen Species and Synbiotics for Effective Management of Ulcerative Colitis. Front. Nutr. 2023, 10, 1126579. [Google Scholar]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar]
- Kang, Y.; Park, H.; Choe, B.-H.; Kang, B. The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease. Front. Med. 2022, 9, 848344. [Google Scholar]
- Otte, M.L.; Tamang, R.L.; Papapanagiotou, J.; Ahmad, R.; Dhawan, P.; Singh, A.B. Mucosal healing and inflammatory bowel disease: Therapeutic implications and new targets. World J. Gastroenterol. 2023, 29, 1157–1172. [Google Scholar] [PubMed]
- Tang, X.; de Vos, P. Structure-function effects of different pectin chemistries and its impact on the gastrointestinal immune barrier system. Crit. Rev. Food Sci. Nutr. 2023, 65, 1201–1215. [Google Scholar]
- Zheng, J.; Gong, S.; Han, J. Arabinogalactan Alleviates Lipopolysaccharide-Induced Intestinal Epithelial Barrier Damage through Adenosine Monophosphate-Activated Protein Kinase/Silent Information Regulator 1/Nuclear Factor Kappa-B Signaling Pathways in Caco-2 Cells. Int. J. Mol. Sci. 2023, 24, 15337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural Polysaccharides with Immunomodulatory Activities. Mini-Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, X.; Cao, L.; Ji, J.; Zheng, Q.; Gao, J. Isolation and structural identification of a novel fructan from Radix Codonopsis. J. Carbohydr. Chem. 2020, 39, 163–174. [Google Scholar]
- Wang, D.; Zhang, Y.; Yang, S.; Zhao, D.; Wang, M. A polysaccharide from cultured mycelium of Hericium erinaceus relieves ulcerative colitis by counteracting oxidative stress and improving mitochondrial function. Int. J. Biol. Macromol. 2019, 125, 572–579. [Google Scholar]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar]
- Zhang, X.; Ding, R.; Zhou, Y.; Zhu, R.; Liu, W.; Jin, L.; Yao, W.; Gao, X. Toll-like receptor 2 and Toll-like receptor 4-dependent activation of B cells by a polysaccharide from marine fungus Phoma herbarum YS4108. PLoS ONE 2013, 8, e60781. [Google Scholar]
- Li, M.; Wen, J.; Huang, X.; Nie, Q.; Wu, X.; Ma, W.; Nie, S.; Xie, M. Interaction between polysaccharides and toll-like receptor 4: Primary structural role, immune balance perspective, and 3D interaction model hypothesis. Food Chem. 2022, 374, 131586. [Google Scholar] [PubMed]
- Meldrum, O.W.; Yakubov, G.E.; Gartaula, G.; McGuckin, M.A.; Gidley, M.J. Mucoadhesive functionality of cell wall structures from fruits and grains: Electrostatic and polymer network interactions mediated by soluble dietary polysaccharides. Sci. Rep. 2017, 7, 15794. [Google Scholar]
- Cardoso, V.M.d.O.; Gremião, M.P.D.; Cury, B.S.F. Mucin-polysaccharide interactions: A rheological approach to evaluate the effect of pH on the mucoadhesive properties. Int. J. Biol. Macromol. 2020, 149, 234–245. [Google Scholar]
- Li, Y.; Chang, R.; Zhu, D.; Lu, L.; Gao, Z.; Wu, Y.; Jiang, W.; Yuan, D. Strengthening the diffusion of sodium ions by interactions between gum Arabic and oral mucin. Food Hydrocoll. 2023, 150, 109691. [Google Scholar]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, Y.; Han, Y.; Zhang, X.; Wu, Y.; Lin, J.; Cao, L.; Wu, M.; Zheng, H.; Fang, Y.; et al. Qing Hua Chang Yin ameliorates chronic colitis in mice by inhibiting PERK-ATF4-CHOP pathway of ER stress and the NF-κB signalling pathway. Pharm. Biol. 2024, 62, 607–620. [Google Scholar]
- Seixas, F.L.; Fukuda, D.L.; Turbiani, F.R.; Garcia, P.S.; Petkowicz, C.L.d.O.; Jagadevan, S.; Gimenes, M.L. Extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) by microwave-induced heating. Food Hydrocoll. 2014, 38, 186–192. [Google Scholar]
- Bueno, L.R.; Soley, B.d.S.; Abboud, K.Y.; França, I.W.; da Silva, K.S.; de Oliveira, N.M.T.; Barros, J.S.; Gois, M.B.; Cordeiro, L.M.C.; Maria-Ferreira, D. Protective Effect of Dietary Polysaccharides from Yellow Passion Fruit Peel on DSS-Induced Colitis in Mice. Oxidative Med. Cell. Longev. 2022, 2022, 6298662. [Google Scholar]
- Cordeiro, G.S.; Góis, M.B.; Santos, L.S.; Espírito-Santo, D.A.; Silva, R.T.; Pereira, M.U.; Santos, J.N.; Conceição-Machado, M.E.P.; Deiró, T.C.B.J.; Barreto-Medeiros, J.M. Perinatal and Post-Weaning Exposure to a High-Fat Diet Causes Histomorphometric, Neuroplastic, and Histopathological Changes in the Rat Ileum. J. Dev. Orig. Health Dis. 2023, 14, 231–241. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar]
- Wzorek França Dos Santos, I.; Sauruk da Silva, K.; Regis Bueno, L.; Suzane Schneider, V.; Silva Schiebel, C.; Mulinari Turin de Oliveira, N.; Cristine Malaquias da Silva, L.; Soares Fernandes, E.; Biondaro Gois, M.; Mach Cortes Cordeiro, L.; et al. Polysaccharide Fraction from Campomanesia adamantium and Campomanesia pubescens Attenuates 5-Fluorouracil-Induced Intestinal Mucosal Inflammation in Mice. Nutr. Cancer 2023, 75, 1382–1398. [Google Scholar] [PubMed]

| Animal | C | DSS | STWA (10 mg/kg) | STWA (30 mg/kg) | STWA (100 mg/kg) |
|---|---|---|---|---|---|
| 1 | − | + | + | − | − |
| 2 | − | + | + | − | − |
| 3 | − | + | − | + | + |
| 4 | − | + | + | + | − |
| 5 | − | + | + | − | + |
| 6 | − | + | − | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio de Mello Braga, L.L.; Silva Schiebel, C.; Simão, G.; Sauruk da Silva, K.; dos Santos Maia, M.H.; Vieira Ulysséa Fernardes, A.C.; do Nascimento, G.E.; Cordeiro, L.M.C.; Adel Issa, T.; Gois, M.B.; et al. Type I Arabinogalactan and Methyl-Esterified Homogalacturonan Polysaccharides from Tamarillo (Solanum betaceum cav.) Fruit Pulp Ameliorate DSS-Induced Ulcerative Colitis. Pharmaceuticals 2025, 18, 461. https://doi.org/10.3390/ph18040461
Valerio de Mello Braga LL, Silva Schiebel C, Simão G, Sauruk da Silva K, dos Santos Maia MH, Vieira Ulysséa Fernardes AC, do Nascimento GE, Cordeiro LMC, Adel Issa T, Gois MB, et al. Type I Arabinogalactan and Methyl-Esterified Homogalacturonan Polysaccharides from Tamarillo (Solanum betaceum cav.) Fruit Pulp Ameliorate DSS-Induced Ulcerative Colitis. Pharmaceuticals. 2025; 18(4):461. https://doi.org/10.3390/ph18040461
Chicago/Turabian StyleValerio de Mello Braga, Lara Luisa, Carolina Silva Schiebel, Gisele Simão, Karien Sauruk da Silva, Mateus Henrique dos Santos Maia, Ana Carolina Vieira Ulysséa Fernardes, Georgia E. do Nascimento, Lucimara Mach Côrtes Cordeiro, Tufik Adel Issa, Marcelo Biondaro Gois, and et al. 2025. "Type I Arabinogalactan and Methyl-Esterified Homogalacturonan Polysaccharides from Tamarillo (Solanum betaceum cav.) Fruit Pulp Ameliorate DSS-Induced Ulcerative Colitis" Pharmaceuticals 18, no. 4: 461. https://doi.org/10.3390/ph18040461
APA StyleValerio de Mello Braga, L. L., Silva Schiebel, C., Simão, G., Sauruk da Silva, K., dos Santos Maia, M. H., Vieira Ulysséa Fernardes, A. C., do Nascimento, G. E., Cordeiro, L. M. C., Adel Issa, T., Gois, M. B., Fernandes Soares, E., & Maria-Ferreira, D. (2025). Type I Arabinogalactan and Methyl-Esterified Homogalacturonan Polysaccharides from Tamarillo (Solanum betaceum cav.) Fruit Pulp Ameliorate DSS-Induced Ulcerative Colitis. Pharmaceuticals, 18(4), 461. https://doi.org/10.3390/ph18040461








